Bacillus subtilis: As an Efficient Bacterial Strain for the Reclamation of Water Loaded with Textile Azo Dye, Orange II
Abstract
1. Introduction
2. Results and Discussion
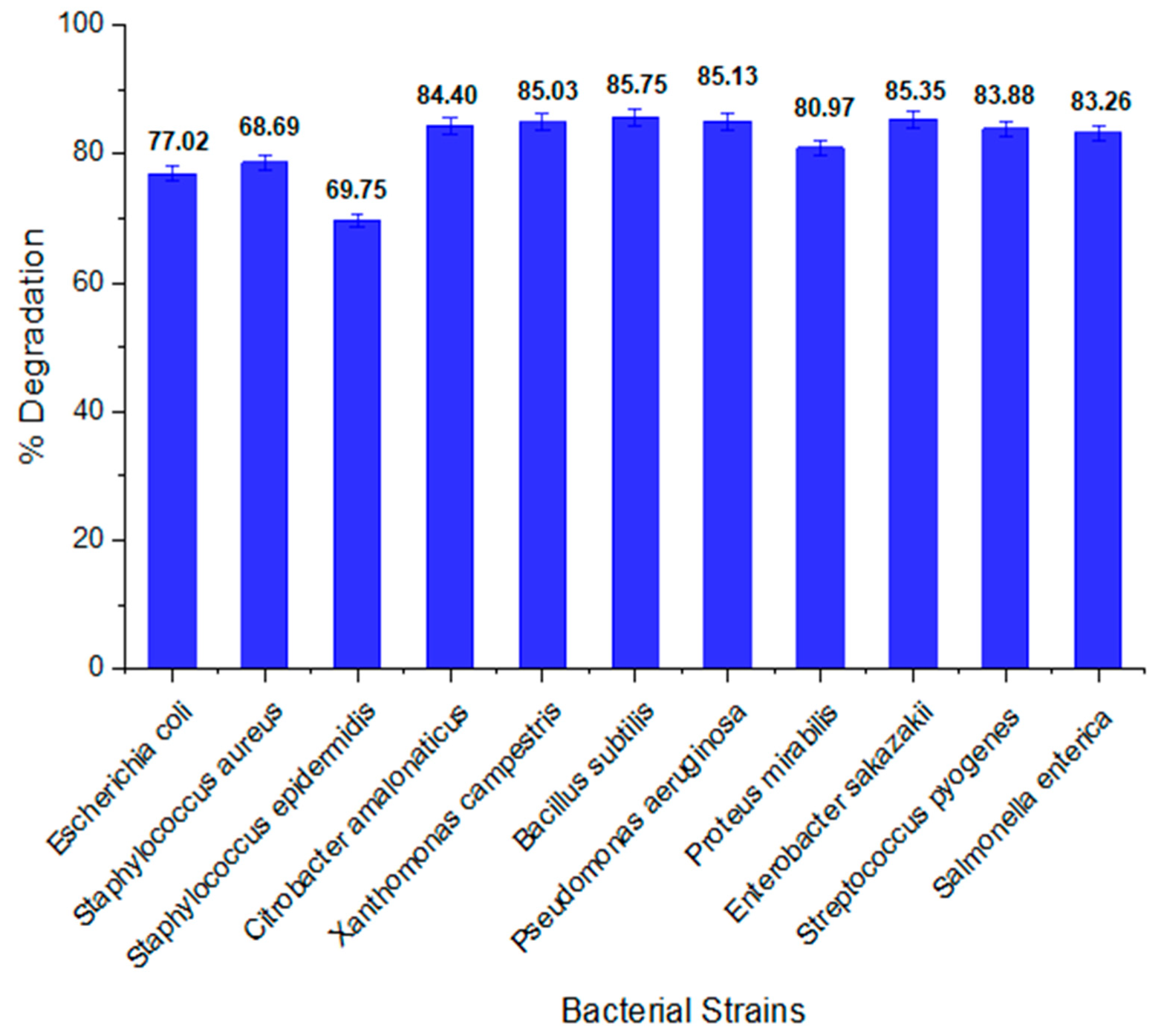
2.1. Highly Potent Bacterium for Orange II Degradation
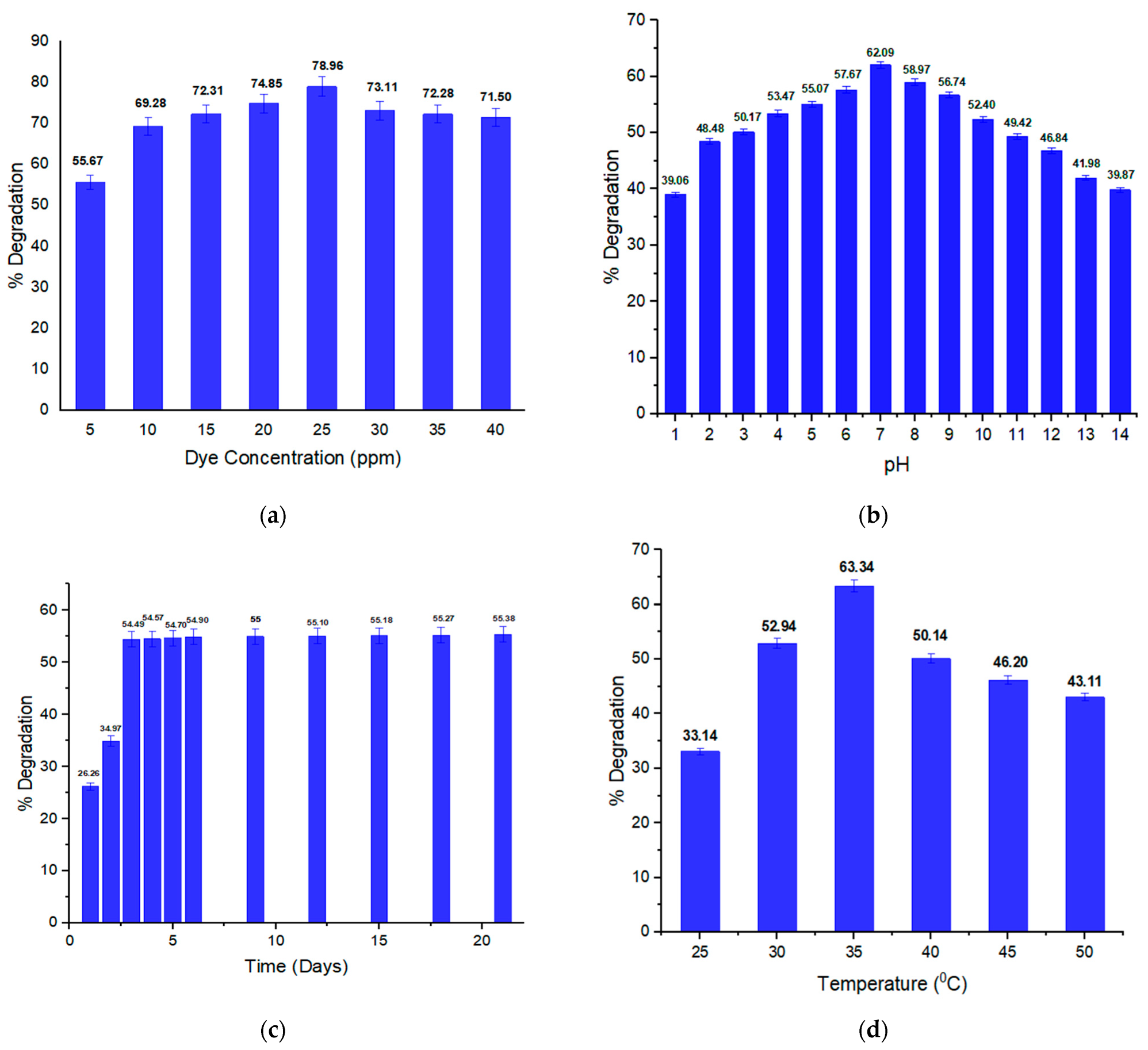
2.2. Optimal Conditions of Orange II Degradation
2.2.1. Impact of Dye Concentration on Orange II Degradation
2.2.2. pH Effect on Orange II Degradation
2.2.3. Impact of Incubation Duration (Time) on Orange II Degradation
2.2.4. Temperature Effect on Orange II Dye Degradation
2.2.5. Impact of Glucose on Orange II Degradation
2.2.6. Impact of Urea on Orange II Dye Degradation
2.2.7. Impact of Sodium Chloride Concentration on Degradation
2.2.8. Redox Mediators and Their Effects on Selected Dye Degradation
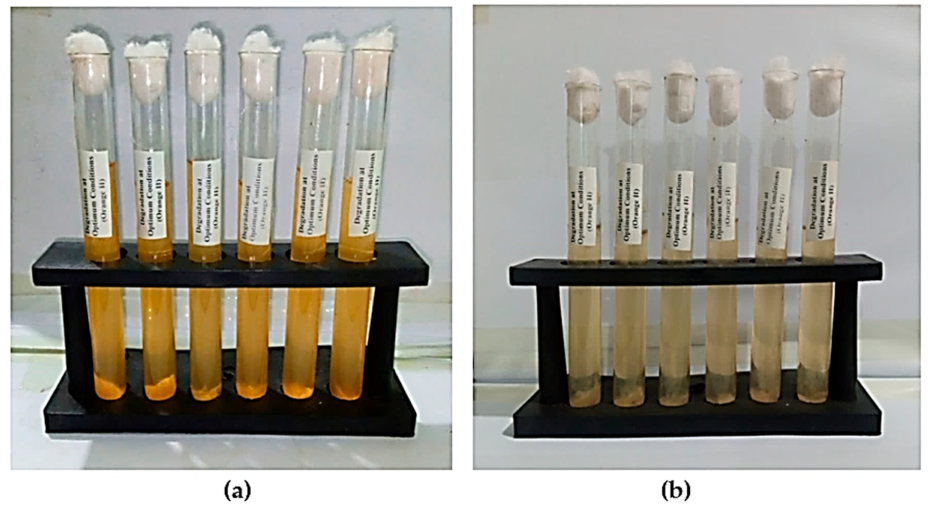
2.3. Orange II Biodegradation at Optimum Conditions
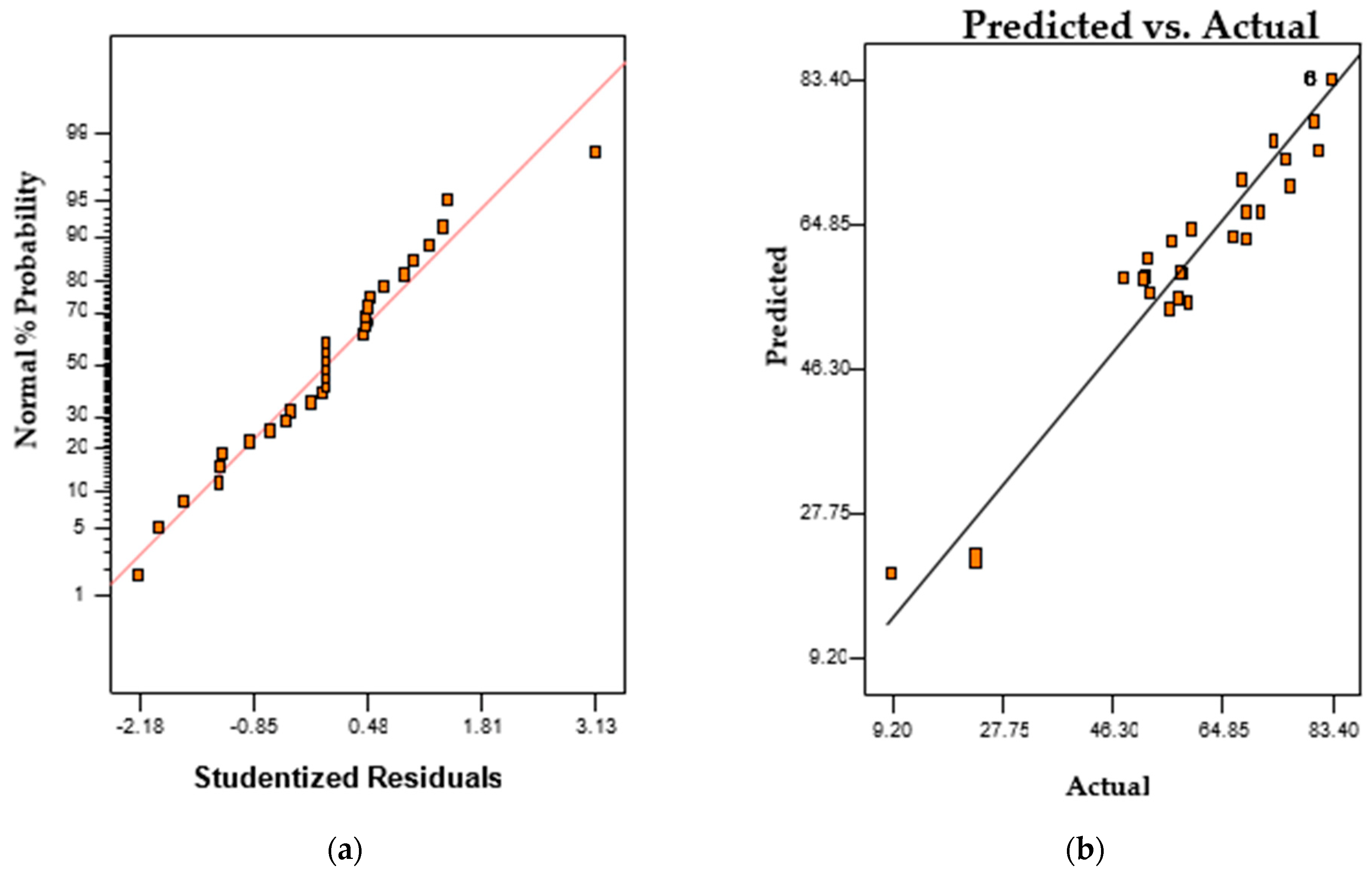
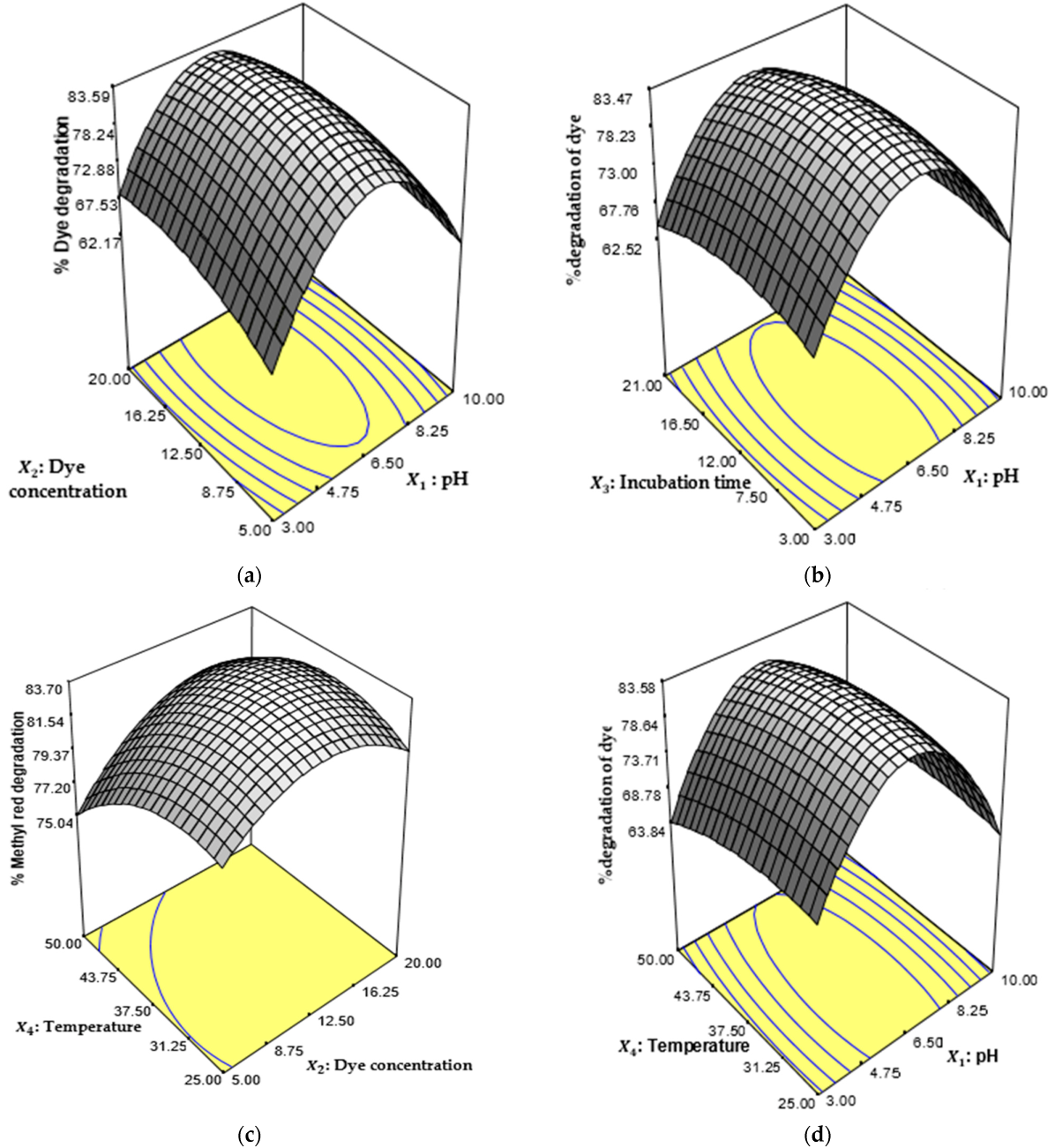
2.4. Most Significant Parameters and Response Surface Optimization for Degradation of Orange II
2.5. Characterization Study of Orange II Biodegradation Products
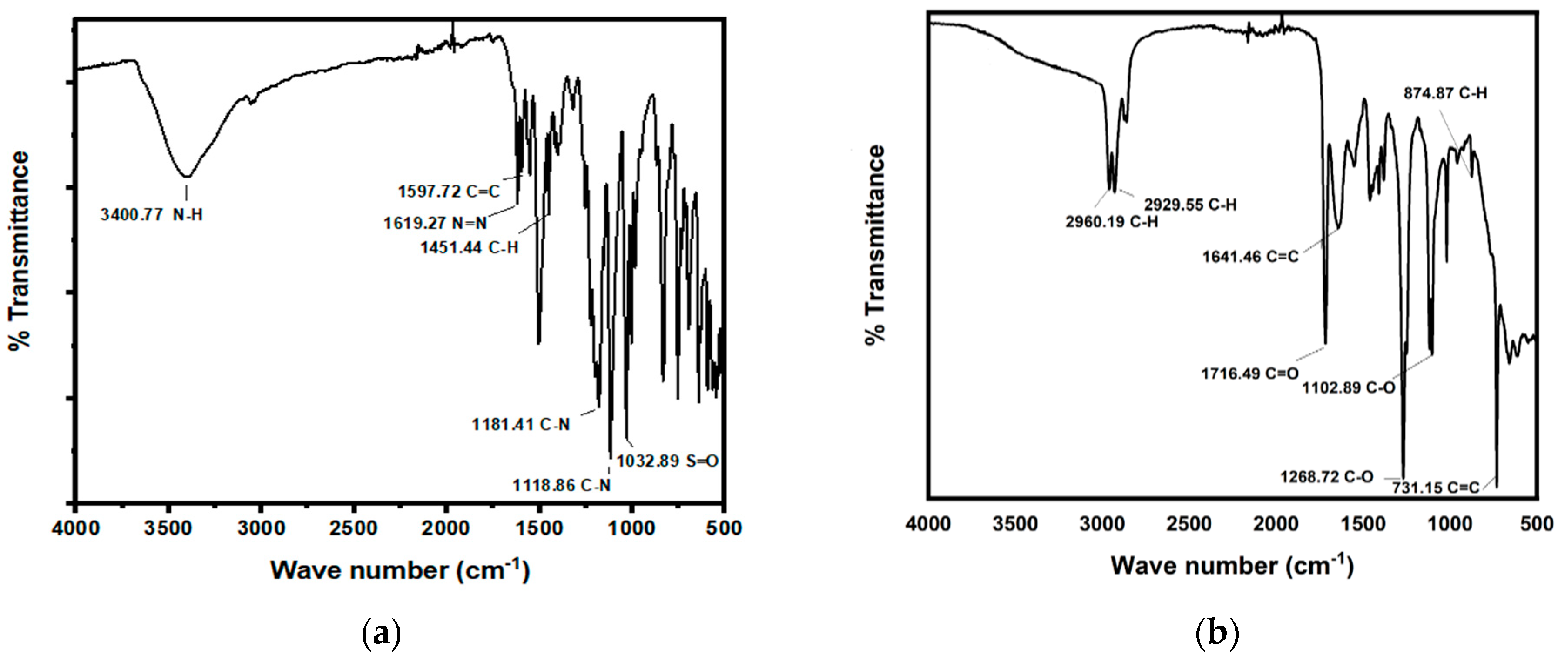
2.5.1. Fourier Transform Infrared Spectroscopic Analysis
2.5.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
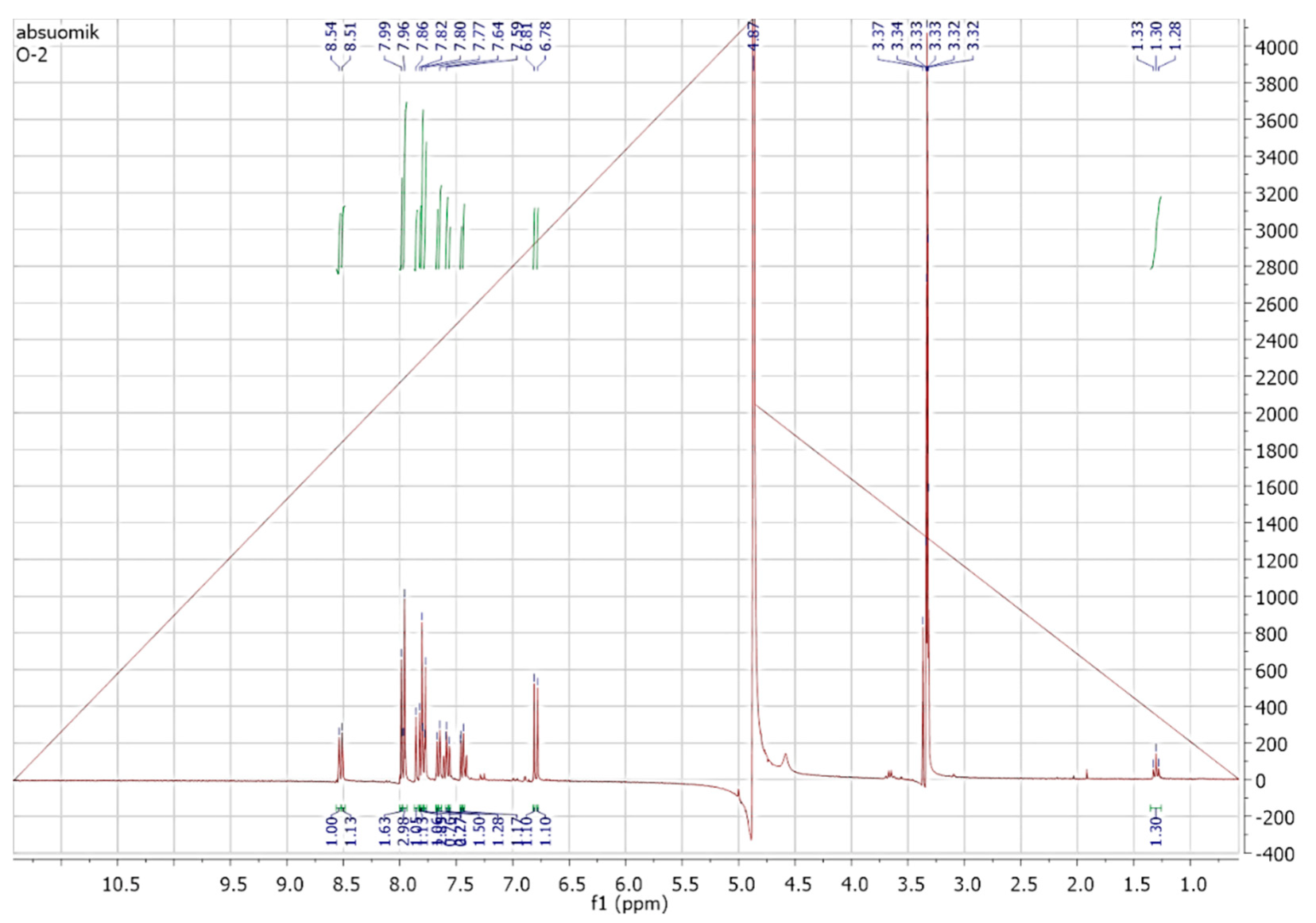
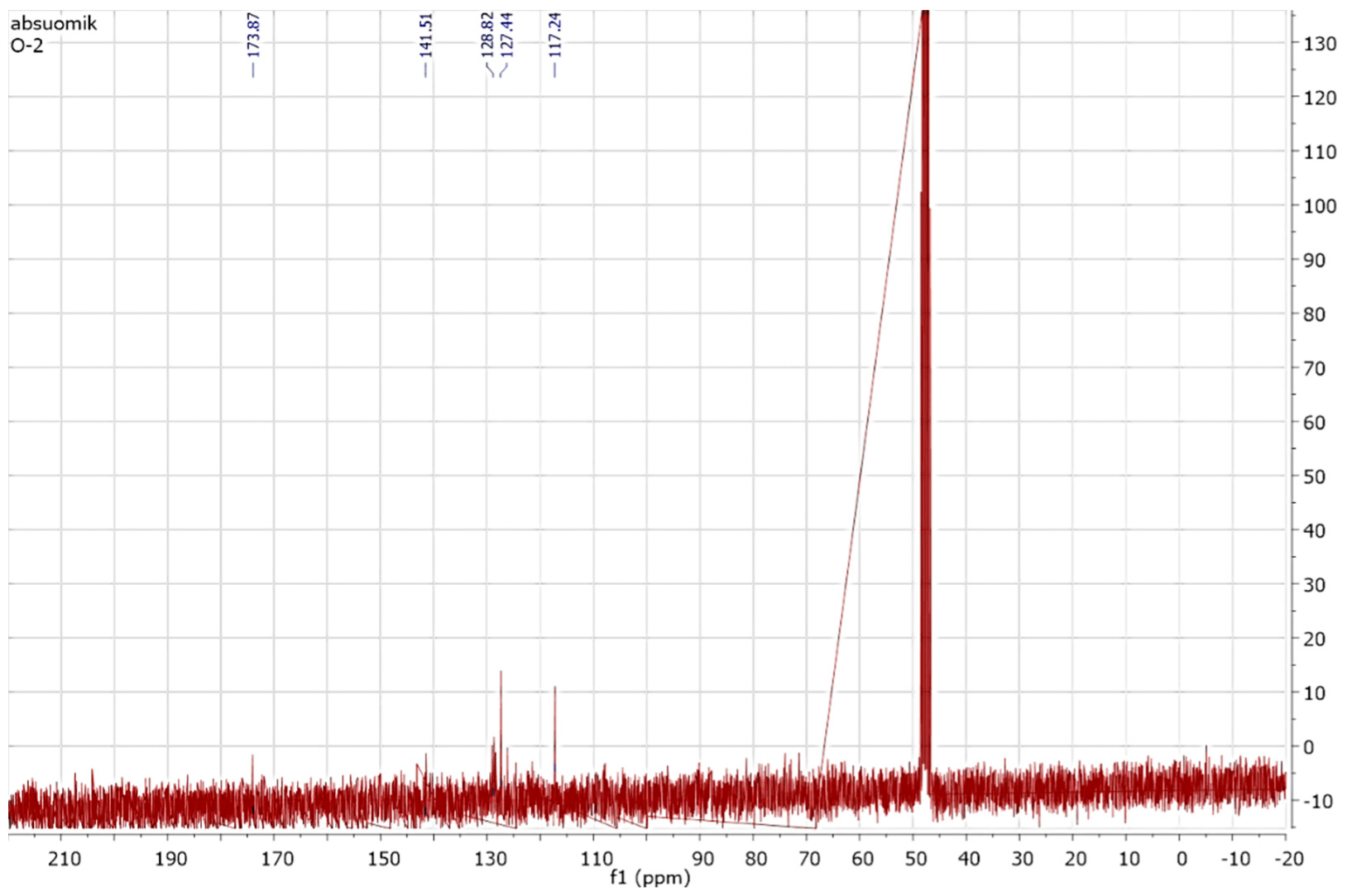
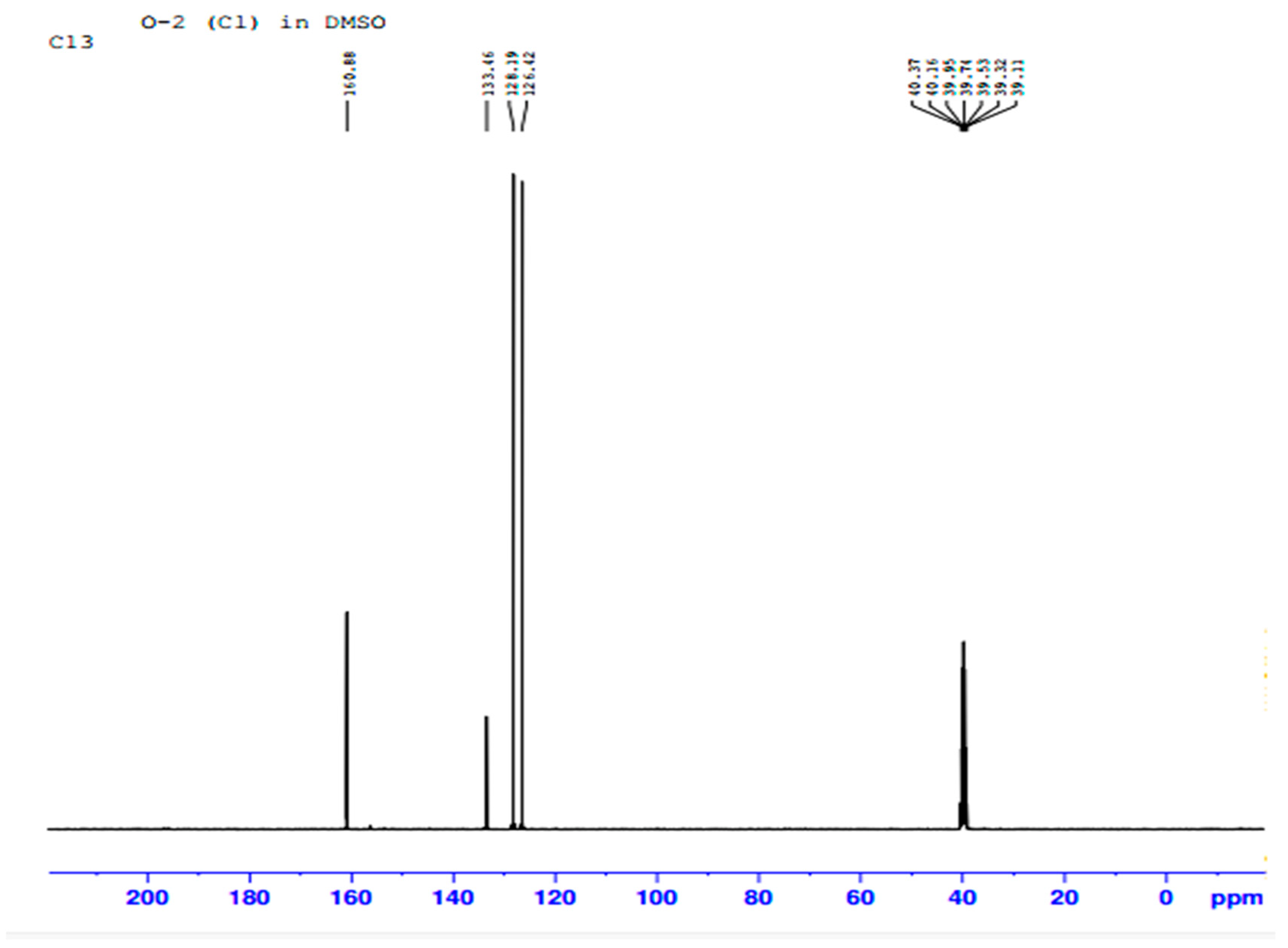
2.5.3. NMR Spectroscopic Analysis
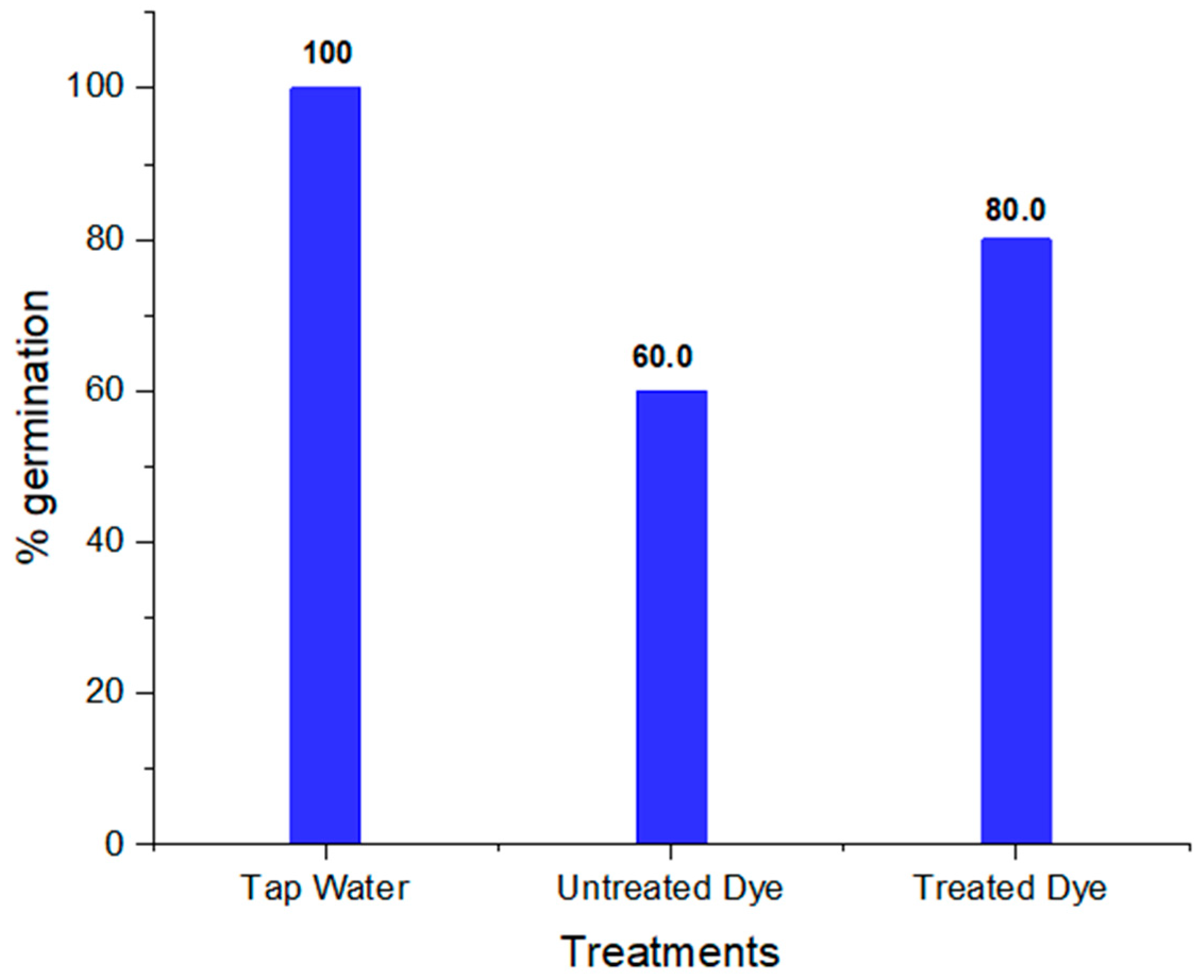
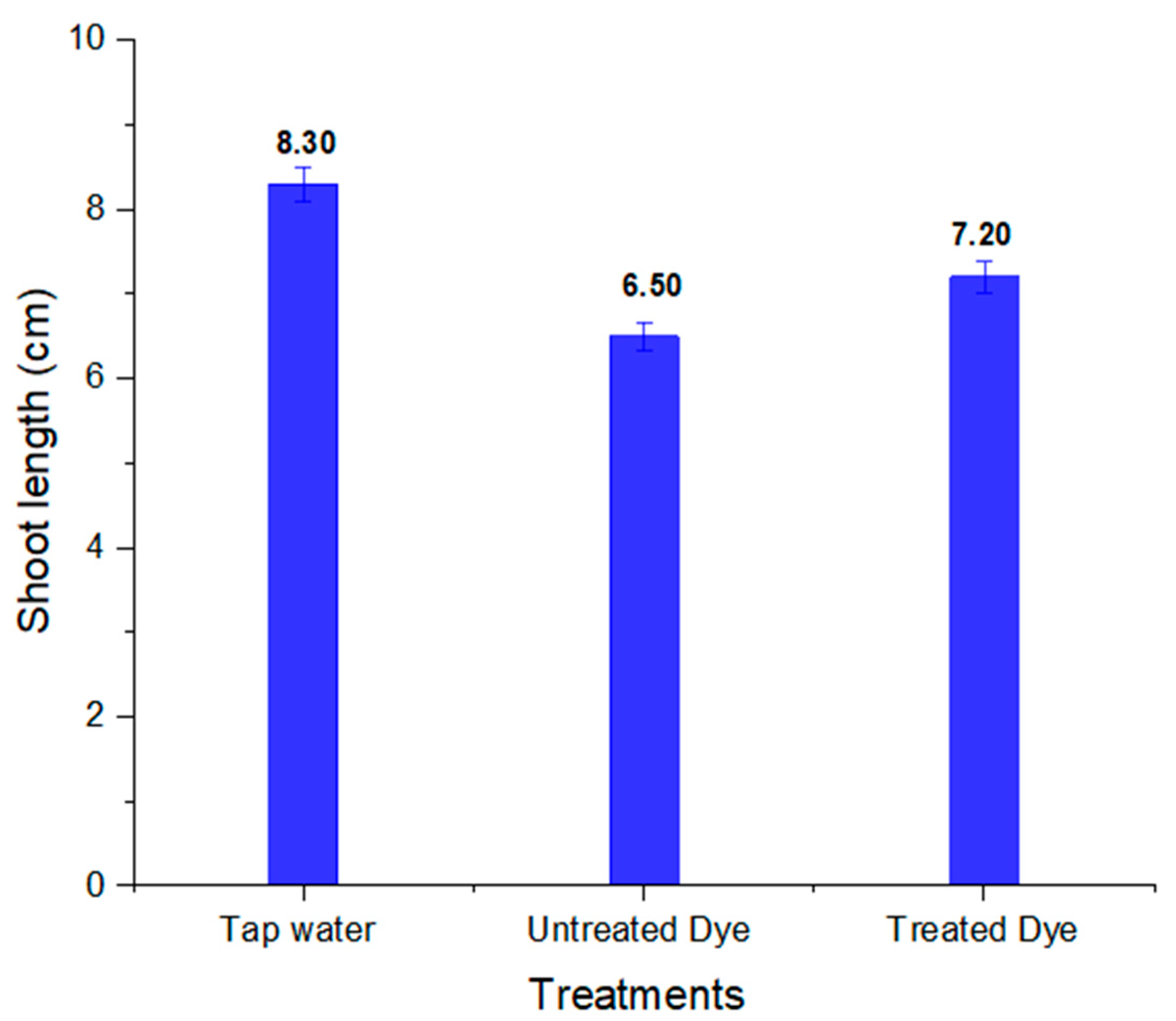
2.6. Phytotoxicity Analysis
2.7. Proposed Mechanism for Orange II Degradation by B. subtilis
3. Materials and Methods
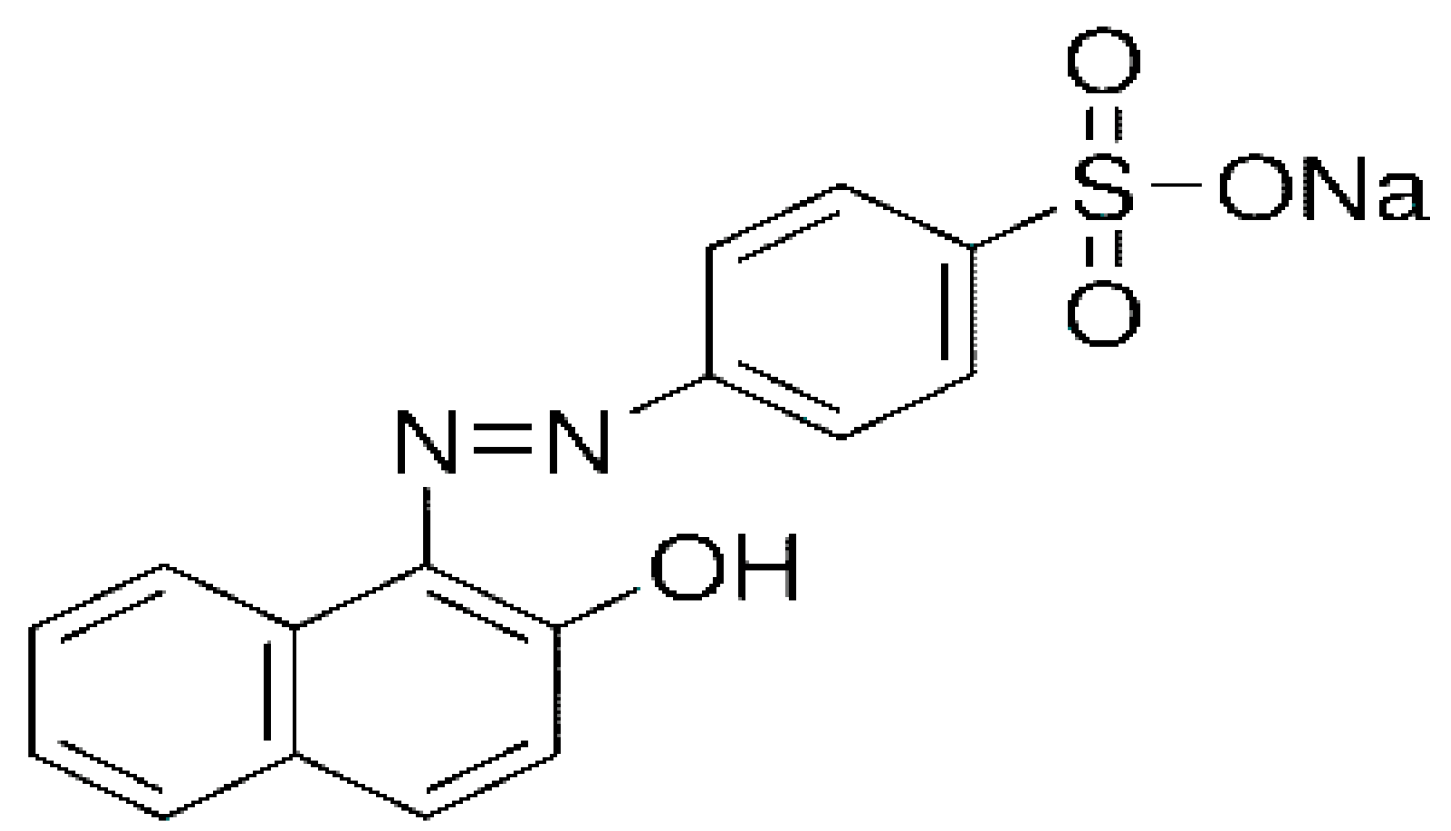
3.1. Dye and Other Reagents
3.2. Bacteria Cultures
3.3. Preparation of Dye Stock Solution and Growth Media (Nutrient Broth)
3.4. Culture Growth
3.5. Degradation/Decolorization Experiments
3.6. Determination of Physiochemical Parameters for Optimization of Degradation Efficiency of B. subtilis
3.6.1. Impact of Orange II Concentration on Degradation
3.6.2. Impact of Time and Temperature on Orange II Degradation
3.6.3. pH Effect on Orange II Degradation
3.6.4. Impact of Glucose and Urea on Degradation of Orange II
3.6.5. Impact of Sodium Chloride Concentration on Orange II Degradation
3.6.6. Redox Mediators’ Effect on Orange II Degradation
3.7. Orange II Degradation at Optimum Conditions
3.8. Extraction and Isolation of Metabolites after Orange II Biodegradation
3.8.1. GCMS and FTIR Analysis of Orange II Metabolites
3.8.2. NMR Analysis of the Metabolites Formed after Biodegradation
3.9. Phytotoxicity Assay
