Treadmill Exercise Prevents Cognitive Impairments in Adolescent Intermittent Ethanol Rats by Reducing the Excessive Activation of Microglia Cell in the Hippocampus
Abstract
:1. Introduction
2. Results
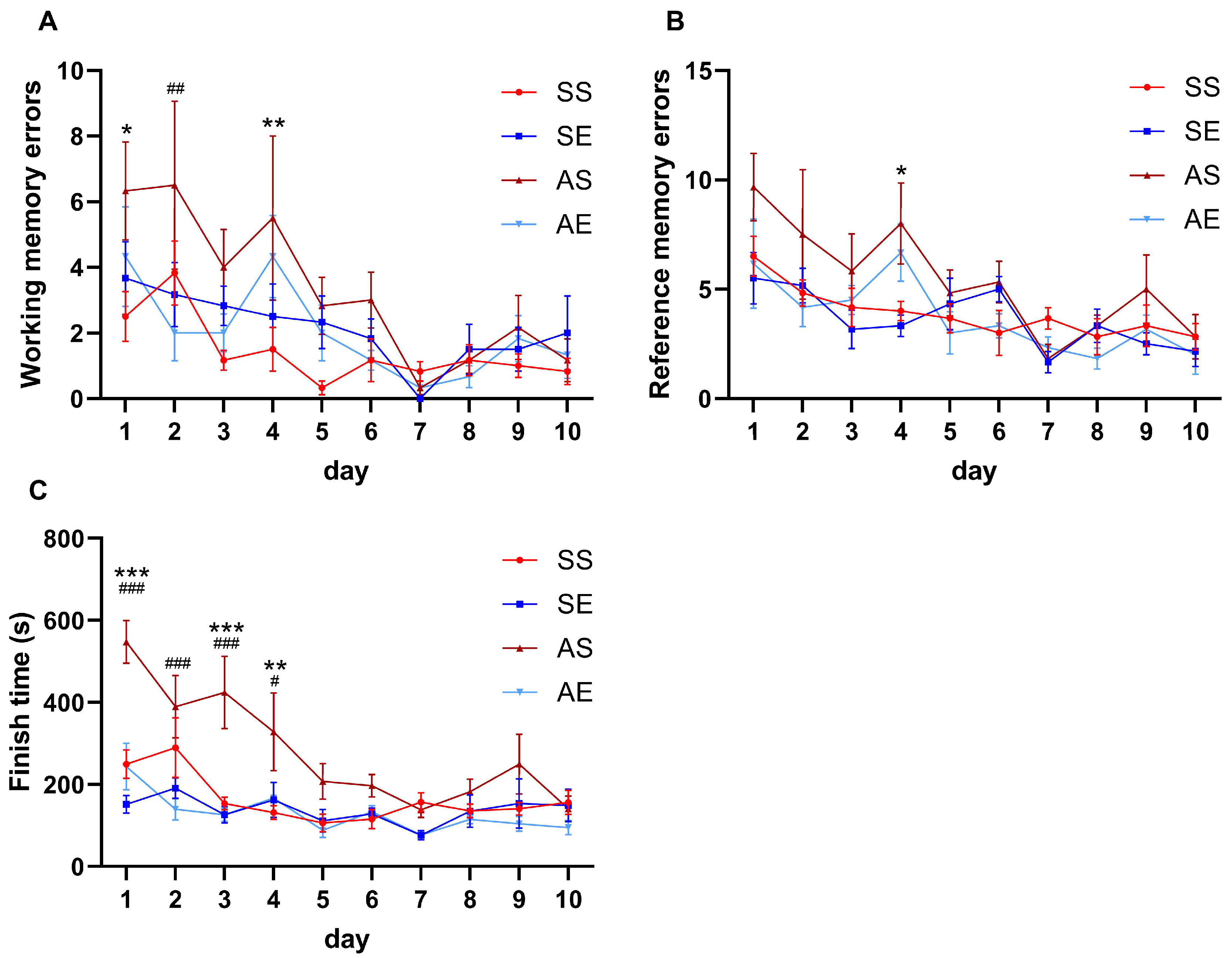
2.1. Treadmill Exercise Prevented AIE-Induced Decline in Spatial Learning and Memory in Young Adult Rats
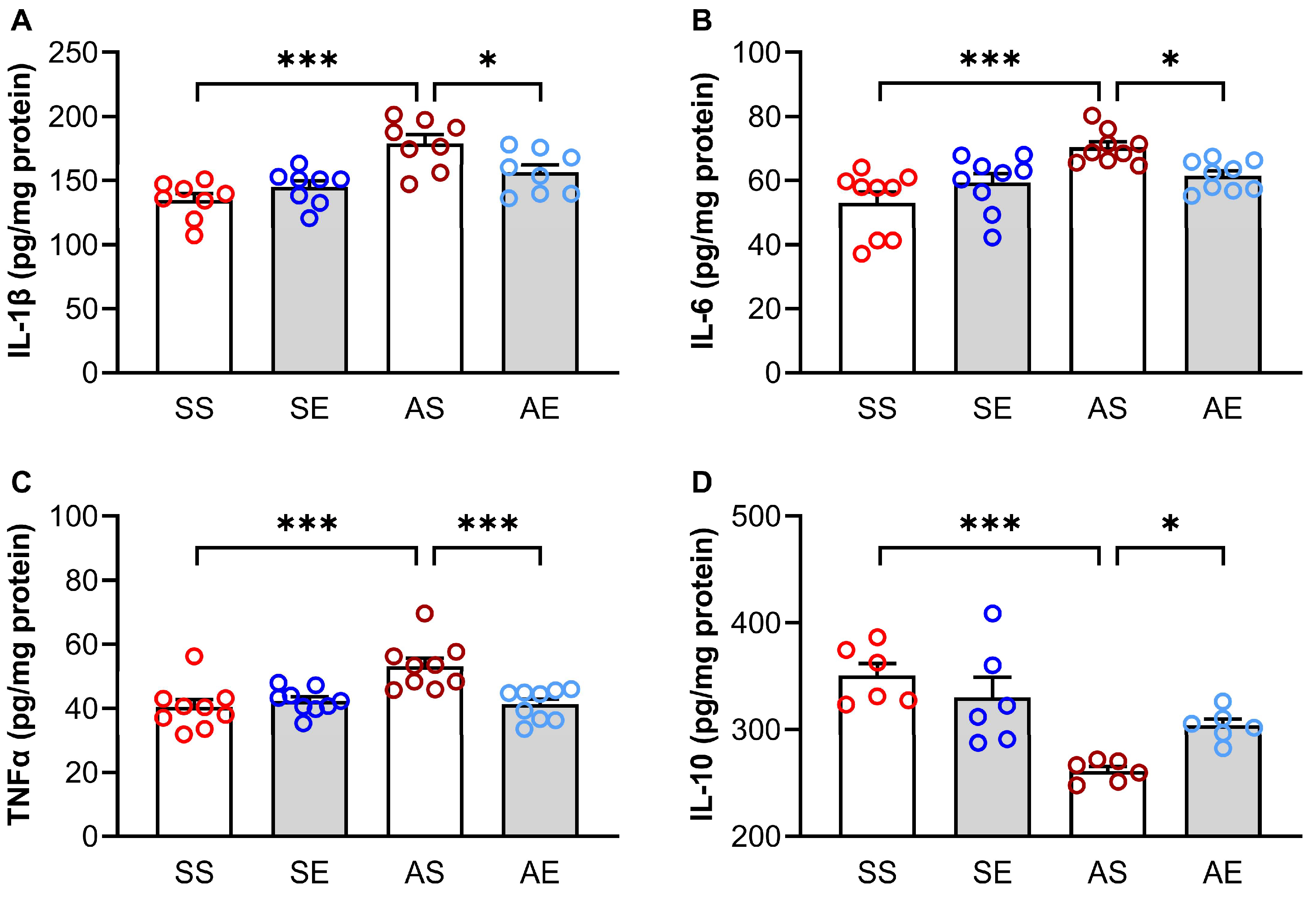
2.2. Treadmill Exercise Reversed AIE-Induced Inflammation in the Hippocampus of Young Adult Rats
