Comparative Analyses of the Self-Sealing Mechanisms in Leaves of Delosperma cooperi and Delosperma ecklonis (Aizoaceae)
Abstract
:1. Introduction
2. Results
2.1. Habitus and Leaf Morphology
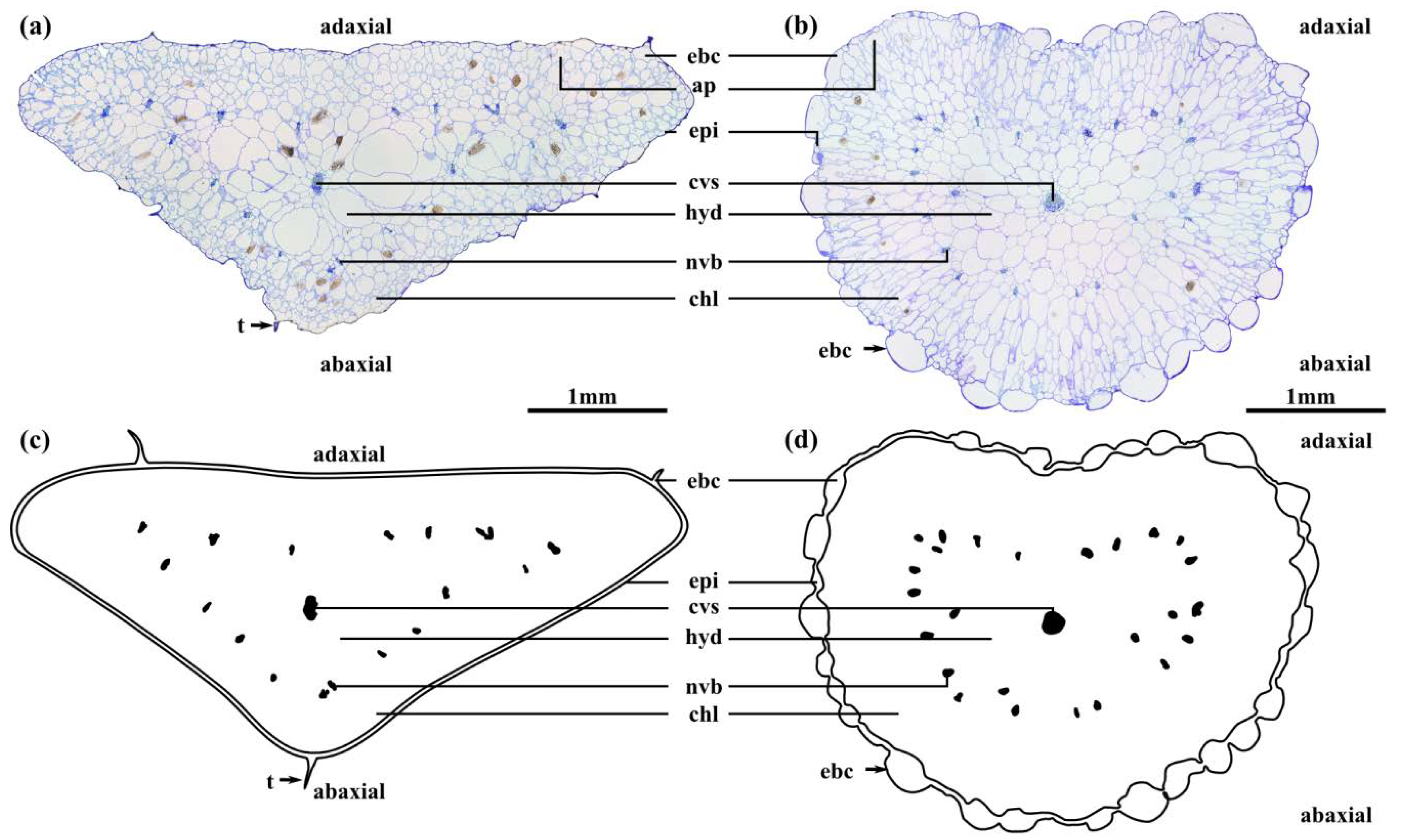
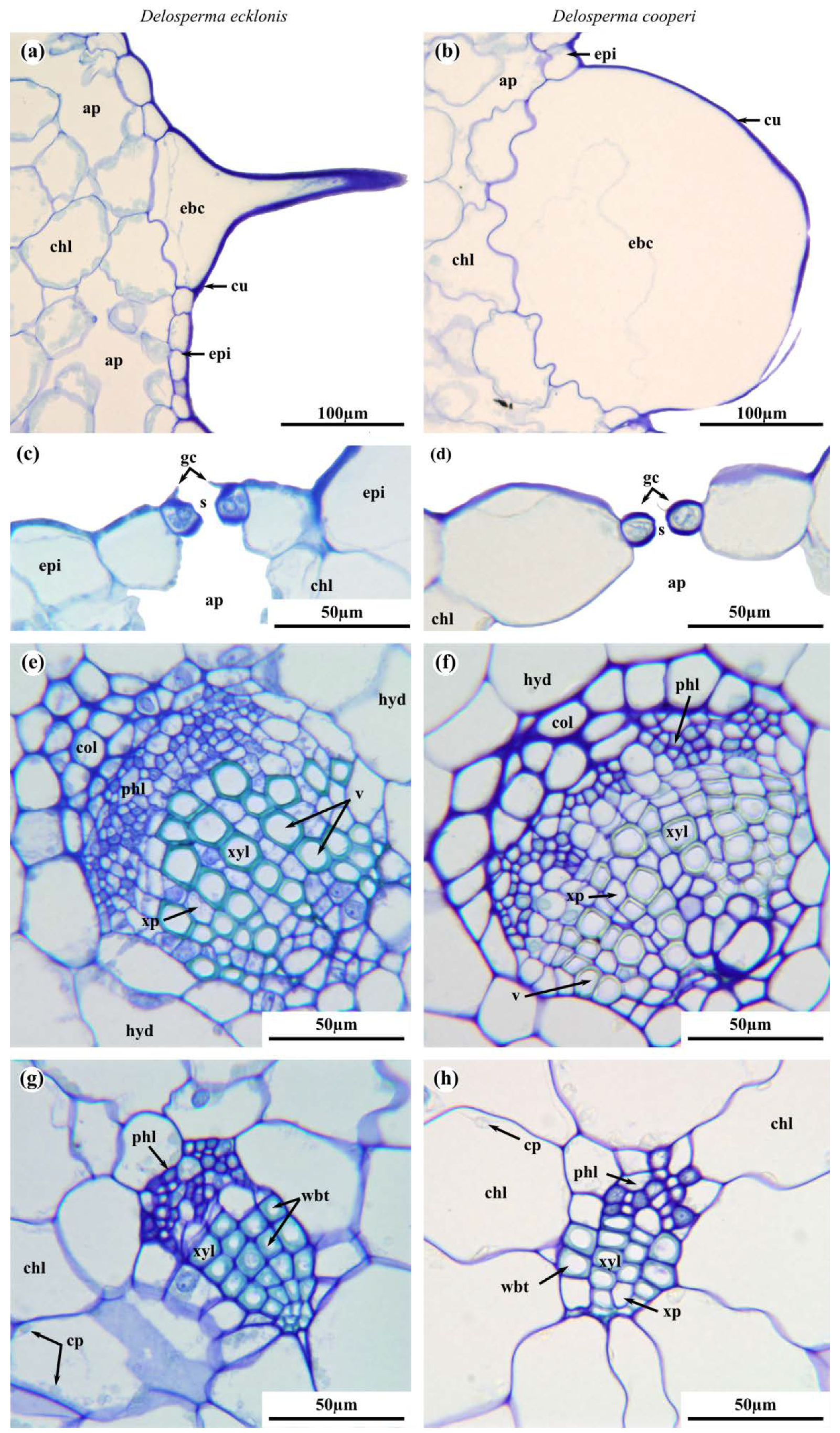
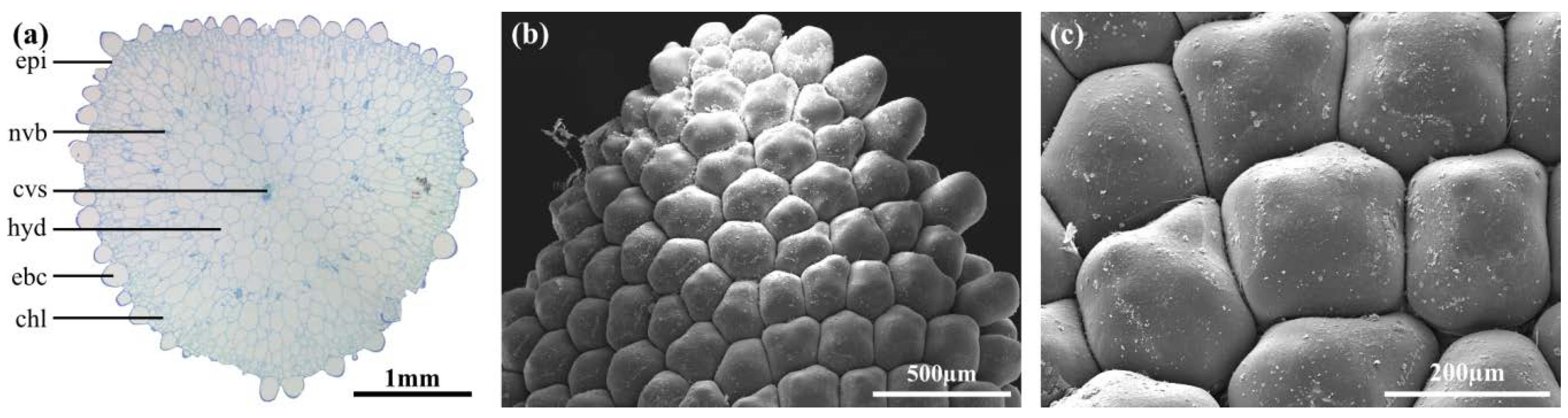
2.2. Leaf Anatomy
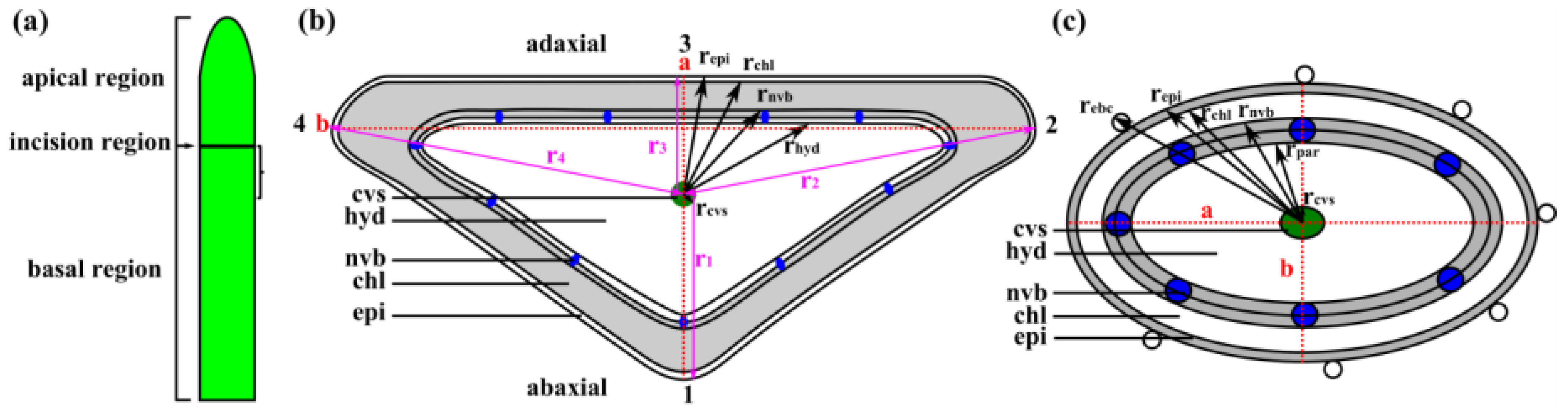
2.3. Leaf Morphometry
2.4. Leaf Biomechanics
2.4.1. Elastic Modulus and Tensile Strength
2.4.2. Elastic Modulus of Parenchymatous Tissues
2.4.3. Poisson’s Ratio and Turgor
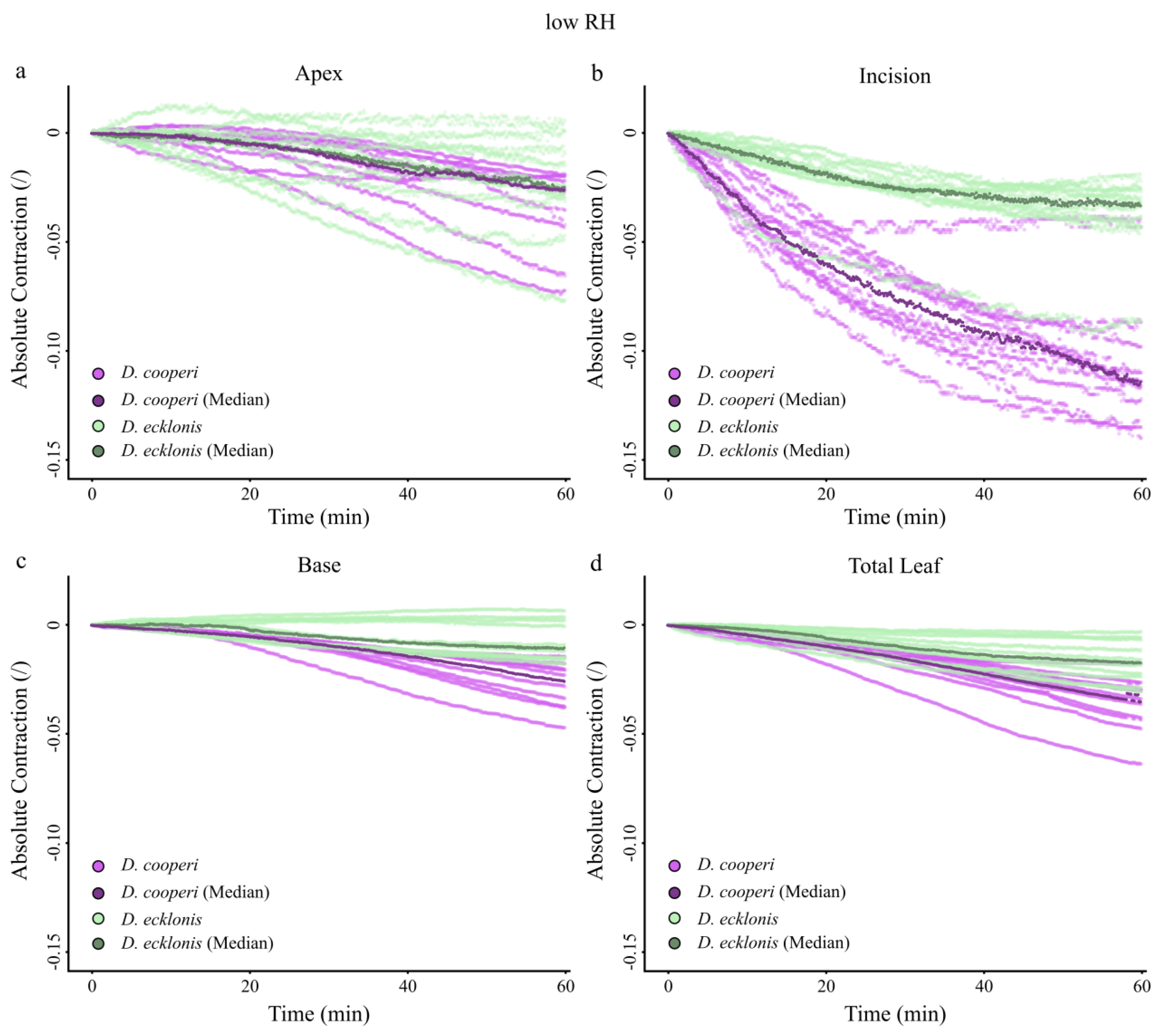
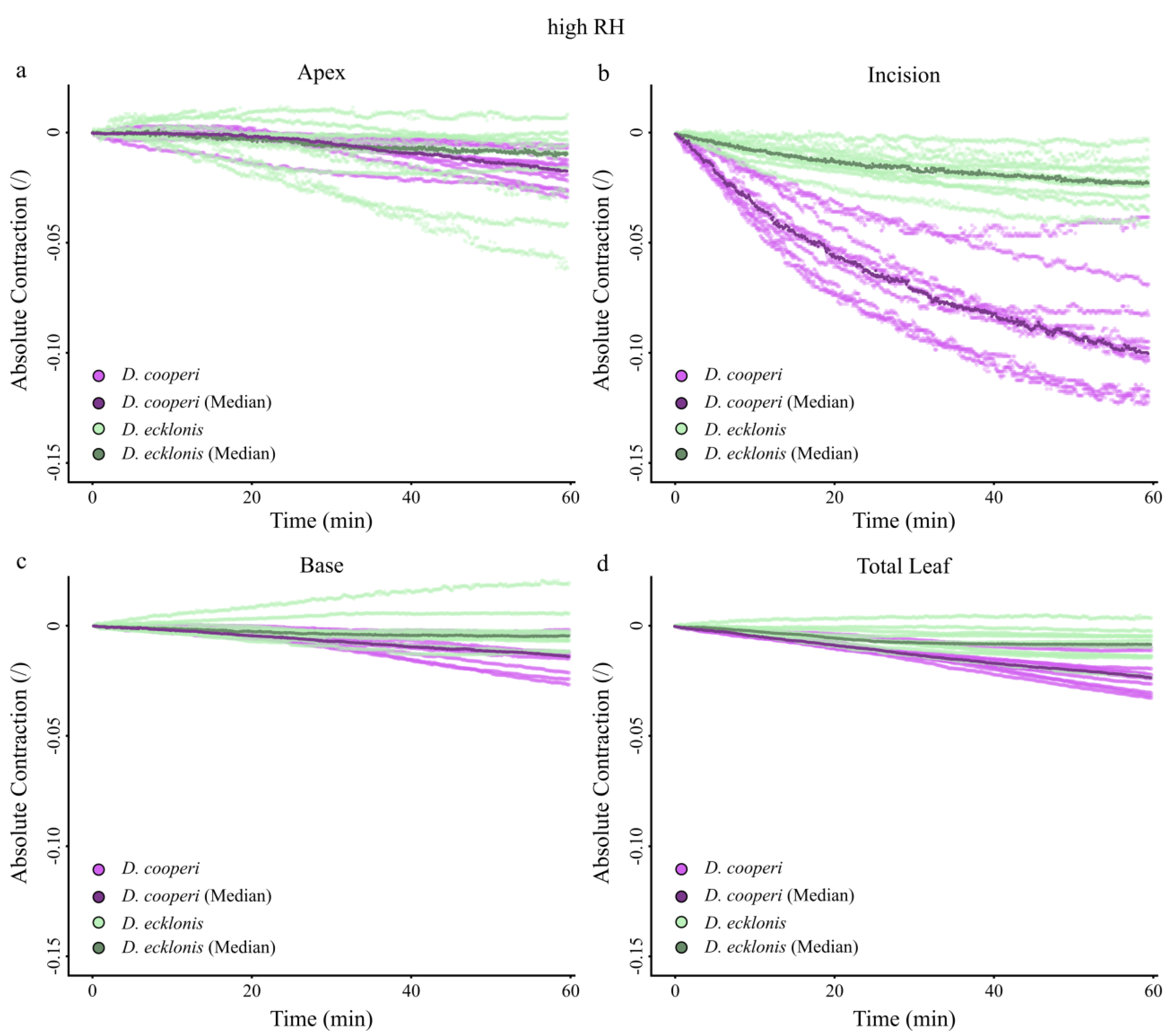
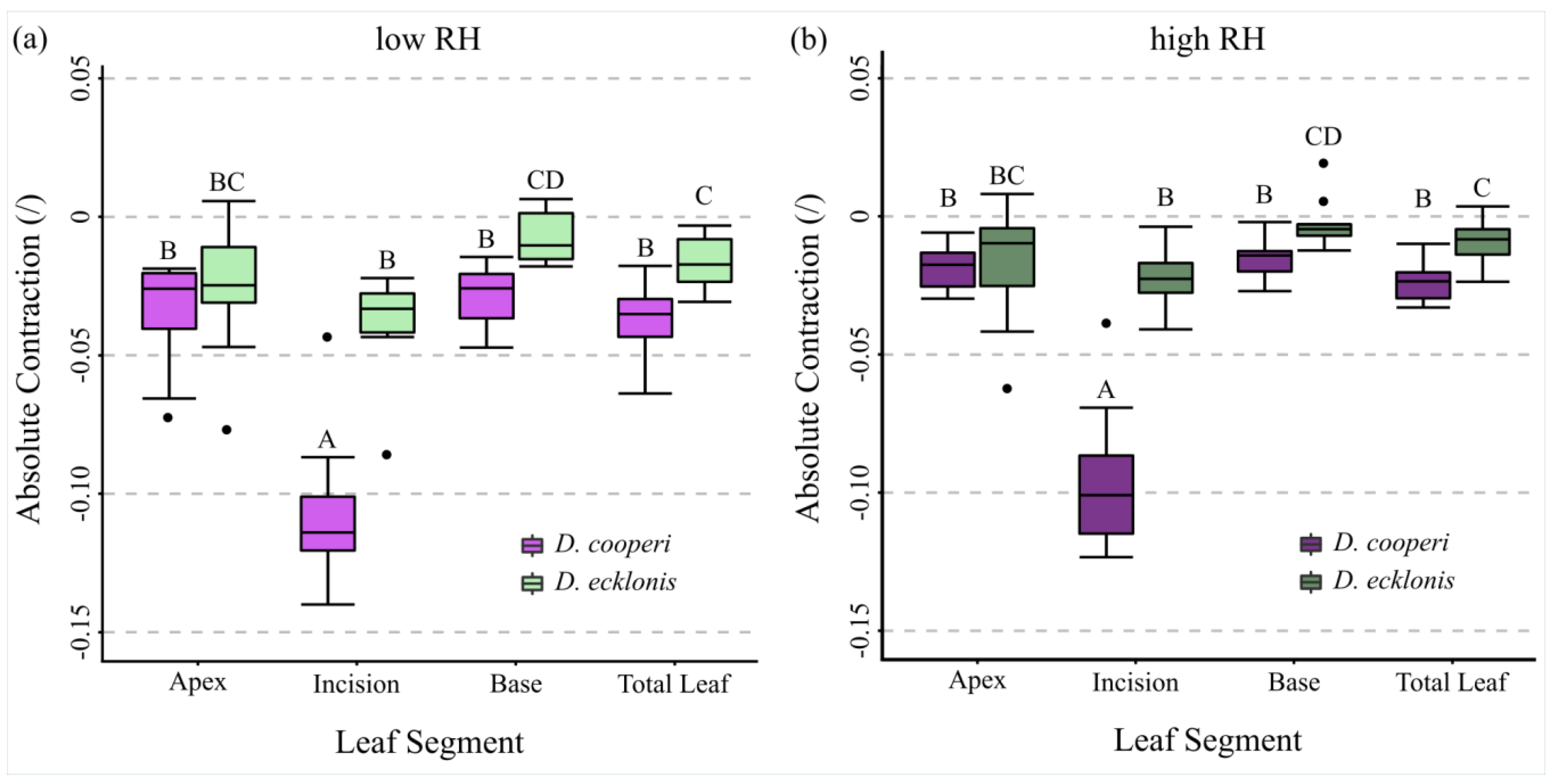
2.4.4. Leaf Kinematics
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Occurrence and Climate Data
4.3. Leaf Morphometry
4.4. Leaf Anatomy
4.4.1. Light Microscopy
4.4.2. Scanning Electron Microscopy
4.4.3. Magnetic Resonance Imaging
4.5. Leaf Biomechanics
4.6. Leaf Kinematics
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Absolute Values of Self-Sealing Kinematics of D. cooperi and D. ecklonis
Appendix B. Climate-Specific Data
| D. ecklonis | D. cooperi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Min | Max | n | Mean | sd | Min | Max | n | |
| Altitude (m) | 283.1 | 274.4 | 10.0 | 838.2 | 10 | 1419.2 | 292.6 | 953.0 | 1920.2 | 10 |
| Annual mean prec. (mm) | 601.7 | 176.1 | 332.0 | 1009.4 | 10 | 585.5 | 77.0 | 420.0 | 713.4 | 10 |
| Annual mean temp. (°C) | 17.9 | 1.1 | 16.0 | 20.4 | 10 | 16.5 | 1.9 | 12.9 | 20.0 | 10 |
| Max. temp. (°C) | 21.6 | 1.1 | 20.0 | 23.2 | 6 | 24.0 | 1.9 | 21.0 | 28.0 | 10 |
| Min. temp. (°C) | 13.0 | 1.0 | 11.5 | 14.5 | 6 | 8.9 | 2.3 | 5.2 | 12.3 | 10 |
| Annual RH (%) | 75.9 | 3.3 | 69.0 | 80.0 | 8 | 57.0 | 2.5 | 52.1 | 59.7 | 8 |
| Morning RH (%) | 86.5 | 0.5 | 86.0 | 87.0 | 2 | 73.5 | 6.5 | 67.0 | 80.0 | 2 |
| Evening RH (%) | 68.0 | 4.0 | 72.0 | 64.0 | 2 | 32.5 | 1.5 | 31.0 | 34.0 | 2 |
Appendix C. Calculation of Radii of Individual Tissue Layers
Appendix D. Percentage Shrinkage
