Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Literature Screening
2.2. Study Assessment
2.3. Study Selection
2.4. Data Extraction
2.5. Endpoint Definition
3. Results
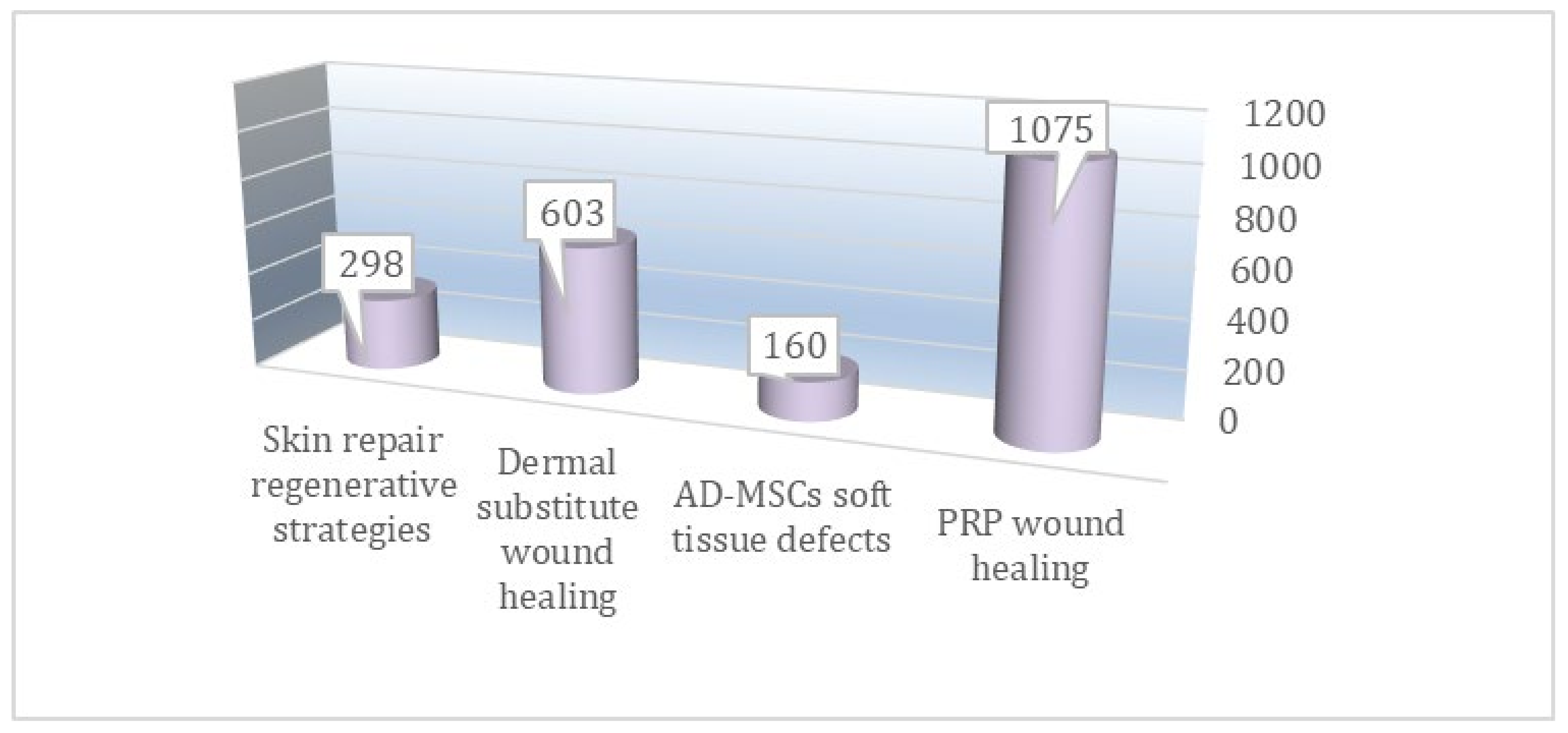
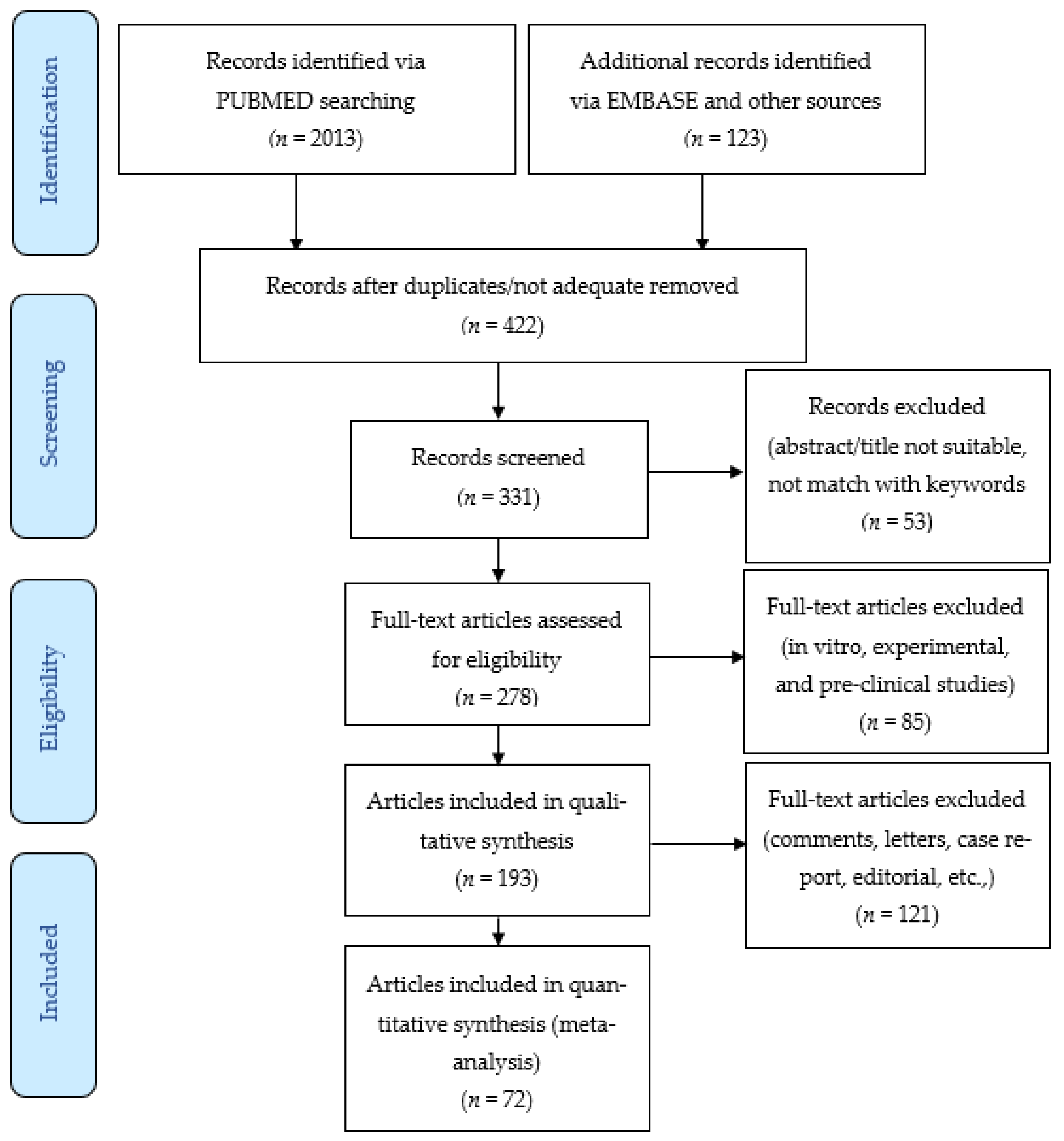
3.1. Literature Search
3.2. Study Subjects
3.3. Platelet-Rich Plasma Preparation
3.4. Adipose-Derived Mesenchymal Stem Cells Preparation
3.5. Biomaterial Application
3.6. Outcome Evaluation Methods and Adverse Effects
3.7. Selected Studies Analyzed
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef]
- Lucich, E.A.; Rendon, J.L.; Valerio, I.L. Advances in addressing full-thickness skin defects: A review of dermal and epidermal substitutes. Regen. Med. 2018, 13, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Kishi, K. Skin graft. Plast. Surg. Int. 2012, 2012, 563493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Diao, J.S.; Xia, W.-S.; Pan, Y.; Han, Y. Clinical application of full-face, whole, full-thickness skin grafting: A case report. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Fábio Lana, J.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Hesseler, M.J.; Shyam, N. Platelet-rich plasma and its utility in the treatment of acne scars: A systematic review. J. Am. Acad. Dermatol. 2019, 80, 1730–1745. [Google Scholar] [CrossRef]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Impact of incubation method on the release of growth factors in non-Ca(2+)- activated PRP, Ca(2+)-activated PRP, PRF and A-PRF. J. Craniomaxillofac. Surg. 2019, 47, 365–372. [Google Scholar] [CrossRef]
- Formigli, L.; Benvenuti, S.; Mercatelli, R.; Quercioli, F.; Tani, A.; Mirabella, C.; Dama, A.; Saccardi, R.; Mazzanti, B.; Cellai, I.; et al. Dermal matrix scaffold engineered with adult mesenchymal stem cells and platelet-rich plasma as a potential tool for tissue repair and regeneration. J. Tissue. Eng. Regen. Med. 2012, 6, 125–134. [Google Scholar] [CrossRef]
- Chang, Q.; Cai, J.; Wang, Y.; Yang, R.; Xing, M.; Lu, F. Large adipose tissue generation in a mussel-inspired bioreactor of elasticmimetic cryogel and platelets. J. Tissue. Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Yang, Y.; Niu, X.; Lin, Q.; Zhao, B.; Wang, Y.; Zhu, L. An in situ photocrosslinkable platelet rich plasma—Complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta. Biomater. 2017, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Nguyen, T.J.; Shahabi, A.; Hwang, B.H.; Chan, L.S.; Wong, A.K. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann. Plast. Surg. 2012, 68, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Wester, J.L.; Pittman, A.L.; Lindau, R.H.; Wax, M.K. AlloDerm with split-thickness skin graft for coverage of the forearm free flap donor site. Otolaryngol. Head Neck Surg. 2014, 150, 47–52. [Google Scholar] [CrossRef]