2.3. Treadmill Exercise Decreased AIE-Induced Excessive Activation of Microglia in the Hippocampus of Young Adult Rats
2.4. Effects of Treadmill Exercise on the Protein Levels of CB1R, CB2R and MAGL in the Hippocampus of Young Adult Rats
3. Discussion
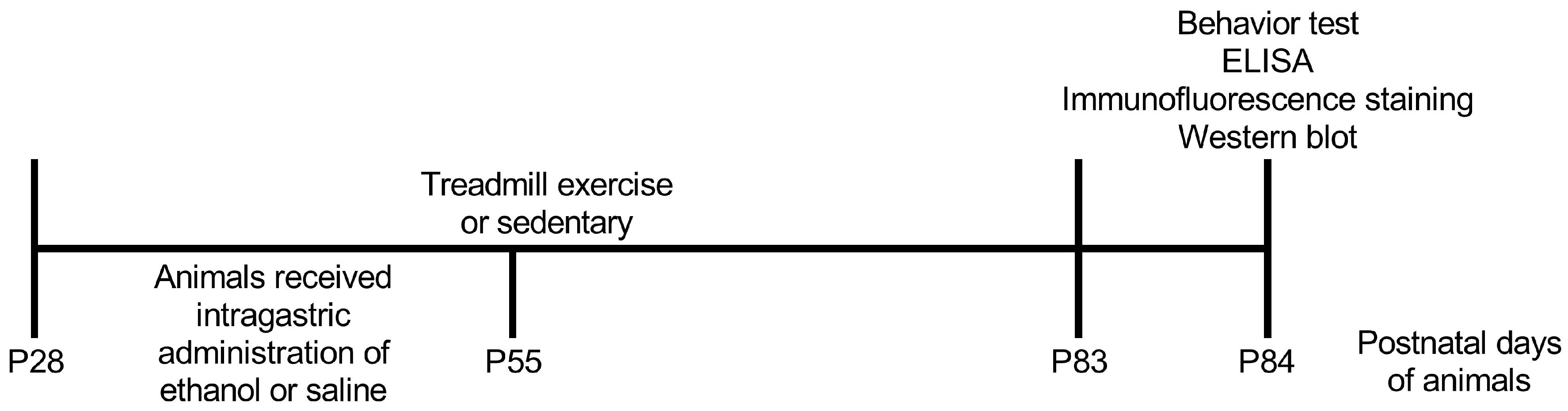
4. Materials and Methods
4.1. Animals
4.2. Adolescent Intermittent Ethanol (AIE) Paradigm
4.3. Treadmill Exercise Protocol
4.4. Eight-Arm Radial Maze Test
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Immunofluorescent Staining
4.7. Western Blots
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections. Nat. Rev. Gastroenterol. Hepatol. 2022; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.A. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar]
- Meda, S.A.; Hawkins, K.A.; Dager, A.D.; Tennen, H.; Khadka, S.; Austad, C.S.; Wood, R.M.; Raskin, S.; Fallahi, C.R.; Pearlson, G.D. Longitudinal effects of alcohol consumption on the hippocampus and parahippocampus in college students. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 610–617. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019.
