Upregulated Guanine Deaminase Is Involved in Hyperpigmentation of Seborrheic Keratosis via Uric Acid Release
Abstract
1. Introduction
2. Results
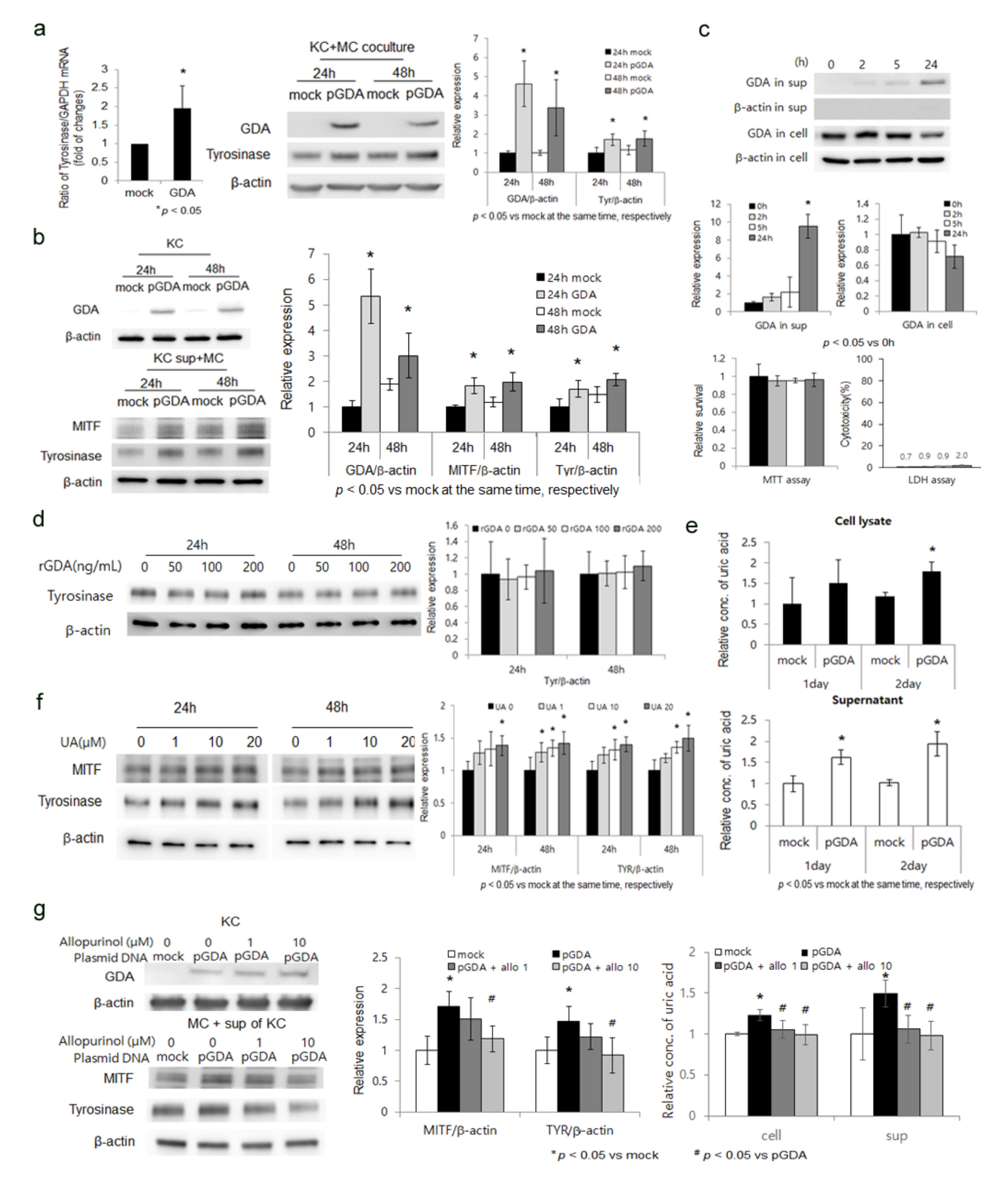
2.1. GDA Upregulation in Keratinocytes Stimulates Melanogenesis by Releasing Uric Acid
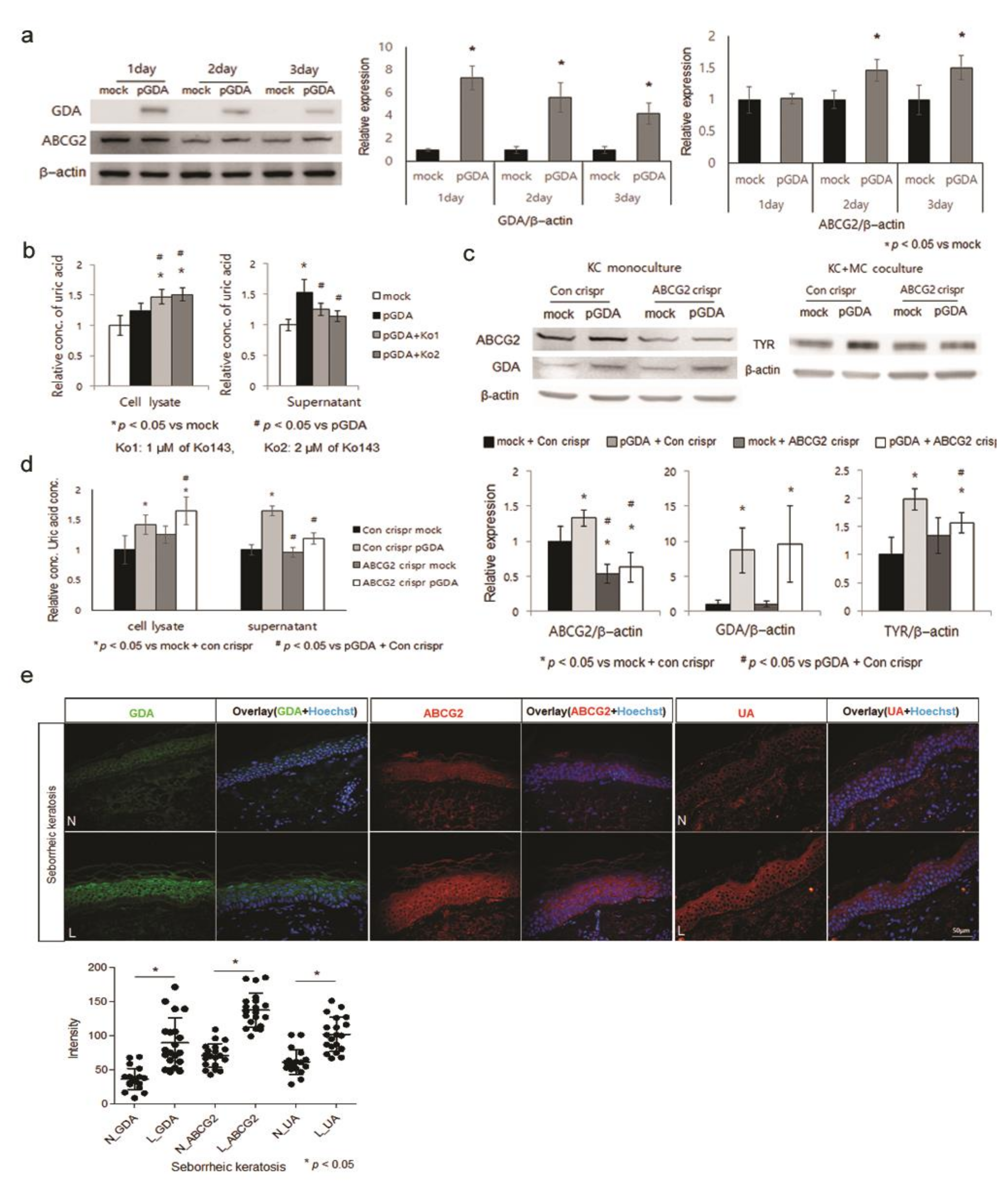
2.2. Uric Acid Is Secreted from Keratinocytes through ABCG2
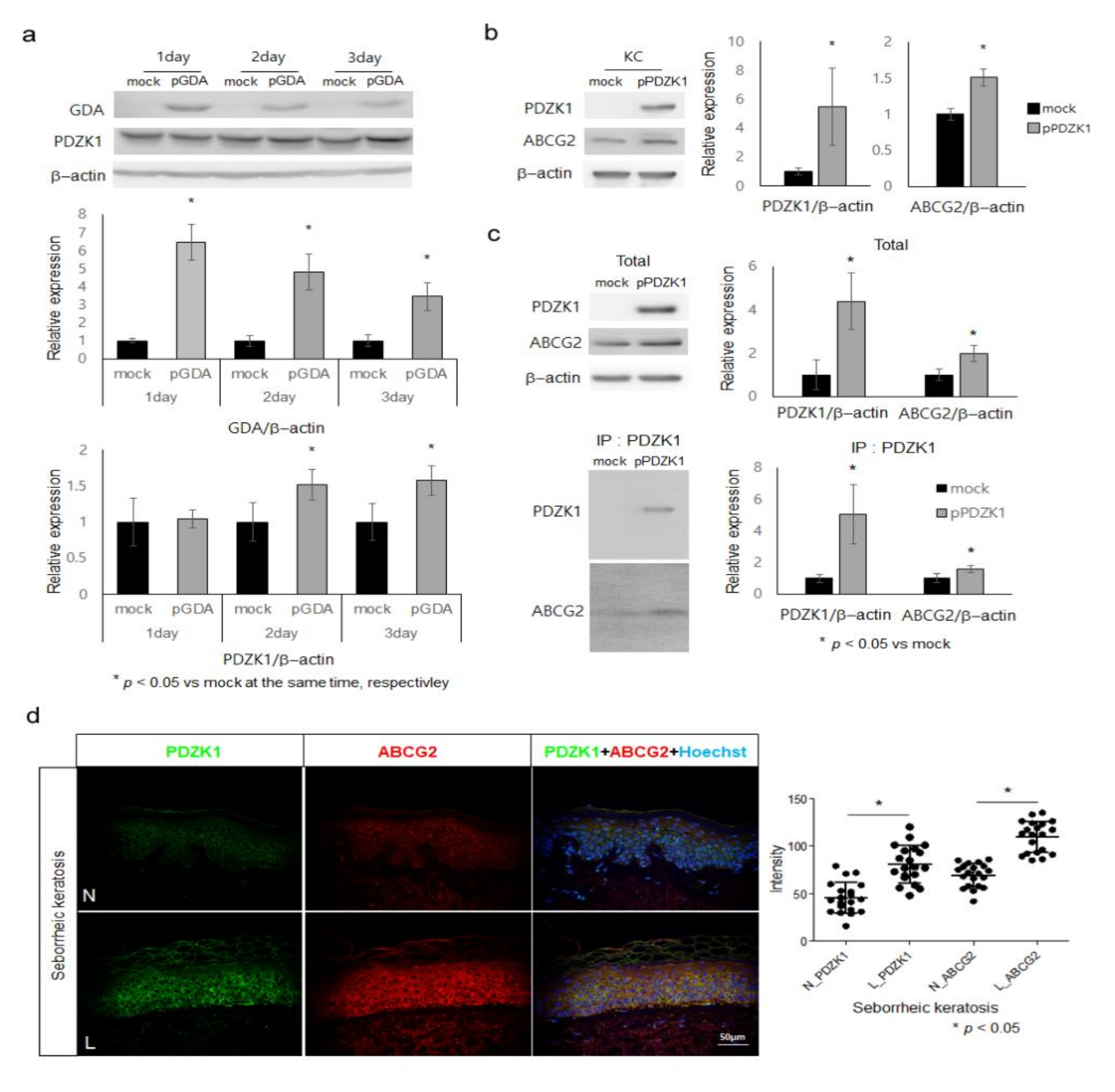
2.3. ABCG2 Action Is Facilitated by PDZK1 in Keratinocytes
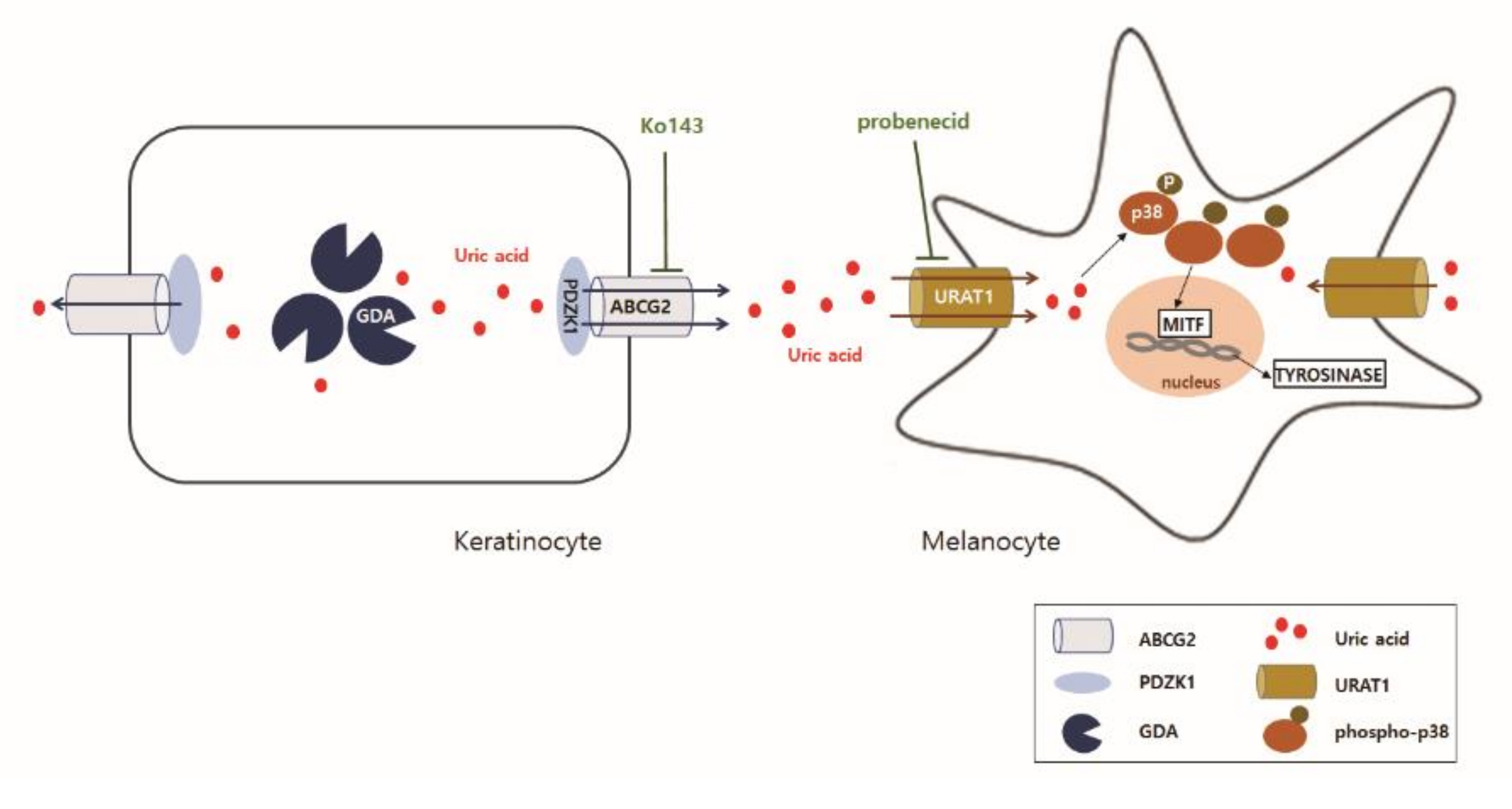
2.4. Uric Acid Uptake through URAT1 in Melanocytes, Stimulating Melanogenesis via p38 MAPK
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Normal Human Epidermal Cell Culture
4.3. Overexpression of GDA and Knockdown of ABCG2, or URAT1
4.4. Cell Viability and Cytotoxicity Test
4.5. Recombinant GDA Treatment
4.6. Uric Acid Assay
4.7. Treatment with Allopurinol or Ko143
4.8. Uric Acid Treatment with/without Probenecid
4.9. Real-Time PCR
4.10. Western Blot Analysis
4.11. Immunoprecipitation
4.12. Immunohistochemistry
4.13. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDA | guanine deaminase |
| UV | ultraviolet |
| SCF | stem cell factor |
| ABCG2 | ATP-binding cassette transporter, sub-family G, member 2 |
| URAT1 | urate transporter 1 |
| PDZK1 | PDZ domain containing 1 |
| MAPK | mitogen-activated protein kinase |
| Ko143 | [3S,6S,12aS]-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-[2-methylpropyl]-1,4-dioxopyrazino [1′,2′:1,6] pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester] |
References
- Hahnel, E.; Blume-Peytavi, U.; Trojahn, C.; Dobos, G.; Jahnke, I.; Kanti, V.; Richter, C.; Lichterfeld-Kottner, A.; Bartels, N.G.; Kottner, J. Prevalence and associated factors of skin diseases in aged nursing home residents: A multicentre prevalence study. BMJ Open 2017, 7, e018283. [Google Scholar] [CrossRef]
