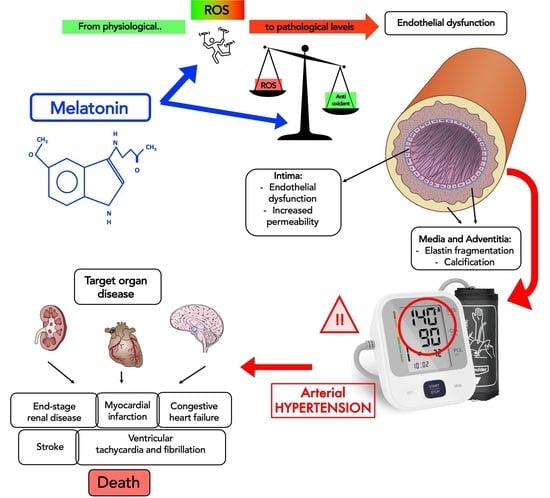
Essential Hypertension and Oxidative Stress: Novel Future Perspectives
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
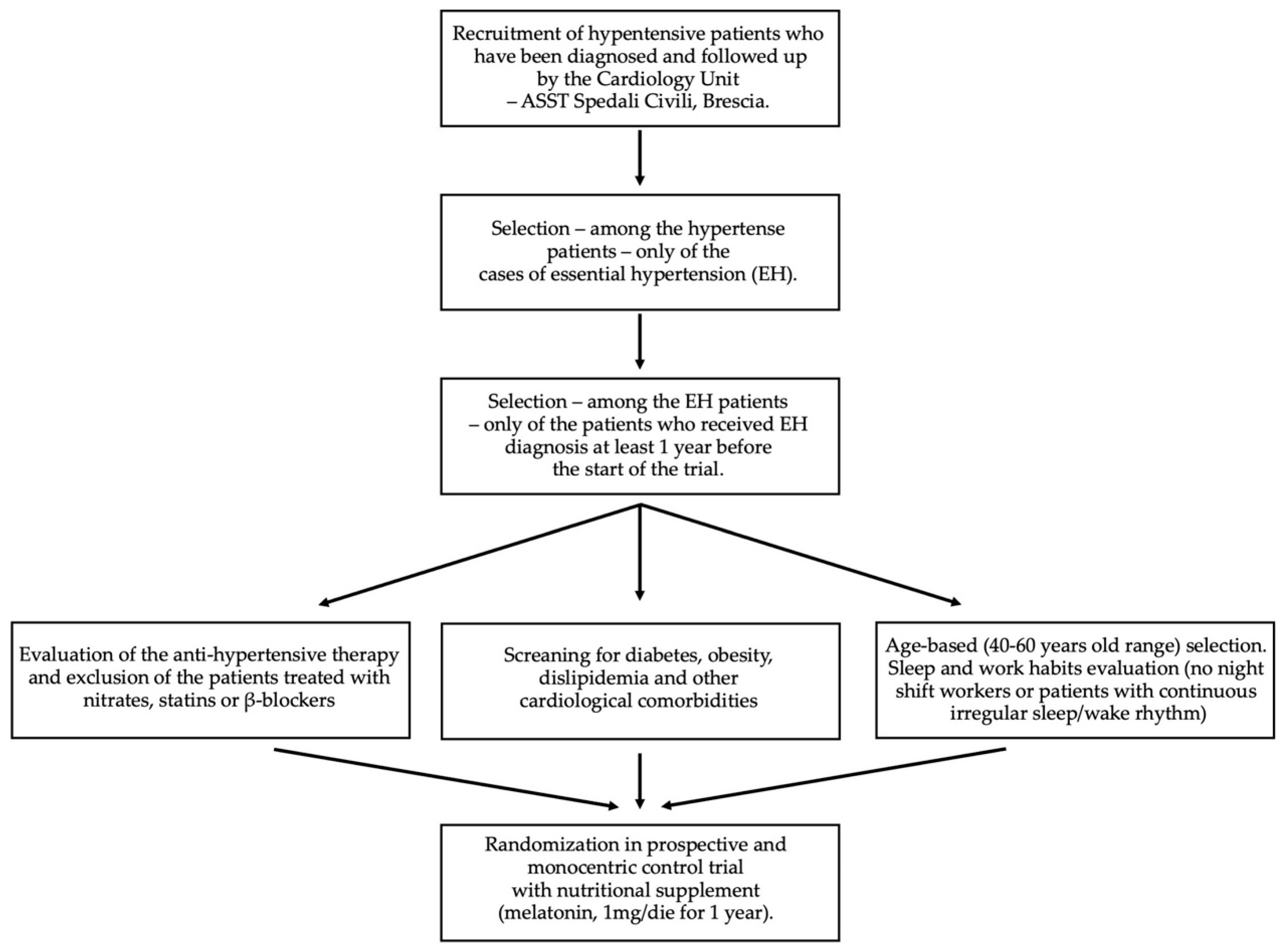
4.1. Study Design
- -
- Any kind of heart disease (e.g., angina pectoris, myocardial infarction, coronary revascularization, congestive heart failure, aortic coarctation);
- -
- Autoimmune, rheumatological, or vascular diseases other than EH;
- -
- Antihypertensive therapies with nitrates, statins, or β-blockers;
- -
- Pregnancy and lactation;
- -
- Obesity;
- -
- Diabetes;
- -
- Irregular sleep/wake rhythm, such as workers with night shifts (for a period of less than 3 months before recruitment).