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef]
- Bava, S.; Tapert, S.F. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol. Rev. 2010, 20, 398–413. [Google Scholar] [CrossRef] [Green Version]
- Crews, F.T.; Vetreno, R.P.; Broadwater, M.A.; Robinson, D.L. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol. Rev. 2016, 68, 1074–1109. [Google Scholar] [CrossRef] [Green Version]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Fontaine, C.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; de Fonseca, F.; Suarez, J.; Barrondo, S.; et al. Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor-dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 2020, 45, 309–318. [Google Scholar] [CrossRef]
- Bohnsack, J.P.; Zhang, H.; Wandling, G.M.; He, D.; Kyzar, E.J.; Lasek, A.W.; Pandey, S.C. Targeted epigenomic editing ameliorates adult anxiety and excessive drinking after adolescent alcohol exposure. Sci. Adv. 2022, 8, eabn2748. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Liput, D.J.; Homanics, G.E.; Lovinger, D.M. Age-dependent impairment of metabotropic glutamate receptor 2-dependent long-term depression in the mouse striatum by chronic ethanol exposure. Alcohol 2020, 82, 11–21. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Nephew, B.C.; Choudhury, A.; Poirier, G.L.; Lim, A.; Mandrekar, P. Chronic alcohol-induced liver injury correlates with memory deficits: Role for neuroinflammation. Alcohol 2020, 83, 75–81. [Google Scholar] [CrossRef]
- Boschen, K.E.; Ruggiero, M.J.; Klintsova, A.Y. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 2016, 324, 355–366. [Google Scholar] [CrossRef]
- Silva-Gotay, A.; Davis, J.; Tavares, E.R.; Richardson, H.N. Alcohol drinking during early adolescence activates microglial cells and increases frontolimbic Interleukin-1 beta and Toll-like receptor 4 gene expression, with heightened sensitivity in male rats compared to females. Neuropharmacology 2021, 197, 108698. [Google Scholar] [CrossRef]
- Gursky, Z.H.; Johansson, J.R.; Klintsova, A.Y. Postnatal alcohol exposure and adolescent exercise have opposite effects on cerebellar microglia in rat. Int. J. Dev. Neurosci. 2020, 80, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Zhu, J.; Song, S.; Huang, Y.; Zhang, W.; Sun, Y.; Hao, J.; Yang, X.; Gao, Q.; et al. Neuroinflammation-mediated mitochondrial dysregulation involved in postoperative cognitive dysfunction. Free Radic. Biol. Med. 2022, 178, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Lawrimore, C.J.; Rowsey, P.J.; Crews, F.T. Persistent adult neuroimmune activation and loss of hippocampal neurogenesis following adolescent ethanol exposure: Blockade by exercise and the anti-inflammatory drug indomethacin. Front. Neurosci. 2018, 12, 200. [Google Scholar] [CrossRef] [Green Version]
- Barnett, A.; David, E.; Rohlman, A.; Nikolova, V.D.; Moy, S.S.; Vetreno, R.P.; Coleman, L.G., Jr. Adolescent binge alcohol enhances early Alzheimer’s disease pathology in adulthood through proinflammatory neuroimmune activation. Front. Pharmacol. 2022, 13, 884170. [Google Scholar] [CrossRef] [PubMed]
- Macht, V.; Vetreno, R.; Elchert, N.; Crews, F. Galantamine prevents and reverses neuroimmune induction and loss of adult hippocampal neurogenesis following adolescent alcohol exposure. J. Neuroinflamm. 2021, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Y.; Gao, M.; Xue, M.; Wang, Z.; Liang, H. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020, 11, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Kalk, N.J.; Guo, Q.; Owen, D.; Cherian, R.; Erritzoe, D.; Gilmour, A.; Ribeiro, A.S.; McGonigle, J.; Waldman, A.; Matthews, P.; et al. Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: A [(11)C]PBR28 PET study. Transl. Psychiatry 2017, 7, e996. [Google Scholar] [CrossRef] [Green Version]
- Nwachukwu, K.N.; King, D.M.; Healey, K.L.; Swartzwelder, H.S.; Marshall, S.A. Sex-specific effects of adolescent intermittent ethanol exposure-induced dysregulation of hippocampal glial cells in adulthood. Alcohol 2022, 100, 31–39. [Google Scholar] [CrossRef]
