A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
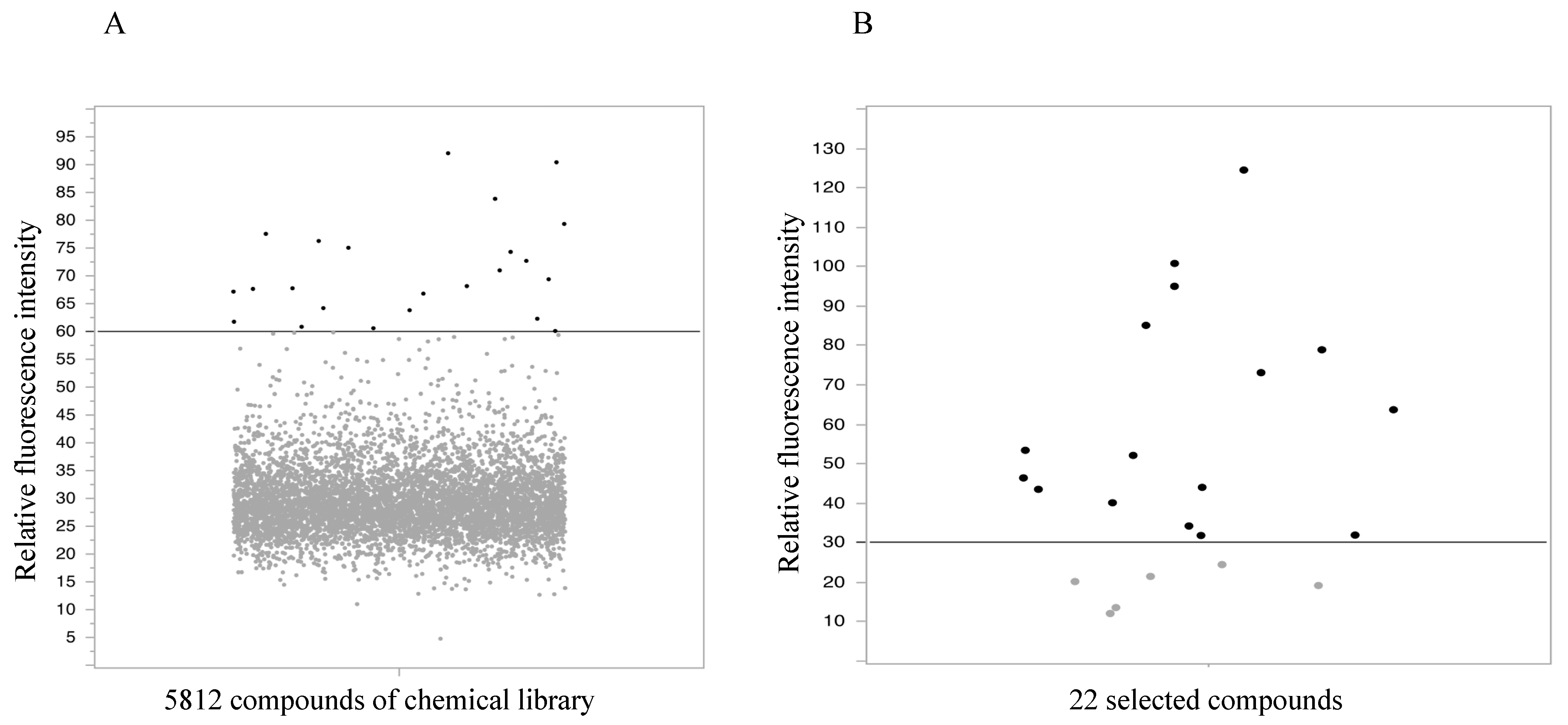
2.1. High-Throughput Screening
2.2. Flow Cytometry Assay
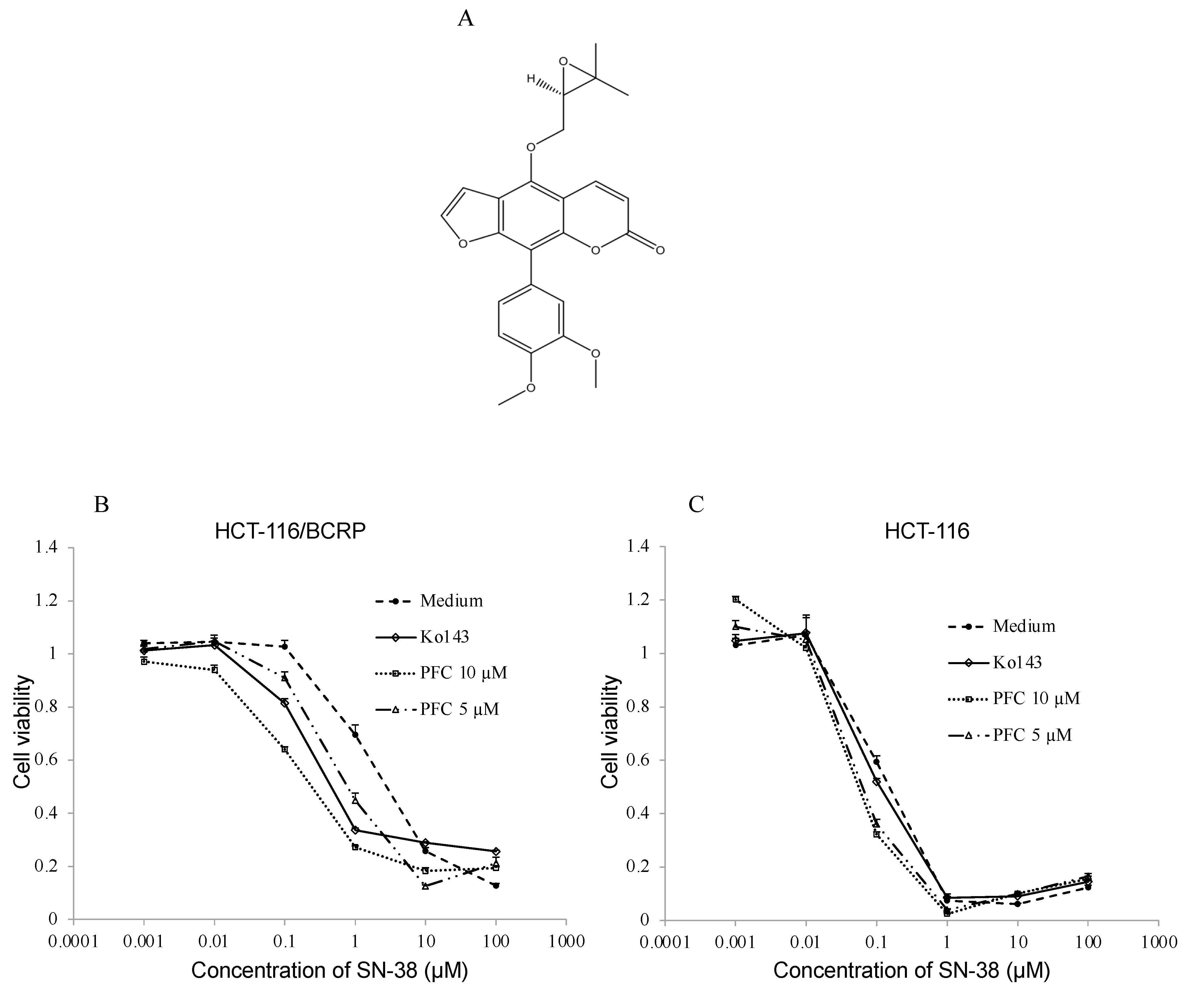
2.3. Cytotoxic Assay
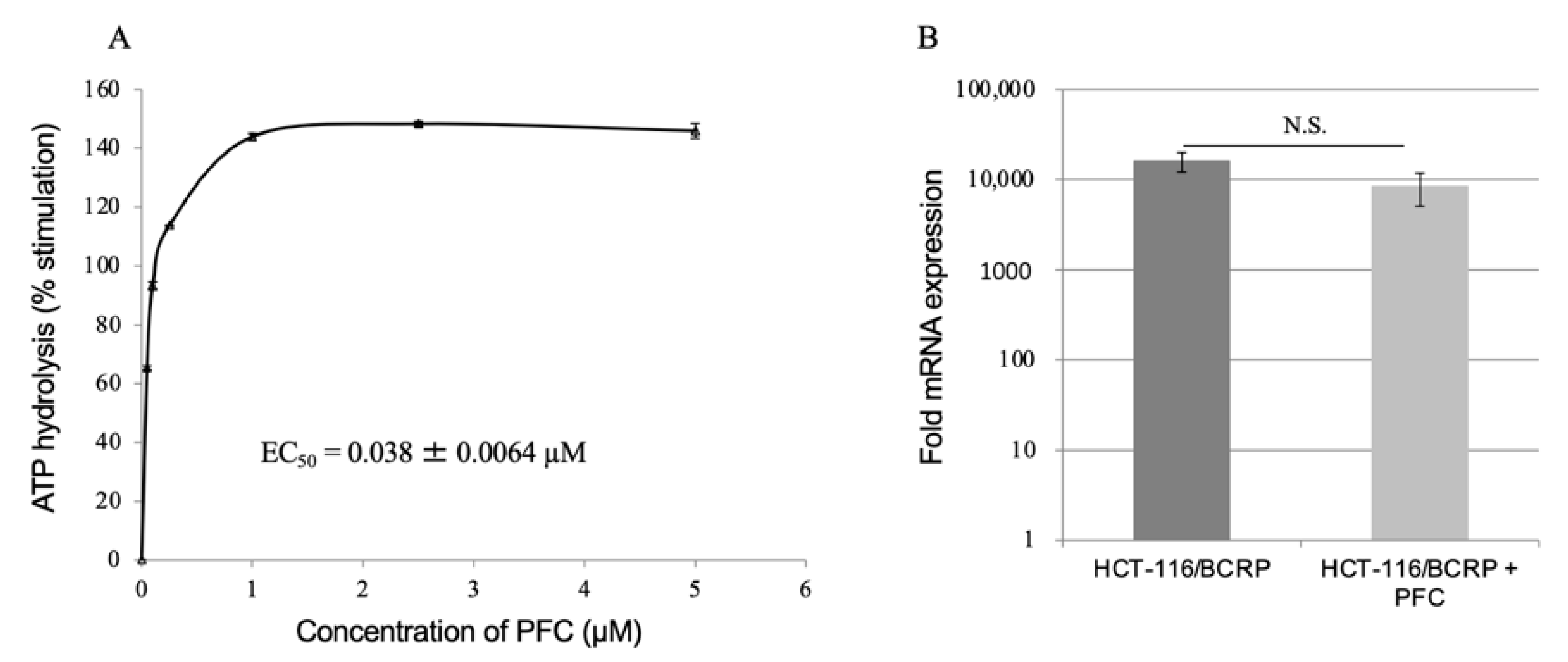
2.4. ATPase Assay
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction
2.6. Antitumor Activity In Vivo
3. Discussion
4. Materials and Methods
4.1. Screening of Chemical Compounds
4.2. Chemicals
4.3. Cell Lines
4.4. High-Throughput Screening
4.5. Flow Cytometry
4.6. Cytotoxic Assay
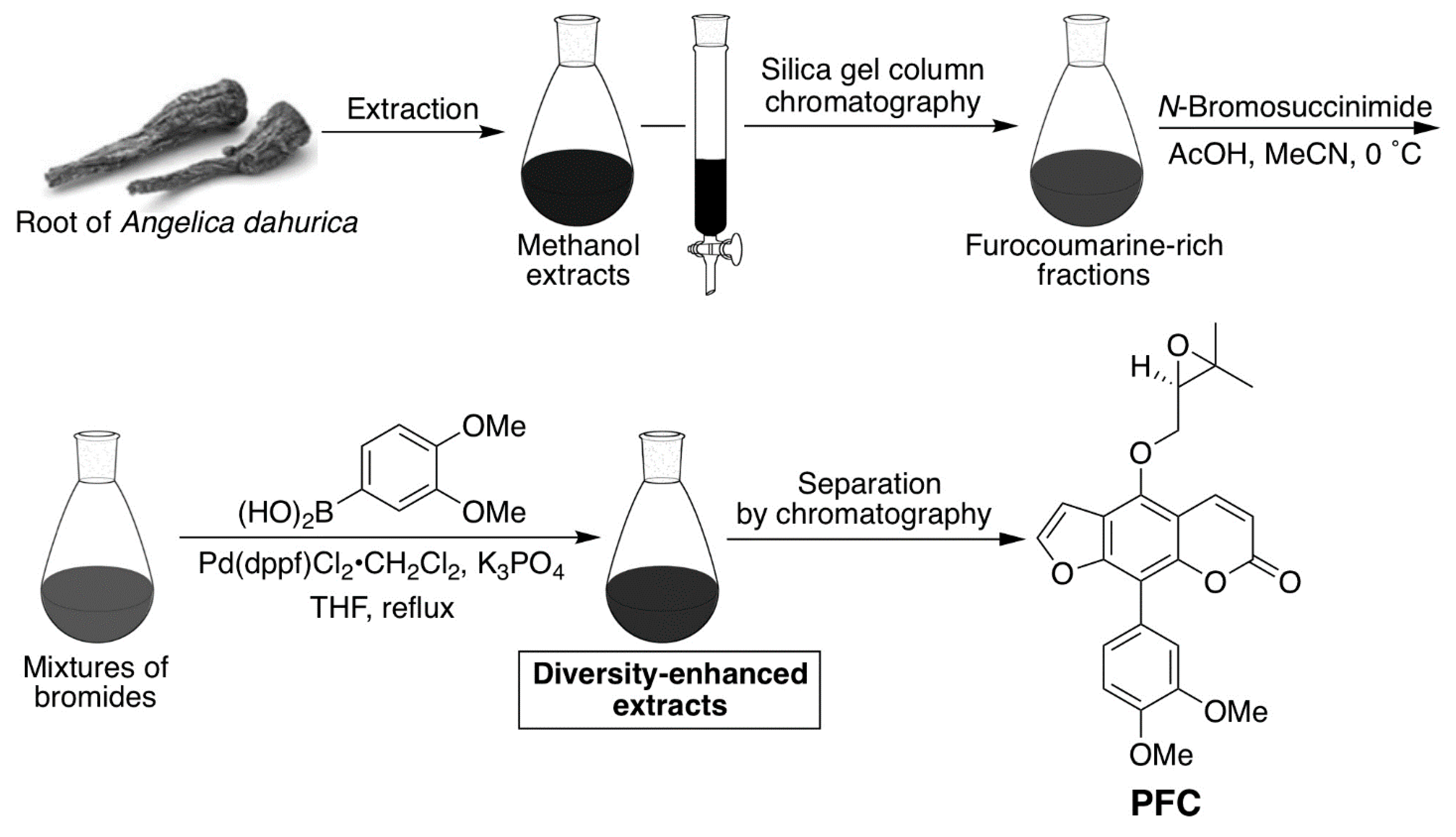
4.7. Preparation of the Phenylfurocoumarin Derivative
4.8. ATPase Assay
4.9. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. In Vivo Antitumor Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Ohnuma, S.; Ambudkar, S.V. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr. Drug Targets 2011, 12, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Allikmets, R.; Schriml, L.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar]
- Aronica, E.; Gorter, J.A.; Redeker, S.; van Vliet, E.; Ramkema, M.; Scheffer, G.L.; Scheper, R.J.; Van Der Valk, P.; Leenstra, S.; Baayen, J.C.; et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 2005, 46, 849–857. [Google Scholar] [CrossRef]
- Fetsch, P.A.; Abati, A.; Litman, T.; Morisaki, K.; Honjo, Y.; Mittal, K.; Bates, S.E. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006, 235, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; van Gastelen, M.A.; Pijnenborg, A.C.; Schinkel, A.H.; van De Vijver, M.J.; Scheper, R.J.; Schellens, J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001, 61, 3458–3464. [Google Scholar]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharm. Pers. Med. 2014, 7, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Robey, R.; Medina-Pérez, W.Y.; Nishiyama, K.; Lahusen, T.; Miyake, K.; Litman, T.; Senderowicz, A.M.; Ross, D.D.; Bates, S.E. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin. Cancer Res. 2001, 7, 145–152. [Google Scholar]
- Maliepaard, M.; van Gastelen, M.; Tohgo, A.; Hausheer, F.H.; van Waardenburg, R.C.; de Jong, L.A.; Pluim, D.; Beijnen, J.H.; Schellens, J.H. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin. Cancer Res. 2001, 7, 935–941. [Google Scholar] [PubMed]
- Burger, H.; van Tol, H.; Boersma, A.W.; Brok, M.; Wiemer, E.A.; Stoter, G.; Nooter, K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 2004, 104, 2940–2942. [Google Scholar] [CrossRef]
- Elkind, N.B.; Szentpétery, Z.; Apáti, A.; Ozvegy-Laczka, C.; Várady, G.; Ujhelly, O.; Szabó, K.; Homolya, L.; Váradi, A.; Buday, L.; et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, gefitinib). Cancer Res. 2005, 65, 1770–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar] [PubMed]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.; Koomen, G.J.; Schinkel, A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar]
- de Bruin, M.; Miyake, K.; Litman, T.; Robey, R.; Bates, S.E. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999, 146, 117–126. [Google Scholar] [CrossRef]
- Robey, R.W.; Steadman, K.; Polgar, O.; Morisaki, K.; Blayney, M.; Mistry, P.; Bates, S.E. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004, 64, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Dai, Y.; Vethanayagam, R.R.; Hebert, M.F.; Thummel, K.E.; Unadkat, J.D.; Ross, D.D.; Mao, Q. Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother. Pharmacol. 2006, 58, 374–383. [Google Scholar] [CrossRef]