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, X.C.; Liu, G.Q.; Xu, Z.H.; Tao, W.Y. Decolorization of a dye industry effluent by Aspergillus fumigatus XC6. Appl. Microbiol. Biotechnol. 2007, 74, 239–243. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in Textile effluent: A critical review on current Treatment technologies with a Proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic azo dyes of textile industry: A sustainable approach using Microbial enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Pinheiro, L.R.S.; Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Degradation of Azo Dyes: Bacterial Potential for Bioremediation. Sustainability 2022, 14, 1510. [Google Scholar] [CrossRef]
- Kamal, I.M.; Abdeltawab, N.F.; Ragab, Y.M.; Farag, M.A.; Ramadan, M.A. Biodegradation, Decolorization, and Detoxification of Di-Azo Dye Direct Red 81 by Halotolerant, Alkali-Thermo-Tolerant Bacterial Mixed Cultures. Microorganisms 2022, 10, 994. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Latiff, A.A.A.; Daud, Z.; Mohamed, R.M.S.R.; Aziz, N.A.A.; Ismail, N.; Hossain, K. Adsorption of pollutants from palm oil mill effluent using natural adsorbents: Optimization and isotherm studies. Desalin. Water Treat. 2019, 169, 181–190. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef]
- Perera, H.J. Removal of acid orange 7 dye from wastewater: Review. Int. J. Waste Resour. 2019, 9, 2. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Ikram, M.; Zahoor, M.; Batiha, G.E.S. Biodegradation and decolorization of textile dyes by bacterial strains: A biological approach for wastewater treatment. Z. Phys. Chem. 2020, 235, 1381–1393. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced bioreactor treatments of hydrocarbon-containing wastewater. Appl. Sci. 2020, 10, 831. [Google Scholar] [CrossRef]
- Rosli, M.A.; Daud, Z.; Ridzuan, M.B.; Abd Aziz, N.; Awang, H.; Oyekanmi Adeleke, A. Equilibrium isotherm and kinetic study of the adsorption of organic pollutants of leachate by using micro peat-activated carbon composite media. Desalin. Water Treat. 2019, 160, 185–192. [Google Scholar] [CrossRef]
- Li, H.X.; Xu, B.; Tang, L.; Zhang, J.H.; Mao, Z.G. Reductive decolorization of indigo carmine dye with Bacillus sp. MZS10. Int. Biodeterior. Biodegrad. 2015, 103, 30–37. [Google Scholar] [CrossRef]
- Ivshina, I.; Tyumina, E.; Vikhareva, E. Biodegradation of emerging pollutants: Focus on pharmaceuticals. Microbiol. Aust. 2018, 39, 117–122. [Google Scholar] [CrossRef]
- Ngo, A.C.R.; Tischler, D. Microbial Degradation of Azo Dyes: Approaches and Prospects for a Hazard-Free Conversion by Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 4740. [Google Scholar] [CrossRef]
- Zerva, I.; Remmas, N.; Kagalou, I.; Melidis, P.; Ariantsi, M.; Sylaios, G.; Ntougias, S. Effect of Chlorination on Microbiological Quality of Effluent of a Full-Scale Wastewater Treatment Plant. Life 2021, 11, 68. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Biodegradation of Methylene Blue Using a Novel Lignin Peroxidase Enzyme Producing Bacteria, Named Bacillus sp. React3, as a Promising Candidate for Dye-Contaminated Wastewater Treatment. Fermentation 2022, 8, 190. [Google Scholar] [CrossRef]
