Enzymatic Construction of DARPin-Based Targeted Delivery Systems Using Protein Farnesyltransferase and a Capture and Release Strategy
Abstract
1. Introduction
2. Results and Discussion
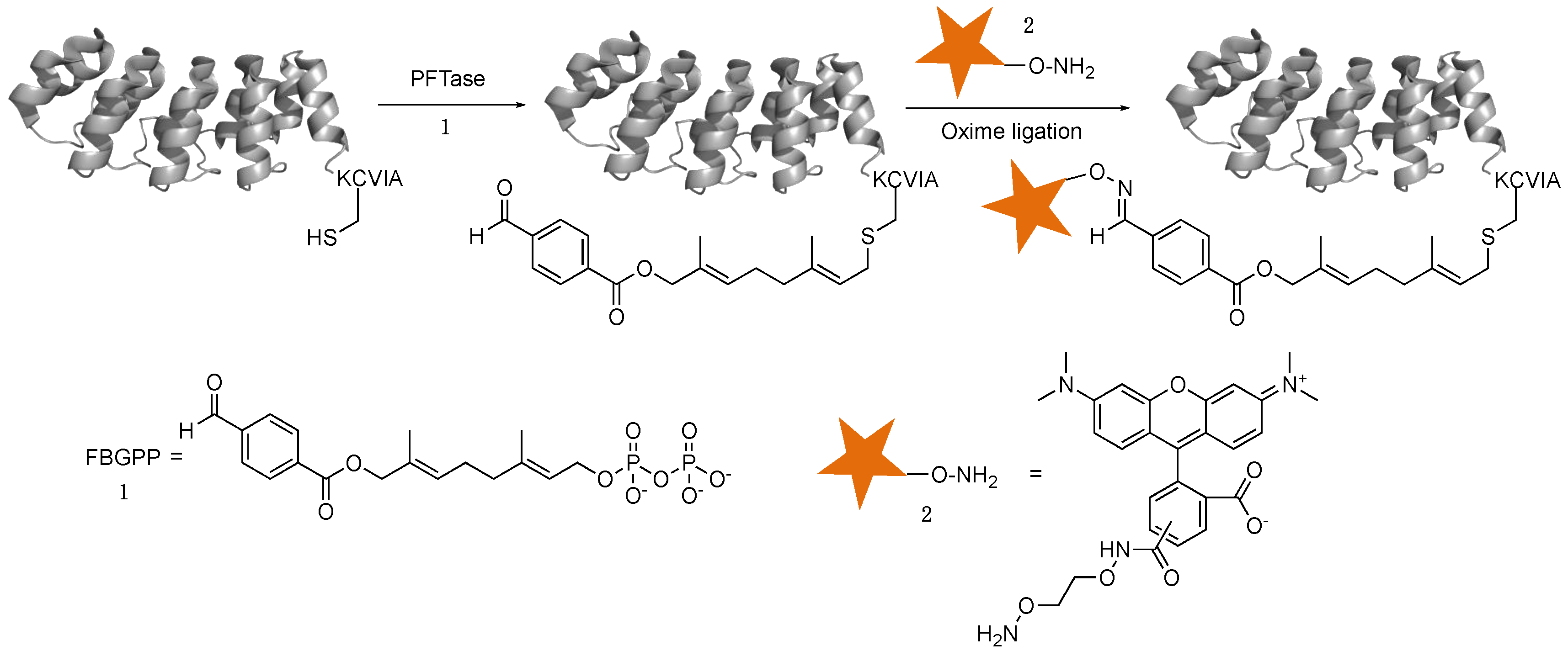
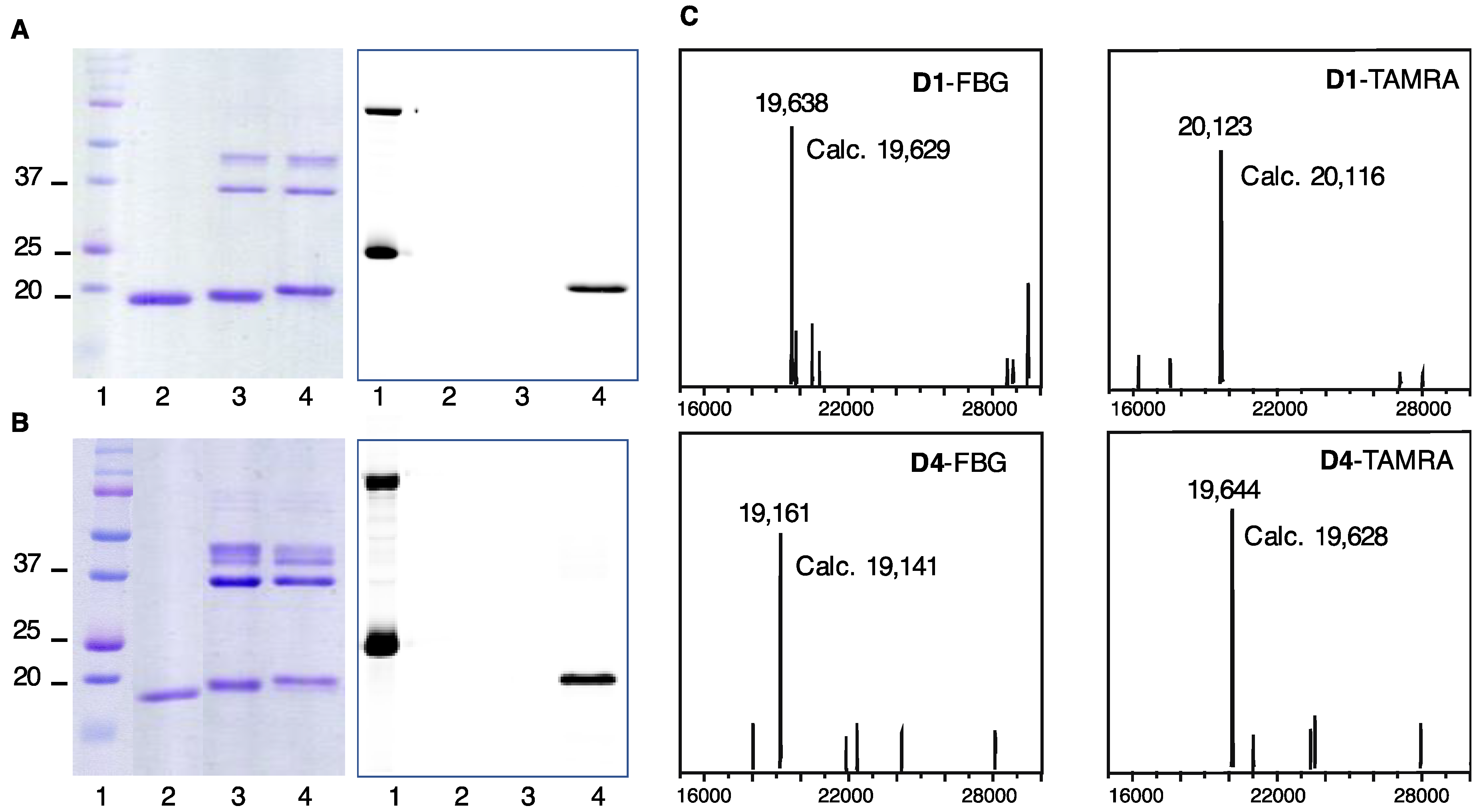
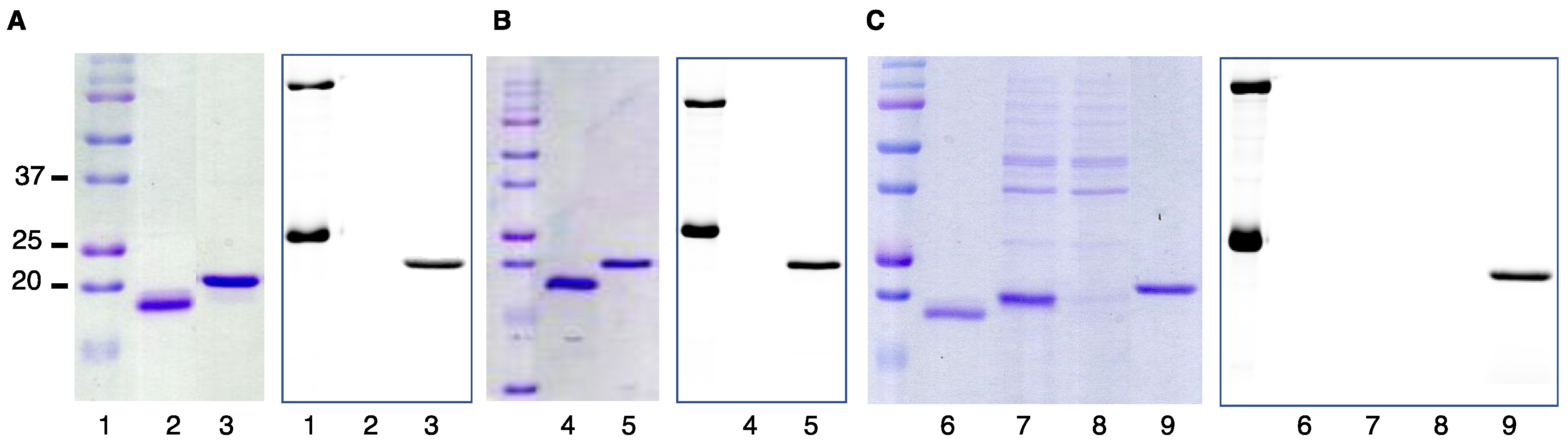
2.1. DARPin Modification by PFTase
2.2. Enzymatic Incorporation of Aldehyde Functionality and Fluorophore Conjugation
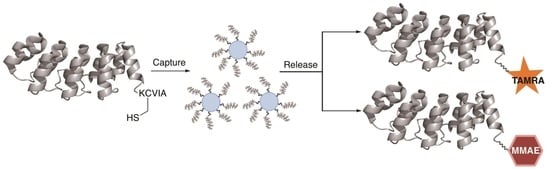
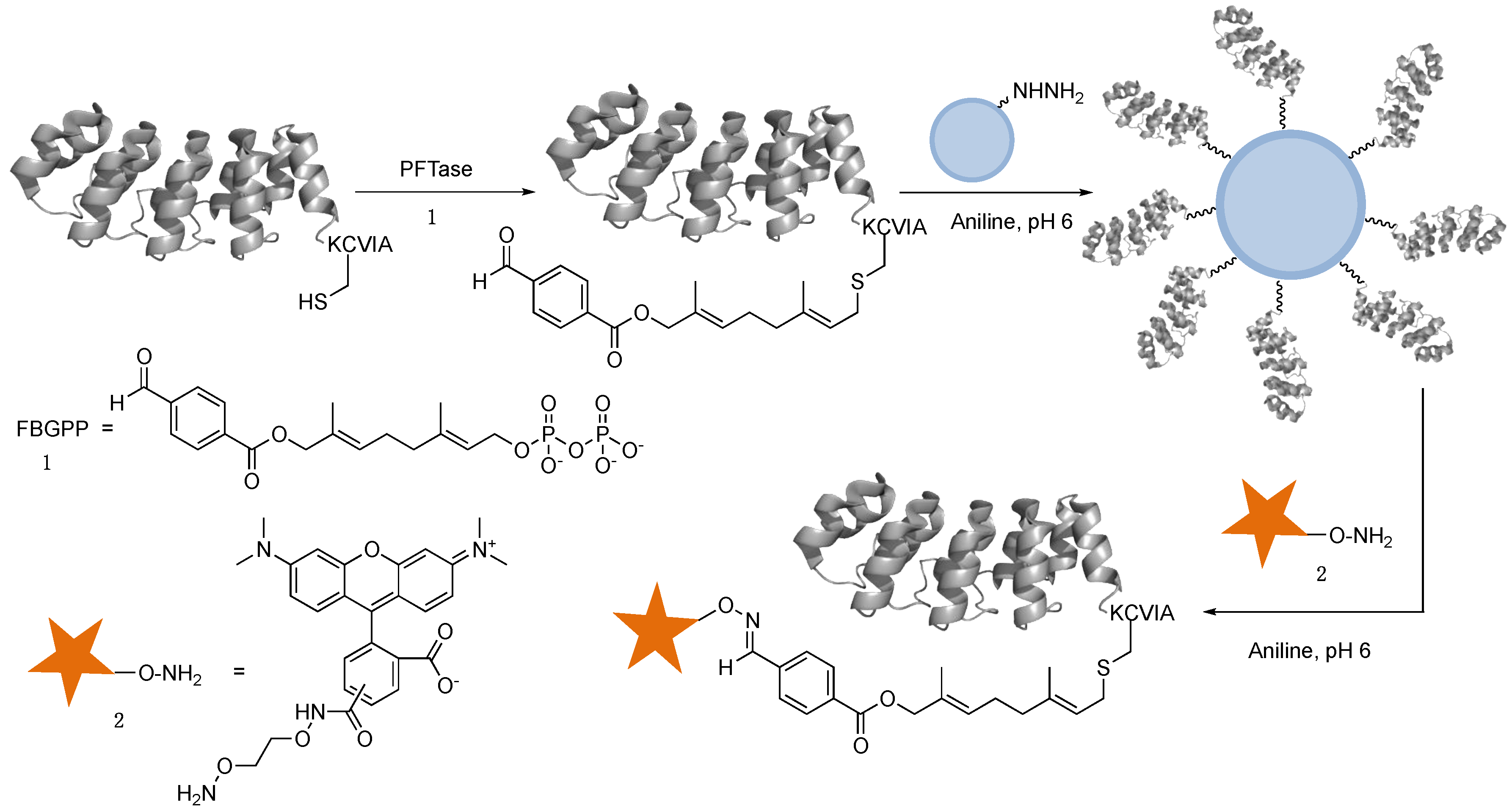
2.3. Capture and Release Strategy Allows Facile Construction of DARPin-TAMRA Conjugates
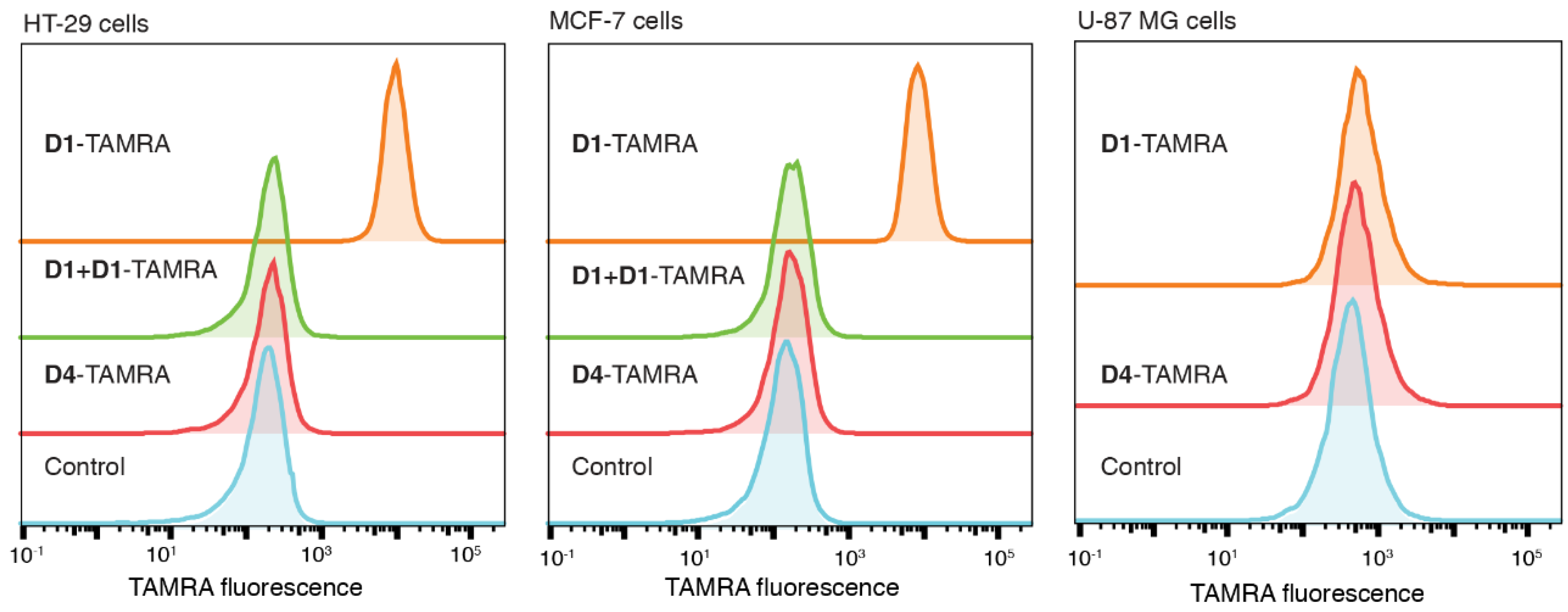
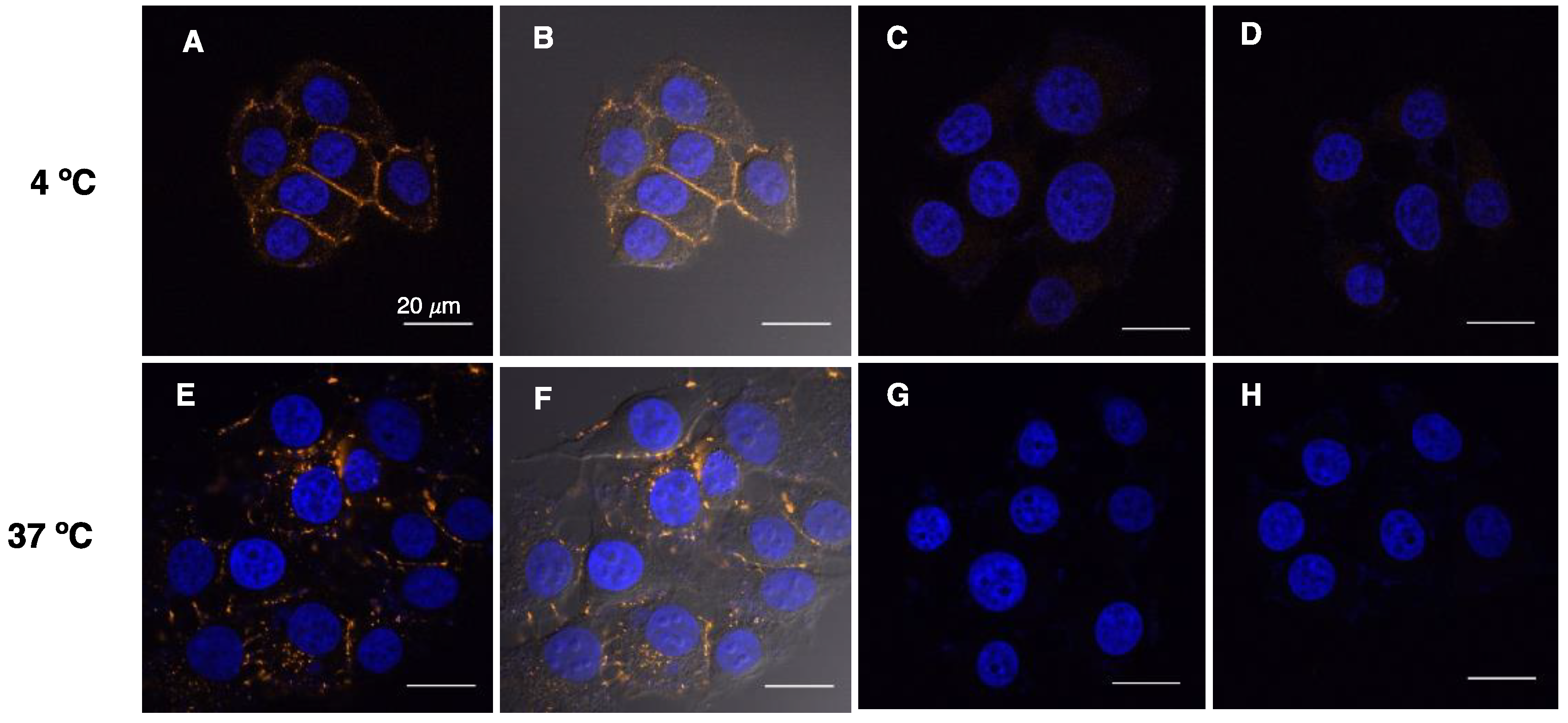
2.4. D1-TAMRA Retains Selective Binding to Cell-Surface EpCAM
2.5. Application of PFTase Labeling to a DARPin Binding another Target
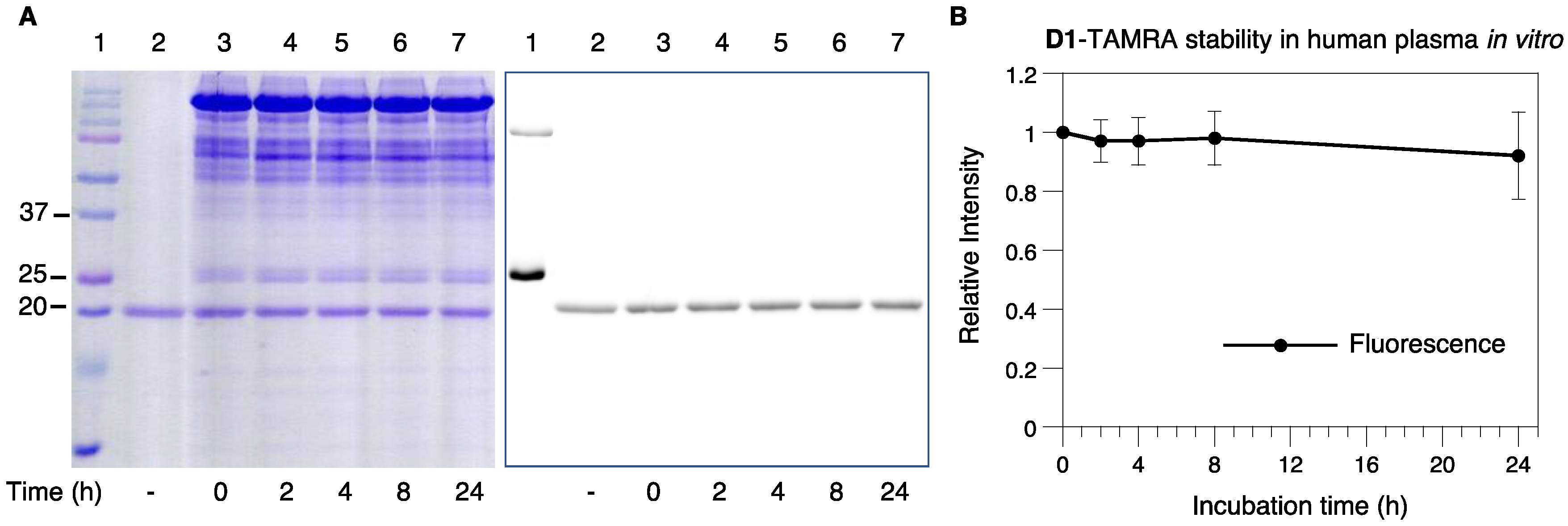
2.6. Serum Stability of D1-TAMRA
2.7. Construction of DARPin-MMAE Conjugates
2.8. Cytotoxicity of DARPin-MMAE Conjugates In Vitro
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Modification of DARPins
3.3. DARPin-FBG Conjugation to TAMRA-Aminooxy (2)
3.4. Capture and Release Strategy to Construct DARPin-TAMRA Conjugates
3.5. Flow Cytometry Analysis of D1-TAMRA Binding to Cell Surface EpCAM
3.6. Visualization of D1-TAMRA Binding and Internalization to MCF-7 Cells
3.7. Serum Stability of D1-TAMRA In Vitro
3.8. Construction of DARPin-MMAE Conjugates
3.9. DARPin-MMAE Cytotoxicity Assay in Cell Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dozier, J.K.; Distefano, M.D. Site-Specific Pegylation of Therapeutic Proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Bhavanasi, S.; Quadir, M.; Singh, K.; Ghosh, G.; Vasamreddy, K.; Ghosh, A.; Siahaan, T.J.; Banerjee, S.; Banerjee, S.K. Protein PEGylation for Cancer Therapy: Bench to Bedside. J. Cell Commun. Signal. 2019, 13, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Stennicke, H.R.; Kjalke, M.; Karpf, D.M.; Balling, K.W.; Johansen, P.B.; Elm, T.; Øvlisen, K.; Möller, F.; Holmberg, H.L.; Gudme, C.N.; et al. A Novel B-Domain O-GlycoPEGylated FVIII (N8-GP) Demonstrates Full Efficacy and Prolonged Effect in Hemophilic Mice Models. Blood 2013, 121, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel Anti-B-Cell Maturation Antigen Antibody-Drug Conjugate (GSK2857916) Selectively Induces Killing of Multiple Myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef]