- -
- A diagnosis of essential hypertension dating back at least 1 year before the start of the study;
- -
- Treatment with antihypertensive drugs already in progress and not modifiable during the course of the study;
- -
- No other cardiological (i.e., dyslipidaemia, heart failure, atrial fibrillation) and metabolic (i.e., diabetes) comorbidities;
- -
- No pregnancy in progress;
- -
- Regular sleep/wake rhythm (no worker with night shifts for a period of less than 3 months before recruitment);
- -
- Fasting blood sugar < 100 mg/dL;
- -
- Total cholesterol < 200 mg/dL and triglycerides < 150 mg/dL;
4.2. Blood Pressure Measurement
4.3. Circulating Total Antioxidant Capacity Levels
4.4. Cardiovascular Evaluation
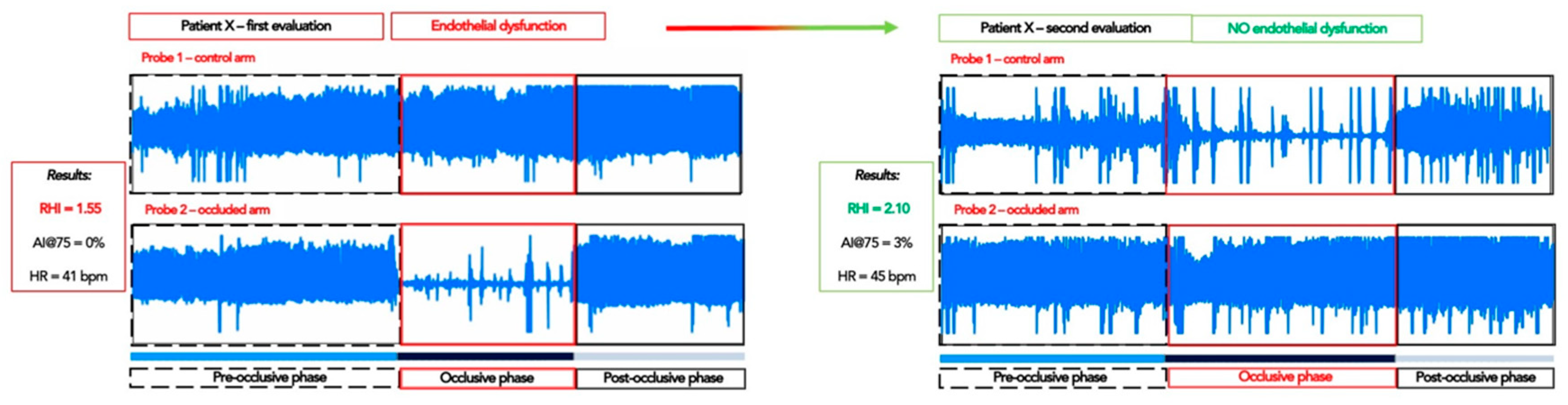
4.4.1. Peripheral Arterial Tonometry: Assessment of the Endothelial Function
4.4.2. SphygmoCor System Evaluation: Study of the Pulse Wave Velocity
4.5. Follow Up Evaluation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maleki, B.; Alani, B.; Zadeh, S.S.T.; Saadat, S.; Rajabi, A.; Ayoubzadeh, S.M.J.; Verdi, J.; Farrokhian, A.; Ghanbarian, H.; Noureddini, M.; et al. MicroRNAs and exosomes: Cardiac stem cells in heart diseases. Pathol.-Res. Pract. 2022, 229, 153701. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Blood Pressure. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 25 August 2021).
- World Health Organization (WHO). Raised Blood Pressure. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 25 August 2021).
- World Health Organization (WHO). A Global Brief on Hypertension. 2013. Available online: www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ (accessed on 10 January 2014).
- Baker, J.; Kimpinski, K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018, 45, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Olczak, K.J.; Taylor-Bateman, V.; Nicholls, H.L.; Traylor, M.; Cabrera, C.P.; Munroe, P.B. Hypertension genetics past, present and future applications. J. Intern. Med. 2021, 290, 1130–1152. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Wickramasinghe, K.; Wilkins, E.; Townsend, N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016, 102, 1945–1952. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Jamal, S.F. Essential Hypertension. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mensah, G.A.; Croft, J.B.; Giles, W.H. The heart, kidney, and brain as target organs in hypertension. Curr. Probl. Cardiol. 2003, 28, 156–193. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Bahammam, A.S.; Ojike, N.I.; Akinseye, O.A.; Kendzerska, T.; Buttoo, K.; Dhandapany, P.S.; Brown, G.M.; Cardinali, D.P. Melatonin and Human Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 122–132. [Google Scholar] [CrossRef]
