Engineering Climate-Change-Resilient Crops: New Tools and Approaches
Abstract
:1. Introduction
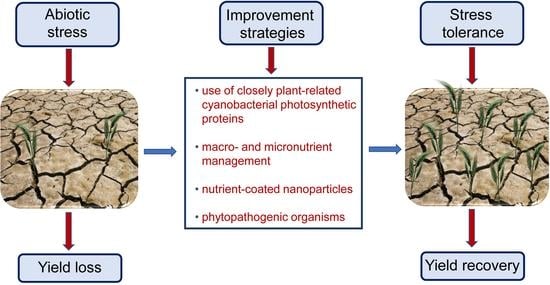
2. Introduction of Alternative Electron Sinks Protects Chloroplast Metabolic Activities under Stress
2.1. Chloroplasts as Targets and Sensors of Environmental Stresses
2.2. Cyanobacterial Flavodoxin as a Tool to Achieve Plant Tolerance to a Broad Range of Environmental Adversities
2.3. Expression of Plastid-Targeted Algal Cytochrome c6 in Plants Increases Growth and Biomass Accumulation
2.4. Flavodi-Iron Proteins Act as Electron Sinks and Enhance Plant Growth under Drought Stress Conditions
3. Increasing Accessibility and Mobilization of Macro- and Micronutrients to Improve Plant Stress Tolerance
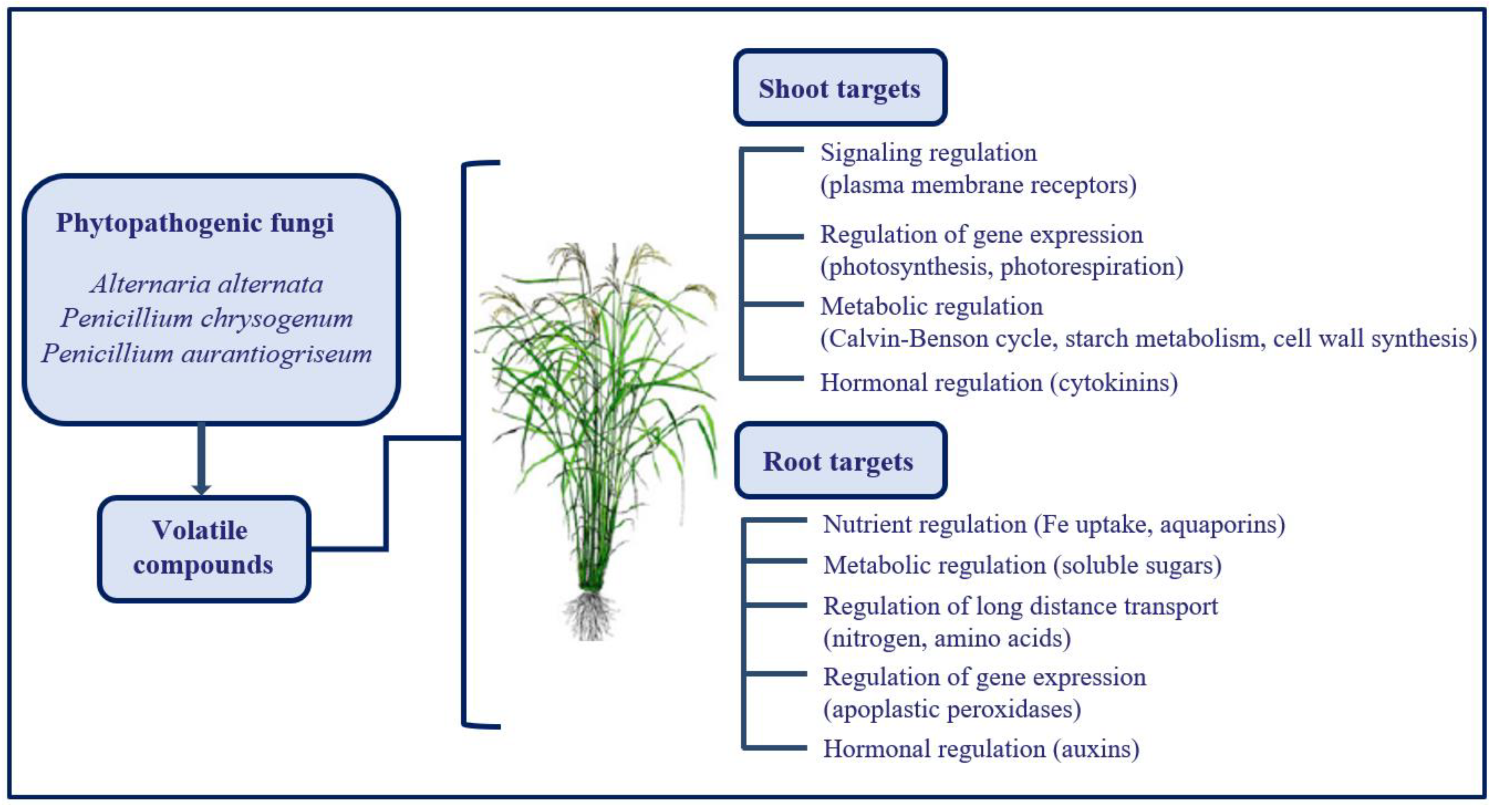
4. Volatile Emission by Phytopathogenic Organisms Represents a Great Opportunity to Improve Plant Tolerance towards Adverse Environmental Conditions
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; American Society of Plant Physiologists: Rockville, MD, USA, 2018. [Google Scholar]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production; scavenging and signaling in plant response to environmental stresses. Free Rad. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Gómez, R.; Vicino, P.; Carrillo, N.; Lodeyro, A.F. Manipulation of oxidative stress responses as a strategy to generate stress-tolerant crops. From damage to signaling to tolerance. Crit. Rev. Biotechnol. 2019, 39, 693–708. [Google Scholar] [CrossRef]
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 2006, 17, 113–122. [Google Scholar] [CrossRef]
- Vij, S.; Tyagi, A.K. Emerging trends in the functional genomics of the abiotic stress response in crop plants. Plant Biotechnol. J. 2007, 5, 361–380. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, C.-Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [Green Version]
- Zurbriggen, M.D.; Tognetti, V.B.; Fillat, M.F.; Hajirezaei, M.R.; Valle, E.M.; Carrillo, N. Combating stress with flavodoxin: A promising route for crop improvement. Trends Biotechnol. 2008, 26, 531–537. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.; Zhang, J.; Xie, M.; Yuan, G.; Tschaplinski, T.J.; Muchero, W.; Chen, J.G. Transcriptional regulation of drought response in Arabidopsis and woody plants. Front. Plant Sci. 2021, 11, 572137. [Google Scholar] [CrossRef] [PubMed]
- Nepal, N.; Yactayo-Chang, J.P.; Gable, R.; Wilkie, A.; Martin, J.; Aniemena, C.L.; Gaxiola, R.A.; Lorence, A. Phenotypic characterization of Arabidopsis thaliana lines overexpressing AVP1 and MIOX4 in response to abiotic stresses. Appl. Plant Sci. 2020, 8, e11384. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Ma, Y.; Zheng, D.; Wisniewski, M.; Cheng, Z.M. Meta-analysis of the effect of overexpression of dehydration-responsive element binding family genes on temperature stress tolerance and related responses. Front. Plant Sci. 2018, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, N.; Zhuang, L.; Yu, J.; Huang, B. Enhanced stolon growth and metabolic adjustment in creeping bentgrass with elevated CO2 concentration. Environ. Exp. Bot. 2018, 155, 87–97. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Hori, C.; Yu, X.; Mortimer, J.C.; Sano, R.; Matsumoto, T.; Kikuchi, J.; Demura, T.; Ohtani, M. Impact of abiotic stress on the regulation of cell wall biosynthesis in Populus trichocarpa. Plant Biotechnol. 2020, 37, 273–283. [Google Scholar] [CrossRef]