- Zammarchi, F.; Corbett, S.; Adams, L.; Tyrer, P.C.; Kiakos, K.; Janghra, N.; Marafioti, T.; Britten, C.E.; Havenith, C.E.G.; Chivers, S.; et al. ADCT-402, a PBD Dimer–Containing Antibody Drug Conjugate Targeting CD19-Expressing Malignancies. Blood 2018, 131, 1094–1105. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, a Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef]
- Rashidian, M.; Keliher, E.J.; Bilate, A.M.; Duarte, J.N.; Wojtkiewicz, G.R.; Jacobsen, J.T.; Cragnolini, J.; Swee, L.K.; Victora, G.D.; Weissleder, R.; et al. Noninvasive Imaging of Immune Responses. Proc. Natl. Acad. Sci. USA 2015, 112, 6146–6151. [Google Scholar] [CrossRef]
- Dammes, N.; Peer, D. Monoclonal Antibody-Based Molecular Imaging Strategies and Theranostic Opportunities. Theranostics 2020, 10, 938–955. [Google Scholar] [CrossRef]
- Wang, Y.; Rozumalski, L.; Lichtenfels, C.; Petersberg, J.R.; Kilic, O.; Distefano, M.D.; Wagner, C.R. Engineering Biomimetic Trogocytosis with Farnesylated Chemically Self-Assembled Nanorings. BioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, K.Y.; Suazo, K.F.; Distefano, M.D. Recent Progress in Enzymatic Protein Labelling Techniques and Their Applications. Chem. Soc. Rev. 2018, 47, 9106–9136. [Google Scholar] [CrossRef]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y.; et al. Site-Selective Modification Strategies in Antibody-Drug Conjugates. Chem. Soc. Rev. 2021, 50, 1305–1353. [Google Scholar] [CrossRef] [PubMed]
- Adusumalli, S.R.; Rawale, D.G.; Singh, U.; Tripathi, P.; Paul, R.; Kalra, N.; Mishra, R.K.; Shukla, S.; Rai, V. Single-Site Labeling of Native Proteins Enabled by a Chemoselective and Site-Selective Chemical Technology. J. Am. Chem. Soc. 2018, 140, 15114–15123. [Google Scholar] [CrossRef] [PubMed]