- Gomez, G.I.; Falcon, R.V.; Maturana, C.J.; Labra, V.C.; Salgado, N.; Rojas, C.A.; Oyarzun, J.E.; Cerpa, W.; Quintanilla, R.A.; Orellana, J.A. Heavy Alcohol Exposure Activates Astroglial Hemichannels and Pannexons in the Hippocampus of Adolescent Rats: Effects on Neuroinflammation and Astrocyte Arborization. Front. Cell. Neurosci. 2018, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar Taklimie, F.; Gasterich, N.; Scheld, M.; Weiskirchen, R.; Beyer, C.; Clarner, T.; Zendedel, A. Hypoxia Induces Astrocyte-Derived Lipocalin-2 in Ischemic Stroke. Int. J. Mol. Sci. 2019, 20, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, G.F.; Jablonski, S.A.; Schiffino, F.L.; St Cyr, S.A.; Stanton, M.E.; Klintsova, A.Y. Exercise and environment as an intervention for neonatal alcohol effects on hippocampal adult neurogenesis and learning. Neuroscience 2014, 265, 274–290. [Google Scholar] [CrossRef] [Green Version]
- Vetreno, R.P.; Patel, Y.; Patel, U.; Walter, T.J.; Crews, F.T. Adolescent intermittent ethanol reduces serotonin expression in the adult raphe nucleus and upregulates innate immune expression that is prevented by exercise. Brain Behav. Immun. 2017, 60, 333–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, G.F.; Criss, K.J.; Klintsova, A.Y. Voluntary exercise partially reverses neonatal alcohol-induced deficits in mPFC layer II/III dendritic morphology of male adolescent rats. Synapse 2015, 69, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Gomes da Silva, S.; Doná, F.; da Silva Fernandes, M.J.; Scorza, F.A.; Cavalheiro, E.A.; Arida, R.M. Physical exercise during the adolescent period of life increases hippocampal parvalbumin expression. Brain Dev. 2010, 32, 137–142. [Google Scholar] [CrossRef]
- Stigger, F.S.; Zago Marcolino, M.A.; Portela, K.M.; Plentz, R.D.M. Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: A systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 616–624. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin. PLoS ONE 2018, 13, e0204500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Baos, A.; Alegre-Zurano, L.; Cantacorps, L.; Martin-Sanchez, A.; Valverde, O. Role of cannabinoids in alcohol-induced neuroinflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110054. [Google Scholar] [CrossRef]
- Sampedro-Piquero, P.; Millon, C.; Moreno-Fernandez, R.D.; Garcia-Fernandez, M.; Diaz-Cabiale, Z.; Santin, L.J. Treadmill exercise buffers behavioral alterations related to ethanol binge-drinking in adolescent mice. Brain Sci. 2020, 10, 576. [Google Scholar] [CrossRef]
- Schulteis, G.; Archer, C.; Tapert, S.F.; Frank, L.R. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol 2008, 42, 459–467. [Google Scholar] [CrossRef]
- Sircar, R.; Basak, A.K.; Sircar, D. Repeated ethanol exposure affects the acquisition of spatial memory in adolescent female rats. Behav. Brain Res. 2009, 202, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macht, V.; Elchert, N.; Crews, F. Adolescent alcohol exposure produces protracted cognitive-behavioral impairments in adult male and female rats. Brain Sci. 2020, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Bohnsack, J.P.; Kusumo, H.; Liu, W.; Pandey, S.C.; Crews, F.T. Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise. Addict. Biol. 2020, 25, e12731. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Cai, J.; Gu, B.; Yu, L.; Li, C.; Liu, Q.S.; Zhao, L. Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3xTg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex. Cells 2022, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Bierlein-De La Rosa, G.; Biggerstaff, N.; Pushpavathi Selvakumar, G.; Wang, R.; Mason, S.; Dailey, M.E.; Marcinkiewcz, C.A. Adolescent ethanol drinking promotes hyperalgesia, neuroinflammation and serotonergic deficits in mice that persist into adulthood. Brain Behav. Immun. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Oppong-Damoah, A.; Gannon, B.M.; Murnane, K.S. The endocannabinoid system and alcohol dependence: Will cannabinoid receptor 2 agonism be more fruitful than cannabinoid receptor 1 antagonism? CNS Neurol. Disord. Drug Targets 2022, 21, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pihlaja, R.; Takkinen, J.; Eskola, O.; Vasara, J.; Lopez-Picon, F.R.; Haaparanta-Solin, M.; Rinne, J.O. Monoacylglycerol lipase inhibitor JZL184 reduces neuroinflammatory response in APdE9 mice and in adult mouse glial cells. J. Neuroinflamm. 2015, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledesma, J.C.; Rodriguez-Arias, M.; Gavito, A.L.; Sanchez-Perez, A.M.; Vina, J.; Medina Vera, D.; Rodriguez de Fonseca, F.; Minarro, J. Adolescent binge-ethanol accelerates cognitive impairment and beta-amyloid production and dysregulates endocannabinoid signaling in the hippocampus of APP/PSE mice. Addict. Biol. 2021, 26, e12883. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Wu, Y.; Wang, D.; Feng, G.; Tang, Y.P.; Teng, Z.; Chen, C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012, 2, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr. Pharm. Des. 2008, 14, 2347–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutierrez, S.O.; van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.; Wen, J.; Li, S.; Zhang, Y. Involvement of ERK1/2, cPLA2 and NF-kappaB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat. 2013, 100–101, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.Y.; Yu, W.B.; Chen, J.; Li, L.; Jiang, L.S.; Xiao, B.G.; Liu, Z.G. Neuroprotective effect is driven through the upregulation of CB1 receptor in experimental autoimmune encephalomyelitis. J. Mol. Neurosci. 2016, 58, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: From pathogenesis to a promising therapeutic target. Front. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aymerich, M.S.; Aso, E.; Abellanas, M.A.; Tolon, R.M.; Ramos, J.A.; Ferrer, I.; Romero, J.; Fernandez-Ruiz, J. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem. Pharmacol. 2018, 157, 67–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecha, M.; Carrillo-Salinas, F.J.; Feliu, A.; Mestre, L.; Guaza, C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol. Ther. 2016, 166, 40–55. [Google Scholar] [CrossRef]
- Desai, S.; Borg, B.; Cuttler, C.; Crombie, K.M.; Rabinak, C.A.; Hill, M.N.; Marusak, H.A. A Systematic review and meta-analysis on the effects of exercise on the endocannabinoid system. Cannabis Cannabinoid Res. 2022, 7, 388–408. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wang, G.W. Effects of treadmill exercise intensity on spatial working memory and long-term memory in rats. Life Sci. 2016, 149, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.G.; Tipton, C.M.; Wilson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various experimental procedures. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Zhao, Z.; Lv, Y.; Gu, B.; Zhao, L. Aerobic exercise regulates synaptic transmission and reactive oxygen species production in the paraventricular nucleus of spontaneously hypertensive rats. Brain Res. 2019, 1712, 82–92. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yan, M.; Li, L.; Zhao, L.; Li, Y. Treadmill Exercise Prevents Cognitive Impairments in Adolescent Intermittent Ethanol Rats by Reducing the Excessive Activation of Microglia Cell in the Hippocampus. Int. J. Mol. Sci. 2022, 23, 14701. https://doi.org/10.3390/ijms232314701
Guo Y, Yan M, Li L, Zhao L, Li Y. Treadmill Exercise Prevents Cognitive Impairments in Adolescent Intermittent Ethanol Rats by Reducing the Excessive Activation of Microglia Cell in the Hippocampus. International Journal of Molecular Sciences. 2022; 23(23):14701. https://doi.org/10.3390/ijms232314701
Chicago/Turabian StyleGuo, Yanxia, Min Yan, Li Li, Li Zhao, and Yan Li. 2022. "Treadmill Exercise Prevents Cognitive Impairments in Adolescent Intermittent Ethanol Rats by Reducing the Excessive Activation of Microglia Cell in the Hippocampus" International Journal of Molecular Sciences 23, no. 23: 14701. https://doi.org/10.3390/ijms232314701