- Shabir, H.W.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Engineering abiotic stress response in plants for biomass production. J. Biol. Chem. 2018, 293, 5035–5043. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.X.; Jiao, Y.; Li, M.X.; Zhong, X.L.; Gu, F.X.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought–sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016. [Google Scholar] [CrossRef] [Green Version]
- García-Gómez, P.; Almagro, G.; Sánchez-López, A.M.; Bahaji, A.; Ameztoy, K.; Ricarte-Bermejo, A.; Baslam, M.; Antolín, M.C.; Urdiain, A.; López-Belchi, M.D.; et al. Volatile compounds other than CO2 emitted by different microorganisms promote distinct posttranscriptionally regulated responses in plants. Plant Cell Environ. 2019, 42, 1729–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, T.; Shao, S.; Nixon, P.J. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar] [PubMed] [Green Version]
- Fernández, A.P.; Strand, Å. Retrograde signaling and plant stress: Plastid signals initiate cellular stress responses. Curr. Opin. Plant Biol. 2008, 11, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Peshev, D.; Van den Ende, W. Sugars as antioxidants in plants. In Crop Improvement under Adverse Conditions; Tuteja, N., Gill, S.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 285–308. [Google Scholar]
- Xu, Z.Z.; Zhou, G.S. Effects of water stress on photosynthesis and nitrogen metabolism in vegetative and reproductive shoots of Leymus chinensis. Photosynthetica 2005, 43, 29–35. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Handa, S.; Bressan, R.A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986, 82, 890–903. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Saglio, P.H.; Pradet, A. Soluble sugars, respiration, and energy charge during aging of excised maize root tips. Plant Physiol. 1980, 66, 516–519. [Google Scholar] [CrossRef] [Green Version]
- Dobrota, C. Energy dependant plant stress acclimation. In Life in Extreme Environments; Amils, R., Ellis-Evans, C., Hinghofer-Szalkay, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 277–285. [Google Scholar]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Takahashi, S.; Milward, S.E.; Fan, D.Y.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef] [Green Version]
- Lehtimaki, N.; Lintala, M.; Allahverdiyeva, Y.; Aro, E.M.; Mulo, P. Drought stress-induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer. J. Plant Physiol. 2010, 167, 1018–1022. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Strand, D.E.; Fisher, N.; Kramer, D.M. The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow. J. Biol. Chem. 2017, 292, 11850–11860. [Google Scholar] [CrossRef] [Green Version]
- Scheibe, R.; Backhausen, J.E.; Emmerlich, V.; Holtgrefe, S. Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J. Exp. Bot. 2005, 56, 1481–1489. [Google Scholar] [CrossRef] [Green Version]
