The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies?
Abstract
:1. Introduction
2. The Human Gut Microbiome: A Major Homeostasis Player
3. The Role of the Gut Microbiome in Pancreatic Cancer
4. Other Host-Associated Microbiomes and Pancreatic Cancer: Oral and Intra-Tumoral Microbial Communities
4.1. Oral Microbiome
4.2. Intratumor Microbiome
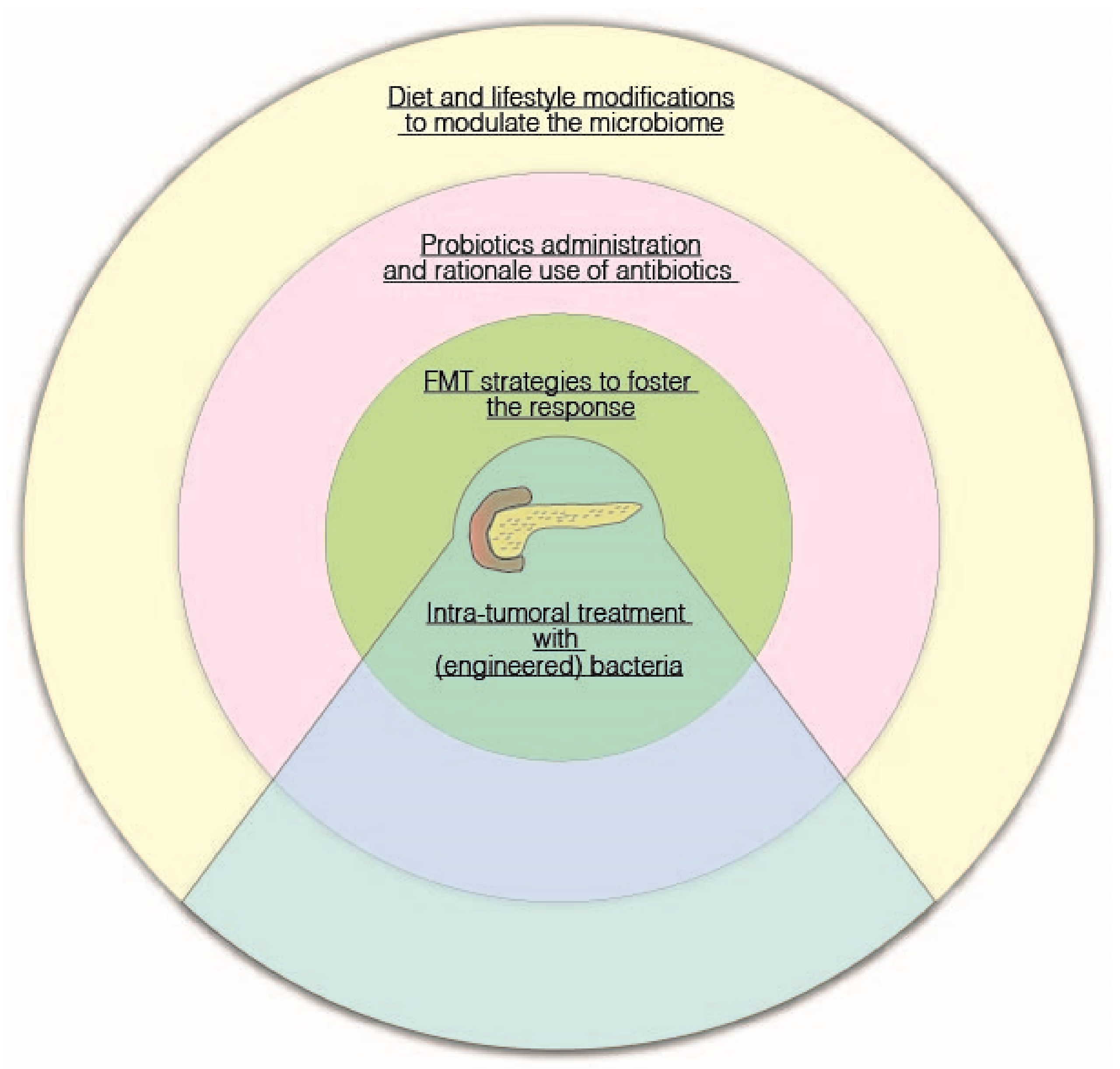
5. Potential Therapeutic Strategies: Diet, Probiotics, Fecal Microbiota Transplantation, and Tumor-Targeting Bacteria
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FMT | Fecal Microbial Transplantation |
| PanIN | Pancreatic Intraepithelial Neoplasia |
| PC | Pancreatic cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| SCFA | Short-chain fatty acids |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota-Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 2018, 61, 47–61. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Brandi, G.; Dabard, J.; Raibaud, P.; Battista, M.D.; Bridonneau, C.; Pisi, A.M.; Labate, A.M.M.; Pantaleo, M.A.; Vivo, A.D.; Biasco, G. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin. Cancer Res. 2006, 12, 1299–1307. [Google Scholar] [CrossRef] [Green Version]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zheng, H.-M.; Li, P.; McDonald, D.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.-X.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnenburg, E.D.; Sonnenburg, J.L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [Green Version]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [Green Version]
- Zaneveld, J.R.; McMinds, R.; Vega Thurber, R. Stress and stability: Applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2017, 2, 17121. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, O.; Dor, Y.; Alsina, J.; Stirman, A.; Lauwers, G.; Trainor, A.; Castillo, C.F.-D.; Warshaw, A.L.; Thayer, S.P. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 2007, 133, 1999–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczny, S.F.; Leach, S.D. Metaplastic metamorphoses in the mammalian pancreas. Gastroenterology 2007, 133, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- De Lisle, R.C.; Logsdon, C.D. Pancreatic acinar cells in culture: Expression of acinar and ductal antigens in a growth-related manner. Eur. J. Cell Biol. 1990, 51, 64–75. [Google Scholar]