- Hsueh, C.-C.; Chen, C.T.; Hsu, A.W.; Wu, C.C.; Chen, B.-Y. Comparative assessment of azo dyes and nitroaromatic compounds reduction using indigenous dye-decolorizing bacteria. J. Taiwan Inst. Chem. Eng. 2017, 79, 134–140. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Rahim, A.; Hanafiah, M.M.; Oyekanmi, A.A.; Shah, A.B.; Mahnashi, M.H.; Al Ali, A.; Jalal, N.A.; et al. Biodegradation of Azo Dye Methyl Red by Pseudomonas aeruginosa: Optimization of Process Conditions. Int. J. Environ. Res. Public Health 2022, 19, 9962. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Negm, F.; Hussain, S.; Ashraf, M.; Ashraf, M.; Ahmad, Z.; Arshad, M.; Crowley, D.E. Characterization and purification of membrane-bound azoreductase from azo dye degrading Shewanella sp. strain IFN4. CLEAN Soil Air Water 2016, 44, 1523–1530. [Google Scholar] [CrossRef]
- Kolekar, Y.M.; Konde, P.D.; Markad, V.L.; Kulkarni, S.V.; Chaudhari, A.U.; Kodam, K.M. Effective bioremoval and detoxification of textile dye mixture by Alishewanella sp. KMK6. Appl. Microbiol. Biotechnol. 2013, 97, 881–889. [Google Scholar] [CrossRef]
- Manjarrez, P.G.; Baldiris, A.R.; Baena, B.D. Application of environmental bacteria as potential methods of azo dye degradation systems. Glob. J. Environ. Sci. 2021, 7, 131–154. [Google Scholar]
- Saranraj, P.; Sumathi, V.; Reetha, D.; Stella, D. Decolourization and degradation of Direct azo dyes and Biodegradation of textile dye effluent by using bacteria isolated from textile dye effluent. J. Ecobiotechnol. 2010, 2, 7–11. [Google Scholar]
- Nadi, A.; Boyer, D.; Charbonnel, N.; Boukhriss, A.; Forestier, C.; Gmouh, S. Immobilisation of bacteria onto magnetic nanoparticles for the decolorisation and degradation of azo dyes. IET Nanobiotechnol. 2019, 13, 144–149. [Google Scholar] [CrossRef]
- Yasuhiko, S.; Tomoko, Y.; Amin, R.; Watura, S. Molecular cloning and characterization of the gene coding for azoreductase from Bacillus sp. OY1-2 isolated from soil. J. Biol. Chem. 2001, 276, 9059. [Google Scholar]
- Zissi, U.; Lyberatos, G.; Pavlou, S. Biodegradation of p-aminoazobenzene by Bacillus subtilis under aerobic conditions. J. Ind. Microbiol. Biotechnol. 1997, 19, 49–55. [Google Scholar] [CrossRef]
- Ponraj, M.; Gokila, K.; Zambare, V. Bacterial decolorization of textile dye-Orange 3R. Int. J Adv. Biotechnol. Res. 2011, 2, 168–177. [Google Scholar]
- Ikram, M.; Zahoor, M.; Khan, E.; Umar Khayam, S.M. Biodegradation of Novacron Turqueiose (Reactive Blue 21) by Pseudomonas aeruginosa. J. Chem. Soc. Pak. 2020, 42, 737–745. [Google Scholar]
- Khayam, S.M.U.; Zahoor, M.; Khan, E.; Shah, M. Reduction of Keto Group in Drimarene Blue by Aspergillus niger: A Predominant Reason for Subsequent Decolorization. Fresenius Environ. Bull. 2020, 29, 1397–1410. [Google Scholar]
