Antimicrobial Peptides with Enhanced Salt Resistance and Antiendotoxin Properties
Abstract
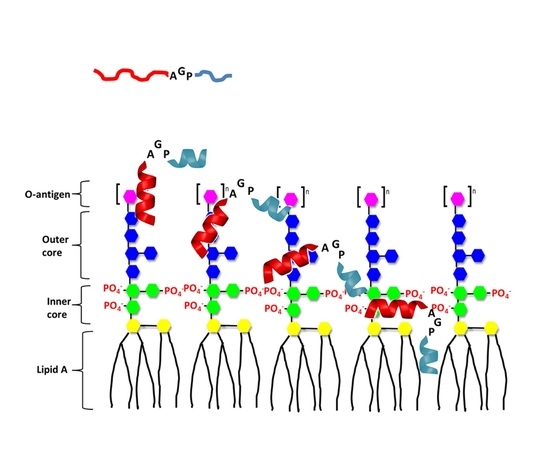
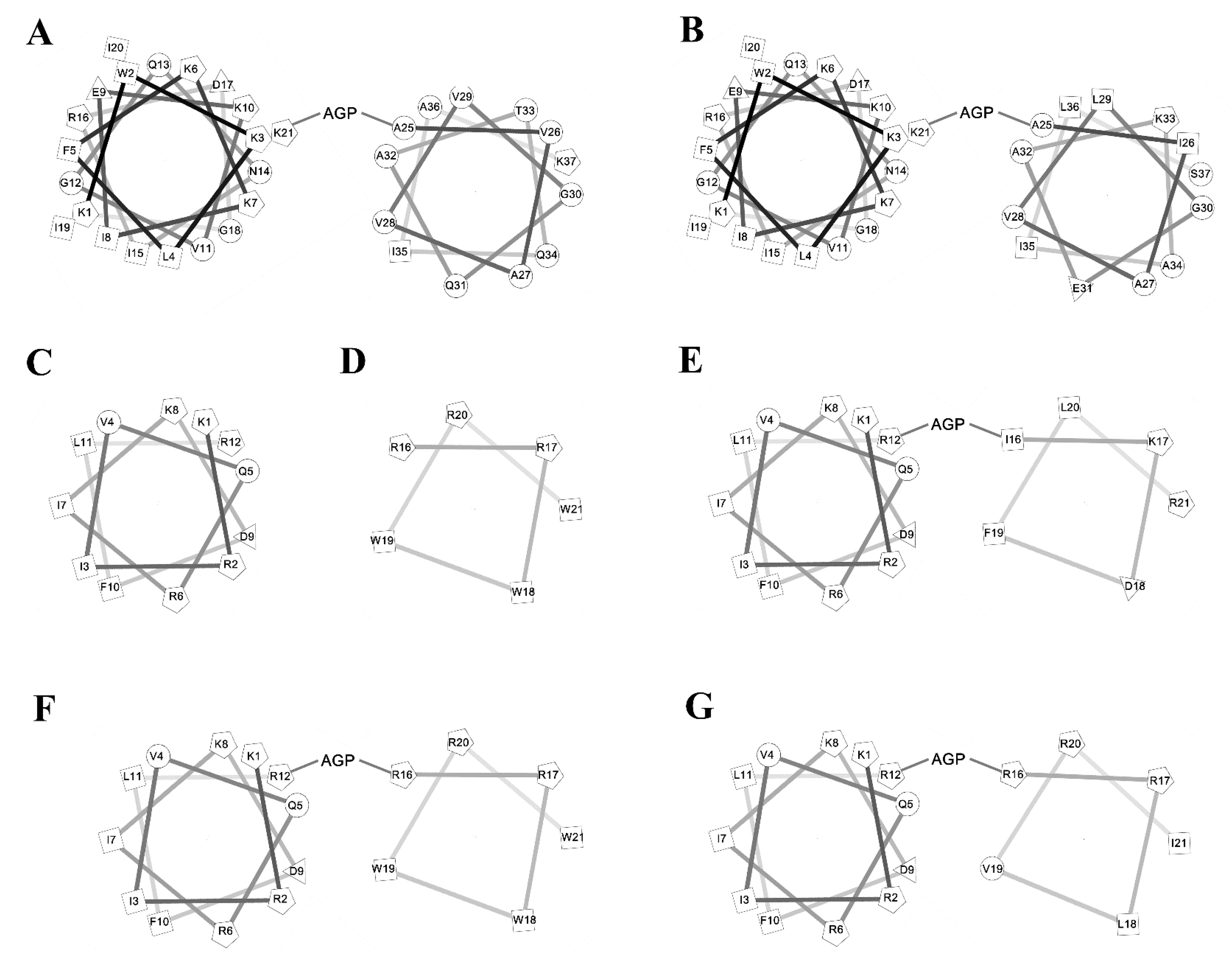
1. Introduction
2. Results
2.1. Antimicrobial Peptides
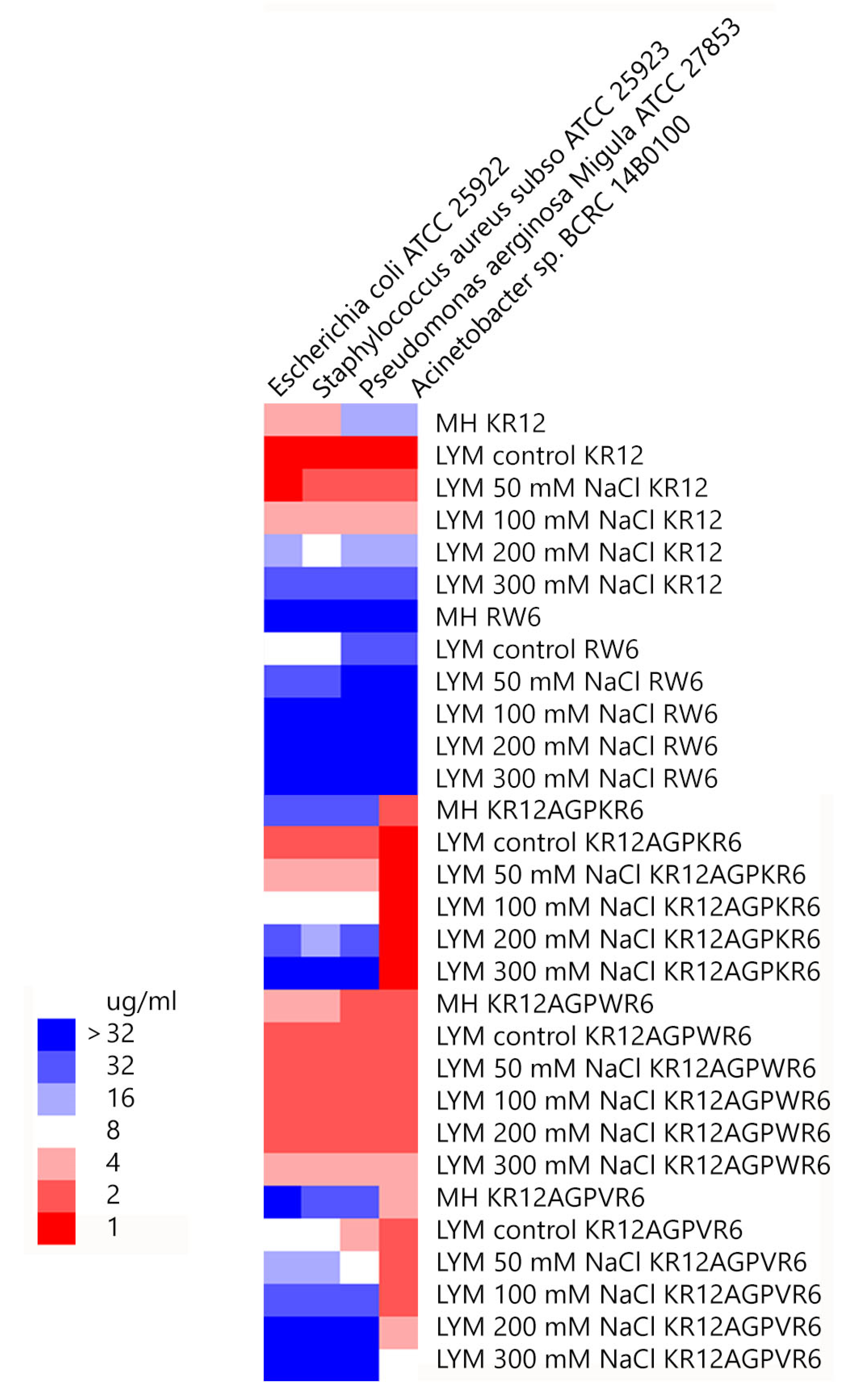
2.2. Antibacterial Activity and Salt Resistance
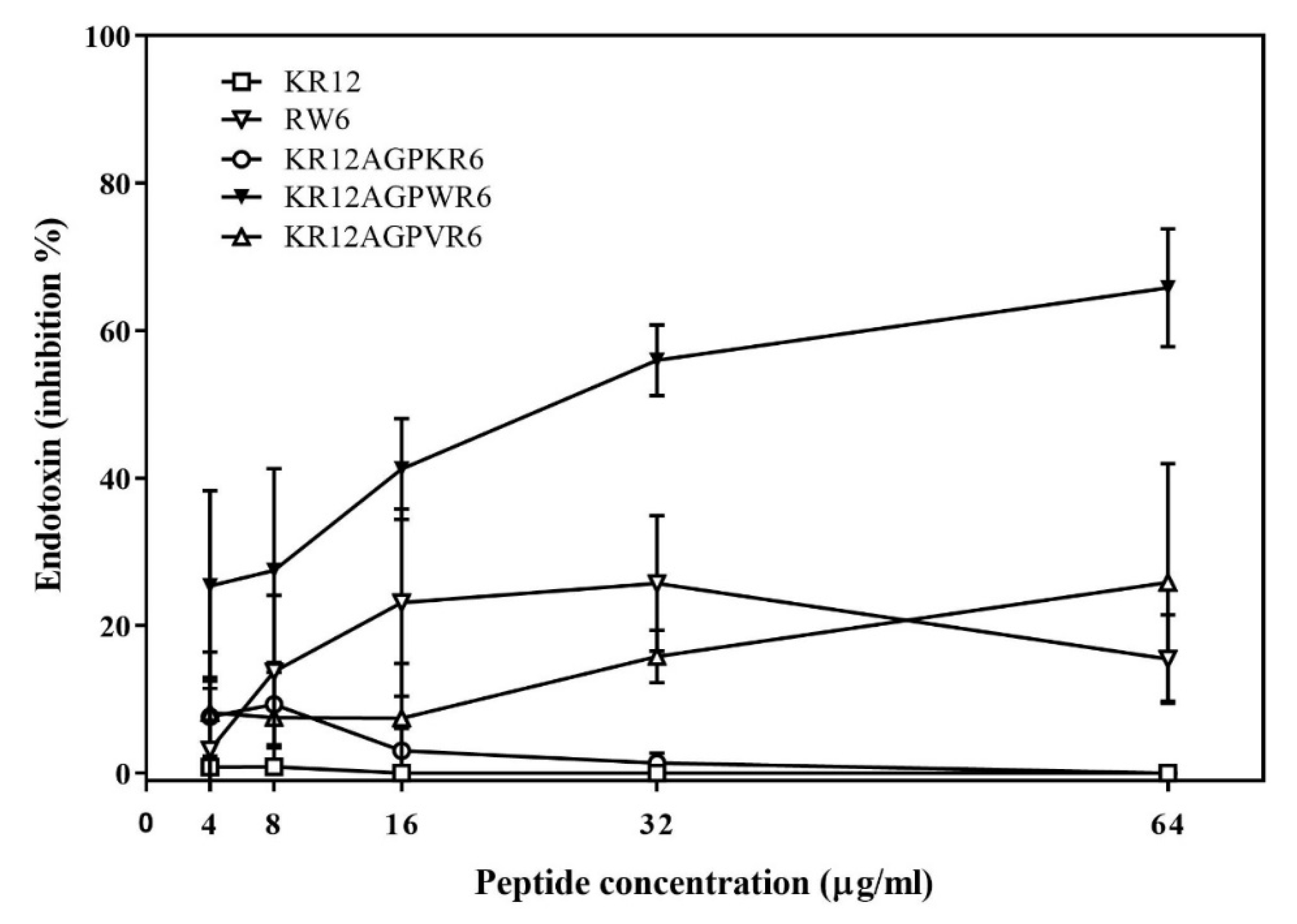
2.3. Limulus Amebocyte Lysate (LAL) Assay
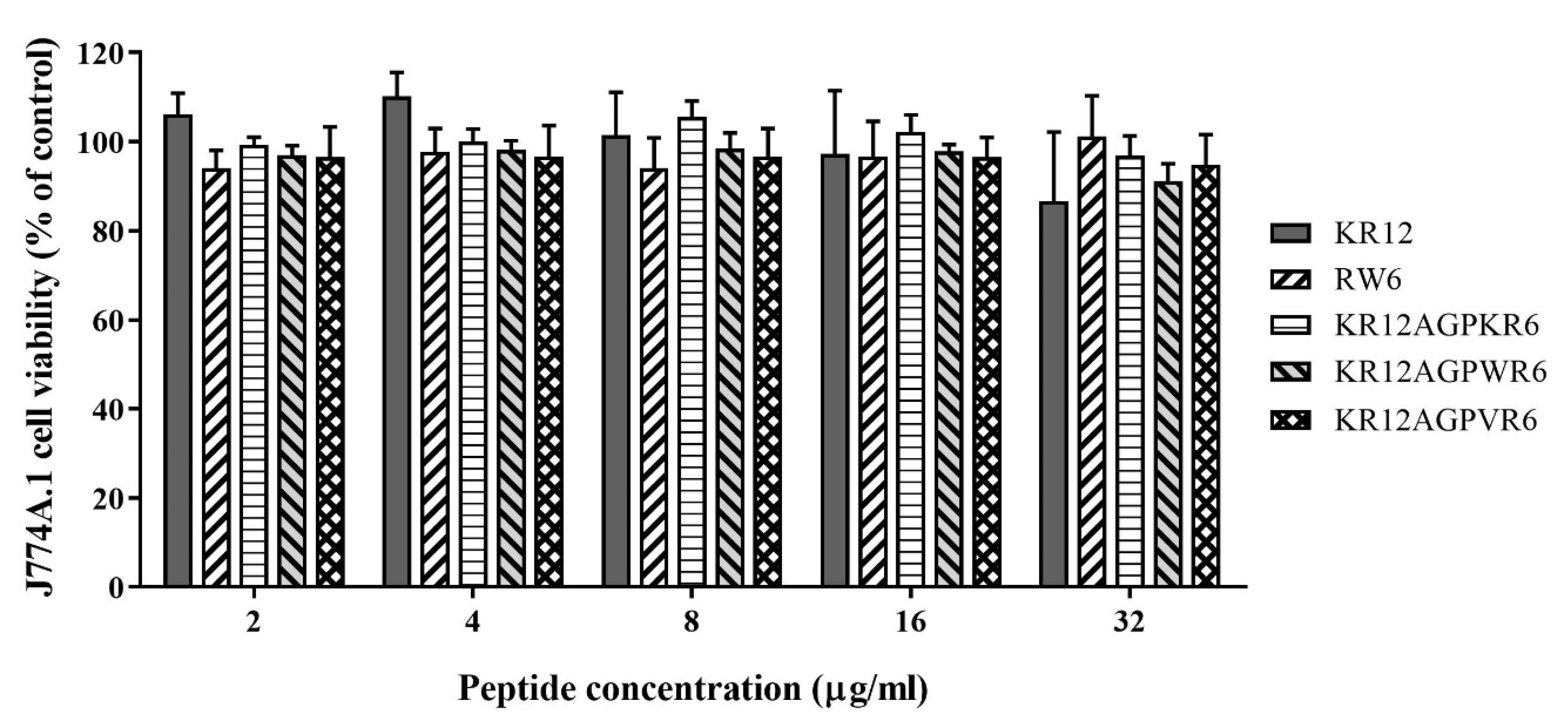
2.4. Cytotoxicity
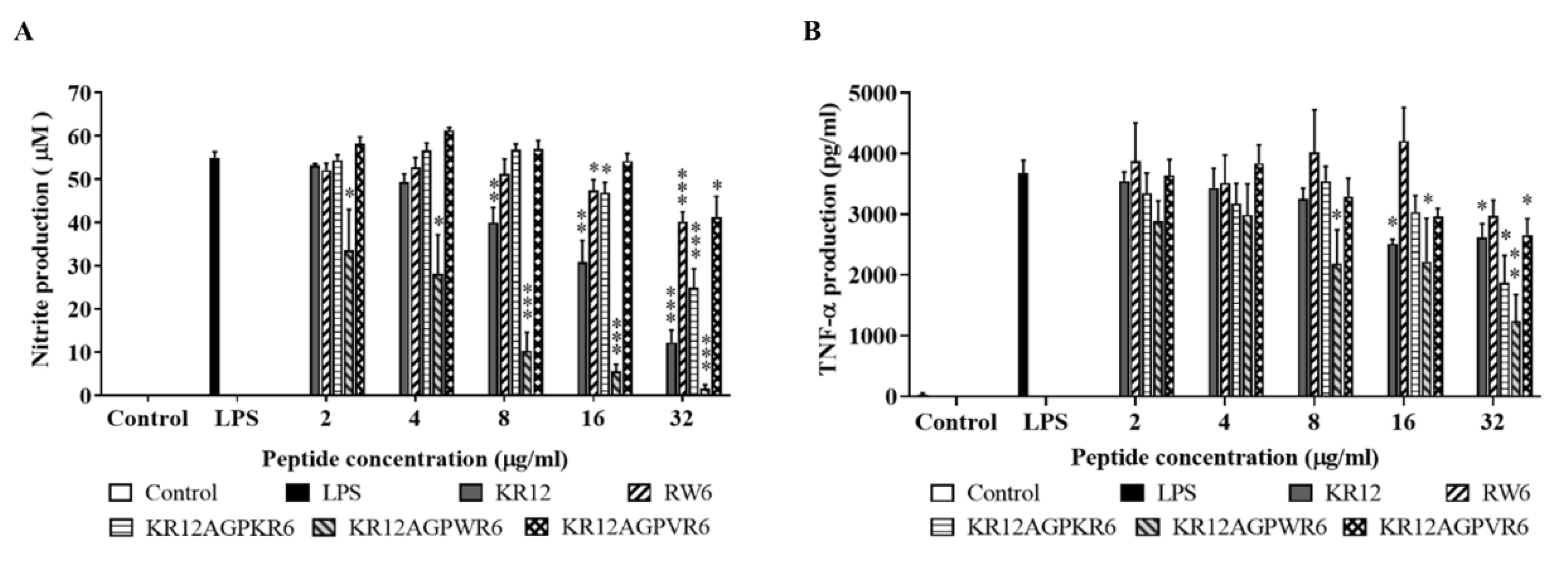
2.5. Inhibition of Endotoxin-Induced Inflammation
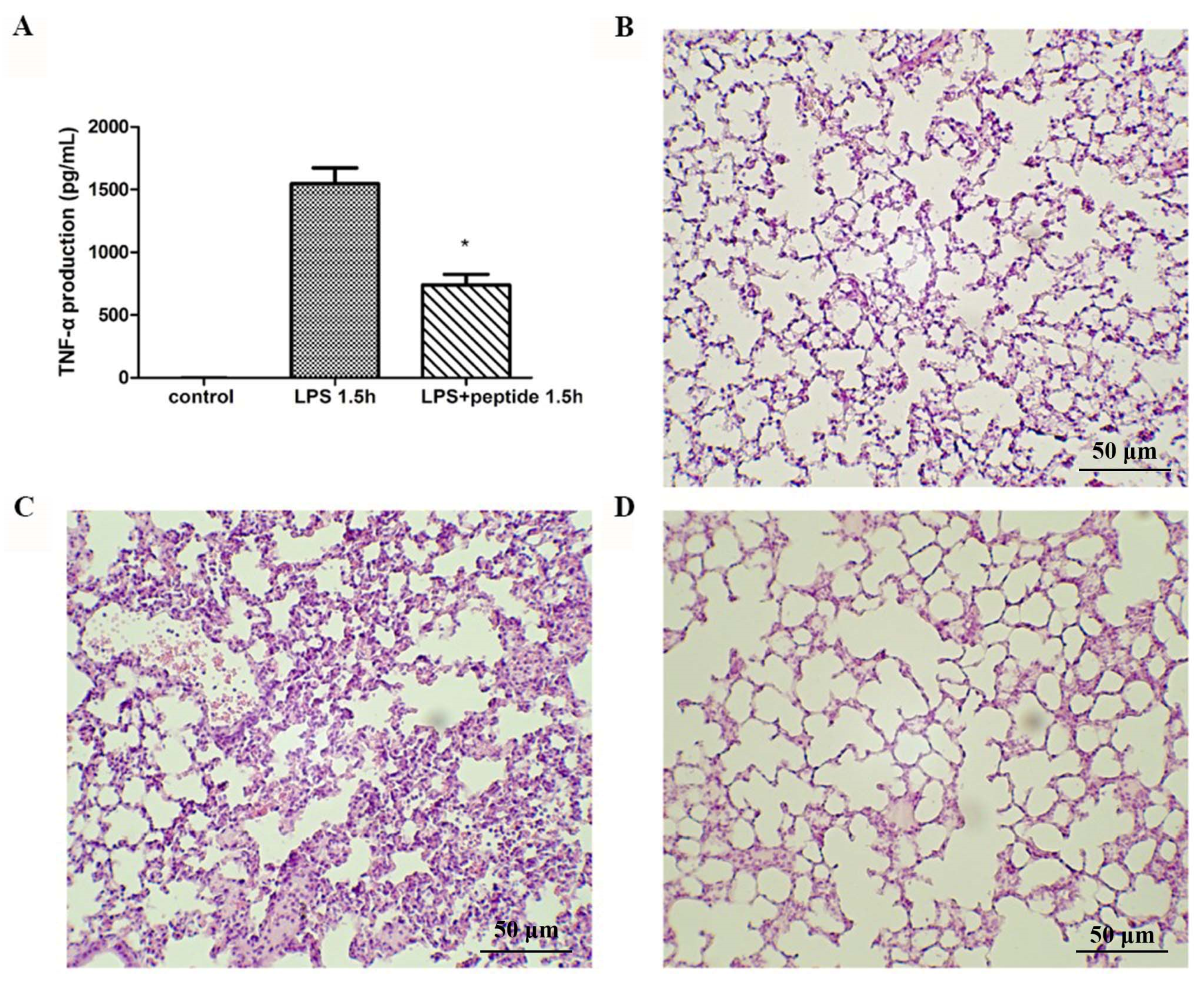
2.6. Endotoxemia Mouse Model
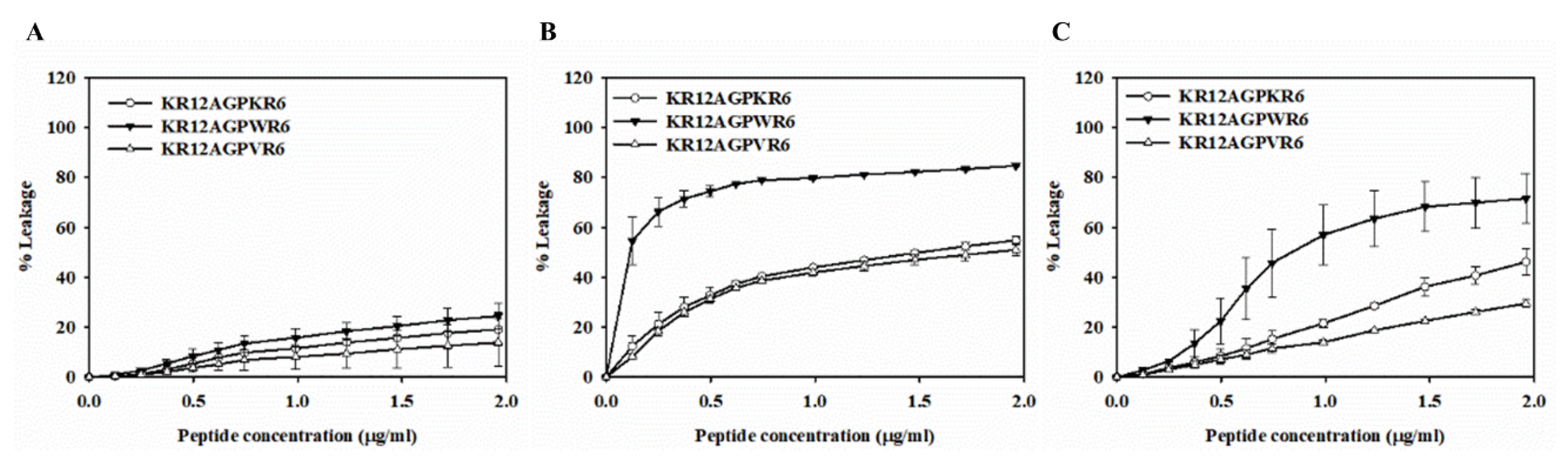
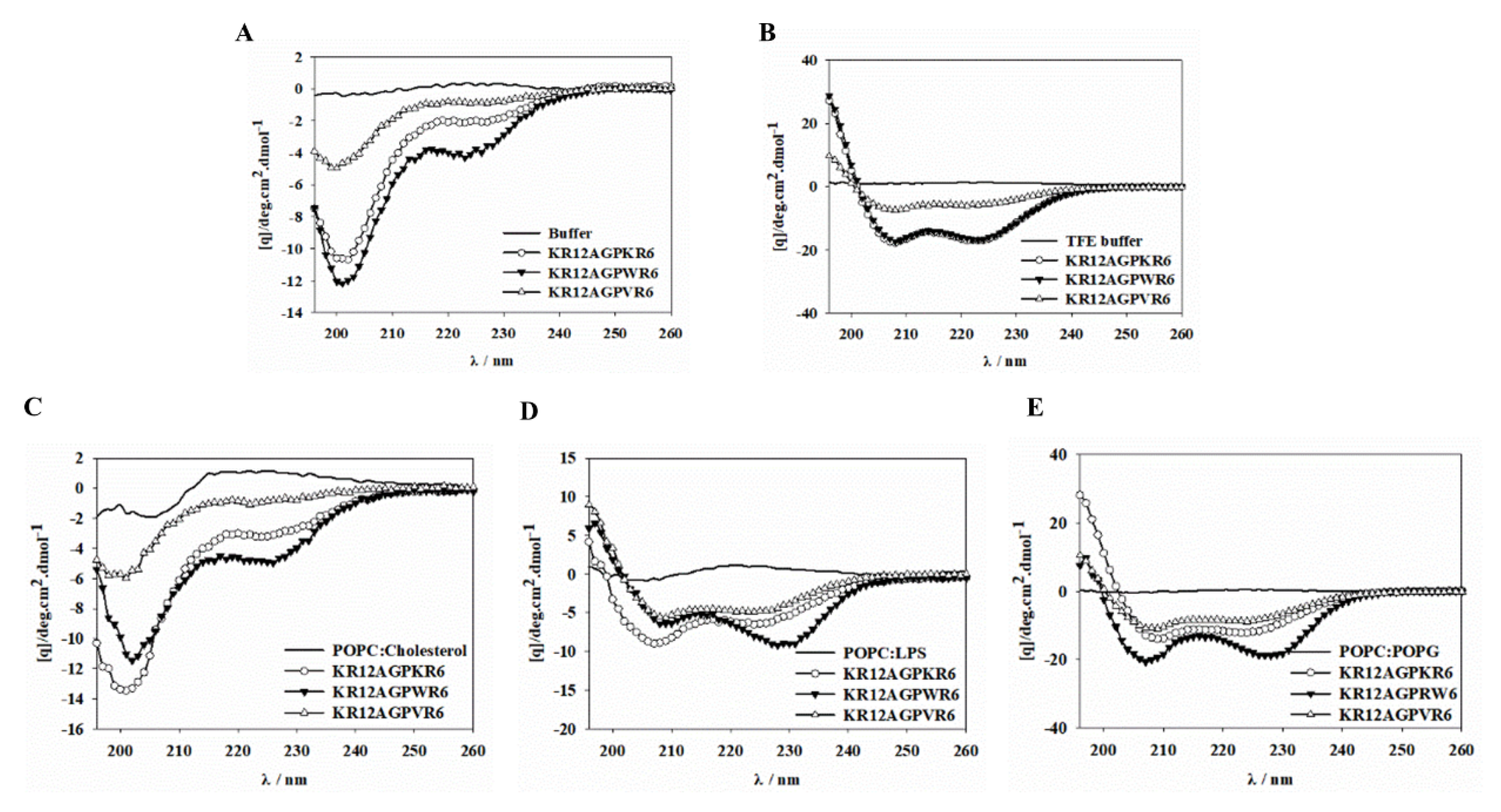
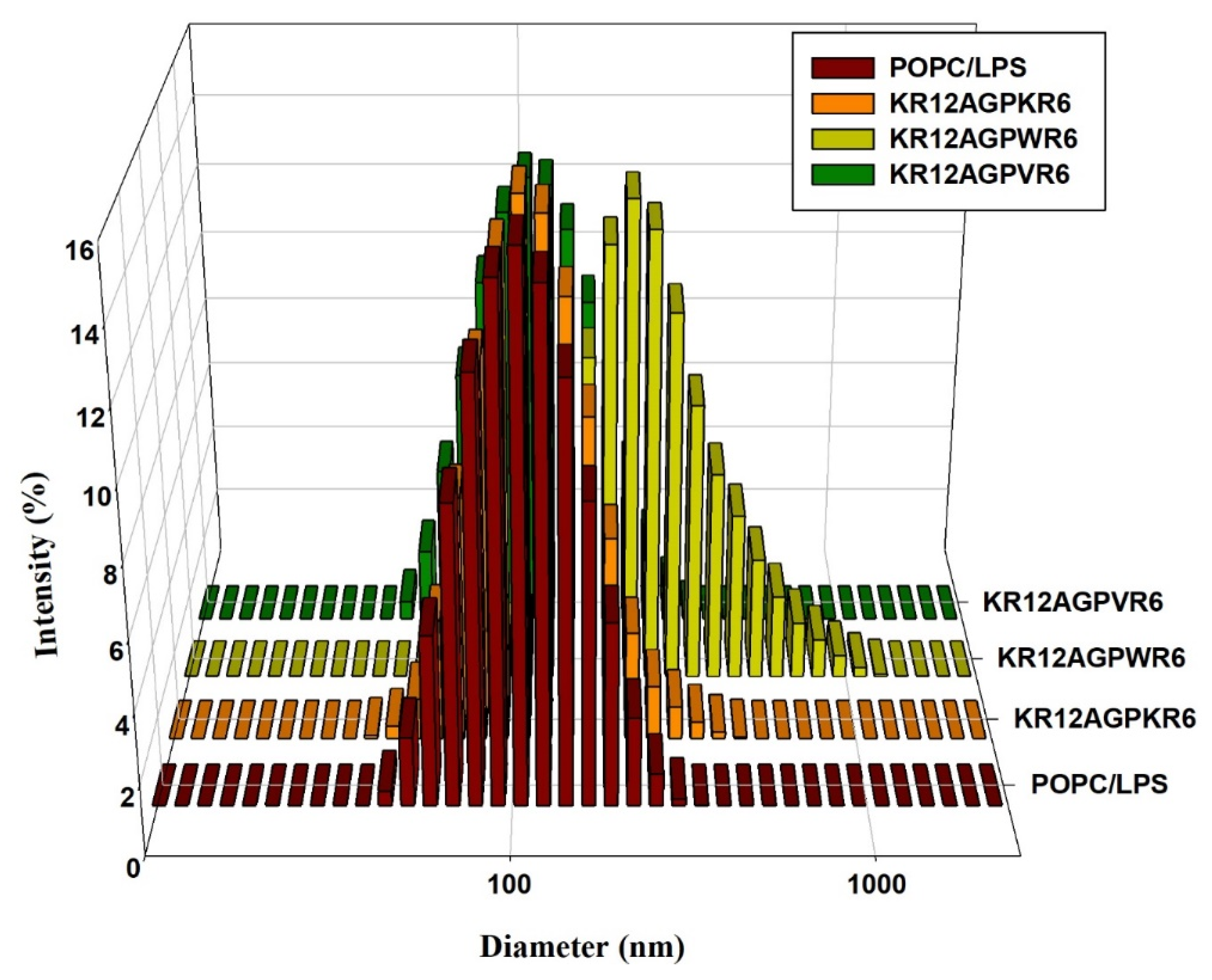
2.7. Peptide-Induced Permeabilization, Circular Dichroism (CD) Spectroscopy, and LPS Aggregation
2.8. Peptide-Induced Permeabilization
2.9. CD Spectroscopy
2.10. LPS Aggregation
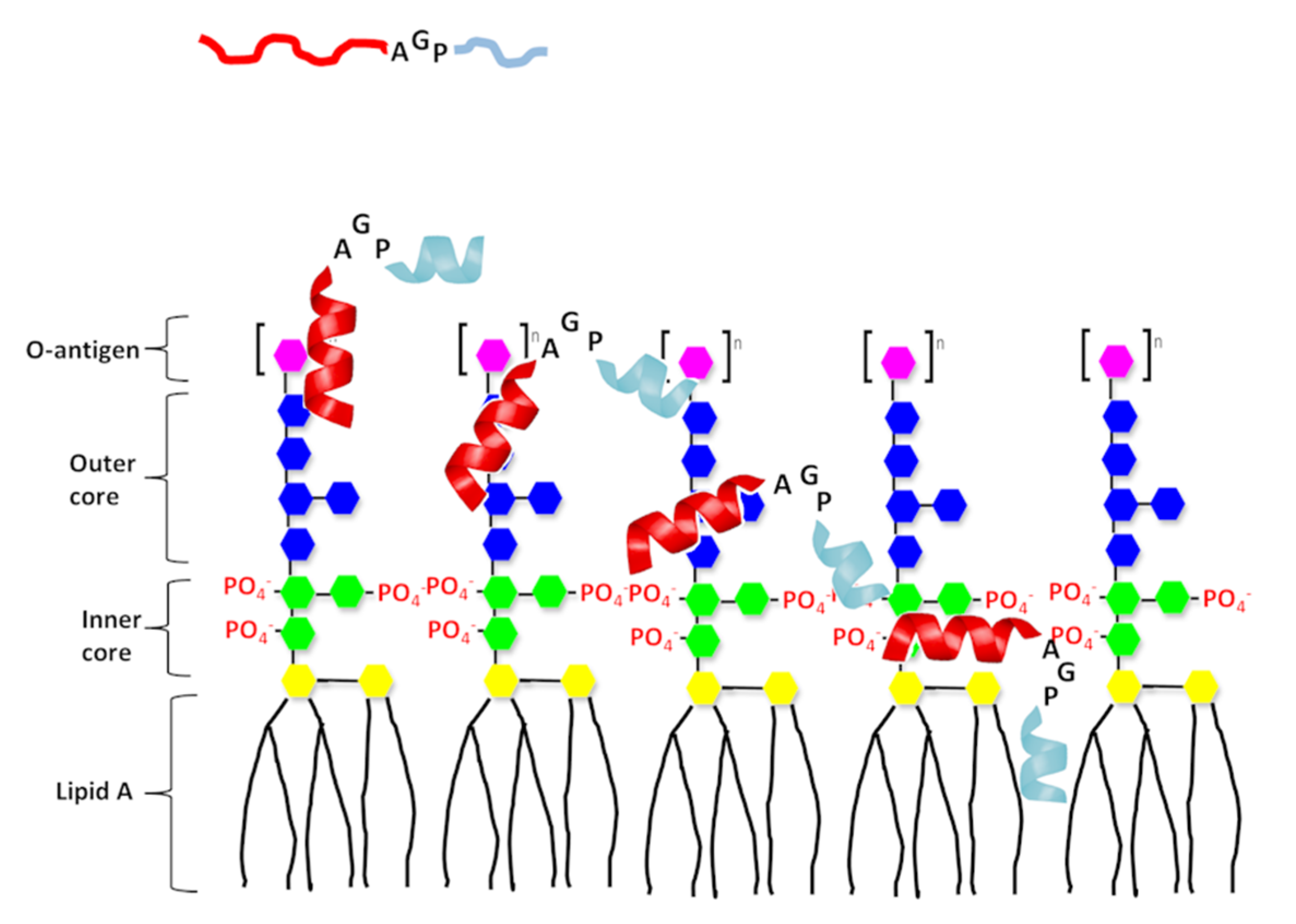
3. Discussion
4. Methods
4.1. Bacteria Culture
4.2. Antimicrobial Activity
4.3. Binding and Neutralization of Peptides to LPS
4.4. Cell Culture
4.5. Cytotoxicity Assay
4.6. Preparation of Large Unilamellar Vesicles (LUVs)
4.7. Dye Leakage Experiments
4.8. Circular Dichroism Spectroscopy
4.9. Dynamic Light Scattering
4.10. Inhibition of Endotoxin-Induced Inflammatory
4.11. Endotoxemia Mouse Model
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPs | antimicrobial peptides |
| ATCC | American Type Culture Collection |
| BCRC | Bioresource Collection and Research Center |
| Bip | β-(4,40-biphenyl)alanine |
| CD | circular dichroism |
| CFU | colony forming unit |
| CSE | control standard endotoxin |
| Dip | β-diphenylalanine |
| DLS | dynamic light scattering |
| DMEM | Dulbecco’s modified eagle’s medium |
| DMSO | dimethyl sulfoxide |
| ELISA | enzyme-linked immunosorbent assay |
| EU | endotoxin unit |
| H&E | hematoxylin and eosin |
| HPLC | high performance liquid chromatography |
| i.p. | intraperitoneal |
| LAL | Limulus amebocyte Lysate |
| LPS | lipopolysaccharide |
| LUV | large unilamellar vesicles |
| MH broth | Mueller−Hinton broth |
| MIC | minimal inhibitory concentration |
| MALDI-TOF | matrix-assisted laser desorption-ionization time-of-flight |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| Nal | β-naphthylalanine |