- Chearwae, W.; Shukla, S.; Limtrakul, P.; Ambudkar, S.V. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 1995–2006. [Google Scholar] [CrossRef] [Green Version]
- Anreddy, N.; Patel, A.; Zhang, Y.; Wang, Y.J.; Shukla, S.; Kathawala, R.J.; Kumar, P.; Gupta, P.; Ambudkar, S.V.; Wurpel, J.N.D.; et al. A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo. Oncotarget 2015, 6, 39276–39291. [Google Scholar] [CrossRef]
- Ji, N.; Yang, Y.; Lei, Z.N.; Cai, C.Y.; Wang, J.Q.; Gupta, P.; Xian, X.; Yang, D.H.; Kong, D.; Chen, Z.S. Ulixertinib (BVD-523) antagonizes ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Biochem. Pharmacol. 2018, 158, 274–285. [Google Scholar] [CrossRef]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of febuxostat as a new strong ABCG2 inhibitor: Potential applications and risks in clinical situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Ohnuma, S.; Fukuda, M.; Chufan, E.E.; Kudoh, K.; Kanehara, K.; Sugisawa, N.; Ishida, M.; Naitoh, T.; Shibata, H.; et al. Synthetic analogs of curcumin modulate the function of multidrug resistance-linked ATP-binding cassette transporter ABCG2. Drug Metab. Dispos. 2017, 45, 1166–1177. [Google Scholar] [CrossRef] [Green Version]
- Westover, D.; Ling, X.; Lam, H.; Welch, J.; Jin, C.; Gongora, C.; Del Rio, M.; Wani, M.; Li, F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol. Cancer 2015, 14, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Fan, Y.F.; Cai, C.Y.; Wang, J.Q.; Teng, Q.X.; Lei, Z.N.; Zeng, L.; Gupta, P.; Chen, Z.S. Olmutinib (BI1482694/HM61713), a novel epidermal growth factor receptor tyrosine kinase inhibitor, reverses ABCG2-mediated multidrug resistance in cancer cells. Front. Pharmacol. 2018, 9, 1097. [Google Scholar] [CrossRef] [Green Version]