- Lobba, M.J.; Fellmann, C.; Marmelstein, A.M.; Maza, J.C.; Kissman, E.N.; Robinson, S.A.; Staahl, B.T.; Urnes, C.; Lew, R.J.; Mogilevsky, C.S.; et al. Site-Specific Bioconjugation through Enzyme-Catalyzed Tyrosine-Cysteine Bond Formation. ACS Cent. Sci. 2020, 6, 1564–1571. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, J.; Liu, S.; Zhang, X.; Sun, J.; Hu, Y.; Yang, Y.; Chen, L. A Chemical Covalent Tactic for Bio-Thiol Sensing and Protein Labeling Agent Design. Chem. Commun. 2020, 56, 11485–11488. [Google Scholar] [CrossRef]
- Rashidian, M.; Dozier, J.K.; Distefano, M.D. Enzymatic Labeling of Proteins: Techniques and Approaches. Bioconjug. Chem. 2013, 24, 1277–1294. [Google Scholar] [CrossRef]
- Spycher, P.R.; Amann, C.A.; Wehrmüller, J.E.; Hurwitz, D.R.; Kreis, O.; Messmer, D.; Ritler, A.; Küchler, A.; Blanc, A.; Béhé, M.; et al. Dual, Site-Specific Modification of Antibodies by Using Solid-Phase Immobilized Microbial Transglutaminase. ChemBioChem 2017, 18, 1923–1927. [Google Scholar] [CrossRef]
- Wang, H.H.; Altun, B.; Nwe, K.; Tsourkas, A. Proximity-Based Sortase-Mediated Ligation. Angew. Chem. Int. Ed. 2017, 56, 5349–5352. [Google Scholar] [CrossRef]
- Bellucci, J.J.; Amiram, M.; Bhattacharyya, J.; McCafferty, D.; Chilkoti, A. Three-in-One Chromatography-Free Purification, Tag Removal, and Site-Specific Modification of Recombinant Fusion Proteins Using Sortase A and Elastin-like Polypeptides. Angew. Chem. Int. Ed 2013, 125, 3791–3796. [Google Scholar] [CrossRef]
- Policarpo, R.L.; Kang, H.; Liao, X.; Rabideau, A.E.; Simon, M.D.; Pentelute, B.L. Flow-Based Enzymatic Ligation by Sortase A. Angew. Chem. Int. Ed. 2014, 126, 9357–9362. [Google Scholar] [CrossRef]
- Chen, I.; Dorr, B.M.; Liu, D.R. A General Strategy for the Evolution of Bond-Forming Enzymes Using Yeast Display. Proc. Natl. Acad. Sci. USA 2011, 108, 11399–11404. [Google Scholar] [CrossRef] [PubMed]
- Heck, T.; Pham, P.H.; Yerlikaya, A.; Thöny-Meyer, L.; Richter, M. Sortase A Catalyzed Reaction Pathways: A Comparative Study with Six SrtA Variants. Catal. Sci. Technol. 2014, 4, 2946–2956. [Google Scholar] [CrossRef]