- Zamboni, G.; Hirabayashi, K.; Castelli, P.; Lennon, A.M. Precancerous lesions of the pancreas. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Andea, A.; Sarkar, F.; Adsay, V.N. Clinicopathological correlates of pancreatic intraepithelial neoplasia: A comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod. Pathol. 2003, 16, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [Green Version]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- De Couck, M.; Maréchal, R.; Moorthamers, S.; Van Laethem, J.-L.; Gidron, Y. Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 2016, 40, 47–51. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capurso, G.; Lahner, E. The interaction between smoking, alcohol and the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 579–588. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-X.; Schwabe, R.F. The gut microbiome and liver cancer: Mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Dapito, D.H.; Mencin, A.; Gwak, G.-Y.; Pradere, J.-P.; Jang, M.-K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Jin, M.; Liu, Y.; Jin, L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front. Cell. Infect. 2020, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-Y.; Shi, S.; Liang, C.; Meng, Q.-C.; Hua, J.; Zhang, Y.-Y.; Liu, J.; Zhang, B.; Xu, J.; Yu, X.-J. The microbiota and microbiome in pancreatic cancer: More influential than expected. Mol. Cancer 2019, 18, 97. [Google Scholar] [CrossRef]
- Ochi, A.; Nguyen, A.H.; Bedrosian, A.S.; Mushlin, H.M.; Zarbakhsh, S.; Barilla, R.; Zambirinis, C.P.; Fallon, N.C.; Rehman, A.; Pylayeva-Gupta, Y.; et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J. Exp. Med. 2012, 209, 1671–1687. [Google Scholar] [CrossRef]
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.M.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012, 122, 4118–4129. [Google Scholar] [CrossRef]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Beesetty, Y.; Lee, W.; Washington, M.K.; Chen, X.; Lockhart, A.C.; Merchant, N.B. Novel Mechanistic Insights into Ectodomain Shedding of EGFR Ligands Amphiregulin and TGF-α: Impact on Gastrointestinal Cancers Driven by Secondary Bile Acids. Cancer Res. 2014, 74, 2062–2072. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Half, E.; Keren, N.; Reshef, L.; Dorfman, T.; Lachter, I.; Kluger, Y.; Reshef, N.; Knobler, H.; Maor, Y.; Stein, A.; et al. Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep. 2019, 9, 16801. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [Green Version]
- Enrichment of Oral Microbiota in Early Cystic Precursors to Invasive Pancreatic Cancer | Gut. Available online: https://gut.bmj.com/content/68/12/2186.long (accessed on 14 January 2020).