- Fourman, M.S.; Phillips, B.T.; Fritz, J.R.; Conkling, N.; McClain, S.A.; Simon, M.; Dagum, A.B. Laser-assisted indocyanine green dye angiography accurately predicts the split-thickness graft timing of integra artificial dermis. Ann. Plast. Surg. 2014, 73, 150–155. [Google Scholar] [CrossRef]
- De Haas, L.E.M.; Gardien, K.L.M.; van Trier, A.J.M.; Vloemans, A.F.P.M.; Buis, D.R. The Use of Integra in Extensive Full-Thickness Scalp Burn Involving Page 24 the Skull in a Child. J. Craniofac. Surg. 2019, 30, 888–890. [Google Scholar] [CrossRef]
- Notodihardjo, S.C.; Morimoto, N.; Munisso, M.C.; Le, T.M.; Mitsui, T.; Kakudo, N.; Kusumoto, K. A comparison of the wound healing process after the application of three dermal substitutes with or without basic fibroblast growth factor impregnation in diabetic mice. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1547–1555. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2020, 6, 579–588. [Google Scholar] [CrossRef]
- Hou, J.; Chen, L.; Liu, Z.; Li, J.; Yang, J.; Zhong, A.; Zhou, M.; Sun, Y.; Guo, L.; Yang, Y.; et al. Sustained release of N-acetylcysteine by sandwich structured polycaprolactone/collagen scaffolds for wound healing. J. Biomed. Mater. Res. A 2019, 107, 1414–1424. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, W.; Tang, C.; Huang, J.; Fan, C.; Yin, Z.; Hu, Y.; Chen, W.; Ouyang, H.; Zhou, Y. Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci. Adv. 2020, 6, eaay9526. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.; Luo, G.; Lei, Q.; Shu, Q.; Yao, Z.; Li, H.; Zhou, J.; Tan, J.; Yang, S.; et al. Biomimetic fibroblast-loaded artificial dermis with “sandwich” structure and designed gradient pore sizes promotes wound healing by favoring granulation tissue formation and wound re epithelialization. Acta Biomater. 2016, 30, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, J.; Gao, Y.; Dong, M.; Zheng, Z.; Li, Y.; Tan, R.; She, Z.; Yang, L. Epithelial differentiation of human adipose-derived stem cells (hASCs) undergoing three-dimensional (3D) cultivation with collagen sponge scaffold (CSS) via an indirect coculture strategy. Stem Cell Res. Ther. 2020, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Roman, S. Matriderm and