The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity
Abstract
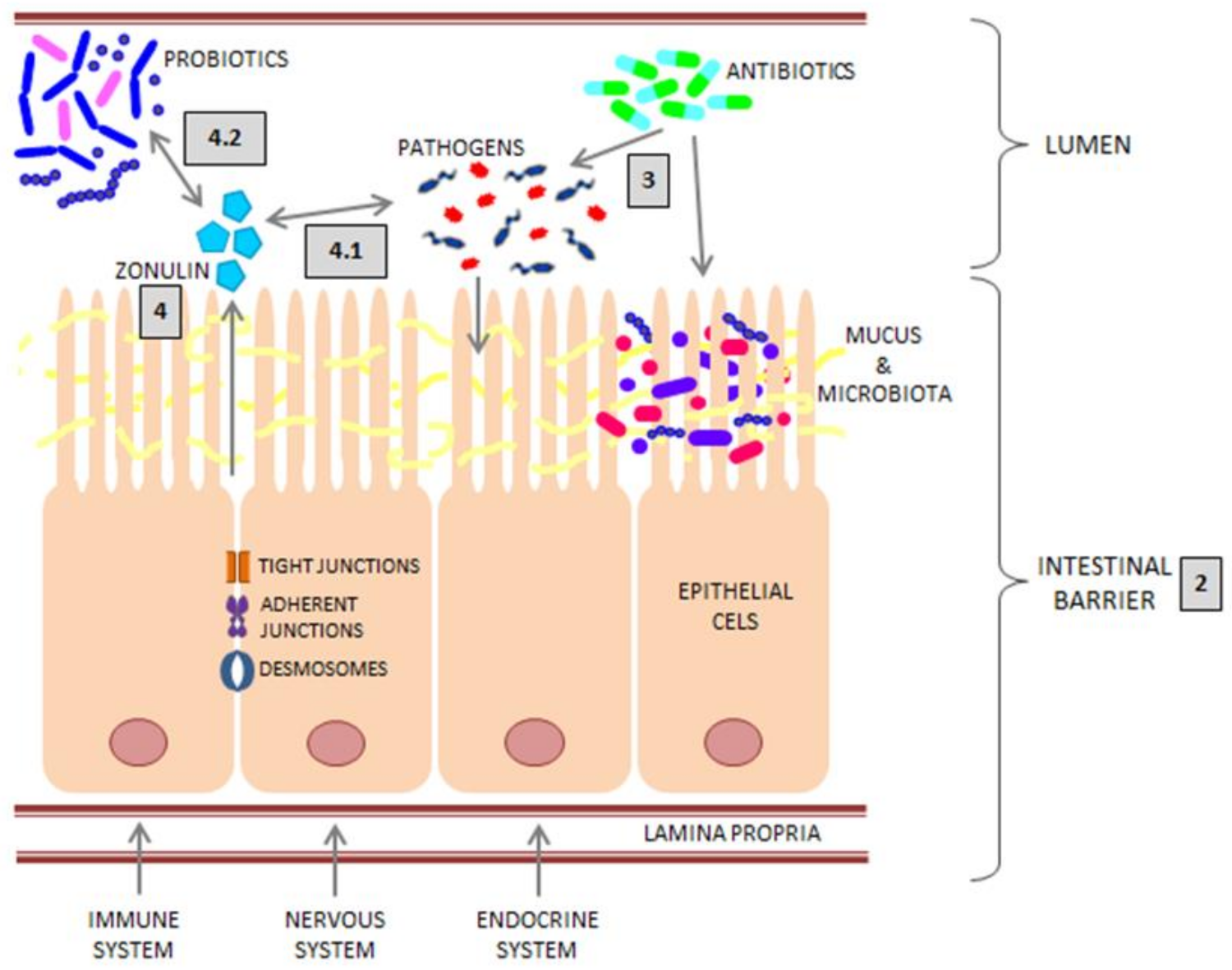
:1. Introduction
2. Intestinal Barrier
2.1. Epithelium
2.2. Transport across the Intestinal Barrier
2.3. Mucus
2.4. Microbiota
3. Intestinal Infections and Antibiotic Therapy
4. Zonulin
4.1. Zonulin and Bacterial Infections
4.2. Zonulin and Probiotics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caviglia, G.P.; Rosso, C.; Ribaldone, D.G.; Dughera, F.; Fagoonee, S.; Astegiano, M.; Pellicano, R. Physiopathology of intestinal barrier and the role of zonulin. Minerva Biotecnol. 2019, 31, 83–92. [Google Scholar] [CrossRef]
- Marchiando, A.M.; Graham, W.V.; Turner, J.R. Epithelial Barriers in Homeostasis and Disease. Annu. Rev. Pathol. 2010, 5, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Meerveld, B.G.V.A.N.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. Intestinal permeability defects: Is it time to treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Keita, Å.V.; Söderholm, J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010, 22, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2012, 70, 631–659. [Google Scholar] [CrossRef]
- Heyman, M.; Abed, J.; Lebreton, C.; Cerf-Bensussan, N. Intestinal permeability in coeliac disease: Insight into mechanisms and relevance to pathogenesis. Gut 2012, 61, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, D.R.; Boe, D.M.; Yu, D.; Weber, C.R.; Marchiando, A.M.; Bradford, E.M.; Wang, Y.; Wu, L.; Schneeberger, E.E.; Shen, L.; et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 2011, 193, 565. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 Stabilizes the Tight Junction Solute Barrier through Coupling to the Perijunctional Cytoskeleton. Mol. Biol. Cell 2009, 20, 3930. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.-M.; De Meester, I.; De Man, J.G.; Winter, B.Y. De Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106. [Google Scholar] [CrossRef]
- Graziani, C.; Talocco, C.; De Sire, R.; Petito, V.; Lopetuso, L.R.; Gervasoni, J.; Persichilli, S.; Franceschi, F.; Ojetti, V.; Gasbarrini, A.; et al. Intestinal permeability in physiological and pathological conditions: Major determinants and assessment modalities. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 795–810. [Google Scholar] [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61. [Google Scholar] [CrossRef]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; MacDonald, T.T.; Collins, J.E. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005, 85, 1139–1162. [Google Scholar] [CrossRef]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) Regulates Claudin-2 Expression and Tight Junction Permeability in Intestinal Epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [Green Version]
- Brose, N.; Rosenmund, C. Move over protein kinase C, you’ve got company: Alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 2002, 115, 4399–4411. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, S. Protein Kinase Cα (PKCα): Regulation and Biological Function. J. Biochem. 2002, 132, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Leaky gut-concept or clinical entity? Curr. Opin. Gastroenterol. 2016, 32, 74–79. [Google Scholar] [CrossRef]