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [Green Version]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Öğrendik, M. Periodontal Pathogens in the Etiology of Pancreatic Cancer. GAT 2016, 3, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Zeller, I.; Hutcherson, J.A.; Lamont, R.J.; Demuth, D.R.; Gumus, P.; Nizam, N.; Buduneli, N.; Scott, D.A. Altered antigenic profiling and infectivity of Porphyromonas gingivalis in smokers and non-smokers with periodontitis. J. Periodontol. 2014, 85, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Zambon, J.J.; Grossi, S.G.; Machtei, E.E.; Ho, A.W.; Dunford, R.; Genco, R.J. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J. Periodontol. 1996, 67, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.-Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.-C.; Vlahovic, G.; et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.B.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, S.; Komatsu, Y.; Kawamoto, Y.; Saito, R.; Ito, K.; Nakatsumi, H.; Yuki, S.; Sakamoto, N. Association between the use of antibiotics and efficacy of gemcitabine plus nab-paclitaxel in advanced pancreatic cancer. Medicine 2020, 99, e22250. [Google Scholar] [CrossRef] [PubMed]
- Mohindroo, C.; Hasanov, M.; Rogers, J.E.; Dong, W.; Prakash, L.R.; Baydogan, S.; Mizrahi, J.D.; Overman, M.J.; Varadhachary, G.R.; Wolff, R.A.; et al. Antibiotic use influences outcomes in advanced pancreatic adenocarcinoma patients. Cancer Med. 2021, 10, 5041–5050. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Wei, A.-L.; Li, M.; Li, G.-Q.; Wang, X.; Hu, W.-M.; Li, Z.-L.; Yuan, J.; Liu, H.-Y.; Zhou, L.-L.; Li, K.; et al. Oral microbiome and pancreatic cancer. World J. Gastroenterol. 2020, 26, 7679–7692. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Rossi, O.; van Berkel, L.A.; Chain, F.; Tanweer Khan, M.; Taverne, N.; Sokol, H.; Duncan, S.H.; Flint, H.J.; Harmsen, H.J.M.; Langella, P.; et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep. 2016, 6, 18507. [Google Scholar] [CrossRef] [Green Version]
- Ulsemer, P.; Toutounian, K.; Kressel, G.; Goletz, C.; Schmidt, J.; Karsten, U.; Hahn, A.; Goletz, S. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFα-specific antibodies in human adults. Benef. Microbes. 2016, 7, 485–500. [Google Scholar] [CrossRef]
- Ulsemer, P.; Henderson, G.; Toutounian, K.; Löffler, A.; Schmidt, J.; Karsten, U.; Blaut, M.; Goletz, S. Specific humoral immune response to the Thomsen-Friedenreich tumor antigen (CD176) in mice after vaccination with the commensal bacterium Bacteroides ovatus D-6. Cancer Immunol. Immunother. 2013, 62, 875–887. [Google Scholar] [CrossRef]
- Motta, J.-P.; Bermúdez-Humarán, L.G.; Deraison, C.; Martin, L.; Rolland, C.; Rousset, P.; Boue, J.; Dietrich, G.; Chapman, K.; Kharrat, P.; et al. Food-Grade Bacteria Expressing Elafin Protect Against Inflammation and Restore Colon Homeostasis. Sci. Transl. Med. 2012, 4, 158ra144. [Google Scholar] [CrossRef]
- Caluwaerts, S.; Vandenbroucke, K.; Steidler, L.; Neirynck, S.; Vanhoenacker, P.; Corveleyn, S.; Watkins, B.; Sonis, S.; Coulie, B.; Rottiers, P. AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil Factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol. 2010, 46, 564–570. [Google Scholar] [CrossRef]