- Kumar, D.; Das, A.; Bandyopadhyay, D.; Chowdhury, S.N.; Das, N.K.; Sharma, P.; Kumar, A. Dermatoses in the Elderly: Clinico-Demographic Profile of Patients Attending a Tertiary Care Centre. Indian J. Dermatol. 2021, 66, 74–80. [Google Scholar]
- Wollina, U. Recent advances in managing and understanding seborrheic keratosis. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef]
- Todorova, K.; Mandinova, A. Novel approaches for managing aged skin and nonmelanoma skin cancer. Adv. Drug. Deliv. Rev. 2020, 153, 18–27. [Google Scholar] [CrossRef]
- Cheong, K.A.; Lee, A.Y. Guanine deaminase stimulates ultraviolet-induced keratinocyte senescence in seborrhoeic keratosis via guanine metabolites. Acta Derm. Venereol. 2020, 100, adv00109. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Noh, M. The regulation of epidermal melanogenesis via cAMP and/or PKC signaling pathways: Insights for the development of hypopigmenting agents. Arch. Pharm. Res. 2013, 36, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Molinar, V.E.; Taylor, S.C.; Pandya, A.G. What’s new in objective assessment and treatment of facial hyperpigmentation? Dermatol. Clin. 2014, 32, 123–135. [Google Scholar] [CrossRef]
- Liu, Z.; Que, S.; Zhou, L.; Zheng, S. Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: A Meta-analysis of Prospective Studies. Sci. Rep. 2015, 5, 14325. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, C.; Li, X.; Sun, L.; Zhu, X.; Zhao, C.; Zhang, Z.; Yang, Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2015, 100, 4198–4207. [Google Scholar] [CrossRef]
- King, C.; Lanaspa, M.A.; Jensen, T.; Tolan, D.R.; Sánchez-Lozada, L.G.; Johnson, R.J. Uric Acid as a Cause of the Metabolic Syndrome. Contrib. Nephrol. 2018, 192, 88–102. [Google Scholar]