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986. [Google Scholar] [CrossRef]
- Chen, S.; Chen, R.; Zhang, T.; Shaowei, L.; Zhou, C.; Bi, Z.; Huangyuan, L.; Siying, W. Relationship of cardiovascular disease risk factors and noncoding RNAs with hypertension: A case-control study. BMC Cardiovasc. Disord. 2018, 18, 58. [Google Scholar] [CrossRef]
- Gavrilova, A.; Bandere, D.; Rutkovska, I.; Šmits, D.; Mauriņa, B.; Poplavska, E.; Urtāne, I. Knowledge about Disease, Medication Therapy, and Related Medication Adherence Levels among Patients with Hypertension. Medicina 2019, 55, 715. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef]
- Mullins, L.J.; Bailey, M.A.; Mullins, J.J. Hypertension, Kidney, and Transgenics: A Fresh Perspective. Physiol. Rev. 2006, 86, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, J.J. Essential hypertension: An approach to its etiology and neurogenic pathophysiology. Int. J. Hypertens. 2013, 2013, 547809. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Sawamura, T. Endothelin and endothelial dysfunction. Proc. Jpn. Acad. Ser. B 2006, 82, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [PubMed]
- Climie, R.E.; Gallo, A.; Picone, D.; Di Lascio, N.; Van Sloten, T.T.; Guala, A.; Mayer, C.C.; Hametner, B.; Bruno, R.M. Measuring the Interaction Between the Macro- and Micro-Vasculature. Front. Cardiovasc. Med. 2019, 6, 169. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Van Buchem, M.A.; Sigurdsson, S.; Gotal, J.D.; Jónsdóttir, M.K.; Kjartansson, Ó.; Garcia, M.; Aspelund, T.; Harris, T.B.; Gudnason, V.; et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility–Reykjavik Study. Brain 2011, 134 Pt 11, 3398–3407. [Google Scholar] [CrossRef]
- González, J.; Valls, N.; Brito, R.; Rodrigo, R. Essential hypertension and oxidative stress: New insights. World J. Cardiol. 2014, 6, 353–366. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Ramprasath, T.; Zou, M.-H. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid. Redox Signal. 2021, 34, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Lamirault, G.; Artifoni, M.; Daniel, M.; Barber-Chamoux, N. Nantes University Hospital Working Group On Hypertension. Resistant Hypertension: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 61–72. [Google Scholar] [CrossRef]
- Parati, G.; Kjeldsen, S.; Coca, A.; Cushman, W.C.; Wang, J. Adherence to Single-Pill Versus Free-Equivalent Combination Therapy in Hypertension: A Systematic Review and Meta-Analysis. Hypertension 2021, 77, 692–705. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef]