- Cai, R.; Cheng, C.; Chen, J.; Xu, X.; Ding, C.; Gu, B. Interactions of commensal and pathogenic microorganisms with the mucus layer in the colon. Gut Microbes 2020, 11, 680–690. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.-C. Facing a new challenge: The adverse effects of antibiotics on gut microbiota and host immunity. Chin. Med. J. 2019, 132, 1135. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Silva, S.; Falony, G.; Darzi, Y.; Lima-Mendez, G.; Garcia Yunta, R.; Okuda, S.; Vandeputte, D.; Valles-Colomer, M.; Hildebrand, F.; Chaffron, S.; et al. Species–function relationships shape ecological properties of the human gut microbiome. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Baloch, Z.; Shah, Z.; Cui, X.; Xia, X. The Intestinal Microbiota: Impacts of Antibiotics Therapy, Colonization Resistance, and Diseases. Int. J. Mol. Sci. 2021, 22, 6597. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Sorg, J.A.; Sonenshein, A.L. Bile Salts and Glycine as Cogerminants for Clostridium difficile Spores. J. Bacteriol. 2008, 190, 2505. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.V.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 2015, 18, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.V.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria Penetrate the Inner Mucus Layer before Inflammation in the Dextran Sulfate Colitis Model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Starkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric Dysbiosis Associated with a Mouse Model of Alcoholic Liver Disease. Hepatology 2011, 53, 96. [Google Scholar] [CrossRef] [Green Version]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef]
- Eliakim, R.; Mahmood, A.; Alpers, D.H. Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim. Biophys. Acta Mol. Cell Res. 1991, 1091, 1–8. [Google Scholar] [CrossRef]
- Nakano, T.; Inoue, I.; Alpers, D.H.; Akiba, Y.; Katayama, S.; Shinozaki, R.; Kaunitz, J.D.; Ohshima, S.; Akita, M.; Takahashi, S.; et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G207. [Google Scholar] [CrossRef] [Green Version]
- Malo, M.S.; Alam, S.N.; Mostafa, G.; Zeller, S.J.; Johnson, P.V.; Mohammad, N.; Chen, K.T.; Moss, A.K.; Ramasamy, S.; Faruqui, A.; et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 2010, 59, 1476–1484. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal Alkaline Phosphatase Detoxifies Lipopolysaccharide and Prevents Inflammation in Response to the Gut Microbiota. Cell Host Microbe 2007, 2, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Millán, J.L.; Mecsas, J.; Guillemin, K. Intestinal Alkaline Phosphatase Deficiency Leads to Lipopolysaccharide Desensitization and Faster Weight Gain. Infect. Immun. 2015, 83, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Rep. 2015, 5, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Hu, D.; Huo, H.; Zhang, W.; Adiliaghdam, F.; Morrison, S.; Ramirez, J.M.; Gul, S.S.; Hamarneh, S.R.; Hodin, R.A. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J. Am. Coll. Surg. 2016, 222, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, K.A.; Gareau, M.G.; Wang, Y.J.J.; Sherman, P.M. Lactobacillus rhamnosus GG attenuates interferon-γ and tumour necrosis factor-α-induced barrier dysfunction and pro-inflammatory signalling. Microbiology 2010, 156, 3288–3297. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Henry, K.C.; Donato, K.A.; Shen-Tu, G.; Gordanpour, M.; Sherman, P.M. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect. Immun. 2008, 76, 1340–1348. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, E.; Morita, H.; Tanabe, S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 2009, 92, 2400–2408. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [CrossRef] [Green Version]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Langen, M.L.; Madsen, K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Chey, W.D.; Shah, E.D.; DuPont, H.L. Mechanism of action and therapeutic benefit of rifaximin in patients with irritable bowel syndrome: A narrative review. Therap. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef] [Green Version]
- Gatta, L.; Scarpignato, C. Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Berkell, M.; Mysara, M.; Xavier, B.B.; Van Werkhoven, C.H.; Monsieurs, P.; Lammens, C.; Ducher, A.; Vehreschild, M.J.; Goossens, H.; De Gunzburg, J.; et al. Microbiota-based markers predictive of development of Clostridioides difficile infection. Nat. Commun. 2021, 12, 2241. [Google Scholar] [CrossRef]
- Kintses, B.; Méhi, O.; Ari, E.; Számel, M.; Györkei, Á.; Jangir, P.K.; Nagy, I.; Pál, F.; Fekete, G.; Tengölics, R.; et al. Phylogenetic barriers to horizontal transfer of antimicrobial peptide resistance genes in the human gut microbiota. Nat. Microbiol. 2019, 4, 447. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [Green Version]
- Roxas, J.L.; Koutsouris, A.; Bellmeyer, A.; Tesfay, S.; Royan, S.; Falzari, K.; Harris, A.; Cheng, H.; Rhee, K.-J.; Hecht, G. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in Shiga toxin independent manner. Lab. Investig. 2010, 90, 1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philpott, D.J.; McKay, D.M.; Mak, W.; Perdue, M.H.; Sherman, P.M. Signal Transduction Pathways Involved in Enterohemorrhagic Escherichia coli-Induced Alterations in T84 Epithelial Permeability. Infect. Immun. 1998, 66, 1680. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.B. Modulation of the enterohemorrhagic E. coli virulence program through the human gastrointestinal tract. Virulence 2013, 4, 315. [Google Scholar] [CrossRef] [Green Version]
- Hazen, T.H.; Donnenberg, M.S.; Panchalingam, S.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; Ramamurthy, T.; Tamboura, B.; Qureshi, S.; et al. Genomic diversity of EPEC associated with clinical presentations of differing severity. Nat. Microbiol. 2016, 1, 15014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, J.; Staunton, L.; O’Connell, K.; Doran, P.; Ohlendieck, K. Phosphoproteomic analysis of aged skeletal muscle. Int. J. Mol. Med. 2008, 22, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Yuhan, R.; Koutsouris, A.; Hecht, G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 1997, 113, 1873–1882. [Google Scholar] [CrossRef]
- Simonovic, I.; Rosenberg, J.; Koutsouris, A.; Hecht, G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell. Microbiol. 2000, 2, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.; Kenny, B. The effector repertoire of enteropathogenic E. coli: Ganging up on the host cell. Curr. Opin. Microbiol. 2009, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Li, Q.; Wang, C.; Liu, X.; Li, N.; Li, J. Enteropathogenic Escherichia coli changes distribution of occludin and ZO-1 in tight junction membrane microdomains in vivo. Microb. Pathog. 2010, 48, 28–34. [Google Scholar] [CrossRef]