- Ajaz, M.; Shakeel, S.; Rehman, A. Microbial Use for Azo Dye Degradation-A Strategy for Dye Bioremediation. Int. Microbiol. 2020, 23, 149–159. [Google Scholar] [CrossRef]
- Zhuang, M.; Sanganyado, E.; Zhang, X.; Xu, L.; Zhu, J.; Liu, W.; Song, H. Azo dye degrading bacteria tolerant to extreme conditions inhabit near shore ecosystems: Optimization and degradation pathways. J. Environ. Manag. 2020, 261, 110222–110231. [Google Scholar] [CrossRef]
- Sunar, N.M.; Mon, Z.K.; Rahim, N.A.; Leman, A.M.; Airish, N.A.M.; Khalid, A.; Ali, R.; Zaidi, E.; Azhar, A.T.S. Bioremediation of Coractive blue Dye by using Pseudomonas spp. Isolated from the Textile dye Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012062. [Google Scholar] [CrossRef]
- Guadie, A.; Tizazu, S.; Melese, M.; Guo, W.; Ngo, H.H.; Xia, S. Biodecolorization of textile azo dye using Bacillus sp. strain CH12 isolated from alkaline lake. Biotechnol. Rep. 2017, 15, 92–100. [Google Scholar] [CrossRef]
- Abo-State, M.A.M.; Saleh, Y.E.; Hazaa, H.A. Decolorization of Congo Red dye by bacterial isolates. J. Ecol. Environ. 2017, 5, 41–48. [Google Scholar]
- Hadibarata, T.; Adnan, L.A.; Yusoff, A.R.M.; Yuniarto, A.; Rubiyatno; Zubir, M.M.F.A.; Khudhair, A.B.; Teh, Z.C.; Naser, M.A. Microbial decolorization of an azo dye reactive black 5 using white-rot fungus Pleurotus eryngii F032. Water Air Soil Pollut. 2013, 224, 1595. [Google Scholar] [CrossRef]
- Solis, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Agrawal, S.; Tipre, D.; Patel, B.; Dave, S. Optimization of triazo Acid Black 210 dye degradation by Providencia sp. SRS82 and elucidation of degradation pathway. Process Biochem. 2014, 49, 110–119. [Google Scholar] [CrossRef]
- Khan, A.U.; Zahoor, M.; Rehman, M.U.; Shah, A.B.; Zekker, I.; Khan, F.A.; Ullah, R.; Albadrani, G.M.; Bayram, R.; Mohamed, H.R.H. Biological Mineralization of Methyl Orange by Pseudomonas aeruginosa. Water 2022, 14, 1551. [Google Scholar] [CrossRef]
- Chang, J.S.; Lin, C.Y. Decolorization kinetics of a Recombinant Escherichia coli strain harboring azo dye decolorizing determinants from Rhodococcus sp. Biotechnol. Lett. 2001, 23, 631–636. [Google Scholar] [CrossRef]
- Kalme, S.; Ghodake, G.; Gowindwar, S. Red HE7B degradation using desulfonation by Pseudomonas desmolyticum NCIM 2112. Int. Biodeter. Biodegr. 2007, 60, 327–333. [Google Scholar] [CrossRef]
- Suzuki, T.; Timofei, S.; Kurunczi, L.; Dietze, U.; Schuurmann, G. Correlation of aerobic biodegradability of Sulfonated azo dyes with the chemical structure. Chemosphere 2001, 45, 1–9. [Google Scholar] [CrossRef]
- Kannan, S.; Dhandayuthapani, K.; Sultana, M. Decolorization and degradation of Azo due—Remazol Black B by newly isolated Pseudomonas putida. Int. J. Curr. Microbiol. 2013, 2, 108–116. [Google Scholar]