| D. ecklonis | D. cooperi | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Embedded | Shrinkage | Fresh | Embedded | Shrinkage | |||||||
| a1/3 | b1/3 | a1/3 | b1/3 | γa (%) | γb (%) | a1/3 | b1/3 | a1/3 | b1/3 | γa (%) | γb (%) | |
| mean | 2.2 | 4.3 | 2.0 | 4.6 | 11.6 | −7.4 | 3.4 | 3.0 | 2.9 | 2.6 | 14.8 | 13.9 |
| sd | 0.2 | 0.3 | 0.2 | 0.2 | 6.1 | 6.5 | 0.2 | 0.1 | 0.3 | 0.2 | 4.0 | 5.0 |
| min | 1.9 | 3.8 | 1.6 | 4.3 | −0.8 | −18.5 | 3.1 | 2.8 | 2.5 | 2.2 | 8.8 | 4.0 |
| max | 2.7 | 4.7 | 2.3 | 5.0 | 23.7 | 3.4 | 4.0 | 3.1 | 3.6 | 2.9 | 22.1 | 19.6 |
References
- Bloch, B. Wound healing in higher plants. II. Bot. Rev. 1952, 18, 655–679. [Google Scholar] [CrossRef]
- Birnbaum, K.D.; Alvarado, A.S. Slicing across kingdoms: Regeneration in plants and animals. Cell 2008, 132, 697–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtner, G.C.; Werner, S.; Barradon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Konrad, W.; Flues, F.; Schmich, F.; Speck, T.; Speck, O. An analytical model of the self-sealing mechanism of the succulent plant Delosperma cooperi. J. Theor. Biol. 2013, 336, 96–109. [Google Scholar] [CrossRef]
- Speck, T.; Bauer, G.; Flues, F.; Oelker, K.; Rampf, M.; Schüssele, A.C.; von Tapavicua, M.; Bertling, J.; Luchsinger, R.; Nellesen, A.; et al. Bio-inspired self-healing materials. In Materials Design Inspired by Nature: Function through Inner Architecture; Fratzl, P., Dunlop, J.W.C., Weinkamer, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 359–389. [Google Scholar]
- Harrington, M.J.; Speck, O.; Speck, T.; Wagner, S.; Weinkamer, R. Biological archetypes for self-healing materials. In Self-Healing Materials. Advances in Polymer Science; Hager, M., van der Zwaag, S., Schubert, U., Eds.; Springer: Cham, Switzerland, 2015; Volume 273, pp. 307–344. [Google Scholar]
- Anandan, S.; Rudolph, A.; Speck, T.; Speck, O. Comparative morphological and anatomical study of self-repair in succulent cylindrical plant organs. Flora 2017, 241, 1–7. [Google Scholar] [CrossRef]