- Cortese, F.; Scicchitano, P.; Cortese, A.M.; Meliota, G.; Andriani, A.; Truncellito, L.; Calculli, G.; Giordano, P.; Ciccone, M.M. Uric Acid in Metabolic and Cerebrovascular Disorders: A Review. Curr. Vasc. Pharmacol. 2020, 18, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Goli, P.; Riahi, R.; Daniali, S.S.; Pourmirzaei, M.; Kelishadi, R. Association of serum uric acid concentration with components of pediatric metabolic syndrome: A systematic review and meta-analysis. J. Res. Med. Sci. 2020, 25, 43. [Google Scholar]
- Cestari, T.F.; Dantas, L.P.; Boza, J.C. Acquired hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef]
- Park, Y.J.; Kang, H.Y.; Lee, E.S.; Kim, Y.C. Differentiating confluent and reticulated papillomatosis from acanthosis nigricans. J. Cutan. Pathol. 2015, 42, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Wang, X.; Li, Y.; Yang, S.; Qu, S. The Clinical Characteristics of Obese Patients with Acanthosis Nigricans and Its Independent Risk Factors. Exp. Clin. Endocrinol. Diabetes 2017, 125, 191–195. [Google Scholar]
- Zhu, C.; Cui, R.; Gao, M.; Rampersad, S.; You, H.; Sheng, C.; Yang, P.; Sheng, H.; Cheng, X.; Bu, L.; et al. The Associations of Serum Uric Acid with Obesity-Related Acanthosis nigricans and Related Metabolic Indices. Int. J. Endocrinol. 2017, 2017, 5438157. [Google Scholar] [CrossRef]
- Fujita, K.; Ichida, K. ABCG2 as a therapeutic target candidate for gout. Expert Opin. Ther. Targets 2018, 22, 123–129. [Google Scholar] [CrossRef]
- Ma, D.; Chua, A.W.; Yang, E.; Teo, P.; Ting, Y.; Song, C.; Lane, E.B.; Lee, S.T. Breast cancer resistance protein identifies clonogenic keratinocytes in human interfollicular epidermis. Stem Cell Res. Ther. 2015, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.E.; Jansson, P.J.; Richardson, D.R. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol. Pharmacol. 2013, 84, 655–669. [Google Scholar] [CrossRef]