- Dubreuil, J.D. STb and AIDA-I: The missing link? Crit. Rev. Microbiol. 2010, 36, 212–220. [Google Scholar] [CrossRef]
- Mukiza, C.N.; Dubreuil, J.D. Escherichia coli Heat-Stable Toxin b Impairs Intestinal Epithelial Barrier Function by Altering Tight Junction Proteins. Infect. Immun. 2013, 81, 2819. [Google Scholar] [CrossRef] [Green Version]
- Turner, S.M.; Scott-Tucker, A.; Cooper, L.M.; Henderson, I.R. Weapons of mass destruction: Virulence factors of the global killer Enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 2006, 263, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Strauman, M.C.; Harper, J.M.; Harrington, S.M.; Boll, E.J.; Nataro, J.P. Enteroaggregative Escherichia coli Disrupts Epithelial Cell Tight Junctions. Infect. Immun. 2010, 78, 4958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Ellis, S.J.; Crossman, L.C.; McGrath, C.J.; Chattaway, M.A.; Hölken, J.M.; Brett, B.; Bundy, L.; Kay, G.L.; Wain, J.; Schüller, S. Identification and characterisation of enteroaggregative Escherichia coli subtypes associated with human disease. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Abdelhalim, K.A.; Uzel, A.; Ünal, N.G. The role of major virulence factors and pathogenicity of adherent-invasive Escherichia coli in patients with Crohn’s disease. Przegla̜d Gastroenterol. 2020, 15, 279. [Google Scholar] [CrossRef]
- Fasano, A.; Uzzau, S.; Fiore, C.; Margaretten, K. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 1997, 112, 839–846. [Google Scholar] [CrossRef]
- Bando, S.Y.; Moreno, A.C.; Albuquerque, J.A.; Amhaz, J.M.; Moreira-Filho, C.A.; Martinez, M.B. Expression of bacterial virulence factors and cytokines during in vitro macrophage infection by enteroinvasive Escherichia coli and Shigella flexneri: A comparative study. Mem. Inst. Oswaldo Cruz 2010, 105, 786–791. [Google Scholar] [CrossRef]
- Nusrat, A.; von Eichel-Streiber, C.; Turner, J.R.; Verkade, P.; Madara, J.L.; Parkos, C.A. Clostridium difficile Toxins Disrupt Epithelial Barrier Function by Altering Membrane Microdomain Localization of Tight Junction Proteins. Infect. Immun. 2001, 69, 1329. [Google Scholar] [CrossRef] [Green Version]
- Ciesla, W.P.; Bobak, D.A. Clostridium difficile Toxins A and B Are Cation-dependent UDP-glucose Hydrolases with Differing Catalytic Activities. J. Biol. Chem. 1998, 273, 16021–16026. [Google Scholar] [CrossRef] [Green Version]
- Nava, P.; Vidal, J.E. The CpAL system regulates changes of the trans-epithelial resistance of human enterocytes during Clostridium perfringens type C infection. Anaerobe 2016, 39, 143–149. [Google Scholar] [CrossRef]
- Seike, S.; Takehara, M.; Takagishi, T.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Delta-toxin from Clostridium perfringens perturbs intestinal epithelial barrier function in Caco-2 cell monolayers. Biochim. Biophys. Acta Biomembr. 2018, 1860, 428–433. [Google Scholar] [CrossRef]
- Eichner, M.; Augustin, C.; Fromm, A.; Piontek, A.; Walther, W.; Bücker, R.; Fromm, M.; Krause, G.; Schulzke, J.-D.; Günzel, D.; et al. In Colon Epithelia, Clostridium perfringens Enterotoxin Causes Focal Leaks by Targeting Claudins Which are Apically Accessible Due to Tight Junction Derangement. J. Infect. Dis. 2018, 217, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-sify, A. A review on Clostridium perfringens toxins with special reference to Beta 2 toxin. J. Curr. Vet. Res. 2015, 9, 85–100. [Google Scholar] [CrossRef]