- Frossard, C.P.; Steidler, L.; Eigenmann, P.A. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitization. J. Allergy Clin. Immunol. 2007, 119, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Whitehead, T.R.; Lan, J.; Dilger, P.; Thorpe, R.; Holland, K.T.; Carding, S.R. Engineering of the gut commensal bacterium Bacteroides ovatus to produce and secrete biologically active murine interleukin-2 in response to xylan. J. Appl. Microbiol. 2005, 98, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Hamady, Z.Z.R.; Scott, N.; Farrar, M.D.; Wadhwa, M.; Dilger, P.; Whitehead, T.R.; Thorpe, R.; Holland, K.T.; Lodge, J.P.A.; Carding, S.R. Treatment of colitis with a commensal gut bacterium engineered to secrete human TGF-β1 under the control of dietary xylan 1. Inflamm. Bowel Dis. 2011, 17, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Tan, H.Q.; Chua, K.J.; Kang, A.; Lim, K.H.; Ling, K.L.; Yew, W.S.; Lee, Y.S.; Thiery, J.P.; Chang, M.W. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2018, 2, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Lopes, L.C.L.; Cordero, R.J.B.; Nosanchuk, J.D. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J. Antimicrob. Chemother. 2011, 66, 2573–2580. [Google Scholar] [CrossRef]
- Bradley, C.A. Tumour microbiome defines outcomes. Nat. Rev. Cancer 2019, 19, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, V.; McAllister, F. Therapeutic potential of microbial modulation in pancreatic cancer. Gut 2021, 70, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
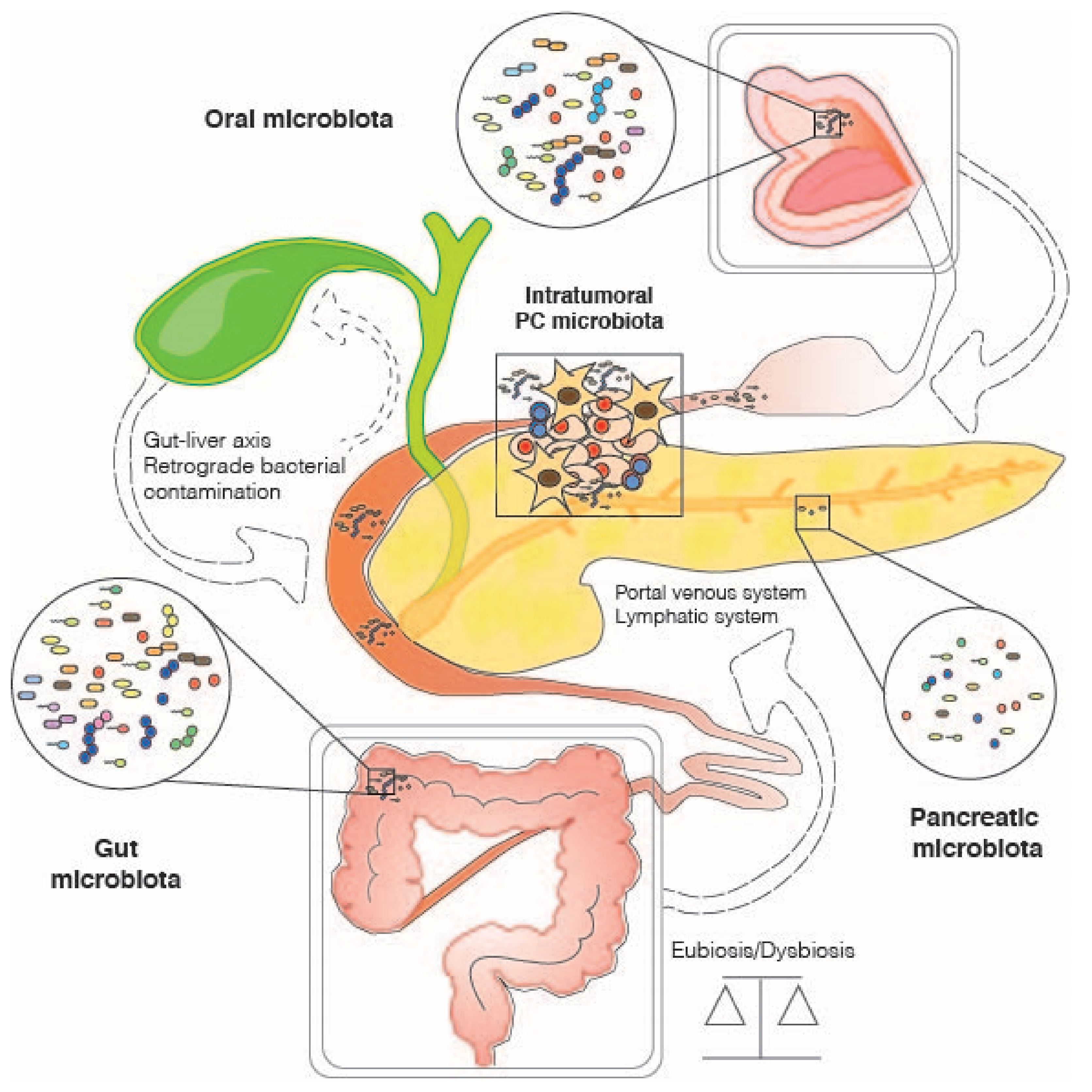
| Ref. | Study Design (Subjects and Samples) | Methods of Microbiota Analysis | Main Results |
|---|---|---|---|
| Gut Microbiome | |||
| Ren et al., 2017 [50] | Prospective cohort study on stool samples from: -85 PC patients -57 matched healthy controls | 16S rRNA gene sequencing (Illumina MiSeq platform) | PC patients showed reduced gut microbiota diversity, increased proportions of some pathogens and LPS-producing bacteria (Veillonella, Klebsiella, Selenomonas, Prevotella, Hallella and Enterobacter), and reduced amounts of beneficial taxa, such as bifidobacteria and butyrate producers (Coprococcus, Flavonifractor, Anaerostipes and Clostridium cluster IV members). The microbial community in obstruction cases was distinct from the unobstructed cases. Through inferred metagenomics, the authors found enrichment in leucine and LPS biosynthesis in PC. Based on 40 genera associated with PC using LDA selection, a high classification power with AUC of 0.842 was achieved. |
| Pushalkar et al., 2018 [31] | Prospective cohort study on: -PDAC patients (33 rectal swabs and 12 tissue samples) -Matched healthy volunteers (31 rectal swabs and 5 tissue samples) -Murine models (WT, KC and KPC mice) | 16S rRNA gene sequencing (Illumina MiSeq platform) FISH qPCR for total bacterial DNA in pancreatic tissues and feces | The cancerous pancreas showed a more abundant microbiome than normal tissue in both mice and humans, with distinct bacterial signatures from the gut. The relative abundance of Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia was greater in the gut of PDAC patients than in healthy controls. Proteobacteria members were also enriched in the intratumor microbiome and associated with advanced disease. Germ-free mice were protected against PDAC, while FMT from PDAC-bearing mice reversed that protection. Microbiota ablation by antibiotics was associated with immunogenic reprogramming of the tumor microenvironment, reduction in myeloid-derived suppressor cells, increase in M1 macrophage differentiation, Th1 differentiation of CD4+ T cells and CD8+ T cell activation. |