Split Skin Grafting for Full-Thickness Pediatric Facial Burns. J. Burn Care Res. 2019, 40, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qiu, C.; Ben, C.; Li, H.; Zhu, S. One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay. Burns Trauma 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Mano, J.F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 2014, 43, 3453–3479. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Concise review: Adipose-derived stem cells (ASCs) and adipocyte-secreted exosomal microRNA (A-SE-miR) modulate cancer growth and proMote wound repair. J. Clin. Med. 2019, 8, 855. [Google Scholar] [CrossRef]
- Gentile, P.; Casella, D.; Palma, E.; Calabrese, C. Engineered fat graft enhanced with adipose-derived stromal vascular fraction cells for regenerative medicine: Clinical, histological and instrumental evaluation in breast reconstruction. J. Clin. Med. 2019, 8, 504. [Google Scholar] [CrossRef]
- Gentile, P.; Piccinno, M.S.; Calabrese, C. Characteristics and potentiality of human adipose-derived stem cells (hASCs) obtained from enzymatic digestion of fat graft. Cells 2019, 8, 282. [Google Scholar] [CrossRef]
- Gentile, P.; de Angelis, B.; di Pietro, V.; Amorosi, V.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Gentle is better: The original “gentle technique” for fat placement in breast lipofilling. J. Cutan. Aesthet. Surg. 2018, 11, 120–126. [Google Scholar] [CrossRef]
- Gentile, P.; Cervelli, V. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery. Methods Mol. Biol. 2018, 1773, 107–122. [Google Scholar] [PubMed]
- Fiaschetti, V.; Pistolese, C.A.; Fornari, M.; Liberto, V.; Cama, V.; Gentile, P.; Floris, M.; Floris, R.; Cervelli, V.; Simonetti, G. Magnetic resonance imaging and ultrasound evaluation after breast autologous fat grafting combined with platelet-rich plasma. Plast. Reconstr. Surg. 2013, 132, 498e–509e. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; di Pasquali, C.; Bocchini, I.; Floris, M.; Eleonora, T.; Fiaschetti, V.; Floris, R.; Cervelli, V. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg. Innov. 2013, 20, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Sarlo, F.; de Angelis, B.; de Lorenzo, A.; Cervelli, V. Obesity phenotypes and resorption percentage after breast autologous fat grafting: Rule of low-grade inflammation. Adv. Biomed. Res. 2016, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Breast reconstruction with enhanced stromal vascular fraction fat grafting: What is the best method? Plast. Reconstr. Surg. Glob. Open 2015, 3, e406. [Google Scholar] [CrossRef]