- Favero, G.; Moretti, E.; Bonomini, F.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Promising Antineoplastic Actions of Melatonin. Front. Pharmacol. 2018, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-J.; Zhong, C.-B.; Wu, W. Cardioprotective effects of melatonin: Focusing on its roles against diabetic cardiomyopathy. Biomed. Pharmacother. 2020, 128, 110260. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F. Potential Use of Melatonin as Adjunct Antihypertensive Therapy. Am. J. Hypertens. 2005, 18 Pt 1, 1619–1620. [Google Scholar] [CrossRef]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and Central Effects of Melatonin on Blood Pressure Regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef]
- Simko, F.; Paulis, L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007, 42, 319–322. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 1409. [Google Scholar] [CrossRef]
- Tembo, M.C.; Holloway-Kew, K.L.; Bortolasci, C.C.; Sui, S.X.; Brennan-Olsen, S.L.; Williams, L.J.; Kotowicz, M.A.; Pasco, J.A. Total Antioxidant Capacity and Frailty in Older Men. Am. J. Men’s Health 2020, 14, 1557988320946592. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Bromfield, S.; Muntner, P. High Blood Pressure: The Leading Global Burden of Disease Risk Factor and the Need for Worldwide Prevention Programs. Curr. Hypertens. Rep. 2013, 15, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Ghaedi, E.; Moradi, S.; Pourmasoumi, M.; Ghavami, A.; Kafeshani, M. Effects of Melatonin Supplementation On Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2019, 51, 157–164. [Google Scholar] [CrossRef]
- Azedi, F.; Tavakol, S.; Ketabforoush, A.H.M.E.; Khazaei, G.; Bakhtazad, A.; Mousavizadeh, K.; Joghataei, M.T. Modulation of autophagy by melatonin via sirtuins in stroke: From mechanisms to therapies. Life Sci. 2022, 307, 120870. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Zhang, S.; Wang, Y.; Liu, Z.; Liu, Z.; Zhou, Y.; Zhou, H.; Xu, X.; Li, Z.; et al. Melatonin improves cardiac remodeling and brain-heart sympathetic hyperactivation aggravated by light disruption after myocardial infarction. J. Pineal Res. 2022, 73, e12829. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.-X. Neurotoxins: Free Radical Mechanisms and Melatonin Protection. Curr. Neuropharmacol. 2010, 8, 194–210. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Fuentes-Broto, L. Melatonin: A Multitasking Molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; Nadruz, W., Jr.; Mónica, F.Z. Endothelial and vascular smooth muscle dysfunction in hypertension. Biochem. Pharmacol. 