| Half et al., 2019 [51] | Cohort study on stool samples of: -30 patients with PDAC -6 patients with precancerous lesions -16 patients with non-alcoholic fatty liver disease -13 healthy subjects | 16S rRNA gene sequencing (Illumina MiSeq platform) | PC patients showed reduced proportions of health-associated bacterial families (Clostridiaceae, Lachnospiraceae and Ruminococcaceae), and increased amounts of Veillonellaceae, Akkermansia and Odoribacter. These microbial signatures were distinct from those of common PC comorbidities, namely bile duct obstruction and liver damage. Based on the discriminating features between PC patients and healthy subjects, a high classification power with AUC of 82.5% was achieved. However, only a few microbial alterations were present in patients with precancerous pancreatic lesions, limiting the potential for early diagnosis. |
| Oral Microbiome | |||
| Farrell et al., 2012 [66] | PRoBE study with microbial profiling of saliva of: -10 resectable PC patients -10 matched healthy controls Validation of bacterial candidates in an independent cohort of 28 resectable PC, 28 matched healthy control and 27 chronic pancreatitis samples | 16S rRNA-based oligonucleotide microarray, the Human Oral Microbe Identification Microarray (HOMIM) qPCR | PC patients showed reduced levels of Neisseria elongata and Streptococcus mitis, and increased levels of Granulicatella adiacens. N. elongata and S. mitis were validated using independent samples. Based on the combination of these two bacterial candidates, a high classification power with AUC of 0.9 was achieved. |
| Michaud et al., 2013 [56] | Prospective cohort study evaluating antibodies to oral bacteria in pre-diagnosis blood samples from: -405 PC cases -416 matched controls | Immunoblot array using a pre-selected panel of whole-cell formalin fixed bacterial antigens | High levels of antibodies against Porphyromonas gingivalis were associated with a two-fold higher risk of PC. High levels of antibodies against commensal bacteria might be associated with reduced risk of PC. |
| Wei et al., 2020 [67] | Prospective cohort study on saliva of: -41 PDAC patients -69 healthy individuals | 16S rRNA gene sequencing (Illumina NovaSeq platform) | Streptococcus and Leptotrichia were associated with a higher risk of PDAC, while Veillonella and Neisseria with a lower risk. Porphyromonas, Fusobacterium and Alloprevotella were enriched in patients with bloating, Prevotella in those reporting jaundice, and Veillonella in those reporting dark brown urine. Neisseria and Campylobacter were depleted in patients with diarrhea, and Alloprevotella in those reporting vomiting. |
| Intratumor Microbiome | |||
| Geller et al., 2017 [30] | Cohort study on: -113 human PDACs -20 samples from normal human pancreas (from organ donors) | qPCR rRNA FISH Immunohistochemistry using an anti-LPS antibody 16S rRNA gene sequencing (on 65 PDACs, Illumina MiSeq platform) | 76% of PDACs were positive for bacteria, mainly Gammaproteobacteria (Enterobacteriaceae and Pseudomonadaceae families). More bacteria were detected in patients undergoing pancreatic duct instrumentation, suggesting possible retrograde bacterial migration from the duodenum to the pancreas. Bacteria cultured from fresh human PDAC tumors made human colon carcinoma cell lines completely resistant to gemcitabine, suggesting that intratumor bacteria might contribute to the drug resistance of these tumors. |