| NF-κB | nuclear factor-kappa B |
| NO | nitrite oxide |
| PBS | phosphate-buffered saline |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(10-rac-glycerol) |
| SD | standard deviation |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor-alpha |
References
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Rishi, P.; Singh, A.P.; Arora, S.; Garg, N.; Kaur, I.P. Revisiting eukaryotic anti-infective biotherapeutics. Crit. Rev. Microbiol. 2014, 40, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; Goncalves, S.; Santos, N.C. Defensins: Antifungal lessons from eukaryotes. Front. Microbiol. 2014, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Rivas, L. Animal antimicrobial peptides: An overview. Biopolymers 1998, 47, 415–433. [Google Scholar] [CrossRef]
- La Rocca, P.; Shai, Y.; Sansom, M.S. Peptide-bilayer interactions: Simulations of dermaseptin B, an antimicrobial peptide. Biophys. Chem. 1999, 76, 145–159. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Drider, D.; Rebuffat, S. Prokaryotic Antimicrobial Peptides; Springer: New York, NY, USA, 2011; p. 451. [Google Scholar]
- Draper, L.A.; Cotter, P.D.; Hill, C.; Ross, R.P. The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol. 2013, 13, 212. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Baah, J.; Hober, D.; Jouy, E.; Rubrecht, C.; Sane, F.; Drider, D. Synergistic effect between colistin and bacteriocins in controlling gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 2719–2725. [Google Scholar] [CrossRef]
- Wu, C.L.; Hsueh, J.Y.; Yip, B.S.; Chih, Y.H.; Peng, K.L.; Cheng, J.W. Antimicrobial peptides display strong synergy with vancomycin against vancomycin-reisstant E. faecium, S. aureus, and wild-type E. coli. Int. J. Mol. Sci. 2020, 21, 4578. [Google Scholar] [CrossRef]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Huang, K.C.; Yip, B.S.; Tu, C.H.; Chen, H.L.; Cheng, H.T.; Cheng, J.W. Rational design of tryptophan-rich antimicrobial peptides with enhanced antimicrobial activities and specificities. Chembiochem 2010, 11, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.L.; Yu, H.Y.; Yip, B.S.; Chih, Y.H.; Liang, C.W.; Cheng, H.T.; Cheng, J.W. Boosting salt resistance of short antimicrobial peptides. Antimcrob. Agents Chemother. 