- Warden-Rothman, R.; Caturegli, I.; Popik, V.; Tsourkas, A. Sortase-Tag Expressed Protein Ligation: Combining Protein Purification and Site-Specific Bioconjugation into a Single Step. Anal. Chem. 2013, 85, 11090–11097. [Google Scholar] [CrossRef]
- Zhang, Y.; Auger, S.; Schaefer, J.V.; Plückthun, A.; Distefano, M.D. Site-Selective Enzymatic Labeling of Designed Ankyrin Repeat Proteins Using Protein Farnesyltransferase. In Methods in Molecular Biology: Bioconjugation; Massa, S., Ed.; Humana: New York, NY, USA, 2019; pp. 207–219. ISBN 9781452289830. [Google Scholar]
- Palsuledesai, C.C.; Distefano, M.D. Protein Prenylation: Enzymes, Therapeutics, and Biotechnology Applications. ACS Chem. Biol. 2015, 10, 51–62. [Google Scholar] [CrossRef]
- Kim, Y.; Park, T.; Woo, S.; Lee, H.; Kim, S.; Cho, J.; Jung, D.; Kim, Y.; Kwon, H.; Oh, K.; et al. Antibody-Active Agent Conjugates and Methods of Use. U.S. Patent No. 9669107 B2, 6 June 2017. [Google Scholar]
- Wang, Y.; Kilic, O.; Csizmar, C.M.; Ashok, S.; Hougland, J.L.; Distefano, M.D.; Wagner, C.R. Engineering Reversible Cell-Cell Interactions Using Enzymatically Lipidated Chemically Self-Assembled Nanorings. Chem. Sci. 2021, 12, 331–340. [Google Scholar] [CrossRef]
- Lee, J.J.; Choi, H.J.; Yun, M.; Kang, Y.; Jung, J.E.; Ryu, Y.; Kim, T.Y.; Cha, Y.J.; Cho, H.S.; Min, J.J.; et al. Enzymatic Prenylation and Oxime Ligation for the Synthesis of Stable and Homogeneous Protein-Drug Conjugates for Targeted Therapy. Angew. Chem. Int. Ed. 2015, 54, 12020–12024. [Google Scholar] [CrossRef]
- Rose, M.W.; Rose, N.D.; Boggs, J.; Lenevich, S.; Xu, J.; Barany, G.; Distefano, M.D. Evaluation of Geranylazide and Farnesylazide Diphosphate for Incorporation of Prenylazides into a CAAX Box-Containing Peptide Using Protein Farnesyltransferase. J. Pept. Res. 2005, 65, 529–537. [Google Scholar] [CrossRef]
- Hosokawa, A.; Wollack, J.W.; Zhang, Z.; Chen, L.; Barany, G.; Distefano, M.D. Evaluation of an Alkyne-Containing Analogue of Farnesyl Diphosphate as a Dual Substrate for Protein-Prenyltransferases. Int. J. Pept. Res. Ther. 2007, 13, 345–354. [Google Scholar] [CrossRef]