2022, 205, 115263. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2022, 34, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Orabona, R.; Sciatti, E.; Vizzardi, E.; Prefumo, F.; Bonadei, I.; Valcamonico, A.; Metra, M.; Lorusso, R.; Ghossein-Doha, C.; Spaanderman, M.E.A.; et al. Inappropriate left ventricular mass after preeclampsia: Another piece of the puzzle Inappropriate LVM and PE. Hypertens. Res. 2019, 42, 522–529. [Google Scholar] [CrossRef]
- Teren, A.; Beutner, F.; Wirkner, K.; Löffler, M.; Scholz, M. Relationship Between Determinants of Arterial Stiffness Assessed by Diastolic and Suprasystolic Pulse Oscillometry: Comparison of Vicorder and Vascular Explorer. Medicine 2016, 95, e2963. [Google Scholar] [CrossRef] [PubMed]
- Orabona, R.; Sciatti, E.; Vizzardi, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Elastic properties of ascending aorta in women with previous pregnancy complicated by early- or late-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2016, 47, 316–323. [Google Scholar] [CrossRef]
- Orabona, R.; Sciatti, E.; Vizzardi, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Endothelial dysfunction and vascular stiffness in women with a previous pregnancy complicated by early or late pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 49, 116–123. [Google Scholar] [CrossRef]
- Sciatti, E.; Orabona, R.; Prefumo, F.; Vizzardi, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Elastic properties of ascending aorta and ventricular–arterial coupling in women with previous pregnancy complicated by HELLP syndrome. J. Hypertens. 2019, 37, 356–364. [Google Scholar] [CrossRef]
- Vizzardi, E.; Sciatti, E.; Bonadei, I.; Menotti, E.; Prati, F.; Scodro, M.; Dallapellegrina, L.; Berlendis, M.; Poli, P.; Padoan, R.; et al. Elastic aortic properties in cystic fibrosis adults without cardiovascular risk factors: A case-control study. Echocardiography 2019, 36, 1118–1122. [Google Scholar] [CrossRef]
- Cagnacci, A.; Arangino, S.; Angiolucci, M.; Melis, G.B.; Facchinetti, F.; Malmusi, S.; Volpe, A. Effect of exogenous melatonin on vascular reactivity and nitric oxide in postmenopausal women: Role of hormone replacement therapy. Clin. Endocrinol. 2001, 54, 261–266. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Calle, M.; Rodríguez-Rodríguez, P.; Pablo, Á.L.L.; López-Giménez, M.R.; Aguilera, Y.; Martín-Cabrejas, M.A.; González, M.D.C.; Arribas, S.M. Maternal Antioxidant Status in Early Pregnancy and Development of Fetal Complications in Twin Pregnancies: A Pilot Study. Antioxidants 2020, 9, 269. [Google Scholar] [CrossRef]