- Bielli, A.; Scioli, M.G.; Gentile, P.; Agostinelli, S.; Tarquini, C.; Cervelli, V.; Orlandi, A. Adult adipose-derived stem cells and breast cancer: A controversial relationship. Springerplus 2014, 3, 1–10. [Google Scholar] [CrossRef]
- Gentile, P.; de Angelis, B.; Pasin, M.; Cervelli, G.; Curcio, C.B.; Floris, M.; di Pasquali, C.; Bocchini, I.; Balzani, A.; Nicoli, F.; et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical evaluation for cell-based therapies in patients with scars on the face. J. Craniofac. Surg. 2014, 25, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Bocchini, I.; di Pasquali, C.; de Angelis, B.; Cervelli, G.; Curcio, C.B.; Orlandi, A.; Scioli, M.G.; Tati, E.; Delogu, P.; et al. platelet rich lipotransfert: Our experience and current state of art in the combined use of fat and PRP. BioMed Res. Int. 2013, 2013, 434191. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Comparing different nanofat procedures on scars: Role of the stromal vascular fraction and its clinical implications. Regen. Med. 2017, 12, 939–952. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Concise review: The use of adipose-derived stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cells 2017, 35, 117–134. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Gentile, P.; Cervelli, V.; Orlandi, A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 2398–2410. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Cervelli, V.; Arcuri, G.; Gentile, P.; Doldo, E.; Bielli, A.; Bonanno, E.; Orlandi, A. High insulin-induced down-regulation of Erk-1/IGF-1R/FGFR-1 signaling is required for oxidative stress-mediated apoptosis of adipose-derived stem cells. J. Cell Physiol. 2014, 229, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Bielli, A.; Gentile, P.; Mazzaglia, D.; Cervelli, V.; Orlandi, A. The biomolecular basis of adipogenic differentiation of adipose-derived stem cells. Int. J. Mol. Sci. 2014, 15, 6517–6526. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Orlandi, A.; Scioli, M.G.; di Pasquali, C.; Bocchini, I.; Cervelli, V. Concise review: Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl. Med. 2012, 1, 230–236. [Google Scholar] [CrossRef]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef]
- Araco, A.; Gravante, G.; Araco, F.; Gentile, P.; Castrì, F.; Delogu, D.; Filingeri, V.; Cervelli, V. Breast asymmetries: A brief review and our experience. Aesthet. Plast. Surg. 2006, 30, 309–319. [Google Scholar] [CrossRef]