- Rashidian, M.; Song, J.M.; Pricer, R.E.; Distefano, M.D. Chemoenzymatic Reversible Immobilization and Labeling of Proteins without Prior Purification. J. Am. Chem. Soc. 2012, 134, 8455–8467. [Google Scholar] [CrossRef] [PubMed]
- Wollack, J.W.; Monson, B.J.; Dozier, J.K.; Dalluge, J.J.; Poss, K.; Hilderbrand, S.A.; Distefano, M.D. Site-Specific Labeling of Proteins and Peptides with Trans-Cyclooctene Containing Handles Capable of Tetrazine Ligation. Chem. Biol. Drug Des. 2014, 84, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Suazo, K.F.; Park, K.; Distefano, M.D. A Not-So-Ancient Grease History: Click Chemistry and Protein Lipid Modifications. Chem. Rev. 2021, 121, 7178–7248. [Google Scholar] [CrossRef] [PubMed]
- Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Stumpp, M.T.; Dawson, K.M.; Binz, H.K. Beyond Antibodies: The DARPin® Drug Platform. BioDrugs 2020, 34, 423–433. [Google Scholar] [CrossRef]
- Bery, N.; Miller, A.; Rabbitts, T. A Potent KRAS Macromolecule Degrader Specifically Targeting Tumours with Mutant KRAS. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Tamaskovic, R.; Simon, M.; Stefan, N.; Schwill, M.; Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins) from Research to Therapy. Methods Enzym. 2012, 503, 101–134. [Google Scholar]
- van den Brand, D.; van Lith, S.A.M.; de Jong, J.M.; Gorris, M.A.J.; Palacio-Castañeda, V.; Couwenbergh, S.T.; Goldman, M.R.G.; Ebisch, I.; Massuger, L.F.; Leenders, W.P.J.; et al. EpCAM-Binding Darpins for Targeted Photodynamic Therapy of Ovarian Cancer. Cancers 2020, 12, 1762. [Google Scholar] [CrossRef]
- Brandl, F.; Busslinger, S.; Zangemeister-Wittke, U.; Plückthun, A. Optimizing the Anti-Tumor Efficacy of Protein-Drug Conjugates by Engineering the Molecular Size and Half-Life. J. Control. Release 2020, 327, 186–197. [Google Scholar] [CrossRef]