| Riquelme et al., 2019 [60] | Cohort study on surgical resected PDACs from: -36 long-term survivors -32 short-term survivors (+9 frozen PDACs) Murine models | 16S rRNA gene sequencing (Illumina MiSeq platform) rRNA FISH Immunohistochemistry using an anti-LPS antibody PCR with species-specific primers for Saccharopolyspora rectivirgula | Long-term survivors showed higher alpha diversity of the intra-tumoral microbiome and enrichment in Pseudoxanthomonas/Streptomyces/Saccharopolyspora/Bacillus clausii (in both discovery and validation cohorts). Based on the combination of these 4 taxa, a high classification power with AUC > 97% was achieved. Through inferred metagenomics, the authors found that long-term survivors were enriched in pathways related to the metabolism of amino acids, xenobiotics, lipids, terpenoids and polyketides, besides other cellular functions. Human-into-mice FMT experiments showed that the gut microbiome can modulate the intra-tumoral microbiome, partly by direct translocation, partly by altering the microbial landscape. |
| Title | Status | Results | Condition | Intervention | Location | URL | |
|---|---|---|---|---|---|---|---|
| Diet | Diet and Exercise After Pancreatic Cancer (PACE) | Recruiting | No results available | PC | Diet counseling delivered using visual communication | U.S. | https://clinicaltrials.gov/ct2/show/NCT03187028 |
| Study Evaluating the Ketogenic Diet in Patients with Metastatic Pancreatic Cancer | Recruiting | No results available | Metastatic PDAC | Ketogenic diet | U.S. | https://clinicaltrials.gov/ct2/show/NCT04631445 | |
| Rehabilitation Strategies Following Esophagogastric and Hepatopancreaticobiliary Cancer (RESTORE II) | Not yet recruiting | No results available | PC, esophageal cancer, gastric cancer, liver cancer | RESTORE II program | Ireland | https://ClinicalTrials.gov/show/NCT03958019 | |
| Nutrition Support to Improve Outcomes in Patients with Unresectable Pancreatic Cancer | Active, not recruiting | No results available | PC | Diet with and without nutrition supplementation (Nutrawell Powder or OmegaRich fish oil supplement) | U.S. | https://clinicaltrials.gov/ct2/show/NCT02681601 | |
| Gemcitabine Hydrochloride, Paclitaxel Albumin-Stabilized Nanoparticle Formulation, Metformin Hydrochloride, and a Standardized Dietary Supplement in Treating Patients with Pancreatic Cancer That Cannot be Removed by Surgery | Active, not recruiting | No results available | PC | Dietary supplements (curcumin, vitamin D, vitamin K2, vitamin K1, B6, high-selenium broccoli sprouts, epigallocatechin gallate, L-carnitine, garlic extract, genistein, zinc amino chelate, mixed tocopherols, ascorbic acid, D-limonene) | U.S. | https://clinicaltrials.gov/ct2/show/NCT02336087 | |
| Prevention of Chyle-leak After Major Pancreatic Surgery | Recruiting | No results available | PC with lymph leakage, chyle into mesentery (extravasation) | No-fat diet including medium-chain fatty acids as oil and protein supplements | Finland | https://clinicaltrials.gov/ct2/show/NCT03167814 | |
| Exercise and Nutrition to Improve Pancreatic Outcomes | Recruiting | No results available | PC | Nutritional counseling | U.S. | https://clinicaltrials.gov/ct2/show/NCT03256201 | |
| Early Oral Versus Enteral Nutrition After Pancreatoduodenectomy | Unknown | No results available | PC, duodenal cancer, cholangiocarcinoma, chronic pancreatitis | Oral vs. enteral nutrition | Poland | https://clinicaltrials.gov/ct2/show/NCT01642875 | |