- Dave, S.R.; Patel, T.L.; Tipre, D.R. Bacterial degradation of azo dye containing wastes. In Microbial Degradation of Synthetic Dyes in Wastewaters; Springer: Cham, Switzerland, 2015; Volume 1, pp. 57–83. [Google Scholar]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Shah, M.P. Microbial degradation of textile dye (Remazol Black B) by Bacillus spp. ETL-2012. J. Appl. Environ. Microbiol. 2013, 1, 6–11. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.L. Bio-removal of azo dyes: A review. Int. J. Appl. Sci. Biotechnol. 2017, 5, 108–126. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J. The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes Pigm. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Yue, P.L. Optimal Decolorization and Kinetic Modeling of Synthetic Dye by Pseudomonas Strains. Water Resour. 2001, 35, 3579–3585. [Google Scholar] [CrossRef]
- Anjaneya, O.; Yogesh, S.O.; Santoshkumar, M.; Karegoudar, T.B. Decolorization of sulfonated azo dye Metanil Yellow by newly isolated bacterial strains: Bacillus sp. Strain AK1 and Lysinibacillus sp. strain AK2. J. Hazard. Mat. 2011, 190, 351–358. [Google Scholar] [CrossRef]
- Sihag, S.; Pathak, H.; Jaroli, D.P. Factors affecting the rate of biodegradation of polyaromatic hydrocarbons. Int. J. Pure Appl. Biosci. 2014, 2, 185–202. [Google Scholar]
- Khan, R.; Bhawana, P.; Fulekar, M.H. Microbial Decolorization and Degradation of Synthetic dyes: A review. Rev. Environ. Sci. Biotechnol. 2012, 12, 75–97. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Ecofriendly degradation of sulfonated diazo dye C.I. Reactive Green 19A using Micrococcus glutamicus NCIM-2168. Bioresour. Technol. 2009, 100, 3897. [Google Scholar] [CrossRef]
- Bheemaraddi, M.C.; Shivannavar, C.T.; Gaddad, S.M. Effect of carbon and nitrogen sources on biodegradation of textile azo dye Reactive Violet 5 by Pseudomonas aeruginosa GSM3. Sch. Acad. J. Biosci. 2014, 2, 285–289. [Google Scholar]
- Chang, J.S.; Kuo, T.S.; Chao, Y.P.; Ho, J.Y.; Lin, P.J. Azo dye decolorization with a mutant Escherichia coli strain. Biotechnol. Lett. 2000, 22, 807–812. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Ullah, R.; Farraj, D.A.A.; Elshikh, M.S.; Zekker, I.; Gulfam, N. Biological Degradation of the Azo Dye Basic Orange 2 by Escherichia coli: A Sustainable and Ecofriendly Approach for the Treatment of Textile Wastewater. Water 2022, 14, 2063. [Google Scholar] [CrossRef]
- Khan, A.U.; Rehman, M.U.; Zahoor, M.; Shah, A.B.; Zekker, I. Biodegradation of Brown 706 Dye by Bacterial Strain Pseudomonas aeruginosa. Water 2021, 13, 2959. [Google Scholar] [CrossRef]
- Cui, D.; Li, G.; Zhao, M.; Han, S. Decolourization of azo dyes by a newly isolated Klebsiella sp. strain Y3, and effects of various factors on biodegradation. Biotechnol. Equip. 2014, 28, 478–486. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Kathiravan, M.N.; Srinivasan, R.; Sankaranarayanan, S. Evaluation and elimination of inhibitory effects of salts and heavy metal ions on biodegradation of Congo red by Pseudomonas sp. mutant. Bioresour. Technol. 2011, 102, 3687–3693. [Google Scholar] [CrossRef]