- Chen, M.; Lu, X.; Lu, C.; Shen, N.; Jiang, Y.; Chen, M.; Wu, H. Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway. Arthritis Res. Ther. 2018, 20, 20. [Google Scholar] [CrossRef]
- Anzai, N.; Miyazaki, H.; Noshiro, R.; Khamdang, S.; Chairoungdua, A.; Shin, H.-J.; Enomoto, A.; Sakamoto, S.; Hirata, T.; Tomita, K.; et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J. Biol. Chem. 2004, 279, 45942–45950. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, T.; Ai, W.; Zalloum, W.A.; Kang, D.; Wu, T.; Liu, X.; Zhan, P. Novel urate transporter 1 (URAT1) inhibitors: A review of recent patent literature (2016–2019). Expert Opin. Ther. Pat. 2019, 29, 871–879. [Google Scholar] [CrossRef]
- Tan, P.K.; Ostertag, T.M.; Miner, J.N. Mechanism of high affinity inhibition of the human urate transporter URAT1. Sci. Rep. 2016, 6, 34995. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, R.; Tsukamoto, K.; Harada, K.; Shimizu, A.; Shimada, S.; Kobayashi, T.; Imokawa, G. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: Role of SCF/KIT protein interactions and the downstream effector, MITF-M. J. Pathol. 2004, 202, 463–475. [Google Scholar] [CrossRef]
- Uong, A.; Zon, L.I. Melanocytes in Development and Cancer. J. Cell Physiol. 2010, 222, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.P. Transcriptional Profiling Shows Altered Expression of Wnt Pathway– and Lipid Metabolism–Related Genes as Well as Melanogenesis-Related Genes in Melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef]
- Chung, B.Y.; Noh, T.K.; Yang, S.H.; Kim, I.H.; Lee, M.W.; Yoon, T.J.; Chang, S.E. Gene Expression Profiling in Melasma in Korean Women. Dermatology 2014, 229, 333–342. [Google Scholar] [CrossRef]
- Jung, J.M.; Noh, T.K.; Jo, S.Y.; Kim, S.Y.; Song, Y.; Kim, Y.H.; Chang, S.E. Guanine Deaminase in Human Epidermal Keratinocytes Contributes to Skin Pigmentation. Molecules 2020, 25, 2637. [Google Scholar] [CrossRef]