- Peña-Solórzano, D.; Stark, S.A.; König, B.; Sierra, C.A.; Ochoa-Puentes, C. ABCG2/BCRP: Specific and nonspecific modulators. Med. Res. Rev. 2017, 37, 987–1050. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.J.; Bokesch, H.R.; Dean, M.; Bates, S.E.; Robey, R.W.; Goncharova, E.I.; Wilson, J.A.; McMahon, J.B. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J. Biomol. Screen. 2006, 11, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, C.J.; Robey, R.W.; Bokesch, H.R.; Bates, S.E.; Shukla, S.; Ambudkar, S.V.; Dean, M.; McMahon, J.B. New inhibitors of ABCG2 identified by high-throughput screening. Mol. Cancer Ther. 2007, 6, 3271–3278. [Google Scholar] [CrossRef] [Green Version]
- Strouse, J.J.; Ivnitski-Steele, I.; Khawaja, H.M.; Perez, D.; Ricci, J.; Yao, T.; Weiner, W.S.; Schroeder, C.E.; Simpson, D.S.; Maki, B.E.; et al. A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry. J. Biomol. Screen. 2013, 18, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Byun, Y.; Ren, Y.R.; Liu, J.O.; Laterra, J.; Pomper, M.G. Identification of inhibitors of ABCG2 by a bioluminescence imaging-based high-throughput assay. Cancer Res. 2009, 69, 5867–5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugisawa, N.; Ohnuma, S.; Ueda, H.; Murakami, M.; Sugiyama, K.; Ohsawa, K.; Kano, K.; Tokuyama, H.; Doi, T.; Aoki, J.; et al. Novel potent ABCB1 modulator, Phenethylisoquinoline alkaloid, reverses multidrug resistance in cancer cell. Mol. Pharm. 2018, 15, 4021–4030. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, M.; Kuga, T. Central effects of the neurotropic mycotoxin fumitremorgin A in the rabbit (I). Effects on the spinal cord. Jpn. J. Pharmacol. 1989, 50, 167–173. [Google Scholar] [CrossRef]