- Bauwe, H.; Hagemann, M.; Kern, R.; Timm, S. Photorespiration has a dual origin and manifold links to central metabolism. Curr. Opin. Plant Biol. 2012, 15, 269–275. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Feilke, K. The dual role of the plastid terminal oxidase PTOX: Between a protective and a pro-oxidant function. Front. Plant Sci. 2016, 6, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Kandoi, D.; Mohanty, S.; Tripathy, B.C. Overexpression of plastidic maize NADP-malate dehydrogenase (ZmNADP-MDH) in Arabidopsis thaliana confers tolerance to salt stress. Protoplasma 2018, 255, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, J.; Buyyarapu, R.; Guttikonda, S.K.; Parliament, K.; Abdurakhmonov, I.Y.; Kumpatla, S.P. Wild relatives of maize, rice, cotton, and soybean: Treasure troves for tolerance to biotic and abiotic stresses. Front. Plant Sci. 2018, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Kim, W.C. A fruitful decade using synthetic promoters in the improvement of transgenic plants. Front. Plant Sci. 2019, 10, 1433. [Google Scholar] [CrossRef]
- Ceccoli, R.C.; Blanco, N.E.; Segretin, M.E.; Melzer, M.; Hanke, G.T.; Scheibe, R.; Hajirezaei, M.R.; Bravo-Almonacid, F.F.; Carrillo, N. Flavodoxin displays dose-dependent effects on photosynthesis and stress tolerance when expressed in transgenic tobacco plants. Planta 2012, 236, 1447–1458. [Google Scholar] [CrossRef]
- Pierella Karlusich, J.J.; Lodeyro, A.F.; Carrillo, N. The long goodbye: The rise and fall of flavodoxin during plant evolution. J. Exp. Bot. 2014, 65, 5161–5178. [Google Scholar] [CrossRef] [Green Version]
- Pierella Karlusich, J.J.; Ceccoli, R.D.; Graña, M.; Romero, H.; Carrillo, N. Environmental selection pressures related to iron utilization are involved in the loss of the flavodoxin gene from the plant genome. Genome Biol. Evol. 2015, 7, 750–767. [Google Scholar] [CrossRef]
- Erdner, D.L.; Price, N.M.; Doucette, G.J.; Peleato, M.L.; Anderson, D.M. Characterization of ferredoxin and flavodoxin as markers of iron limitation in marine phytoplankton. Mar. Ecol. Progr. Ser. 1999, 184, 43–53. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Kim, H.S.; Mittenthal, J.E. The origin of modern metabolic networks inferred from phylogenomic analysis of protein architecture. Proc. Natl Acad. Sci. USA 2007, 104, 9358–9363. [Google Scholar] [CrossRef] [Green Version]
- Zurbriggen, M.D.; Tognetti, V.B.; Carrillo, N. Stress-inducible flavodoxin from photosynthetic microorganisms. The mystery of flavodoxin loss from the plant genome. IUBMB Life 2007, 59, 355–360. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Palatnik, J.F.; Fillat, M.F.; Melzer, M.; Hajirezaei, M.R.; Valle, E.M.; Carrillo, N. Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurbriggen, M.D.; Carrillo, N.; Tognetti, V.B.; Melzer, M.; Peisker, M.; Hause, B.; Hajirezaei, M.R. Chloroplast-generated reactive oxygen species contribute decisively to localized cell death during a plant-microorganism nonhost interaction. Plant J. 2009, 60, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.R.; Krapp, A.R.; Bisaro, F.; Maiale, S.J.; Pieckenstain, F.L.; Carrillo, N. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant J. 2017, 95, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, V.B.; Zurbriggen, M.D.; Morandi, E.N.; Fillat, M.F.; Valle, E.M.; Hajirezaei, M.R.; Carrillo, N. Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc. Natl. Acad. Sci. USA 2007, 104, 11495–11500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, N.E.; Ceccoli, R.D.; Segretin, M.E.; Poli, H.O.; Voss, I.; Melzer, M.; Bravo-Almonacid, F.F.; Scheibe, R.; Hajirezaei, M.R.; Carrillo, N. Cyanobacterial flavodoxin complements ferredoxin deficiency in knocked-down transgenic tobacco plants. Plant J. 2011, 65, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Figueroa, N.; Melzer, M.; Hajirezaei, M.R.; Carrillo, N.; Lodeyro, A.F. Photosynthetic characterization of flavodoxin-expressing tobacco plants reveals a high light acclimation-like phenotype. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148211. [Google Scholar] [CrossRef]
- Coba de la Peña, T.; Redondo, F.J.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Nitrogen fixation persists under conditions of salt stress in transgenic Medicago truncatula plants expressing a cyanobacterial flavodoxin. Plant Biotechnol. J. 2010, 8, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, S.; Jia, H.; Gao, F.; Zhou, M.; Yuan, N.; Wu, P.; Hu, Q.; Sun, D.; Luo, H. Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnol. J. 2017, 15, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Pierella Karlusich, J.J.; Arce, R.C.; Shahinnia, F.; Sonnewald, S.; Sonnewald, U.; Zurbriggen, M.D.; Hajirezaei, M.R.; Carrillo, N. Transcriptional and metabolic profiling of potato plants expressing a plastid-targeted electron shuttle reveal modulation of genes associated to drought tolerance by chloroplast redox poise. Int. J. Mol. Sci. 2020, 21, 7199. [Google Scholar] [CrossRef] [PubMed]
- Lodeyro, A.F.; Ceccoli, R.D.; Pierella-Karlusich, J.J.; Carrillo, N. The importance of flavodoxin for environmental stress tolerance in photosynthetic microorganisms and transgenic plants. Mechanism, evolution and biotechnological potential. FEBS Lett. 2012, 586, 2917–2924. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, M.A.; Molina-Heredia, F.P.; Hervás, M.; Navarro, J.A. Convergent evolution of cytochrome c6 and plastocyanin. The evolutionary pathways of the two proteins are connected to the geochemical changes in iron and copper availabilities. In Photosystem I: The Light Driven Plastocyanin:Ferredoxin Oxidoreductase. Advances in Photosynthesis and Respiration; Golbeck, J.H., Ed.; Springer: Dordrecht, The Netherlands, 2006; Volume 24, pp. 683–696. [Google Scholar]
- Castell, C.; Rodríguez-Lumbreras, L.A.; Hervás, M.; Fernández-Recio, J.; Navarro, J.A. New insights into the evolution of the electron transfer from cytochrome f to Photosystem I in the green and red branches of photosynthetic eukaryotes. Plant Cell Physiol. 2021, 27, pcab044. [Google Scholar] [CrossRef]