- Deyev, S.; Vorobyeva, A.; Schulga, A.; Proshkina, G.; Güler, R.; Löfblom, J.; Mitran, B.; Garousi, J.; Altai, M.; Buijs, J.; et al. Comparative Evaluation of Two DARPin Variants: Effect of Affinity, Size, and Label on Tumor Targeting Properties. Mol. Pharm. 2019, 16, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Sosabowski, J.; Livanos, M.; Leyton, J.; Vigor, K.; Bhavsar, G.; Nagy-Davidescu, G.; Rashid, M.; Miranda, E.; Yeung, J.; et al. Development of the Designed Ankyrin Repeat Protein (DARPin) G3 for HER2 Molecular Imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Lorenzer, C.; Streußnig, S.; Tot, E.; Winkler, A.M.; Merten, H.; Brandl, F.; Sayers, E.J.; Watson, P.; Jones, A.T.; Zangemeister-Wittke, U.; et al. Targeted Delivery and Endosomal Cellular Uptake of DARPin-SiRNA Bioconjugates: Influence of Linker Stability on Gene Silencing. Eur. J. Pharm. Biopharm. 2019, 141, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.A.; Mason, M.; Christie, L.A.; Hansen, C.; Hernandez, L.M.; Burke, J.; Luhrs, K.A.; Hohman, T.C. Functional Characterization of Abicipar-Pegol, an Anti-VEGF DARPin Therapeutic That Potently Inhibits Angiogenesis and Vascular Permeability. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5836–5846. [Google Scholar] [CrossRef]
- Sokolova, E.; Proshkina, G.; Kutova, O.; Shilova, O.; Ryabova, A.; Schulga, A.; Stremovskiy, O.; Zdobnova, T.; Balalaeva, I.; Deyev, S. Recombinant Targeted Toxin Based on HER2-Specific DARPin Possesses a Strong Selective Cytotoxic Effect in Vitro and a Potent Antitumor Activity in Vivo. J. Control. Release 2016, 233, 48–56. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Konovalova, E.; Xu, T.; Schulga, A.; Altai, M.; Garousi, J.; Rinne, S.S.; Orlova, A.; Tolmachev, V.; Deyev, S. Feasibility of Imaging Epcam Expression in Ovarian Cancer Using Radiolabeled Darpin Ec1. Int. J. Mol. Sci. 2020, 21, 3310. [Google Scholar] [CrossRef]
- Stefan, N.; Martin-Killias, P.; Wyss-Stoeckle, S.; Honegger, A.; Zangemeister-Wittke, U.; Plückthun, A. DARPins Recognizing the Tumor-Associated Antigen EpCAM Selected by Phage and Ribosome Display and Engineered for Multivalency. J. Mol. Biol. 2011, 413, 826–843. [Google Scholar] [CrossRef]