- Speck, O.; Speck, T. An overview of bioinspired and biomimetic self-repairing materials. Biomimetics 2019, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Klak, C.; Reeves, G.; Hedderson, T. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature 2004, 427, 63–65. [Google Scholar] [CrossRef]
- Valente, L.M.; Britton, A.W.; Powell, M.P.; Papadopulos, A.S.; Burgoyne, P.M.; Savolainen, V. Correlates of hyperdiversity in southern African ice plants (Aizoaceae). Bot. J. Linn. Soc. 2014, 174, 110–129. [Google Scholar] [CrossRef]
- Mauseth, J.D. Wide-band tracheids are present in almost all species of Cactaceae. J. Plant Res. 2004, 117, 69–76. [Google Scholar] [CrossRef]
- Arruda, E.; Melo-de-Pinna, G.F. Wide-band tracheids (WBTs) of photosynthetic and non-photosynthetic stems in species of Cactaceae. J. Torrey Bot. Soc. 2010, 137, 16–29. [Google Scholar] [CrossRef]
- Tüffers, A.V.; Martin, C.E.; von Willert, D.J. Possible water movement from older to younger leaves and photosynthesis during drought stress in two succulent species from South Africa, Delosperma tradescantioides Brg. and Prenia sladeniana L. Bol. (Mesembryanthemaceae). J. Plant Physiol. 1995, 146, 177–182. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. Adv. Bot. Res. 2010, 55, 179–225. [Google Scholar]
- Klein, H.; Hesse, L.; Boljen, M.; Kampowski, T.; Butschek, I.; Speck, T.; Speck, O. Finite element modelling of complex movements during self-sealing of ring incisions in leaves of Delosperma cooperi. J. Theor. Biol. 2018, 458, 184–206. [Google Scholar] [CrossRef] [PubMed]
- Speck, O.; Schlechtendahl, M.; Borm, F.; Kampowski, T.; Speck, T. Humidity-dependent wound-sealing in succulent leaves of Delosperma cooperi—An adaptation to seasonal drought stress. Beilstein J. Nanotechnol. 