| Ascorbic Acid and Combination Chemotherapy in Treating Patients with Locally Advanced or Recurrent Pancreatic Cancer That Cannot Be Removed by Surgery | Completed | Has results | PC, PDAC | Ascorbic acid as dietary supplement | U.S. | https://clinicaltrials.gov/ct2/show/NCT02896907 | |
| Improving Outcomes in Cancer Patients with a Nutritional and Physical Conditioning Prehabilitation Program | Recruiting | No results available | PC, liver cancer, bile duct cancer, hepatobiliary cancer | A high-protein diet, possibly with a whey-protein supplement | Canada | https://clinicaltrials.gov/ct2/show/NCT03475966 | |
| Enteral Nutrition After Pancreaticoduodenectomy | Completed | No results available | PC, duodenal cancer, ampulla of Vater cancer, cholangiocarcinoma | Enteral diet administered through a nasojejunal tube vs. oral intake | China | https://clinicaltrials.gov/ct2/show/NCT03150615 | |
| High Dose Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | Withdrawn (regulatory review needed) | No results available | Metastatic PC | Ascorbic acid as dietary supplement | U.S. | https://clinicaltrials.gov/ct2/show/NCT03797443 | |
| Enhancing Fitness Before Pancreatic Surgery (MedEx) | Completed | No results available | PC, chronic pancreatitis | Nutritional supplementation (based on components of the Mediterranean diet) | United Kingdom | https://clinicaltrials.gov/ct2/show/NCT02940067 | |
| Whipple Protein Study (WPS) | Not yet recruiting | No results available | PC, malnutrition | High-protein nutritional supplementation | U.S. | https://clinicaltrials.gov/ct2/show/NCT04306874 | |
| A Study to See Whether a Nutritional Supplement is Beneficial for Patients with Pancreatic Cancer | Withdrawn (change in concept) | No results available | PC | Nutritional supplement | Canada | https://clinicaltrials.gov/ct2/show/NCT02745197 | |
| A Study of the Efficacy of ONS to Reduce Postoperative Complications Associated with Pancreatic Surgery (INSPIRE) | Terminated (Closed due to low enrollment) | No results available | PC, chronic pancreatitis | Dietary counseling with and without oral nutritional supplementation (Ensure Surgical) | U.S. | https://clinicaltrials.gov/ct2/show/NCT03244683 | |
| Zinc Supplements in Lowering Cadmium Levels in Smokers | Completed | No results available | PC, bladder, cervical, esophageal, gastric, head and neck, kidney, liver, lung cancer, leukemia | Dietary supplement (zinc oxide) | U.S. | https://clinicaltrials.gov/ct2/show/NCT00376987 | |
| Effect of Preoperative Immunonutrition in Upper Digestive Tract | Recruiting | No results available | PC, gastric, esophageal cancer | Immunomodulatory oral nutritional supplement (enriched in arginine, nucleotides, omega-3 fatty acids, olive oil polyphenols, L-carnitine, and antioxidants) | Spain | https://clinicaltrials.gov/ct2/show/NCT04027088 | |
| Resistance Training Intervention to Improve Physical Function in Patients with Pancreatic Cancer Receiving Combination Chemotherapy or Have Undergone Surgery, PancStrength Study | Recruiting | No results available | PC, PDAC | Individualized recommendations for daily protein intake and information about healthy protein supplementation during chemotherapy | U.S. | https://clinicaltrials.gov/ct2/show/NCT04837118 | |
| Gemcitabine and Capecitabine with or Without T-ChOS as Adjuvant Therapy for Patients with Resected Pancreatic Cancer (CHIPAC) | Terminated (Poor accrual and change of SOC) | No results available | PC | T-ChOS as dietary supplement (a blend of chit oligosaccharides from shellfish-derived chitin) | Denmark | https://clinicaltrials.gov/ct2/show/NCT02767752 | |