- Simon, M.; Stefan, N.; Plückthun, A.; Zangemeister-Wittke, U. Epithelial Cell Adhesion Molecule-Targeted Drug Delivery for Cancer Therapy. Expert Opin Drug Deliv. 2013, 10, 451–468. [Google Scholar] [CrossRef]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial Cell Adhesion Molecule Expression (CD326) in Cancer: A Short Review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- Bryant, K.L.; Mancias, J.D.; Kimmelman, A.C.; Der, C.J. KRAS: Feeding Pancreatic Cancer Proliferation. Trends Biochem. Sci. 2014, 39, 91–100. [Google Scholar] [CrossRef]
- Binz, H.K.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Plückthun, A. Designing Repeat Proteins: Well-Expressed, Soluble and Stable Proteins from Combinatorial Libraries of Consensus Ankyrin Repeat Proteins. J. Mol. Biol. 2003, 332, 489–503. [Google Scholar] [CrossRef]
- Boersma, Y.L.; Chao, G.; Steiner, D.; Wittrup, K.D.; Plückthun, A. Bispecific Designed Ankyrin Repeat Proteins (DARPins) Targeting Epidermal Growth Factor Receptor Inhibit A431 Cell Proliferation and Receptor Recycling. J. Biol. Chem. 2011, 286, 41273–41285. [Google Scholar] [CrossRef] [PubMed]
- Rae, J.M.; Scheys, J.O.; Clark, K.M.; Chadwick, R.B.; Kiefer, M.C.; Lippman, M.E. EGFR and EGFRvIII Expression in Primary Breast Cancer and Cell Lines. Breast Cancer Res. Treat. 2004, 87, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Maderna, A.; Leverett, C.A. Recent Advances in the Development of New Auristatins: Structural Modifications and Application in Antibody Drug Conjugates. Mol. Pharm. 2015, 12, 1798–1812. [Google Scholar] [CrossRef]
- Doronina, S.O.; Toki, B.E.; Torgov, M.Y.; Mendelsohn, B.A.; Cerveny, C.G.; Chace, D.F.; Deblanc, R.L.; Gearing, R.P.; Bovee, T.D.; Siegall, C.B.; et al. Development of Potent Monoclonal Antibody Auristatin Conjugates for Cancer Therapy. Nat. Biotech. 2003, 21, 778–784. [Google Scholar] [CrossRef]
- Miller, J.T.; Vitro, C.N.; Fang, S.; Benjamin, S.R.; Tumey, L.N. Enzyme-Agnostic Lysosomal Screen Identifies New Legumain-Cleavable ADC Linkers. Bioconjug. Chem. 2021, 32, 842–858. [Google Scholar] [CrossRef]
- Rashidian, M.; Mahmoodi, M.M.; Shah, R.; Dozier, J.K.; Wagner, C.R.; Distefano, M.D. A Highly Efficient Catalyst for Oxime Ligation and Hydrazone-Oxime Exchange Suitable for Bioconjugation. Bioconjug. Chem. 2013, 24, 333–342. [Google Scholar] [CrossRef]