- Galiñanes, M.; Casós, K.; Blasco-Lucas, A.; Permanyer, E.; Máñez, R.; Le Tourneau, T.; Barquinero, J.; Schwartz, S.; Bottio, T.; Roussel, J.C.; et al. Oxidative Stress in Structural Valve Deterioration: A Longitudinal Clinical Study. Biomolecules 2022, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Waśkiewicz, A.; Witkowska, A.M.; Cicha-Mikołajczyk, A.; Zujko, K.; Drygas, W. Dietary Total Antioxidant Capacity—A New Indicator of Healthy Diet Quality in Cardiovascular Diseases: A Polish Cross-Sectional Study. Nutrients 2022, 14, 3219. [Google Scholar] [CrossRef] [PubMed]
- Sciatti, E.; Bernardi, N.; Dallapellegrina, L.; Valentini, F.; Fabbricatore, D.; Scodro, M.; Cotugno, A.; Alonge, M.; Munari, F.; Zanini, B.; et al. Evaluation of systo-diastolic cardiac function and arterial stiffness in subjects with new diagnosis of coeliac disease without cardiovascular risk factors. Intern. Emerg. Med. 2020, 15, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Proenca, M.; Bonnier, G.; Ferrario, D.; Verjus, C.; Lemay, M. PPG-Based Blood Pressure Monitoring by Pulse Wave Analysis: Calibration Parameters are Stable for Three Months. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2019, 2019, 5560–5563. [Google Scholar] [CrossRef]
- Pauca, A.L.; O’Rourke, M.F.; Kon, N.D. Prospective Evaluation of a Method for Estimating Ascending Aortic Pressure From the Radial Artery Pressure Waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef]
- Butlin, M.; Qasem, A. Large Artery Stiffness Assessment Using SphygmoCor Technology. Pulse 2017, 4, 180–192. [Google Scholar] [CrossRef]
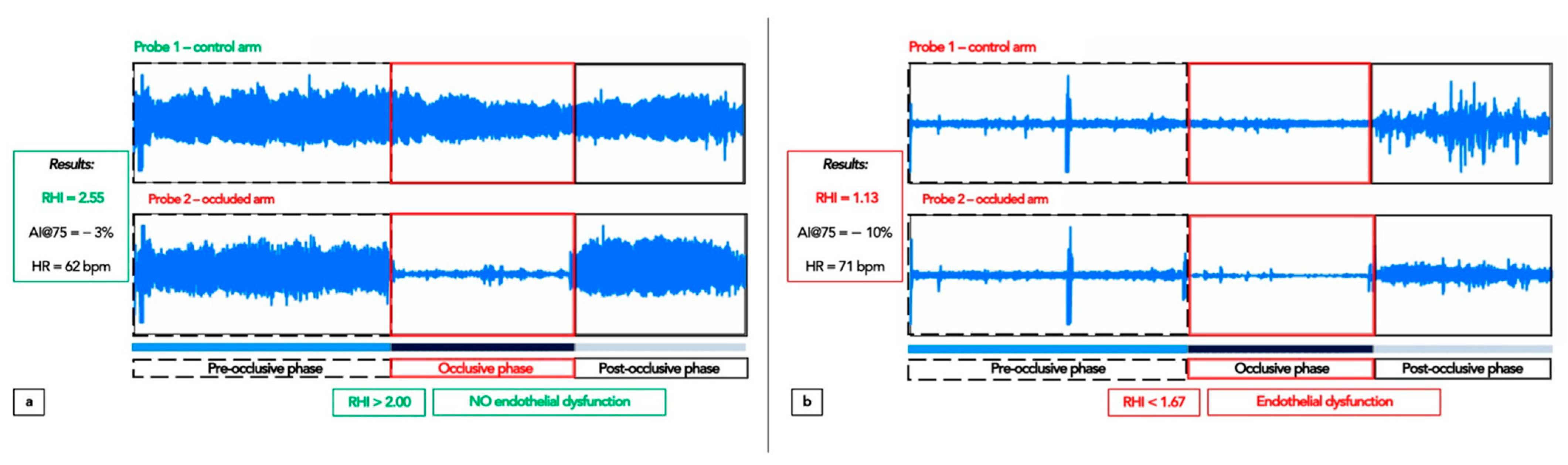
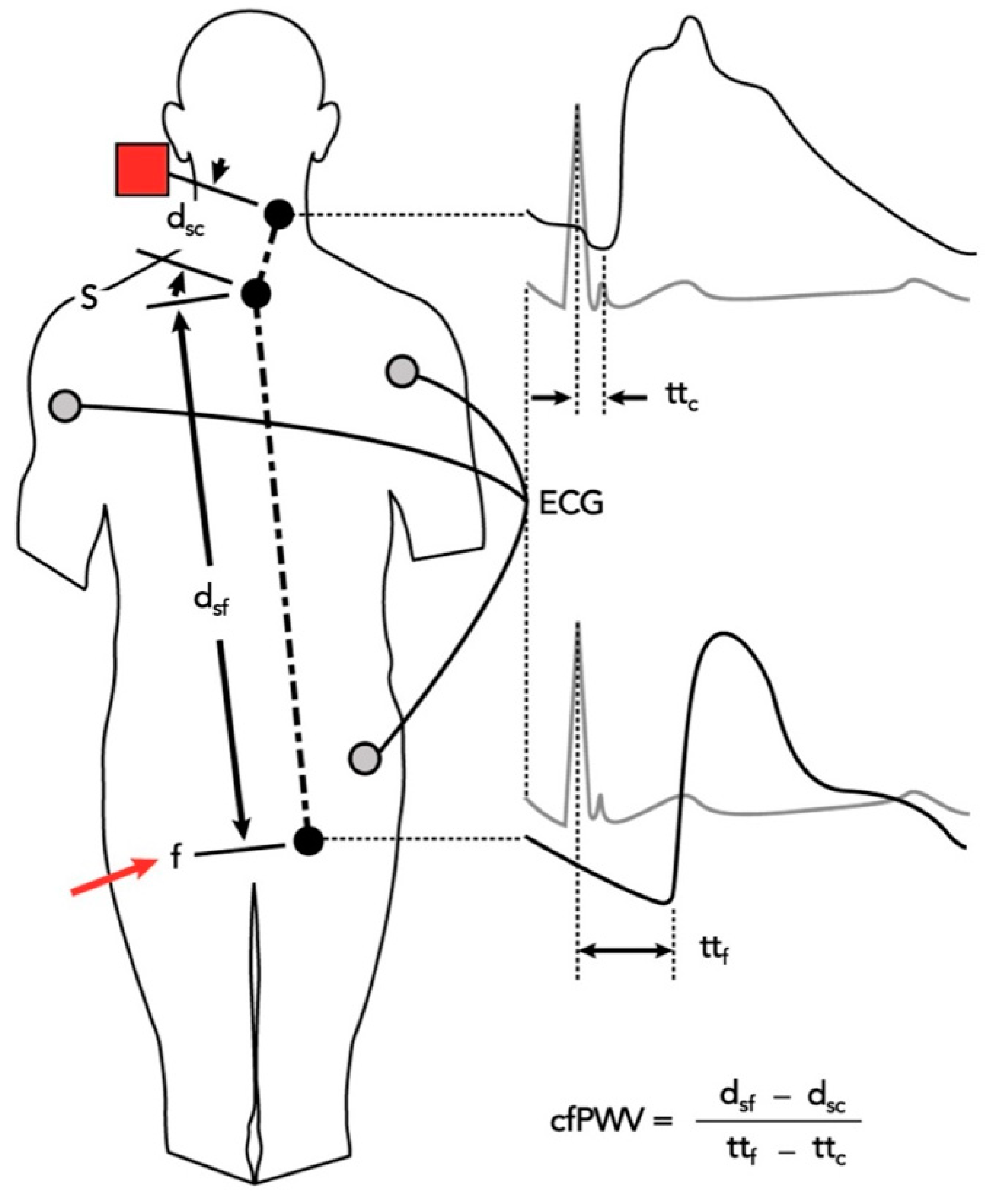
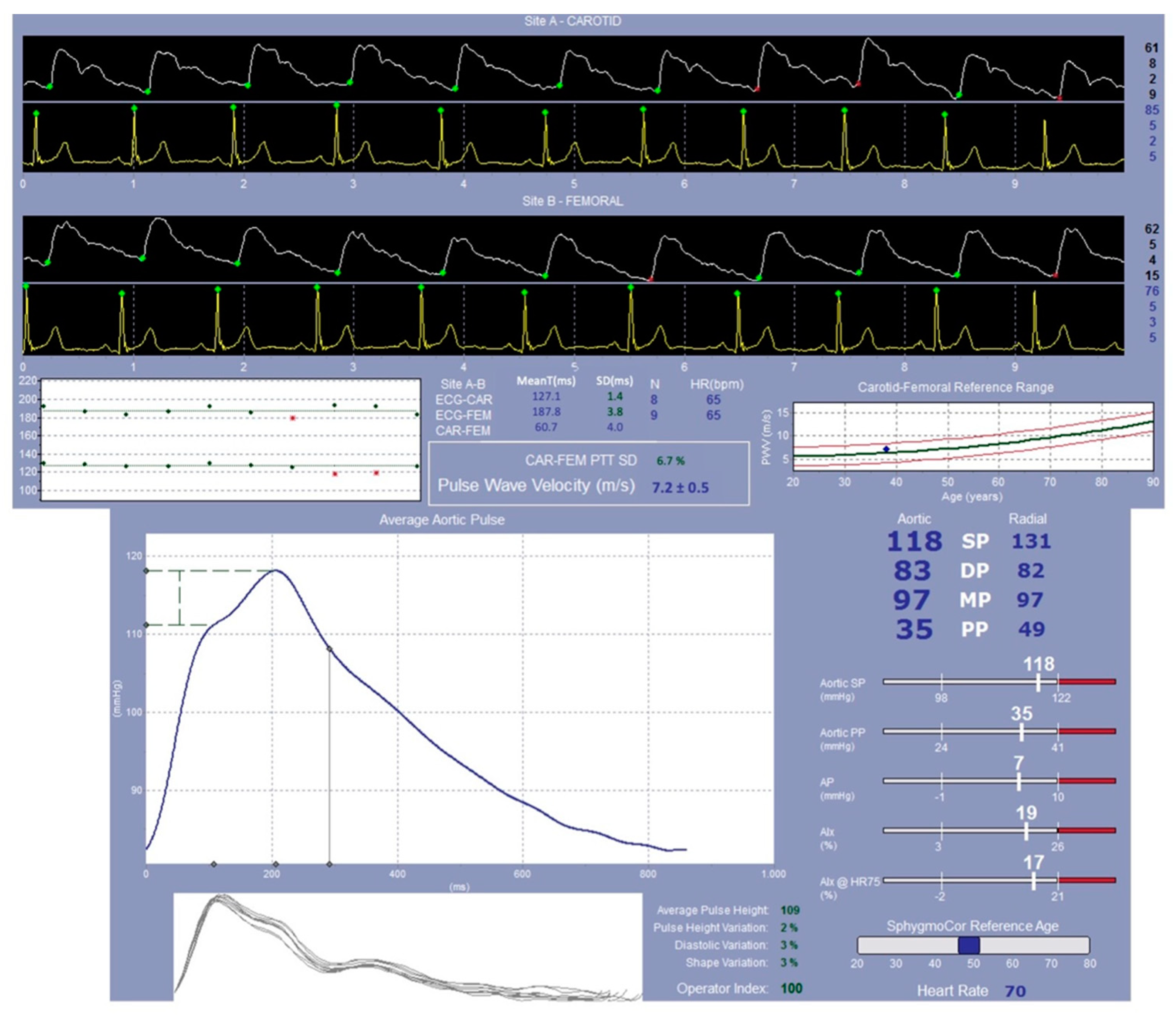
| Characteristics | Melatonin T0 (n = 16) | Control T0 (n = 7) | Intergroup ANOVA (p) |
|---|---|---|---|
| Age at the time of recruitment—T0 (years) | 53 ± 9 | 48 ± 6 | 0.089 |
| Sex | F: 10 (63%) M: 6 (37%) | F: 3 (42%) M: 4 (77%) | 0.0650 |
| BMI (Kg/m2) | 26 ± 4 | 25 ± 3 | 0.452 |
| SBP (mmHg) | 133 ± 10 | 128 ± 5 | 0.278 |
| DBP (mmHg) | 83 ± 7 | 84 ± 8 | 0.820 |
| HR (bpm) | 67 ± 12 | 71 ± 7 | 0.680 |
| Variable | Melatonin T0 (n = 15) | Control T0 (n = 7) | Intergroup ANOVA (p) |
|---|---|---|---|
| TAC (CRE) | 1.49 ± 0.53 | 1.40 ± 0.66 | 1.000 |
| RHI RHI ≤ 1.67 RHI ≤ 2.00 | 1.86 ± 0.71 7 (46.7%) 8 (53.3%) | 2.00 ± 0.58 1 (14.3%) 3 (42.9%) | 0.731 0.193 1.000 |
| Aix@75 (%) | 12.33 ± 16.38 | 9.43 ± 17.98 | 0.731 |
| Aix@75 ≥ 17% | 6 (40.0%) | 2 (28.6%) | 1.000 |
| SBP (mmHg) | 133 ± 10 | 128 ± 5 | 0.278 |