2018, 9, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Busch, S.; Seidel, R.; Speck, O.; Speck, T. Morphological aspects of self-repair of lesions caused by internal growth stresses in stems of Aristolochia macrophylla and Aristolochia rigens. Proc. Roy. Soc. B Biol. Sci. 2010, 277, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Bauer, G.; Speck, T. Restoration of tensile strength in bark samples of Ficus benjamina due to coagulation of latex during fast self-sealing of fissures. Ann. Bot 2012, 109, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Speck, O.; Schmauder, K.; Speck, T.; Paul-Victor, C. Wound reaction in stems of Leonurus cardiaca: A morphological, anatomical and biomechanical study. Botany 2019, 98, 81–89. [Google Scholar] [CrossRef]
- Dumais, J.; Forterre, Y. Vegetable dynamics: The role of water in plant movements. Annu. Rev. Fluid Mech. 2012, 44, 453–478. [Google Scholar] [CrossRef] [Green Version]
- Skotheim, J.M.; Mahadevan, L. Physical limits and design principles for plant and fungal movements. Science 2005, 308, 1308–1310. [Google Scholar] [CrossRef] [Green Version]
- Van der Zwaag, S. Self Healing Materials: An Alternative Approach to 20 Centuries of Material Science; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Rampf, M.; Speck, O.; Speck, T.; Luchsinger, R.H. Self-repairing membranes for inflatable structures inspired by a rapid wound sealing process of climbing plants. J. Bionic Eng. 2011, 8, 242–250. [Google Scholar] [CrossRef]
- Rampf, M.; Speck, O.; Speck, T.; Luchsinger, R.H. Structural and mechanical properties of flexible polyurethane fomes cured under pressure. J. Cell. Plast. 2012, 48, 53–69. [Google Scholar] [CrossRef]
- Rampf, M.; Speck, O.; Speck, T.; Luchsinger, R.H. Investigation of a fast mechanical self-repair mechanism for inflatable structures. Int. J. Eng. Sci. 2013, 63, 61–70. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic self-healing. Angew. Chem. Int. Ed. 2015, 54, 10428–10447. [Google Scholar] [CrossRef] [PubMed]
- Huss, J.C.; Spaeker, O.; Gierlinger, N.; Merritt, D.J.; Miller, B.P.; Neinhuis, C.; Fratzl, P.; Eder, M. Temperature-induced self-sealing capability of Banksia follicles. J. R. Soc. Interface 2018, 15, 20180190. [Google Scholar] [CrossRef]