- Groussman, R.D.; Parker, M.S.; Armbrust, E.V. Diversity and evolutionary history of iron metabolism genes in diatoms. PLoS ONE 2015, 10, e0129081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chida, H.; Nakazawa, A.; Akazaki, H.; Hirano, T.; Suruga, K.; Ogawa, M.; Satoh, T.; Kadokura, K.; Yamada, S.; Hakamata, W.; et al. Expression of the algal cytochrome c6 gene in Arabidopsis enhances photosynthesis and growth. Plant Cell Physiol. 2007, 48, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Khatri, K.; Rathore, M.S.; Jha, B. Introgression of UfCyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata Delile enhanced photosynthesis and growth in tobacco. Mol. Biol. Rep. 2018, 45, 1745–1758. [Google Scholar] [CrossRef]
- López-Calcagno, P.; Brown, K.; Simkin, A.; Fisk, S.J.; Vialet-Chabrand, S.; Lawson, T.; Raines, C. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat. Plants 2020, 6, 1054–1063. [Google Scholar] [CrossRef]
- Ilík, P.; Pavlovič, A.; Kouřil, R.; Alboresi, A.; Morosinotto, T.; Allahverdiyeva, Y.; Aro, E.M.; Yamamoto, H.; Shikanai, T. Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol. 2017, 214, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Vicente, J.B.; Justino, M.C.; Goncalves, V.L.; Saraiva, L.M.; Teixeira, M. Biochemical, spectroscopic and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 2008, 437, 21–45. [Google Scholar] [PubMed]
- Zhang, P.; Eisenhut, M.; Brandt, A.M.; Carmel, D.; Siléna, I.V.H.M.; Allahverdiyeva, Y.; Salminen, T.A.; Aro, E.M. Operon flv4-flv2 provides cyanobacterial Photosystem II with flexibility of electron transfer. Plant Cell 2012, 24, 1952–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sétif, P.; Shimakawa, G.; Krieger-Liszkay, A.; Miyake, C. Identification of the electron donor to flavodiiron proteins in Synechocystis sp. PCC 6803 by in vivo spectroscopy. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148256. [Google Scholar] [CrossRef] [PubMed]
- Bersanini, L.; Battchicova, N.; Jokel, M.; Rehman, A.; Vass, I.; Allahverdiyeva, Y.; Aro, E.M. Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol. 2014, 164, 805–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bersanini, L.; Allahverdiyeva, Y.; Battchikova, N.; Heinz, S.; Lespinasse, M.; Ruohisto, E.; Mustila, H.; Nickelsen, J.; Vass, I.; Aro, E.M. Dissecting the photoprotective mechanism encoded by the flv4-2 operon: A distinct contribution of Sll0218 in photosystem II stabilization. Plant Cell Environ. 2017, 40, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Sánchez, A.; Solymosi, D.; Mustila, H.; Bersanini, L.; Aro, E.M.; Allahverdiyeva, Y. Flavodiiron proteins 1–to-4 function in versatile combinations in O2 photoreduction in cyanobacteria. Elife 2019, 8, e45766. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, H.; Ishizak, K.; Nohira, K.; Takagi, D.; Shimakawa, G.; Sejima, T.; Shaku, K.; Makino, A.; Miyake, C. Land plants drive photorespiration as higher electron-sink: Comparative study of post-illumination transient O2 uptake rates from liverworts to angiosperms through ferns and gymnosperms. Physiol. Plant 2017, 161, 138–149. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; ShikanaI, T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef]