- Cervelli, V.; Gentile, P.; de Angelis, B.; Calabrese, C.; di Stefani, A.; Scioli, M.G.; Curcio, B.C.; Felici, M.; Orlandi, A. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res. 2011, 6, 103–111. [Google Scholar] [CrossRef]
- Gentile, P.; Sterodimas, A.; Calabrese, C.; De Angelis, B.; Trivisonno, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Garcovich, S. Regenerative application of stromal vascular fraction cells enhanced fat graft maintenance: Clinical assessment in face rejuvenation. Expert Opin. Biol. Ther. 2020, 20, 1503–1513. [Google Scholar] [CrossRef]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Calabrese, C.; Garcovich, S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 4982. [Google Scholar] [CrossRef]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int. J. Mol. Sci. 2019, 20, 5471. [Google Scholar] [CrossRef]
- Gentile, P.; Kothari, A.; Casella, D.; Calabrese, C. Fat Graft Enhanced With Adipose-Derived Stem Cells in Aesthetic Breast Augmentation: Clinical, Histological, and Instrumental Evaluation. Aesthet. Surg. J. 2020, 40, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Dionisi, L.; Pizzicannella, J.; Kothari, A.; De Fazio, D.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Autologous Non-Activated Platelet-Rich Plasma (A-PRP) and Activated Platelet-Rich Plasma (AA-PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation. Int. J. Mol. Sci. 2020, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Insalaco, C.; Cervelli, V. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int. J. Mol. Sci. 2017, 18, 408. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Dionisi, L.; Pizzicannella, J.; de Angelis, B.; de Fazio, D.; Garcovich, S. A randomized blinded retrospective study: The combined use of micro-needling technique, low-level laser therapy and autologous non-activated platelet-rich plasma improves hair re-growth in patients with androgenic alopecia. Expert Opin. Biol. Ther. 2020, 20, 1099–1109. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; De Angelis, B.; De Sio, C.; De Fazio, D.; Ceccarelli, G.; Trivisonno, A.; Orlandi, A.; Cervelli, V.; et al. Platelet-Rich Plasma and Micrografts Enriched with Autologous Human Follicle Mesenchymal Stem Cells Improve Hair Re-Growth in Androgenetic Alopecia. Biomolecular Pathway Analysis and Clinical Evaluation. Biomedicines 2019, 7, 27. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The cochrane collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 2 February 2021).