- Rabindran, S.K.; He, H.; Singh, M.; Brown, E.; Collins, K.I.; Annable, T.; Greenberger, L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998, 58, 5850–5858. [Google Scholar] [PubMed]
- Weidner, L.D.; Zoghbi, S.S.; Lu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Mulder, J.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The inhibitor Ko143 is not specific for ABCG2. J. Pharmacol. Exp. Ther. 2015, 354, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Aslanis, V.; Zhang, J.; Lomeli, B.; Grosch, K.; Ouatas, T. Effect of cyclosporine coadministration on the pharmacokinetics of eltrombopag in healthy volunteers. Cancer Chemother. Pharmacol. 2018, 82, 847–855. [Google Scholar] [CrossRef]
- Huguet, J.; Lu, J.; Gaudette, F.; Chiasson, J.L.; Hamet, P.; Michaud, V.; Turgeon, J. No effects of pantoprazole on the pharmacokinetics of rosuvastatin in healthy subjects. Eur. J. Clin. Pharmacol. 2016, 72, 925–931. [Google Scholar] [CrossRef]
- Zhang, X.N.; Ma, Z.J.; Wang, Y.; Sun, B.; Guo, X.; Pan, C.Q.; Chen, L.M. Angelica dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS ONE 2017, 12, e0177862. [Google Scholar]
- Kim, Y.K.; Kim, Y.S.; Ryu, S.Y. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytotherapy Res. 2007, 21, 288–290. [Google Scholar] [CrossRef]
- Lechner, D.; Stavri, M.; Oluwatuyi, M.; Pereda-Miranda, R.; Gibbons, S. The anti-staphylococcal activity of Angelica dahurica (Bai Zhi). Phytochemistry 2004, 65, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.S.; Lim, S.S.; Suzuki, K.; Jung, S.H.; Lee, S.; Lee, Y.S.; Shin, K.H.; Ohuchi, K. Inhibitory effects of furanocoumarins isolated from the roots of Angelica dahurica on prostaglandin E2 production. Planta Med. 2003, 69, 408–412. [Google Scholar]