2013, 57, 4050–4052. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Tu, C.H.; Yip, B.S.; Chen, H.L.; Cheng, H.T.; Huang, K.C.; Lo, H.J.; Cheng, J.W. Easy strategy to increase salt resistance of Antimicrobial Peptides. Antimcrob. Agents Chemother. 2011, 55, 4918–4921. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human b-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997, 65, 2898–2903. [Google Scholar] [CrossRef]
- Wang, C.W.; Yip, B.S.; Cheng, H.T.; Wang, A.H.; Chen, H.L.; Cheng, J.W.; Lo, H.J. Increased potency of a novel D-b-naphthylalanine-substituted antimicrobial peptide against fluconazole-resistant fungal pathogens. FEMS Yeast Res. 2009, 9, 967–970. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Brade, H.; Holst, O.; Brade, L.; Muller-Loennies, S.; Mamat, U.; Zahringer, U.; Beckmann, F.; Seydel, U.; Brandenburg, K.; et al. Bacterial endotoxin: Chemical constitution, biological recognition, host response, and immunological detoxification. Curr. Top. Microbiol. Immunol. 1996, 216, 39–81. [Google Scholar]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef]
- Trent, M.S.; Stead, C.M.; Tran, A.X.; Hankins, J.V. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006, 12, 205–223. [Google Scholar] [CrossRef]
- Rachoin, J.S.; Schorr, C.A.; Dellinger, R.P. Targeting endotoxin in the treatment of sepsis. Subcell. Biochem. 2010, 53, 323–338. [Google Scholar] [PubMed]
- Fisher, C.J., Jr.; Opal, S.M.; Dhainaut, J.F.; Stephens, S.; Zimmerman, J.L.; Nightingale, P.; Harris, S.J.; Schein, R.M.; Panacek, E.A.; Vincent, J.L.; et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. Crit. Care Med. 1993, 21, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Andra, J.; Lohner, K.; Blondelle, S.; Jerala, R.; Moriyon, I.; Koch, M.H.J.; Garidel, P.; Brandenburg, K. Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide. Biochem. J. 2005, 385, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, S. De novo designed lipopolysaccharide binding peptides: Structure based development of antiendotoxic and antimicrobial drugs. Curr. Med. Chem. 2010, 17, 3080–3093. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Castanho, M.A.; Santos, N.C. rBPI21 promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes. PLoS ONE 2009, 4, e8385. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, H.; Bhattacharjya, S. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimcrob. Agents Chemother. 2014, 58, 1987–1996. [Google Scholar] [CrossRef]