- Yang, Y.; Davydovich, D.; Hornat, C.C.; Liu, X.; Urban, M.U. Leaf-inspired self-healing polymers. Chem 2018, 4, 1928–1936. [Google Scholar] [CrossRef]
- Metzler, W. Beiträge zur vergleichenden Anatomie blattsukkulenter Pflanzen. Bot. Arch 1924, 6, 50–83. [Google Scholar]
- Melo-de-Pinna, G.F.A.; Ogura, A.S.; Arruda, E.C.P.; Klak, C. Repeated evolution of endoscopic peripheral vascular bundles in succulent leaves of Aizoaceae (Caryophyllales). Taxon 2014, 6, 1037–1052. [Google Scholar] [CrossRef]
- Nilsson, S.B.; Hertz, C.H.; Falk, S. On the relation between turgor pressure and tissue rigidity. II: Theoretical calculations on model systems. Physiol. Plant 1958, 11, 818–837. [Google Scholar] [CrossRef]
- Milani, P.; Gholamirad, M.; Traas, J.; Arnéodo, A.; Boudaoud, A.; Argoul, F.; Hamant, O. In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 2011, 67, 1116–1123. [Google Scholar] [CrossRef]
- Schlechtendahl, M. Ontogenie Und Biomechanik der Selbstreparatur bei Verschiedenen Delosperma Arten. Unpublished Diploma Thesis, University of Freiburg, Freiburg im Breisgau, Germany, 2013. [Google Scholar]
- Schmich, F. Selbstreparaturverhalten sukkulenter Pflanzen nach äußeren Verletzungen. Unpublished Diploma Thesis, University of Freiburg, Freiburg im Breisgau, Germany, 2012. [Google Scholar]
- Hartmann, H.E.K. Delosperma Ruschioideae. In Aizoaceae. Illustrated Handbook of Succulent Plants; Hartmann, H.E.K., Eggli, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 395–443. [Google Scholar]
- Ihlenfeldt, H.-D. Lebensformen und Überlebensstrategien bei Sukkulenten. Ber. Deutsch. Bot. Ges. 1985, 98, 409–423. [Google Scholar]
- Ogburn, R.M.; Edwards, E.J. Repeated origin of three-dimensional leaf venation releases constraints on the evolution of succulence in plants. Curr. Biol. 2013, 23, 722–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, R853–R909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrum, J.V. Wide-band tracheids in leaves of genera in Aizoaceae: The systematic occurence of a novel cell type and its implications for the monophyly of the subfamily Ruschioideae. Plant Syst. Evol. 2001, 227, 49–61. [Google Scholar] [CrossRef]