- Shukla, S.; Wu, C.-P.; Nandigama, K.; Ambudkar, S.V. The naphthoquinones, vitamin K3 and its structural analogue plumbagin, are substrates of the multidrug resistance linked ATP binding cassette drug transporter ABCG2. Mol. Cancer Ther. 2007, 6, 3279–3286. [Google Scholar] [CrossRef] [Green Version]
- Ambudkar, S.V. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol 1998, 292, 504–514. [Google Scholar]
- Kage, K.; Tsukahara, S.; Sugiyama, T.; Asada, S.; Ishikawa, E.; Tsuruo, T.; Sugimoto, Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 2002, 97, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Shibata, K.; Mitsuhashi, J.; Noguchi, K.; Sugimoto, Y. Pharmacological interplay between breast cancer resistance protein and gefitinib in epidermal growth factor receptor signaling. Anticancer Res. 2009, 29, 1059–1065. [Google Scholar] [PubMed]
- Oshima, Y.; Kikuchi, H. Developments toward the production of diverse natural-product-like compounds: Diversity-oriented synthesis and diversity-enhanced extracts. Heterocycles 2018, 96, 1509. [Google Scholar] [CrossRef]
- Kikuchi, H.; Sakurai, K.; Oshima, Y. Development of Diversity-Enhanced Extracts of Curcuma zedoaria and Their New Sesquiterpene-like compounds. Org. Lett. 2014, 16, 1916–1919. [Google Scholar] [CrossRef]
- Kobayashi, M.; Funayama, R.; Ohnuma, S.; Unno, M.; Nakayama, K. Wnt-beta-catenin signaling regulates ABCC3 (MRP3) transporter expression in colorectal cancer. Cancer Sci. 2016, 107, 1776–1784. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, R.; Nishiyama, Y.; Furuta, T.; Hatano, H.; Igarashi, Y.; Asakawa, N.; Kodaira, H.; Takahashi, H.; Aiyama, R.; Matsuzaki, T.; et al. Novel acrylonitrile derivatives, YHO-13177 and YHO-13351, reverse BCRP/ABCG2-mediated drug resistance in vitro and in vivo. Mol. Cancer Ther. 2011, 10, 1252–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| IC50 ± SE (µM) a | Fold Reversal b | IC50 ± SE (µM) a | Fold Reversal b | |