- Pulido, D.; Nogues, M.V.; Boix, E.; Torrent, M. Lipopolysaccharide neutralization by antimicrobial peptides: A gambit in the innate host defense strategy. J. Innate Immun. 2012, 4, 327–336. [Google Scholar] [CrossRef]
- Ren, J.; Gao, H.; Tang, M.; Gu, J.; Xia, P.; Xiao, G. Lipopolysaccharide (LPS) detoxification of analogue peptides derived from limulus anti-LPS factor. Peptides 2010, 31, 1853–1859. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Sahl, H.G.; Shai, Y. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry 2008, 47, 6468–6478. [Google Scholar] [CrossRef]
- Singh, S.; Kasetty, G.; Schmidtchen, A.; Malmsten, M. Membrane and lipopolysaccharide interactions of C-terminal peptides from S1 peptidases. Biochim. Biophys. Acta 2012, 1818, 2244–2251. [Google Scholar] [CrossRef]
- Singh, S.; Papareddy, P.; Kalle, M.; Schmidtchen, A.; Malmsten, M. Importance of lipopolysaccharide aggregate disruption from the anti-endotoxic effects of heparin cofactor II peptides. Biochim. Biophys. Acta 2013, 1828, 2709–2719. [Google Scholar] [CrossRef]
- Srivastava, S.; Ghosh, J.K. Introduction of a lysine residue promotes aggregation of temporin L in lipopolysaccharides and augmentation of its antiendotoxin property. Antimcrob. Agents Chemother. 2013, 57, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Herrera, A.I.; Bommineni, Y.R.; Soulages, J.L.; Prakash, O.; Zhang, G. The central kink region of Fowlicidin-2, an a-helical host defense peptide, is critically involved in bacterial killing and endotoxin neutralizationon. J. Innate Immun. 2009, 1, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Ghosh, A.; Airoldi, C.; Sperandeo, P.; Mroue, K.H.; Jimenez-Barbero, J.; Kundu, P.; Ramamoorthy, A.; Bhunia, A. Antimicrobial peptides: Insights into membrane permeabilization, lipopolysaccharide fragmentation and application in plant disease control. Sci. Rep. 2015, 5, 11951. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Shai, Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: Role in bacterial resistance and prevention of sepsis. Biochim. Biophys. Acta 2006, 1758, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Chen, Y.A.; Yip, B.S.; Wang, S.Y.; Wei, H.J.; Chih, Y.H.; Chen, K.H.; Cheng, J.W. Role of b-naphthylalanine end-tags in the enhancement of antiendotoxin activities: Solution structure of the antimicrobial peptide S1-Nal-Nal in complex with lipopolysaccharide. Biochim. Biophys. Acta 2017, 1859, 1114–1123. [Google Scholar] [CrossRef]
- Chih, Y.H.; Wang, S.Y.; Yip, B.S.; Cheng, K.T.; Hsu, S.Y.; Wu, C.L.; Yu, H.Y.; Cheng, J.W. Dependence on size and shape of non-nature amino acids in the enhancement of lipopolysaccharide (LPS) neutralizing activities of antimicrobial peptides. J. Colliod Interface Sci. 2019, 533, 492–502. [Google Scholar] [CrossRef]
- Wu, J.M.; Jan, P.S.; Yu, H.C.; Haung, H.Y.; Fang, H.J.; Chang, Y.I.; Cheng, J.W.; Chen, H.M. Structure and function of a custom anticancer peptide, CB1a. Peptides 2009, 30, 839–848. [Google Scholar] [CrossRef]
- Cavallarin, L.; Andreu, D.; San Segundo, B. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol. Plant-Microbe Interact. 1998, 11, 218–227. [Google Scholar] [CrossRef]
- Moore, A.J.; Beazley, W.D.; Bibby, M.C.; Devine, D.A. Antimicrobial activity of cecropins. J. Antimicrob. Chemother. 1996, 37, 1077–1089. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, E.; Shin, S.; Jeong, K.W.; Lee, J.Y.; Bae, S.Y.; Kim, S.H.; Lee, J.; Kim, S.R.; Lee, D.G.; et al. Structure and function of papiliocin with antimicrobial and anti-inflammatory activities isolated from the swallowtail butterfly, Papilio xuthus. J. Biol. Chem. 2011, 286, 41296–41311. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Shin, A.; Kim, Y. Anti-inflammatory activities of cecropin A and its mechanism of action. Arch. Insect Biochem. Physiol. 2015, 88, 31–44. [Google Scholar] [CrossRef]
- Wu, C.; Geng, X.; Wan, S.; Hou, H.; Yu, F.; Jia, B.; Wang, L. Cecropin-P17, an analog of Cecropin B, inhibits human hepatocellular carcinoma cell HepG-2 proliferation via regulation of ROS, Caspase, Bax, and Bcl-2. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2015, 21, 661–668. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, W.; Smith, D.; Chan, S.C. Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim. Biophys. Acta 1997, 1336, 171–179. [Google Scholar] [CrossRef]