- Bobich, E.G.; Nobel, P.S. Vegetative reproduction as related to biomechanics, morphology and anatomy of four cholla cactus species in the Sonoran Desert. Ann. Bot. 2001, 87, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Agarie, S.; Shimoda, T.; Shimizu, Y.; Baumann, K.; Sunagawa, H.; Kondo, A.; Ueno, O.; Nakahara, T.; Nose, A.; Cushman, J.C. Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J. Ex. Bot. 2007, 58, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Kiani-Pouya, A.; Roessner, U.; Jayasinghe, N.S.; Lutz, A.; Rupasinghe, T.; Bazihizina, N.; Bohm, J.; Alharbi, S.; Hedrich, R.; Shabala, S. Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant Cell Environ. 2017, 40, 1900–1915. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, H.E.K. A revision of the species of Delosperma (Aizoaceae) in Gauteng, South Africa. Haseltonia 2009, 15, 53–68. [Google Scholar] [CrossRef]
- Liede-Schumann, S.; Newton, L.E. Notes on the Delosperma-clade (Aizoaceae). Haseltonia 2018, 25, 100–114. [Google Scholar] [CrossRef]
- Köppen, W. Versuch einer Klassifikation der Klimate, vorzugsweise nach ihren Beziehungen zur Pflanzenwelt. Geogr. Z. 1900, 6, 593–611. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 256–263. [Google Scholar] [CrossRef]
- Speck, T. Die relative Leitfläche einiger Landpflanzen aus dem Unterdevon. In Palaeovegetational Development in Europe and Regions Relevant to Its Palaeofloristic Evolution; Kovar-Eder, J., Ed.; Museum of Natural History: Vienna, Auatria, 1992; pp. 401–411. [Google Scholar]
- Tinder-Smith, T.; Ranwashe, F. Bolus Herbarium Specimens Collection (1865–2009); Version 1.4; South African National Biodiversity Institute: Pretoria, South Africa, 2017; Occurrence Dataset. [Google Scholar] [CrossRef]