| Effects of Prehabilitation and Early Mobilization for Patients Undergoing Pancreas Surgery (PreMob) | Completed | No results available | PC | Dietary advice | Sweden | https://clinicaltrials.gov/ct2/show/NCT03466593 | |
| Preoperative Prehabilitation for Sarcopenic Patients Prior to Pancreatic Surgery for Cancer (PSOAS) | Not yet recruiting | No results available | PC | Dietary supplement (Oral Impact®) | France | https://clinicaltrials.gov/ct2/show/NCT04469504 | |
| Cost Effectiveness of an Intervention in Hospitalized Patients with Disease-related Malnutrition | Recruiting | No results available | PC, acute pancreatitis, IBD, esophagus, gastric, colorectal cancer | Dietary advice, oral nutritional supplementation vs. no explicit intervention | Spain | https://clinicaltrials.gov/ct2/show/NCT04188990 | |
| The Pancreatic and Periampullary Resection Arginine Immunomodulation (PRIMe) Trial (PRIMe) | Recruiting | No results available | PC | Dietary supplements (powdered formula containing whey protein and arginine, omega-3 fatty acids) | Canada | https://clinicaltrials.gov/ct2/show/NCT04549662 | |
| Effects on Quality of Life with Zinc Supplementation in Patients with Gastrointestinal Cancer | Recruiting | No results available | PC, gastric, esophageal, liver and intrahepatic bile duct carcinoma | Dietary supplement (zinc) | U.S. | https://clinicaltrials.gov/ct2/show/NCT03819088 | |
| Evaluation of Ocoxin-Viusid® in Advanced Pancreatic Adenocarcinoma | Recruiting | No results available | PC, pancreatic diseases, digestive system neoplasms and diseases, endocrine system neoplasms and diseases | Dietary supplement (Ocoxin-Viusid®) | Cuba | https://clinicaltrials.gov/ct2/show/NCT03717298 | |
| Survivorship Promotion in Reducing IGF-1 Trial (SPIRIT) | Completed | Has results | PC, breast, prostate, lung, colon, skin, endometrial, liver, rectal, kidney cancer and other solid malignant tumors | Changes in dietary intake | U.S. | https://clinicaltrials.gov/ct2/show/NCT02431676 | |
| Prevention of Cancer-associated Malnutrition Through Oral Nutritional Supplements | Unknown | No results available | PC, hepatocellular carcinoma | Oral nutritional supplement (Fortimel Compact/Fortimel Compact Fiber, Nutricia) | Germany | https://clinicaltrials.gov/ct2/show/NCT02312674 | |
| FMT | Fecal Microbial Transplants for the Treatment of Pancreatic Cancer | Not yet recruiting | No results available | PDAC | FMT during colonoscopy and capsules | U.S. | https://clinicaltrials.gov/ct2/show/NCT04975217 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandi, G.; Turroni, S.; McAllister, F.; Frega, G. The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies? Int. J. Mol. Sci. 2021, 22, 9914. https://doi.org/10.3390/ijms22189914
Brandi G, Turroni S, McAllister F, Frega G. The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies? International Journal of Molecular Sciences. 2021; 22(18):9914. https://doi.org/10.3390/ijms22189914
Chicago/Turabian StyleBrandi, Giovanni, Silvia Turroni, Florencia McAllister, and Giorgio Frega. 2021. "The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies?" International Journal of Molecular Sciences 22, no. 18: 9914. https://doi.org/10.3390/ijms22189914
APA StyleBrandi, G., Turroni, S., McAllister, F., & Frega, G. (2021). The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies? International Journal of Molecular Sciences, 22(18), 9914. https://doi.org/10.3390/ijms22189914