- Wada, S.; Yamamoto, H.; Suzuki, Y.; Yamori, W.; Shikanai, T.; Makino, A. Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol. 2018, 176, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Vicino, P.; Carrillo, J.; Gómez, R.; Shahinnia, F.; Tula, S.; Melzer, M.; Rutten, T.; Carrillo, N.; Hajirezaei, M.R.; Lodeyro, A.F. Expression of flavodiiron proteins Flv2-Flv4 in chloroplasts of Arabidopsis and tobacco plants provides multiple stress tolerance. Int. J. Mol. Sci. 2021, 22, 1178. [Google Scholar] [CrossRef]
- Shahinnia, F.; Tula, S.; Hensel, G.; Reiahisamani, N.; Nasr, N.; Kumlehn, J.; Gómez, R.; Lodeyro, A.F.; Carrillo, N.; Hajirezaei, M.R. Plastid-targeted cyanobacterial flavodiiron proteins maintain carbohydrate turnover rand enhance drought stress tolerance in barley. Front. Plant Sci. 2021, 11, 613731. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.C.; Custodio Nogueira, R.J.M.; da Silva, M.A.; de Albuquerque, M.B. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Ul-Allah, U.; Ijaz, M.; Nawaz, A.; Sattar, A.; Sher, A.; Naeem, M.; Shahzad, U.; Farooq, U.; Nawaz, F.; Mahmood, K. Potassium application improves grain yield and alleviates drought susceptibility in diverse maize hybrids. Plants 2020, 9, v9010075. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Khalilzadeh, R.; Khan, S.; Zaib-un-Nisa, K.; Bashir, R.; Pirzad, A.; Malik, A. Mitigation of drought stress and yield improvement in wheat by zinc foliar spray relates to enhanced water use efficiency and zinc contents. Int. J. Plant Prod. 2021, 1–13. [Google Scholar]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 00168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.A. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, N.; Bazrafshan, F.; Zare, M.; Amiri, B.; Bahrani, A. Nano-fertilizer prevents environmental pollution and improves physiological traits of wheat grown under drought stress conditions. Sci. Agropecu. 2021, 12, 41–47. [Google Scholar] [CrossRef]
- Ahmadian, K.; Jalilian, J.; Pirzad, A. Nano-fertilizers improved drought tolerance in wheat under deficit irrigation. Agric. Water Manag. 2021, 244, 106544. [Google Scholar] [CrossRef]
- Kato, M.; Aoyama, T.; Maeshima, M. The Ca2+-binding protein PCaP2 located on the plasma membrane is involved in root hair development as a possible signal transducer. Plant J. 2013, 74, 690–700. [Google Scholar] [CrossRef]
- Tanaka, N.; Kato, M.; Tomioka, R.; Kurata, R.; Fukao, Y.; Aoyama, T.; Maeshima, M. Characteristics of a root hair-less line of Arabidopsis thaliana under physiological stresses. J. Exp. Bot. 2014, 65, 1497–1512. [Google Scholar] [CrossRef] [Green Version]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, J.P.; Foyer, C.H. Field phenotyping of soybean roots for drought stress tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef] [Green Version]
- Ramireddy, R.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, K.; von Wirén, N.; Schmülling, T. Root engineering in barley: Increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef] [Green Version]