- Fox, M.A.; Thwaite, J.E.; Ulaeto, D.O.; Atkins, T.P.; Atkins, H.S. Design and characterization of novel hybrid antimicrobial peptides based on cecropin A, LL-37 and magainin II. Peptides 2012, 33, 197–205. [Google Scholar] [CrossRef]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Mishra, B.; Epand, R.F.; Epand, R.M.; Wang, G. Structural location determines functional roles of the basic amino acids of KR-12, the smallest antimicrobial peptide from human cathelicidin LL-37. RSC Adv. 2013, 42, 19560–19571. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Park, I.S.; Bang, J.K.; Shin, S.Y. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J. Pept. Sci. 2013, 19, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Steckbeck, J.D.; Craigo, J.K.; Doi, Y.; Mietzner, T.A.; Montelaro, R.C. Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrob. Agents Chemother. 2013, 57, 2511–2521. [Google Scholar] [CrossRef]
- Roslansky, P.F.; Novitsky, T.J. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J. Clin. Microbiol. 1991, 29, 2477–2483. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. Anti-inflammatory action of sulfated glucosamine on cytokine regulation in LPS-activated PMA-differentiated THP-1 macrophages. Inflamm. Res. 2011, 60, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Inacio, R.G.; Raimundo, J.M.; Martins, M.; Castanho, M.A.; Santos, N.C. Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems. Biopolym. Pept. Sci. 2012, 98, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chih, Y.H.; Lin, Y.S.; Yip, B.S.; Wei, H.J.; Chu, H.L.; Yu, H.Y.; Cheng, H.T.; Chou, Y.T.; Cheng, J.W. Ultrashort antimicrobial peptides with antiendotoxin properties. Antimicrob. Agents Chemother. 2015, 59, 5052–5056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andra, J.; Koch, M.H.J.; Bartels, R.; Brandenburg, K. Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimcrob. Agents Chemother. 2004, 48, 1593–1599. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Lev, N.; Shai, Y. Effect of the hydrophobicity to net positive charge ratio on antibacterial and anti-endotoxin activities of structurally similar antimicrobial peptides. Biochemistry 2010, 49, 853–861. [Google Scholar] [CrossRef]