- Sharma, L.; Riva, A. Intestinal Barrier Function in Health and Disease—Any Role of SARS-CoV-2? Microorganisms 2020, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lian, J.-S.; Hu, J.-H.; Gao, J.; Zheng, L.; Zhang, Y.-M.; Hao, S.-R.; Jia, H.-Y.; Cai, H.; Zhang, X.-L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002. [Google Scholar] [CrossRef] [Green Version]
- Assimakopoulos, S.F.; Mastronikolis, S.; Lastic, A.-L.D.E.; Aretha, D.; Papageorgiou, D.; Chalkidi, T.; Oikonomou, I.; Triantos, C.; Mouzaki, A.; Marangos, M. Intestinal Barrier Biomarker ZO1 and Endotoxin Are Increased in Blood of Patients with COVID-19-associated Pneumonia. In Vivo 2021, 35, 2483. [Google Scholar] [CrossRef]
- Prasad, R.; Patton, M.J.; Floyd, J.L.; Vieira, C.P.; Fortmann, S.; DuPont, M.; Harbour, A.; Jeremy, C.S.; Wright, J.; Lamendella, R.; et al. Plasma microbiome in COVID-19 subjects: An indicator of gut barrier defects and dysbiosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y.; Xu, H.; Deng, L. Herbal Medicine, Gut Microbiota, and COVID-19. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Yu, W.; Ou, X.; Liu, X.; Zhang, S.; Gao, X.; Cheng, H.; Zhu, B.; Yan, J. ACE2 contributes to the maintenance of mouse epithelial barrier function. Biochem. Biophys. Res. Commun. 2020, 533, 1276. [Google Scholar] [CrossRef]
- Uzzan, M.; Corcos, O.; Martin, J.C.; Treton, X.; Bouhnik, Y. Why is SARS-CoV-2 infection more severe in obese men? The gut lymphatics—Lung axis hypothesis. Med. Hypotheses 2020, 144, 110023. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, R.; Wang, Y.; Deng, P.; Song, T.; Zhang, M.; Wang, P.; Zhang, X.; Cui, K.; Tao, T.; et al. SARS-CoV-2 induced intestinal responses with a biomimetic human gut-on-chip. Sci. Bull. 2021, 66, 783. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Original research: Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, Y.; He, Q.; Zhu, P.; Liu, M.; Xu, J.; Zhao, M. Intestinal Microbiota—A Promising Target for Antiviral Therapy? Front. Immunol. 2021, 12, 1771. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal Permeability and Its Regulation by Zonulin: Diagnostic and Therapeutic Implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Asmar, R.; Panigrahi, P.; Bamford, P.; Berti, I.; Not, T.; Coppa, G.V.; Catassi, C.; Fasano, A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002, 123, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Fasano, A. Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments. Dig. Dis. Sci. 2019, 64, 1748. [Google Scholar] [CrossRef] [PubMed]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Clemente, M.G.; Sapone, A.T.A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; Not, T.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2009, 41, 408–419. [Google Scholar] [CrossRef]