|---|---|---|---|---|
| HCT-116 | HCT-116/BCRP | |||
| Medium | 0.11 ± 0.0037 | 1.0 | 2.59 ± 0.24 | 1.0 |
| Ko143 1 µM | 0.11 ± 0.0036 | 1.0 | 0.26 ± 0.027 | 10.1 |
| PFC 5 µM | 0.077 ± 0.022 | 1.4 | 0.36 ± 0.11 | 7.3 |
| PFC 10 µM | 0.057 ± 0.013 | 1.9 | 0.19 ± 0.018 | 13.8 |
| IC50 ± SE (µM) a | ||
|---|---|---|
| HCT-116 | HCT-116/BCRP | |
| Ko143 1 µM | 77.97 ± 2.33 | 115.79 ± 4.85 |
| PFC 10 µM | 42.70 ± 2.42 | 43.07 ± 3.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokubo, S.; Ohnuma, S.; Murakami, M.; Kikuchi, H.; Funayama, S.; Suzuki, H.; Kajiwara, T.; Yamamura, A.; Karasawa, H.; Sugisawa, N.; et al. A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 12502. https://doi.org/10.3390/ijms222212502
Kokubo S, Ohnuma S, Murakami M, Kikuchi H, Funayama S, Suzuki H, Kajiwara T, Yamamura A, Karasawa H, Sugisawa N, et al. A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(22):12502. https://doi.org/10.3390/ijms222212502
Chicago/Turabian StyleKokubo, Shoji, Shinobu Ohnuma, Megumi Murakami, Haruhisa Kikuchi, Shota Funayama, Hideyuki Suzuki, Taiki Kajiwara, Akihiro Yamamura, Hideaki Karasawa, Norihiko Sugisawa, and et al. 2021. "A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 22: 12502. https://doi.org/10.3390/ijms222212502
APA StyleKokubo, S., Ohnuma, S., Murakami, M., Kikuchi, H., Funayama, S., Suzuki, H., Kajiwara, T., Yamamura, A., Karasawa, H., Sugisawa, N., Ohsawa, K., Kano, K., Aoki, J., Doi, T., Naitoh, T., Ambudkar, S. V., & Unno, M. (2021). A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo. International Journal of Molecular Sciences, 22(22), 12502. https://doi.org/10.3390/ijms222212502