- Arnusch, C.J.; Ulm, H.; Josten, M.; Shadkchan, Y.; Osherov, N.; Sahl, H.G.; Shai, Y. Ultrashort peptide bioconjugates are exclusively antifungal agents and synergize with cyclodextrin and amphotericin B. Antimcrob. Agents Chemother. 2012, 56, 1–9. [Google Scholar] [CrossRef]
- Avrahami, D.; Shai, Y. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 2002, 41, 2254–2263. [Google Scholar] [CrossRef]
- Makovitzki, A.; Avrahami, D.; Shai, Y. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 15997–16002. [Google Scholar] [CrossRef]
- Serrano, G.N.; Zhanel, G.G.; Schweizer, F. Antibacterial activity of ultrashort cationic lipo-b-peptides. Antimcrob. Agents Chemother. 2009, 53, 2215–2217. [Google Scholar] [CrossRef]
- Yu, H.Y.; Yip, B.S.; Tu, C.H.; Chen, H.L.; Chu, H.L.; Chih, Y.H.; Cheng, H.T.; Sue, S.C.; Cheng, J.W. Correlations between membrane immersion depth, orientation, and salt-resistance of tryptophan-rich antimicrobial peptides. Biochim. Biophys. Acta 2013, 1828, 2720–2728. [Google Scholar] [CrossRef]
- Bhunia, A.; Ramamoorthy, A.; Bhattacharjya, S. Helical hairpin structure of a potent antimicrobial peptide MSI-594 in lipopolysaccharide micelles by NMR spectroscopy. Chemistry 2009, 15, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Tack, B.F.; Sawai, M.V.; Kearney, W.R.; Robertson, A.D.; Sherman, M.A.; Wang, W.; Hong, T.; Boo, L.M.; Wu, H.; Waring, A.J.; et al. SMAP-29 has two LPS-binding sites and a central hinge. Eur. J. Biochem. 2002, 269, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Domadia, P.N.; Torres, J.; Hallock, K.J.; Ramamoorthy, A.; Bhattacharjya, S. NMR structure of pardaxin, a pore-forming antimicrobial peptide in lipopolysaccharide micelles: Mechanism of outer membrane permeabilization. J. Biol. Chem. 2010, 285, 3883–3895. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, J.K.; Jeon, D.; Jeong, K.W.; Shin, A.; Kim, Y. Functional roles of aromatic residues and helices of papilioncin in its antimicrobial and anti-inflammatory activities. Sci. Rep. 2015, 5, 12048. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, Y.; Bommineni, Y.R.; Fernando, S.C.; Prakash, O.; Gilliland, S.E.; Zhang, G. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 2006, 281, 2858–2867. [Google Scholar] [CrossRef]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef]