- Moir, D.; Masson, S.; Chu, I. Structure-activity relationship study on the bioreduction of AZO dyes by Clostridium paraputrificum. Environ. Toxicol. Chem. SETAC 2001, 20, 479–484. [Google Scholar] [CrossRef]
- Rau, J.; Stolz, A. Oxygen-insensitive nitroreductases NfsA and NfsB of Escherichia coli function under anaerobic conditions as lawsone-dependent Azo reductases. Appl. Environ. Microbiol. 2003, 69, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Olukanni, O.; Awotula, A.; Osuntoki, A.; Govindwar, S. Influence of redox mediators and media on methyl red decolorization and its biodegradation by Providencia rettgeri. SN Appl. Sci. 2019, 1, 697. [Google Scholar] [CrossRef]
- Van der Zee, F.; Bouwman, R.; Strik, D.; Lettinga, G.; Field, J. Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnol. Bioeng. 2001, 75, 691–701. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Ahmad, A.; Mohd Setapar, S.H.; Alshammari, M.B.; Jawaid, M.; Hanafiah, M.M.; Abdul Khalil, H.P.S.; Vaseashta, A. Sustainable Durio zibethinus-derived biosorbents for congo red removal from aqueous solution: Statistical optimization, isotherms and mechanism studies. Sustainability 2021, 13, 13264. [Google Scholar] [CrossRef]
- Haq, I.; Raj, A. Biodegradation of Azure-B dye by Serratia liquefaciens and its validation by phytotoxicity, genotoxicity and cytotoxicity studies. Chemosphere 2018, 196, 58–68. [Google Scholar] [CrossRef]
- Barathi, S.; Aruljothi, K.N.; Karthik, C.; Padikasan, I.A. Optimization for enhanced ecofriendly decolorization and detoxification of Reactive Blue160 textile dye by Bacillus subtilis. Biotechnol. Rep. 2020, 28, 522. [Google Scholar] [CrossRef]
- Ogola, H.J.O.; Ashida, H.; Ishikawa, T.; Sawa, Y. Explorations and Applications of Enzyme-linked Bioremediation of Synthetic Dyes. In Advances in Bioremediation of Wastewater and Polluted Soil; Shiomi, N., Ed.; Intech: London, UK, 2015; pp. 111–144. [Google Scholar]
- Masarbo, R.S.; Karegoudar, T. Decolourisation of toxic azo dye Fast Red E by three bacterial strains: Process optimization and Toxicity assessment. Int. J. Environ. Anal. Chem. 2020, 102, 2686–2696. [Google Scholar] [CrossRef]
- Amin, S.; Rastogi, R.P.; Chaubey, M.G.; Jain, K.; Divecha, J.; Desai, C.; Madamwar, D. Degradation and toxicity analysis of a reactive textile diazo dye-Direct Red 81 by newly isolated Bacillus sp. DMS2. Front. Microbiol. 2020, 11, 2280. [Google Scholar] [CrossRef]
- Elfarash, A.; Mawad, A.M.; Yousef, N.M.; Shoreit, A.A. Azoreductase kinetics and gene expression in the synthetic dyes-degrading Pseudomonas. Egypt. J. Basic Appl. Sci. 2017, 4, 315–322. [Google Scholar] [CrossRef]