- Clemente, M.G.; De Virgiliis, S.; Kang, J.S.; Macatagney, R.; Musu, M.P.; Di Pierro, M.R.; Drago, S.; Congia, M.; Fasano, A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003, 52, 218–223. [Google Scholar] [CrossRef]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Khaleghi, S.; Ju, J.M.; Lamba, A.; Murray, J.A. The potential utility of tight junction regulation in celiac disease: Focus on larazotide acetate. Therap. Adv. Gastroenterol. 2016, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.Y.; Chen, S.; Wang, X. Apple-Derived Pectin Modulates Gut Microbiota, Improves Gut Barrier Function, and Attenuates Metabolic Endotoxemia in Rats with Diet-Induced Obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Cui, T.; Liu, S.; Chen, B.; Wang, Y.; Yang, T.; Li, T.; Chen, J. Vitamin A supplementation improves the intestinal mucosal barrier and facilitates the expression of tight junction proteins in rats with diarrhea. Nutrition 2019, 57, 97–108. [Google Scholar] [CrossRef]
- Su, L.; Shen, L.; Clayburgh, D.R.; Nalle, S.C.; Sullivan, E.A.; Meddings, J.B.; Abraham, C.; Turner, J.R. Targeted Epithelial Tight Junction Dysfunction Causes Immune Activation and Contributes to Development of Experimental Colitis. Gastroenterology 2009, 136, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Laukoetter, M.G.; Nava, P.; Lee, W.Y.; Severson, E.A.; Capaldo, C.T.; Babbin, B.A.; Williams, I.R.; Koval, M.; Peatman, E.; Campbell, J.A.; et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007, 204, 3067–3076. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Feygin, A.; Wang, D.; Kuemmerle, J.F.; Harris, G.; Conti, M.A.; Adelstein, R.S.; Ivanov, A.I. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role during Experimental Colitis. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin Upregulation Is Associated with Increased Gut Permeability in Subjects with Type 1 Diabetes and Their Relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavón, E.J.; Muñoz, P.; Lario, A.; Longobardo, V.; Carrascal, M.; Abián, J.; Martin, A.B.; Arias, S.A.; Callejas-Rubio, J.-L.; Sola, R.; et al. Proteomic analysis of plasma from patients with systemic lupus erythematosus: Increased presence of haptoglobin α2 polypeptide chains over the α1 isoforms. Proteomics 2006, 6, S282–S292. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, P.; Peng, J.; Li, K.; Du, J.; Gu, J.; Ou, Y. Identification of disease-associated proteins by proteomic approach in ankylosing spondylitis. Biochem. Biophys. Res. Commun. 2007, 357, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.; Berti, I.; Sapone, A.; Gerarduzzi, T.; Not, T.; Zielke, R.; Fasano, A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc. Natl. Acad. Sci. USA 2005, 102, 2916. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Gao, M.; Zhang, W.; Chen, C.; Zhou, F.; Hu, Z.; Zeng, C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci. Rep. 2016, 6, 29142. [Google Scholar] [CrossRef] [PubMed]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.R.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of intestinal barrier dysfunction in sepsis. Shock 2016, 46, 52. [Google Scholar] [CrossRef]