| DBP (mmHg) | 83 ± 7 | 84 ± 8 | 0.820 |
| MAP (mmHg) | 104 ± 9 | 101 ± 8 | 0.308 |
| PP (mmHg) | 49 ± 6 | 44 ± 6 | 0.154 |
| HR (bpm) | 67 ± 12 | 71 ± 7 | 0.680 |
| SBPao (mmHg) | 127 ± 12 | 120 ± 6 | 0.175 |
| DBPao (mmHg) | 90 ± 12 | 86 ± 8 | 0.413 |
| PPao (mmHg) | 36 ± 7 | 36 ± 5 | 0.802 |
| APao (mmHg) | 10 ± 8 | 12 ± 5 | 0.680 |
| Aix@75 (%) | 26.08 ± 19.84 | 23.67 ± 11.23 | 0.368 |
| Aix@75 ≥ 35% | 7 (50.00%) | 1 (16.7%) | 0.325 |
| cfPWV (m/s) | 5.36 ± 2.65 | 8.91 ± 10.13 | 0.588 |
| cfPWV > 9.6 m/s | 0 (0.00%) | 1 (14.7%) | 0.350 |
| Variable | Melatonin T1 (n = 9) | Control T1 (n = 6) | Intergroup ANOVA (p) |
|---|---|---|---|
| TAC (CRE) | 0.92 ± 0.39 | 1.50 ± 0.69 | 0.885 |
| RHI RHI ≤ 1.67 RHI ≤ 2.00 | 1.54 ± 0.67 6 (60.0%) 6 (60.0%) | 1.81 ± 0.32 1 (33.3%) 2 (66.7%) | 0.573 0.559 1.000 |
| Aix@75 (%) | 5.30 ± 11.09 | −4.67 ± 21.50 | 0.469 |
| Aix@75 ≥ 17% | 2 (20.0%) | 1 (33.3%) | 1.000 |
| SBP (mmHg) | 129 ± 14 | 133 ± 22 | 0.600 |
| DBP (mmHg) | 86 ± 6 | 92 ± 2 | 0.145 |
| MAP (mmHg) | 102 ± 9 | 101 ± 16 | 0.921 |
| PP (mmHg) | 42 ± 10 | 47 ± 11 | 0.497 |
| HR (bpm) | 71 ± 13 | 62 ± 9 | 0.373 |
| SBPao (mmHg) | 120 ± 14 | 121 ± 19 | 0.776 |
| DBPao (mmHg) | 87 ± 7 | 86 ± 11 | 1.000 |
| PPao (mmHg) | 33 ± 9 | 35 ± 8 | 0.497 |
| APao (mmHg) | 8 ± 5 | 8 ± 1 | 0.630 |
| Aix@75 (%) | 23.88 ± 10.02 | 16.67 ± 5.03 | 0.133 |
| Aix@75 ≥ 35% | - | - | - |
| cfPWV (m/s) | 6.20 ± 2.95 | 1.8 * | 0.500 |
| cfPWV > 9.6 m/s | - | - | - |
| Variable | Melatonin T0 | Melatonin T1 | Intergroup ANOVA (p) |
|---|---|---|---|
| TAC (CRE) | 1.41 ± 0.61 | 0.92 ± 0.39 | 0.041 |
| RHI RHI ≤ 1.67 RHI ≤ 2.00 | 1.97 ± 0.74 7 (46.7%) 8 (53.3%) | 1.54 ± 0.67 6 (60.0%) 6 (69.0%) | 0.241 0.688 1.000 |
| Aix@75 (%) | 12.40 ± 18.43 | 5.30 ± 11.09 | 0.020 |
| Aix@75 ≥ 17% | 6 (40.0%) | 2 (20.0%) | 0.402 |
| SBP (mmHg) | 135 ± 10 | 129 ± 14 | 0.401 |
| DBP (mmHg) | 86 ± 6 | 86 ± 6 | 1.000 |
| MAP (mmHg) | 104 ± 9 | 102 ± 9 | 0.779 |
| PP (mmHg) | 48 ± 10 | 42 ± 10 | 0.204 |
| HR (bpm) | 73 ± 11 | 71 ± 13 | 0.944 |
| SBPao (mmHg) | 125 ± 9 | 120 ± 14 | 0.484 |
| DBPao (mmHg) | 89 ± 11 | 87 ± 7 | 0.799 |
| PPao (mmHg) | 35 ± 8 | 33 ± 9 | 0.674 |
| APao (mmHg) | 9 ± 8 | 9 ± 5 | 1.000 |
| Aix@75 (%) | 26.57 ± 21.07 | 23.43 ± 10.73 | 0.345 |
| Aix@75 ≥ 35% | 7 (50.0%) | 0 (0.0%) | 0.022 # |
| cfPWV (m/s) | 4.60 ± 2.90 | 6.33 ± 3.20 | 0.345 |
| cfPWV > 9.6 m/s | - | - | - |
| Variable | Control T0 | Control T1 | Intergroup ANOVA (p) |
|---|---|---|---|
| TAC (CRE) | 1.63 ± 0.34 | 1.50 ± 0.68 | 0.539 |
| RHI RHI ≤ 1.67 RHI ≤ 2.00 | 2.19 ± 0.30 1 (14.3%) 3 (42.0%) | 1.81 ± 0.32 1 (33.3%) 2 (66.7%) | 0.109 1.000 1.000 |