- Sahasrabudhe, M.M.; Saratale, R.G.; Saratale, G.D.; Pathade, G.R. Decolorization and detoxification of sulfonated toxic diazo dye CI Direct Red 81 by Enterococcus faecalis YZ 66. J. Environ. Health Sci. Eng. 2014, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Mani, P.; Fidal, V.T.; Bowman, K.; Breheny, M.; Chandra, T.S.; Keshavarz, T.; Kyazze, G. Degradation of azo dye (acid orange 7) in a Microbial fuel cell: Comparison between anodic microbial-mediated reduction and cathodic laccase-mediated oxidation. Front. Energy Res. 2019, 7, 101. [Google Scholar] [CrossRef]
- Maniyam, M.N.; Ibrahim, A.L.; Cass, A.E.G. Decolourization and biodegradation of azo dye methyl red by Rhodococcus strain UCC 0016. Environ. Technol. 2020, 41, 71–85. [Google Scholar] [CrossRef]
- Veni, P.; Satish, C.P.; Tushar, J.; Diksha, S.; Saurabh, G.; Saurabh, K.; Mukesh, S. Biodegradation of toxic dyes: A comparative study of enzyme action in a microbial system. In Smart Bioremediation Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 255–287. [Google Scholar]
- Leelakriangsak, M.; Borisut, S. Characterization of the decolorizing activity of azo dyes by Bacillus subtilis azoreductase AzoR1. Songklanakarin J. Sci. Technol. 2012, 34, 509–516. [Google Scholar]
- Gomaa, E.Z. Biodegradation and detoxification of azo dyes by some bacterial strains. Microbiol. J. 2016, 6, 15–24. [Google Scholar] [CrossRef][Green Version]
- Muliadi, F.N.A.; Halmi, M.I.E.; Wahid, S.B.A.; Gani, S.S.A.; Zaidan, U.H.; Mahmud, K.; Abd Shukor, M.Y. Biostimulation of Microbial Communities from Malaysian Agricultural Soil for Detoxification of Metanil Yellow Dye; a Response Surface Methodological Approach. Sustainability 2020, 13, 138. [Google Scholar] [CrossRef]





| Factors | Units | Code | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| pH | - | 3 | 6.5 | 10 | |
| Dye concentration | ppm | 5 | 22.5 | 40 | |
| Incubation time | day | 3 | 21 | 12 | |
| Temperature | °C | 25 | 37.5 | 50 | |
| Run | Observed Value | Predicted Value | ||||
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 2 | 0 | 47.61 | 36.95 |
| 2 | −1 | −1 | −1 | 1 | 37.20 | 68.30 |
| 3 | 1 | −1 | 1 | 1 | 31.40 | 56.10 |
| 4 | 0 | −2 | 0 | 0 | 37.95 | 76.40 |
| 5 | −1 | −1 | −1 | −1 | 56.60 | 52.80 |
| 6 | −1 | 1 | 1 | −1 | 58.20 | 68.90 |
| 7 | 1 | 1 | 1 | −1 | 57.89 | 52.70 |
| 8 | 0 | 0 | 0 | 2 | 64.20 | 75.60 |
| 9 | −1 | 1 | −1 | −1 | 58.43 | 71.40 |
| 10 | 0 | 0 | 0 | 0 | 75.40 | 83.40 |
| 11 | 0 | 0 | 0 | 0 | 68.30 | 83.40 |
| 12 | 0 | 2 | 0 | 0 | 68.20 | 73.60 |
| 13 | −1 | 1 | 1 | 1 | 70.12 | 66.60 |
| 14 | 1 | −1 | 1 | −1 | 72.90 | 80.30 |
| 15 | −1 | −1 | −1 | 1 | 67.80 | 81.20 |
| 16 | 0 | 0 | 0 | 0 | 63.21 | 83.40 |
| 17 | 1 | −1 | 1 | −1 | 42.30 | 56.40 |
| 18 | −1 | 1 | −1 | 1 | 61.80 | 68.90 |
| 19 | −2 | 0 | 0 | 0 | 72.15 | 19.2 |
| 20 | −1 | 1 | 1 | 1 | 81.20 | 57.80 |
| 21 | 1 | −1 | −1 | −1 | 71.23 | 58.20 |