- Armitage, A.M. Herbaceous Perennial Plants: A treatise on Their Identification, Culture and Garden Attributes, 3rd ed.; Stipes Publishing: Champaign, IL, USA, 1997. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 11 August 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Neinhuis, C.; Edelmann, H.G. Methanol as a rapid fixative for the investigation of plant surfaces by SEM. J. Microsc. 1996, 184, 14–16. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D slicer as an image computing platform for the quantitative imaging network. Mag. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2018; Available online: https://CRAN.R-project.org/package=psych (accessed on 11 August 2020).
- Speck, T.; Speck, O. Process sequences in biomimetic research. In Design and Nature IV; Brebbia, C.A., Ed.; WIT Press: Southampton, UK, 2008; pp. 3–11. [Google Scholar]

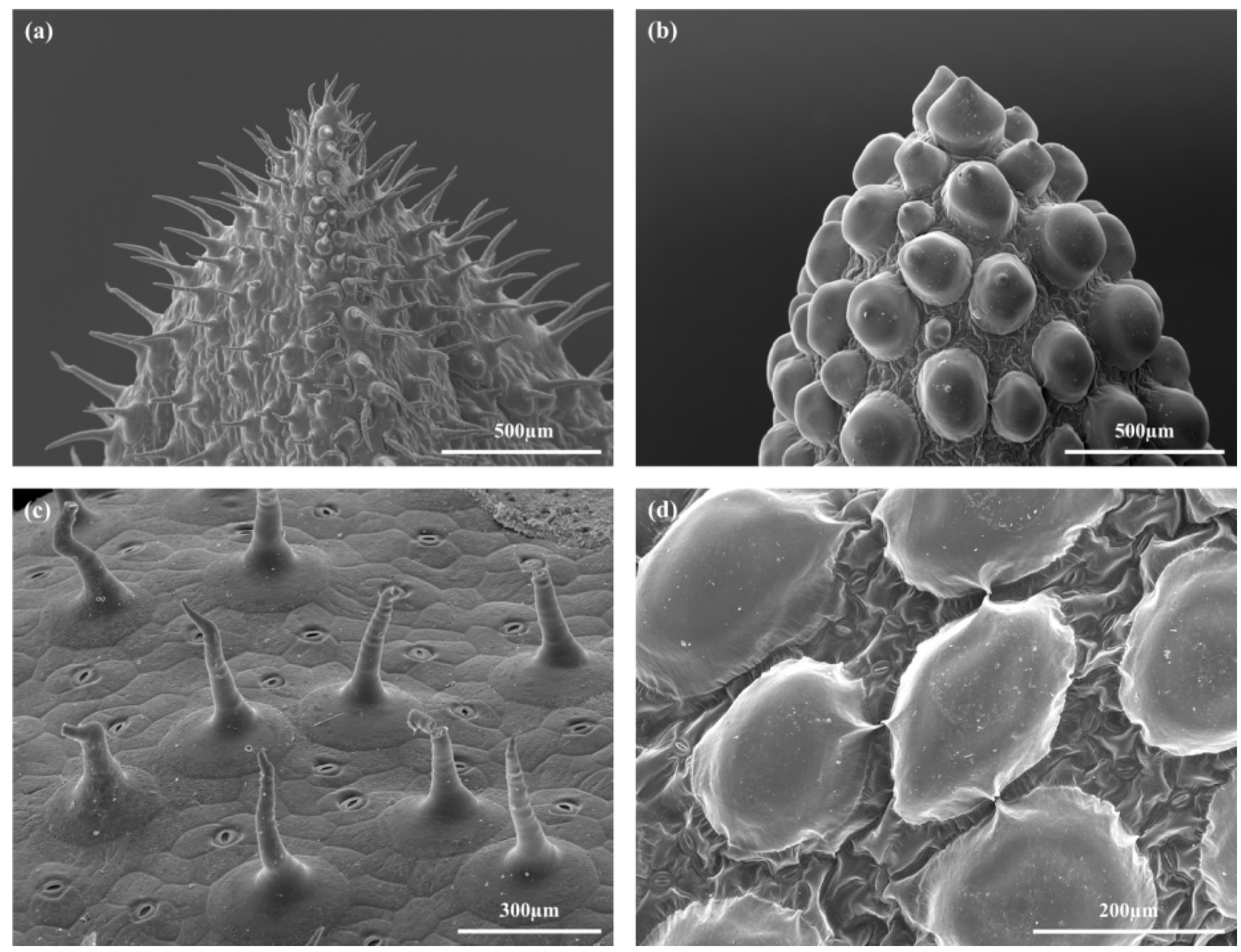
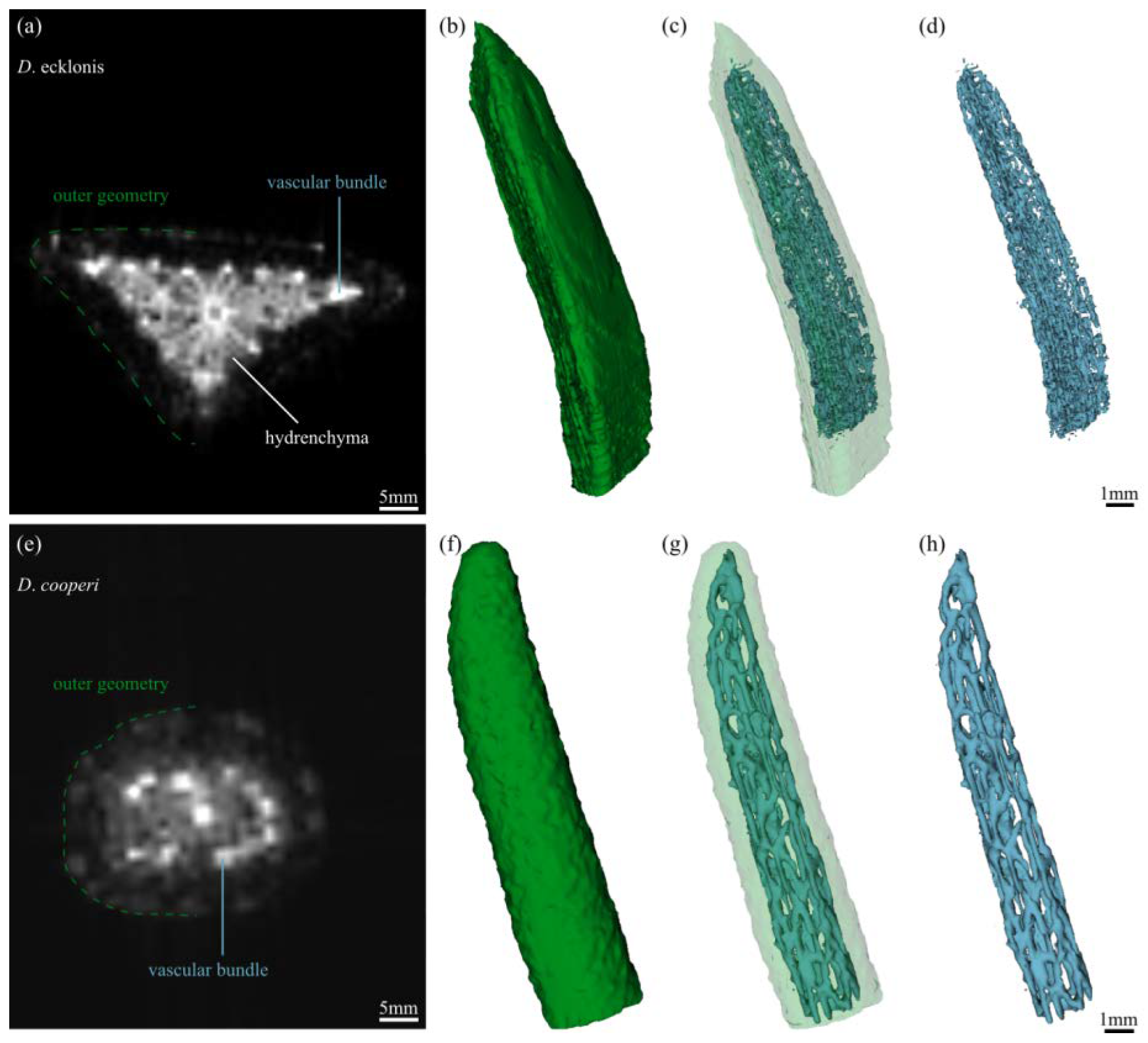
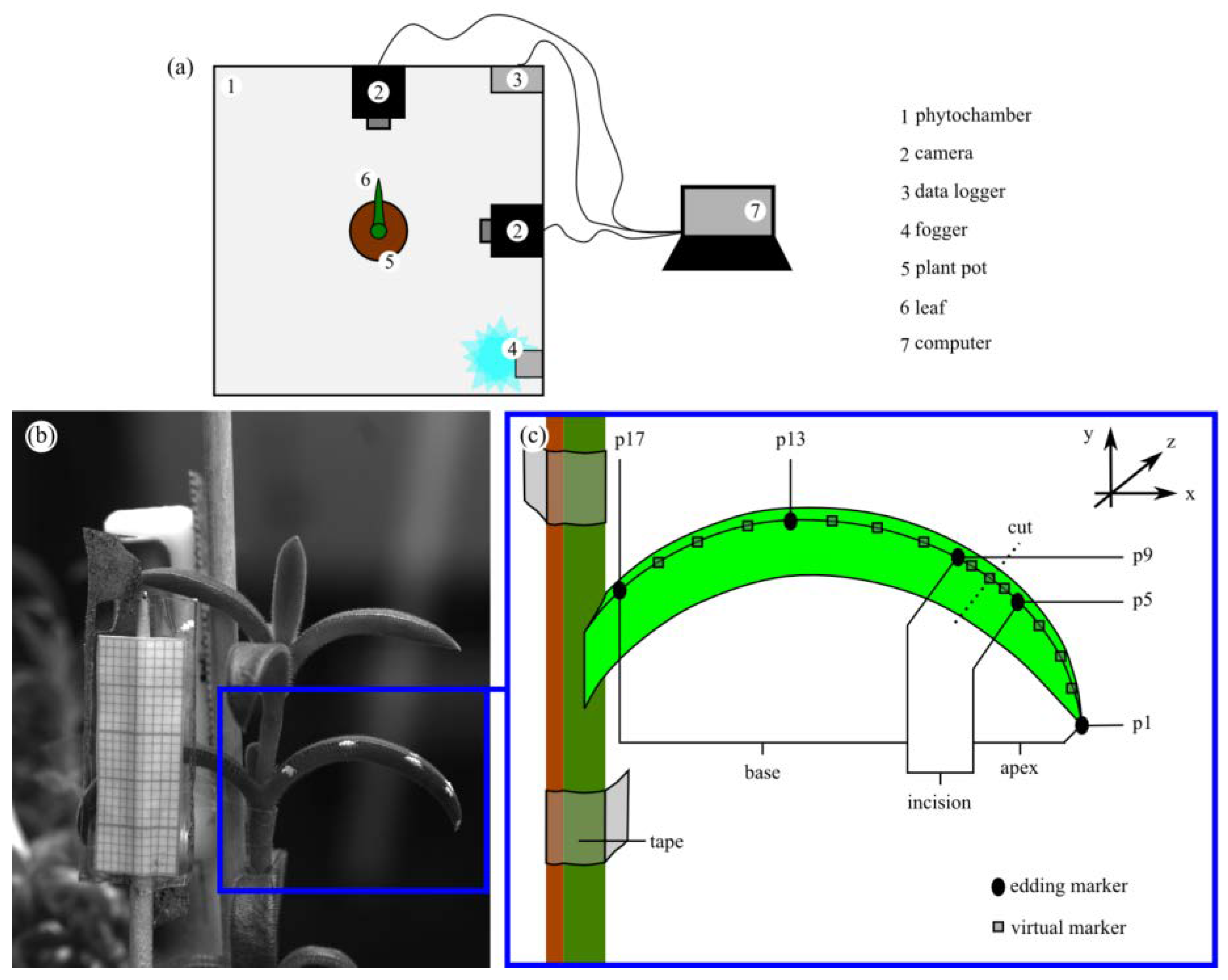


| D. ecklonis | D. cooperi | Interspecific Statistics | |||||
|---|---|---|---|---|---|---|---|
| mean ± s.e. | n | mean ± s.e. | n | t | d.f. | Significance | |
| Lf (mm) | 27.4 ± 1.3 | 20 | 45.5 ± 0.6 | 10 | −12.7 | 26.1 | *** |
| da, f (mm) | 2.5 ± 0.1 *** | 20 | 3.4 ± 0.1 *** | 10 | −7.2 | 27.0 | *** |
| db, f (mm) | 5.4 ± 0.2 *** | 20 | 3.0 ± 0.03 *** | 10 | 13.1 | 20.3 | *** |
| tebc, a (µm) | 82.6 ± 5.5 ns | 20 | 249.5 ± 11.1 ns | 20 | −14.1 | 27.9 | *** |
| tebc, b (µm) | 84.3 ± 3.7 ns | 20 | 258.4 ± 9.5 ns | 20 | −16.3 | 24.5 | *** |
| tepi, a (µm) | 24.4 ± 1.9 ns | 20 | 40.1 ± 2.4 ns | 20 | −5.1 | 36.0 | *** |
| tepi, b (µm) | 26.7 ± 1.9 ns | 20 | 40.2 ± 3.2 ns | 20 | −3.6 | 31.1 | *** |
| tchl, a (µm) | 364.3 ± 19.6 *** | 20 | 524.9 ± 22.1 ns | 20 | −5.4 | 37.5 | *** |
| tchl, b (µm) | 775.3 ± 25.0 *** | 20 | 566.6 ± 16.7 ns | 20 | 7.0 | 33.1 | *** |
| tnvb, a (µm) | 55.4 ± 2.3 * | 20 | 64.4 ± 1.8 ns | 20 | −3.0 | 36.2 | ** |
| tnvb, b (µm) | 63.2 ± 3.0 * | 20 | 65.6 ± 1.8 ns | 20 | −0.7 | 31.5 | ns |
| thyd, a (µm) | 443.7 ± 30.8 *** | 20 | 586.8 ± 28.8 ns | 20 | −3.4 | 37.8 | ** |
| thyd, b (µm) | 1450.6 ± 32.8 *** | 20 | 671.2 ± 25.0 ns | 20 | 18.9 | 35.5 | *** |