- Du, S.Y.; Wang, H.J.; Cheng, H.H.; Chen, S.D.; Wang, L.H.; Wang, W.C. Cholesterol glucosylation by Helicobacter pylori delays internalization and arrests phagosome maturation in macrophages. J. Microbiol. Immunol. Infect. 2014, 49, 636–645. [Google Scholar] [CrossRef]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large unilamellar vesicles. Biochim. Biophys. Acta 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Mayer, L.D.; Hope, M.J.; Cullis, P.R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta 1986, 858, 161–168. [Google Scholar] [CrossRef]
- Stewart, J.C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
| Peptide | Sequence | Charge | Hydrophobicity <H> | Molecular Weight (Da) |
|---|---|---|---|---|
| KR12 | Ac-KRIVQRIKDFLR-NH2 | +4 | 0.193 | 1517.93 |
| RW6 | Ac-RRWWRW-NH2 | +3 | 0.62 | 1045.22 |
| KR12AGPKR6 | Ac-KRIVQRIKDFLR-AGP-IKDFLR-NH2 | +4  +3 +3 | 0.193  0.62 0.62 | 2611.2 |
| KR12AGPWR6 | Ac-KRIVQRIKDFLR-AGP-RRWWRW-NH2 | +4  +3 +3 | 0.193  0.282 0.282 | 2865.46 |
| KR12AGPVR6 | Ac-KRIVQRIKDFLR-AGP-RRLVRI-NH2 | +4  +3 +3 | 0.193  0.282 0.282 | 2632.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.-L.; Chih, Y.-H.; Peng, K.-L.; Wu, C.-L.; Yu, H.-Y.; Cheng, D.; Chou, Y.-T.; Cheng, J.-W. Antimicrobial Peptides with Enhanced Salt Resistance and Antiendotoxin Properties. Int. J. Mol. Sci. 2020, 21, 6810. https://doi.org/10.3390/ijms21186810
Chu H-L, Chih Y-H, Peng K-L, Wu C-L, Yu H-Y, Cheng D, Chou Y-T, Cheng J-W. Antimicrobial Peptides with Enhanced Salt Resistance and Antiendotoxin Properties. International Journal of Molecular Sciences. 2020; 21(18):6810. https://doi.org/10.3390/ijms21186810
Chicago/Turabian StyleChu, Hung-Lun, Ya-Han Chih, Kuang-Li Peng, Chih-Lung Wu, Hui-Yuan Yu, Doris Cheng, Yu-Ting Chou, and Jya-Wei Cheng. 2020. "Antimicrobial Peptides with Enhanced Salt Resistance and Antiendotoxin Properties" International Journal of Molecular Sciences 21, no. 18: 6810. https://doi.org/10.3390/ijms21186810
APA StyleChu, H.-L., Chih, Y.-H., Peng, K.-L., Wu, C.-L., Yu, H.-Y., Cheng, D., Chou, Y.-T., & Cheng, J.-W. (2020). Antimicrobial Peptides with Enhanced Salt Resistance and Antiendotoxin Properties. International Journal of Molecular Sciences, 21(18), 6810. https://doi.org/10.3390/ijms21186810