- Klaus, D.A.; Motal, M.C.; Burger-Klepp, U.; Marschalek, C.; Schmidt, E.M.; Lebherz-Eichinger, D.; Krenn, C.G.; Roth, G.A. Increased plasma zonulin in patients with sepsis. Biochem. Med. 2013, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A. Physiological, Pathological, and Therapeutic Implications of Zonulin-Mediated Intestinal Barrier Modulation: Living Life on the Edge of the Wall. Am. J. Pathol. 2008, 173, 1243. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.A.R.; Zaharuddin, L.; Chan, S.-N.; Wong, Z.; Ngiu, C.S.; Mokhtar, N.M. Sa1838—The Clinical and Circulating Inflammatory Cytokines Effects of Probiotic Containing Lactobacillus and Bifidobacterium Strains in Patients with Colorectal Cancer: A Randomized Double Blind Controlled Trial. Gastroenterology 2018, 154, S414. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Nawawi, K.N.M.; Ali, R.A.R. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [Green Version]
- Ramezani Ahmadi, A.; Sadeghian, M.; Alipour, M.; Ahmadi Taheri, S.; Rahmani, S.; Abbasnezhad, A. The effects of probiotic/synbiotic on serum level of zonulin as a biomarker of intestinal permeability: A systematic review and meta-analysis. Iran J. Public Health 2020, 49, 1222–1231. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef]
- Su, T.; Liu, R.; Lee, A.; Long, Y.; Du, L.; Lai, S.; Chen, X.; Wang, L.; Si, J.; Owyang, C.; et al. Altered Intestinal Microbiota with Increased Abundance of Prevotella Is Associated with High Risk of Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterol. Res. Pract. 2018, 2018, 6961783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokkala, K.; Pussinen, P.; Houttu, N.; Koivuniemi, E.; Vahlberg, T.; Laitinen, K. The impact of probiotics and n-3 long-chain polyunsaturated fatty acids on intestinal permeability in pregnancy: A randomised clinical trial. Benef. Microbes 2018, 9, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; de Roos, N.M.; Hovenier, R.; Meijerink, J.; der Vaart, I.B.; van Hemert, S.; Witteman, B.J.M. Intestinal Permeability Measured by Urinary Sucrose Excretion Correlates with Serum Zonulin and Faecal Calprotectin Concentrations in UC Patients in Remission. J. Nutr. Metab. 2019, 2019, 2472754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.-L.; Linros, J.; et al. Probiotic with or without Fiber Controls Body Fat Mass, Associated with Serum Zonulin, in Overweight and Obese Adults—Randomized Controlled Trial. EBioMedicine 2016, 13, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability–results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef]
- Wilms, E.; Gerritsen, J.; Smidt, H.; der Vaart, I.B.; Rijkers, G.T.; Fuentes, A.R.G.; Masclee, A.A.M.; Troost, F.J. Effects of Supplementation of the Synbiotic Ecologic® 825/FOS P6 on Intestinal Barrier Function in Healthy Humans: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0167775. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serek, P.; Oleksy-Wawrzyniak, M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021, 22, 11359. https://doi.org/10.3390/ijms222111359
Serek P, Oleksy-Wawrzyniak M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. International Journal of Molecular Sciences. 2021; 22(21):11359. https://doi.org/10.3390/ijms222111359
Chicago/Turabian StyleSerek, Paweł, and Monika Oleksy-Wawrzyniak. 2021. "The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity" International Journal of Molecular Sciences 22, no. 21: 11359. https://doi.org/10.3390/ijms222111359
APA StyleSerek, P., & Oleksy-Wawrzyniak, M. (2021). The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. International Journal of Molecular Sciences, 22(21), 11359. https://doi.org/10.3390/ijms222111359