- Lee, A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660. [Google Scholar] [CrossRef]
- Lee, B.W.; Schwartz, R.A.; Janniger, C.K. Melasma. G. Ital. Dermatol. Venereol. 2017, 152, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Picardo, M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Na, J.I.; Choi, J.Y.; Park, K.C. Melasma: Updates and perspectives. Exp. Dermatol. 2019, 28, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Rajanala, S.; de Castro Maymone, M.B.; Vashi, N.A. Melasma pathogenesis: A review of the latest research, pathological findings, and investigational therapies. Dermatol. Online. J. 2019, 25, 13030. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.H.; Lee, T.R.; Lee, C.H.; Lee, A.Y. Cadherin 11 Involved in Basement Membrane Damage and Dermal Changes in Melasma. Acta Derm. Venereol. 2016, 96, 635–640. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.H.; Yi, N.; Lee, T.R.; Lee, A.Y. Arginase-2.; a miR-1299 target.; enhances pigmentation in melasma by reducing melanosomedegradation via senescence-induced autophagy inhibition. Pigment Cell Melanoma Res. 2017, 30, 521–530. [Google Scholar] [CrossRef]
- Kim, N.H.; Cheong, K.A.; Lee, T.R.; Lee, A.Y. PDZK1 upregulation in estrogen-related hyperpigmentation in melasma. J. Investig. Dermatol. 2012, 132, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, S.; Xu, F.; Ma, Z.; Liu, X.; Wang, L. Recent Advances in the Pharmacological Activities of Dioscin. Biomed. Res. Int. 2019, 14, 5763602. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Takada, T.; Saito, H.; Hirata, H.; Ota-Kontani, A.; Kobayashi, N.; Tsuchiya, Y.; Suzuki, H. Inhibitory effect of Citrus flavonoids on the in vitro transport activity of human urate transporter 1 (URAT1/SLC22A12), A renal re-absorber of urate. NPJ Sci. Food 2020, 4, 3. [Google Scholar] [CrossRef]