- De Angelis, B.; Orlandi, F.; Morais D’Autilio, M.F.L.; Di Segni, C.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Vasculogenic Chronic Ulcer: Tissue Regeneration with an Innovative Dermal Substitute. J. Clin. Med. 2019, 8, 525. [Google Scholar] [CrossRef]
- De Angelis, B.; Morais D’Autilio, M.F.L.; Orlandi, F.; Pepe, G.; Garcovich, S.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019, 8, 1486. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.H.; Pazzini, J.M.; Álvarez, J.L.G.; Cassino, P.C.; Bustamante, C.C.; Bernardes, F.J.L.; Kajiura, C.Y.; De Nardi, A.B. Evaluation of angiogenesis, inflammation, and healing on irradiated skin graft with low-level laser therapy in rats (Rattus norvegicus albinus wistar). Lasers Med. Sci. 2020, 35, 1103–1109. [Google Scholar] [CrossRef]
- Alexandrushkina, N.; Nimiritsky, P.; Eremichev, R.; Popov, V.; Arbatskiy, M.; Danilova, N.; Malkov, P.; Akopyan, Z.; Tkachuk, V.; Makarevich, P. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int. J. Mol. Sci. 2020, 21, 5567. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xing, Q.; Zhai, Q.; Tahtinen, M.; Zhou, F.; Chen, L.; Xu, Y.; Qi, S.; Zhao, F. Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics 2017, 7, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.; Shen, J. Factors controlling the microstructure of collagen-based dermis regeneration scaffold. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2004, 21, 311–315. [Google Scholar]
- Qiu, X.; Wang, J.; Wang, G.; Wen, H. Vascularization of Lando((R)) dermal scaffold in an acute full-thickness skin defect porcine model. J. Plast. Surg. Hand Surg. 2018, 52, 204–209. [Google Scholar] [CrossRef]
- Watts, V.; Attie, M.D.; McClure, S. Reconstruction of Complex Full-Thickness Scalp Defects After Dog-Bite Injuries Using Dermal Regeneration Template (Integra): Case Report and Literature Review. J. Oral Maxillofac. Surg. 2019, 77, 338–351. [Google Scholar] [CrossRef]
- Demircan, M.; Cicek, T.; Yetis, M.I. Preliminary results in single-step wound closure procedure of full-thickness facial burns in children by using the collagen-elastin matrix and review of pediatric facial burns. Burns 2015, 41, 1268–1274. [Google Scholar] [CrossRef]
- Heimbach, D.; Luterman, A.; Burke, J.; Cram, A.; Herndon, D.; Hunt, J.; Jordan, M.; McManus, W.; Solem, L.; Warden, G.; et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann. Surg. 1988, 208, 313–320. [Google Scholar] [CrossRef]
- Harrison, S.; Vavken, P.; Kevy, S.; Jacobson, M.; Zurakowski, D.; Murray, M.M. Platelet activation by collagen provides sustained release of anabolic cytokines. Am. J. Sports Med. 2011, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
| Strategies Analyzed | Advantages | Disadvantages | Strategies Available | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Dermal substitutes (DS) | Granulation tissue and angiogenesis for wound healing with less scarring | Costs | Skin grafting or skin flap transplantation | Skin grafts offered less damage to the donor site and easy operation compared with skin flap transplantation | Skin grafts are easily hindered by uncontrollable scar hyperplasia and lower mechanical resistance, due to the lack of sufficient dermal matrix |
| Faster wound healing compared with control groups (hyaluronic acid, skin grafts, advanced dressing, etc.) | Need to be followed by autologous dermal epidermal graft (two-step surgical procedure) | ||||
| Re-epithelialization and formed new tissue architecture analogous to normal skin physiology | |||||
| Platelet-Rich Plasma (PRP) | Autologous treatment | Need to have authorization by the transfusion service | Hyaluronic acid, and/or advanced dressing | Lower cost compared with PRP | Lower regenerative potential in terms of epidermal proliferation and dermal renewal compared with PRP |
| Faster wound healing compared with control groups (hyaluronic acid, skin grafts, advanced dressing etc.) | Need to repeat the treatment more times | ||||
| Mini-invasive procedure | |||||
| Improving neo-angiogenetic vascularization and various activities of fibroblasts | |||||
| Adipose-derived Mesenchymal Stem Cells (AD-MSCs) | Autologous treatment | Need to repeat the treatment more times | Fat grafting or hyaluronic acid | Fat grafting is considered an autologous treatment | Hyaluronic acid is synthetic dressing |
| Faster wound healing compared with control groups (hyaluronic acid, skin grafts, advanced dressing etc.) | Not an easy procedure, which requires consolidated clinical practice | Hyaluronic acid application is an easy and fast procedure | Fat grafting presents a lower soft tissue volume maintenance compared with enriched AD-MSCs fat grafting | ||
| Better soft tissue volume maintenance when they have been used to enrich fat graft, compared with fat graft alone | Costs | ||||
| Improving vascularity via the secretion of growth factors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. https://doi.org/10.3390/ijms22041538
Gentile P, Garcovich S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. International Journal of Molecular Sciences. 2021; 22(4):1538. https://doi.org/10.3390/ijms22041538
Chicago/Turabian StyleGentile, Pietro, and Simone Garcovich. 2021. "Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects" International Journal of Molecular Sciences 22, no. 4: 1538. https://doi.org/10.3390/ijms22041538
APA StyleGentile, P., & Garcovich, S. (2021). Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. International Journal of Molecular Sciences, 22(4), 1538. https://doi.org/10.3390/ijms22041538