| 22 | 1 | 1 | −1 | −1 | 69.20 | 52.30 |
| 23 | 1 | 1 | −1 | 1 | 61.20 | 59.70 |
| 24 | 1 | −1 | −1 | 1 | 68.93 | 48.30 |
| 25 | 0 | 0 | 0 | 0 | 69.87 | 83.40 |
| 26 | 1 | 1 | 1 | 1 | 69.20 | 32.60 |
| 27 | 0 | 0 | 0 | 0 | 65.80 | 83.40 |
| 28 | 2 | 0 | 0 | 0 | 69.73 | 61.70 |
| 29 | −1 | −1 | 1 | 1 | 59.87 | 59.20 |
| 30 | 0 | 0 | 0 | 0 | 69.20 | 83.40 |
| Source | Sum of Squares | DF | Square Values | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 7509.07 | 14 | 536.36 | 9.13 | <0.0001 |
| 12.61 | 1 | 12.61 | 0.21 | 0.0498 | |
| 51.63 | 1 | 51.63 | 0.88 | 0.0363 | |
| 434.09 | 1 | 434.09 | 8.77 | 0.0097 | |
| 207.74 | 1 | 207.74 | 7.59 | 0.0147 | |
| 36.69 | 1 | 36.69 | 3.67 | 0.0074 | |
| Lack of fit | - | - | - | - | 0.6246 |
| Mean | PRESS | Adeq Precision | R-Squared | Adj R-Squared | Std. Dev. |
| 64.51 | 5076.88 | 12.233 | 0.89.50 | 0.7969 | 7.67 |
| S. No | Metabolite | Peak Area | Retention Time | Chemical Formula | Molecular Weight |
|---|---|---|---|---|---|
| 1. | Naphthalene | 2.29 | 6.99 | C10H8 | 128 |
| 2. | o-Xylene | 1.42 | 2.24 | C8H10 | 106 |
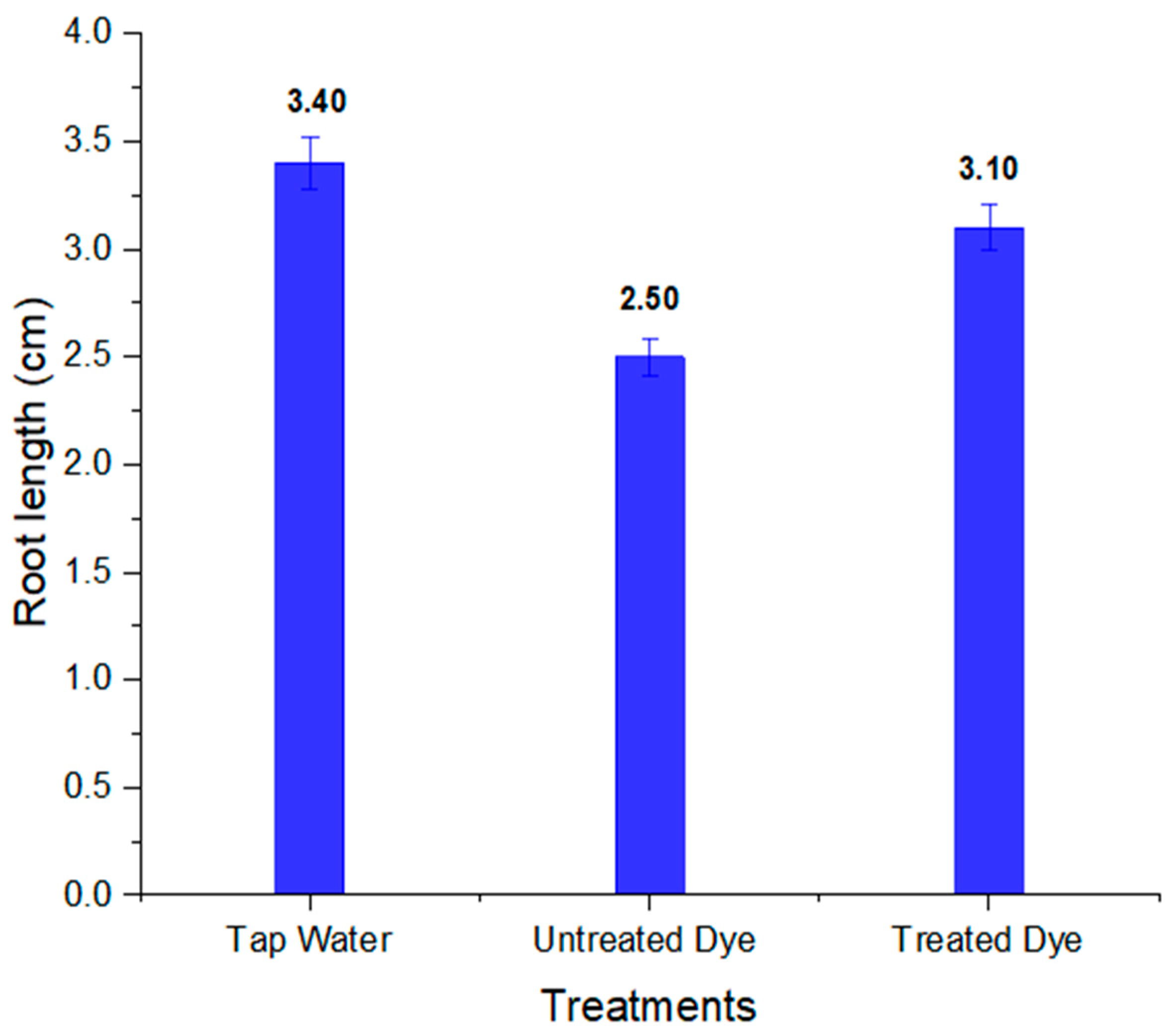
| S.No | Parameters | Tap Water | Dye | Treated Dye |
|---|---|---|---|---|
| 1 | % germination | 100 | 60 | 80 |
| 2 | Shoot length (cm) | 8.30 ± 0.20 | 6.50 ± 0.16 | 7.20 ± 0.18 |
| 3 | Root length (cm) | 3.40 ± 0.11 | 2.50± 0.08 | 3.10 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Islam, N.U.; Ullah, M.; Mahnashi, M.H.; Ali, A.A.; Jalal, N.A.; et al. Bacillus subtilis: As an Efficient Bacterial Strain for the Reclamation of Water Loaded with Textile Azo Dye, Orange II. Int. J. Mol. Sci. 2022, 23, 10637. https://doi.org/10.3390/ijms231810637
Ikram M, Naeem M, Zahoor M, Hanafiah MM, Oyekanmi AA, Islam NU, Ullah M, Mahnashi MH, Ali AA, Jalal NA, et al. Bacillus subtilis: As an Efficient Bacterial Strain for the Reclamation of Water Loaded with Textile Azo Dye, Orange II. International Journal of Molecular Sciences. 2022; 23(18):10637. https://doi.org/10.3390/ijms231810637
Chicago/Turabian StyleIkram, Muhammad, Mohammad Naeem, Muhammad Zahoor, Marlia Mohd Hanafiah, Adeleke Abdulrahman Oyekanmi, Noor Ul Islam, Midrar Ullah, Mater H. Mahnashi, Amer Al Ali, Naif A. Jalal, and et al. 2022. "Bacillus subtilis: As an Efficient Bacterial Strain for the Reclamation of Water Loaded with Textile Azo Dye, Orange II" International Journal of Molecular Sciences 23, no. 18: 10637. https://doi.org/10.3390/ijms231810637
APA StyleIkram, M., Naeem, M., Zahoor, M., Hanafiah, M. M., Oyekanmi, A. A., Islam, N. U., Ullah, M., Mahnashi, M. H., Ali, A. A., Jalal, N. A., Bantun, F., Momenah, A. M., & Sadiq, A. (2022). Bacillus subtilis: As an Efficient Bacterial Strain for the Reclamation of Water Loaded with Textile Azo Dye, Orange II. International Journal of Molecular Sciences, 23(18), 10637. https://doi.org/10.3390/ijms231810637