| Aix@75 (%) | −1.33 ± 17.55 | −4.67 ± 21.50 | 0.180 |
| Aix@75 ≥ 17% | 2 (28.6%) | 1 (33.3%) | 1.000 |
| SBP (mmHg) | 131 ± 8 | 133 ± 2 | 0.655 |
| DBP (mmHg) | 88 ± 10 | 92 ± 2 | 0.593 |
| MAP (mmHg) | 105 ± 13 | 101 ± 16 | 1.000 |
| PP (mmHg) | 43 ± 2 | 47 ± 11 | 0.414 |
| HR (bpm) | 68 ± 7 | 62 ± 9 | 0.109 |
| SBPao (mmHg) | 122 ± 10 | 121 ± 9 | 1.000 |
| DBPao (mmHg) | 89 ± 11 | 86 ± 11 | 1.000 |
| PPao (mmHg) | 32 ± 2 | 35 ± 8 | 0.414 |
| APao (mmHg) | 10 ± 2 | 8 ± 1 | 0.414 |
| Aix@75 (%) | 27.33 ± 9.07 | 16.67 ± 5.03 | 0.109 |
| Aix@75 ≥ 35% | 1 (16.7%) | 0 (0.0%) | 1.000 |
| cfPWV (m/s) | 6.90 # | 1.80 # | 0.317 |
| cfPWV > 9.6 m/s | 1 (14.3%) | 0 (0.0%) | 1.000 |
| Variable | RHO Correlation | Intergroup ANOVA (p) |
|---|---|---|
| ΔRHI/ΔAix EndoPAT | 0.416 | 0.204 |
| ΔRHI/ΔAix SphygmoCor | 0.299 | 0.471 |
| ΔRHI/ΔPWV | 0.800 | 0.200 |
| ΔRHI/ΔTAC (CRE) | 0.442 | 0.174 |
| ΔAix EndoPAT/ΔAix SphygmoCor | −0.571 | 0.139 |
| ΔAix EndoPAT/ΔPWV | −0.800 | 0.200 |
| ΔAix EndoPAT/ΔTAC (CRE) | 0.483 | 0.132 |
| ΔAix EndoPAT/ΔMelatonin | 0.232 | 0.658 |
| ΔAix SphygmoCor/ΔPWV | −0.158 | 0.663 |
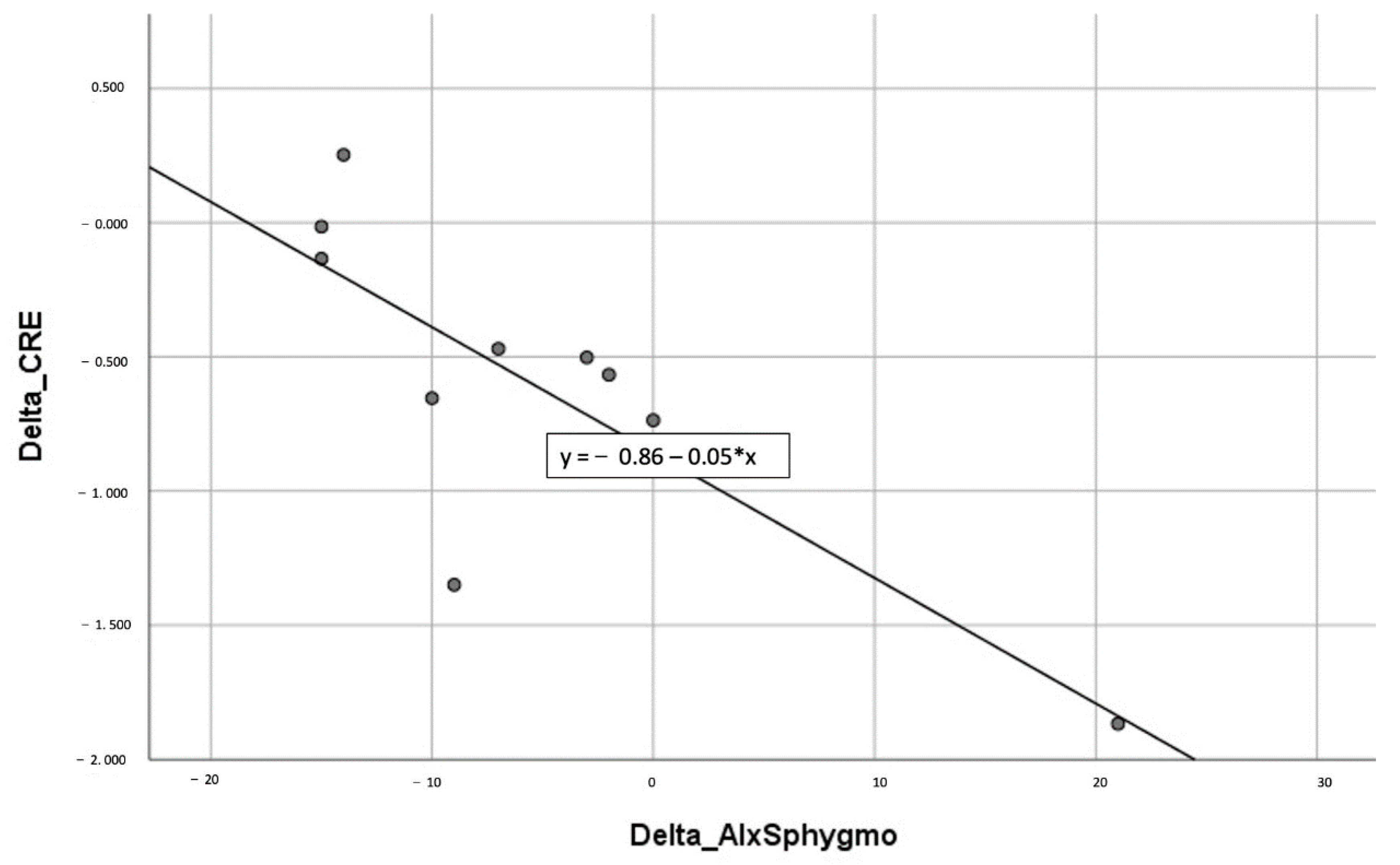
| ΔAix SphygmoCor/ΔTAC (CRE) | −0.729 | 0.017 |
| Name of the Procedure | Laboratory Test/ Cardiological Evaluation | Type of Sample | Time Point |
|---|---|---|---|
| Blood pressure measurement | Cardiological evaluation | N/A | T0 and T1 |
| Total antioxidant capacity level | Laboratory test (ELISA test) | Plasma | T0 and T1 |
| Peripheral arterial tonometry—endothelial function | Cardiological evaluation (EndoPAT-2000) | N/A | T0 and T1 |
| Pulse wave velocity | Cardiological evaluation (SphygmoCor system) | N/A | T0 and T1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. https://doi.org/10.3390/ijms232214489
Franco C, Sciatti E, Favero G, Bonomini F, Vizzardi E, Rezzani R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. International Journal of Molecular Sciences. 2022; 23(22):14489. https://doi.org/10.3390/ijms232214489
Chicago/Turabian StyleFranco, Caterina, Edoardo Sciatti, Gaia Favero, Francesca Bonomini, Enrico Vizzardi, and Rita Rezzani. 2022. "Essential Hypertension and Oxidative Stress: Novel Future Perspectives" International Journal of Molecular Sciences 23, no. 22: 14489. https://doi.org/10.3390/ijms232214489
APA StyleFranco, C., Sciatti, E., Favero, G., Bonomini, F., Vizzardi, E., & Rezzani, R. (2022). Essential Hypertension and Oxidative Stress: Novel Future Perspectives. International Journal of Molecular Sciences, 23(22), 14489. https://doi.org/10.3390/ijms232214489