- Dozier, J.K.; Distefano, M.D. An Enzyme-Coupled Continuous Fluorescence Assay for Farnesyl Diphosphate Synthases. Anal. Biochem. 2012, 421, 158–163. [Google Scholar] [CrossRef]




| Molecule | IC50 (nM) | Selectivity Ratio * | |||
|---|---|---|---|---|---|
| HT29 | MCF-7 | U87-MG | HT-29 | MCF-7 | |
| 4 | 42.8 ± 6.2 | 34.8 ± 8.1 | 29.7 ± 3.9 | n.a. | n.a. |
| D4-MMAE | 40.6 ± 3.0 | 36.9 ± 5.7 | 53.6 ± 3.9 | n.a. | n.a. |
| D1-MMAE | 8.3 ± 0.8 | 8.8 ± 2.2 | 67.8 ± 4.1 | 8 | 8 |
| D10-MMAE | 1.6 ± 0.1 | 1.3 ± 0.2 | 47.4 ± 3.4 | 30 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Uslu, S.; Venkatachalapathy, S.; Rashidian, M.; Schaefer, J.V.; Plückthun, A.; Distefano, M.D. Enzymatic Construction of DARPin-Based Targeted Delivery Systems Using Protein Farnesyltransferase and a Capture and Release Strategy. Int. J. Mol. Sci. 2022, 23, 11537. https://doi.org/10.3390/ijms231911537
Zhang Y, Wang Y, Uslu S, Venkatachalapathy S, Rashidian M, Schaefer JV, Plückthun A, Distefano MD. Enzymatic Construction of DARPin-Based Targeted Delivery Systems Using Protein Farnesyltransferase and a Capture and Release Strategy. International Journal of Molecular Sciences. 2022; 23(19):11537. https://doi.org/10.3390/ijms231911537
Chicago/Turabian StyleZhang, Yi, Yiao Wang, Safak Uslu, Sneha Venkatachalapathy, Mohammad Rashidian, Jonas V. Schaefer, Andreas Plückthun, and Mark D. Distefano. 2022. "Enzymatic Construction of DARPin-Based Targeted Delivery Systems Using Protein Farnesyltransferase and a Capture and Release Strategy" International Journal of Molecular Sciences 23, no. 19: 11537. https://doi.org/10.3390/ijms231911537
APA StyleZhang, Y., Wang, Y., Uslu, S., Venkatachalapathy, S., Rashidian, M., Schaefer, J. V., Plückthun, A., & Distefano, M. D. (2022). Enzymatic Construction of DARPin-Based Targeted Delivery Systems Using Protein Farnesyltransferase and a Capture and Release Strategy. International Journal of Molecular Sciences, 23(19), 11537. https://doi.org/10.3390/ijms231911537