- AbdElgawad, H.; Avramova, V.; Baggerman, G.; Van Raemdonck, G.; Valkenborg, D.; Van Ostade, X.; Guisez, Y.; Prinsen, E.; Asard, H.; Van den Ende, W.; et al. Starch biosynthesis contributes to the maintenance of photosynthesis and leaf growth under drought stress in maize. Plant Cell Environ. 2020, 43, 2254–2271. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Splivallo, R.; Bossi, S.; Maffei, M.; Bonfante, P. Discrimination of truffle fruiting body versus mycelial aromas by stir bar sorptive extraction. Phytochemistry 2007, 68, 2584–2598. [Google Scholar] [CrossRef]
- Splivallo, R.; Nover, M.; Bertea, C.M.; Bossi, S.; Bonfante, P. Truffle volatiles inhibit growth and induce and oxidative burst in Arabidopsis thaliana. New Phytol. 2007, 175, 417–424. [Google Scholar] [CrossRef]
- Ezquer, I.; Li, J.; Ovecka, M.; Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Díaz de Cerio, J.; Hidalgo, M.; Sesma, M.T.; Bahaji, A.; et al. Microbial volatile emissions promote accumulation of exceptionally high levels of starch in leaves in mono and dicotyledonous plants. Plant Cell Physiol. 2010, 51, 1674–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ezquer, I.; Bahaji, A.; Montero, M.; Ovecka, M.; Baroja-Fernández, E.; Muñoz, F.J.; Mérida, A.; Almagro, G.; Hidalgo, M.; et al. Microbial volatiles induced accumulation of exceptionally high levels of starch in Arabidopsis leaves is a process involving NTRC and starch synthases class III and IV. Mol. Plant Microbe Interact. 2011, 24, 1165–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-López, Á.M.; Baslam, M.; De Diego, N.; Muñoz, F.J.; Bahaji, A.; Almagro, G.; Ricarte-Bermejo, A.; García-Gómez, P.; Li, J.; Humplik, J.F.; et al. Volatile compounds emitted by diverse phytopathogenic microorganisms promote plant growth and flowering through cytokinin action. Plant Cell Environ. 2016, 39, 2592–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-López, Á.M.; Bahaji, A.; De Diego, N.; Baslam, M.; Li, J.; Muñoz, F.J.; Almagro, G.; García-Gómez, P.; Ameztoy, K.; Ricarte-Bermejo, A.; et al. Arabidopsis responds to Alternaria alternata volatiles by triggering plastid phosphoglucose isomerase independent mechanisms. Plant Physiol. 2016, 172, 1989–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Gómez, P.; Bahaji, A.; Gámez-Arcas, S.; Muñoz, F.J.; Sánchez-López, A.M.; Almagro, G.; Baroja-Fernández, E.; Ameztoy, K.; De Diego, N.; Ugena, L.; et al. Volatiles from the fungal phytopathogen Penicillium aurantiogriseum modulate root metabolism and architecture through proteome resetting. Plant Cell Environ. 2020, 43, 2551–2570. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahinnia, F.; Carrillo, N.; Hajirezaei, M.-R. Engineering Climate-Change-Resilient Crops: New Tools and Approaches. Int. J. Mol. Sci. 2021, 22, 7877. https://doi.org/10.3390/ijms22157877
Shahinnia F, Carrillo N, Hajirezaei M-R. Engineering Climate-Change-Resilient Crops: New Tools and Approaches. International Journal of Molecular Sciences. 2021; 22(15):7877. https://doi.org/10.3390/ijms22157877
Chicago/Turabian StyleShahinnia, Fahimeh, Néstor Carrillo, and Mohammad-Reza Hajirezaei. 2021. "Engineering Climate-Change-Resilient Crops: New Tools and Approaches" International Journal of Molecular Sciences 22, no. 15: 7877. https://doi.org/10.3390/ijms22157877