- Huang, Y.B.; Lee, K.F.; Huang, C.T.; Tsai, Y.H.; Wu, P.C. The effect of component of cream for topical delivery of hesperetin. Chem. Pharm. Bull. 2010, 58, 611–614. [Google Scholar] [CrossRef][Green Version]
- Murata, K.; Takahashi, K.; Nakamura, H.; Itoh, K.; Matsuda, H. Search for Skin-whitening Agent from Prunus Plants and the Molecular Targets in Melanogenesis Pathway of Active Compounds. Nat. Prod. Commun. 2014, 9, 185–188. [Google Scholar] [CrossRef]
- Campos, P.M.; Prudente, A.S.; da Silva Horinouchi, C.D.; Cechinel-Filho, V.; Fávero, G.M.; Cabrini, D.A.; Otuki, M.F. Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J. Ethnopharmacol. 2015, 174, 224–229. [Google Scholar] [CrossRef]
- Kim, H.J.; Yonezawa, T.; Teruya, T.; Woo, J.T.; Cha, B.Y. Nobiletin, a polymethoxy flavonoid, reduced endothelin-1 plus SCF-induced pigmentation in human melanocytes. Photochem. Photobiol. 2015, 91, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Nishina, A.; Ebina, K.; Ukiya, M.; Fukatsu, M.; Koketsu, M.; Ninomiya, M.; Sato, D.; Kimura, H. Dioscin Derived from Solanum melongena L. “Usukawamarunasu” Attenuates α-MSH-Induced Melanogenesis in B16 Murine Melanoma Cells via Downregulation of Phospho-CREB and MITF. J. Food Sci. 2015, 80, H2354–H2359. [Google Scholar] [CrossRef]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Roles of inflammation factors in melanogenesis. Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef]
- Han, H.J.; Lim, M.J.; Lee, Y.J.; Lee, J.H.; Yang, I.S.; Taub, M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am. J. Physiol. Renal Physiol. 2007, 292, F373–F381. [Google Scholar] [CrossRef]
- Tang, L.; Xu, Y.; Wei, Y.; He, X. Uric acid induces the expression of TNF-α via the ROS-MAPK-NF-κB signaling pathway in rat vascular smooth muscle cells. Mol. Med. Rep. 2017, 16, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Doğru, S.; Yaşar, E.; Yeşilkaya, A. Uric acid can enhance MAPK pathway-mediated proliferation in rat primary vascular smooth muscle cells via controlling of mitochondria and caspase-dependent cell death. J. Recept. Signal Transduct. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Cario, M.; Taieb, A. Isolation and Culture of Epidermal Melanocytes. Methods Mol. Biol. 2019, 1993, 33–46. [Google Scholar] [PubMed]
- Ścieżyńska, A.; Nogowska, A.; Sikorska, M.; Konys, J.; Karpińska, A.; Komorowski, M.; Ołdak, M.; Malejczyk, J. Isolation and culture of human primary keratinocytes-a methods review. Exp. Dermatol. 2019, 28, 107–112. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, K.A.; Kil, I.S.; Ko, H.W.; Lee, A.-Y. Upregulated Guanine Deaminase Is Involved in Hyperpigmentation of Seborrheic Keratosis via Uric Acid Release. Int. J. Mol. Sci. 2021, 22, 12501. https://doi.org/10.3390/ijms222212501
Cheong KA, Kil IS, Ko HW, Lee A-Y. Upregulated Guanine Deaminase Is Involved in Hyperpigmentation of Seborrheic Keratosis via Uric Acid Release. International Journal of Molecular Sciences. 2021; 22(22):12501. https://doi.org/10.3390/ijms222212501
Chicago/Turabian StyleCheong, Kyung Ah, In Sup Kil, Hyuk Wan Ko, and Ai-Young Lee. 2021. "Upregulated Guanine Deaminase Is Involved in Hyperpigmentation of Seborrheic Keratosis via Uric Acid Release" International Journal of Molecular Sciences 22, no. 22: 12501. https://doi.org/10.3390/ijms222212501
APA StyleCheong, K. A., Kil, I. S., Ko, H. W., & Lee, A.-Y. (2021). Upregulated Guanine Deaminase Is Involved in Hyperpigmentation of Seborrheic Keratosis via Uric Acid Release. International Journal of Molecular Sciences, 22(22), 12501. https://doi.org/10.3390/ijms222212501