| tcvs, a (µm) | 50.9 ± 2.9 *** | 20 | 54.9 ± 1.1 * | 20 | 1.6 | 37.3 | ns |
| tcvs, b (µm) | 66.9 ± 2.2 *** | 20 | 60.6 ± 2.6 * | 20 | −1.7 | 27.9 | ns |
| D. ecklonis | D. cooperi | Ratio | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| med ± s.e. | n | med ± s.e. | n | medecklonis/medcooperi | |||||
| Elastic Modulus in Tension (MPa) | |||||||||
| Leaf | 1.21 ± 0.11 | 14 | 0.72 ± 0.14 | 18 | 1.68 | *** | |||
| Central Vascular Strand | 63.68 ± 7.52 | 9 | 32.80 ± 5.96 | 8 | 1.94 | ** | |||
| Epidermis (trans.) | 2.88 ± 0.40 ns | 6 | 3.62 ± 0.27 ns | 10 | 0.80 | * | |||
| Epidermis (long.) | 3.51 ± 1.21 ns | 8 | 5.27 ± 0.85 ns | 10 | 0.67 | ns | |||
| Hydrenchyma † | 0.26 | ‒ | 0.23 | ‒ | 1.13 | ‒ | |||
| Chlorenchyma † | 0.26 | ‒ | 0.27 | ‒ | 0.96 | ‒ | |||
| Tensile Strength (MPa) | |||||||||
| Leaf | 0.16 ± 0.01 | 14 | 0.09 ± 0.01 | 13 | 1.78 | *** | |||
| Central Vascular Strand | 10.99 ± 0.70 | 9 | 8.80 ± 0.76 | 8 | 1.25 | ** | |||
| Epidermis (trans.) | 0.58 ± 0.10 ns | 6 | 1.25 ± 0.07 * | 10 | 0.46 | ** | |||
| Epidermis (long.) | 0.70 ± 0.26 ns | 8 | 1.54 ± 0.11 * | 10 | 0.45 | ** | |||
| Poisson’s Ratio | |||||||||
| Leaf | 0.35 ± 0.03 | 14 | 0.29 ± 0.03 | 18 | 1.21 | * | |||
| Turgor (MPa) | |||||||||
| Hydrenchyma and Chlorenchyma | 0.05 ± 0.002 | 36 | 0.04 ± 0.003 | 44 | 1.25 | ns | |||
| Feature | D. ecklonis | D. cooperi |
|---|---|---|
| Habitat and Climate | ||
| Habitat | coastal regions in South Africa | highland regions in South Africa |
| Köppen-Geiger Climate Classification | Cfb = oceanic climate (warm temperate climate, fully humid) | Cwb = dry-winter subtropical highland climate (warm temperate climate with dry winter) |
| Morphology and Anatomy | ||
| Plant Habitus | branched shrub, erect branches, height: 129.6 ± 27.2 mm, diameter: 149.5 ± 57.0 mm | branched shrub, decumbent, height: 68.0 ± 13.7 mm, diameter: 150.0 ± 44.0 mm |
| Leaf Shape | triquetrous | semi-terete to terete |
| Leaf Morphology | pubescent, recurved | straight |
| Leaf Length | 27.4 ± 26.5 mm | 45.5 ± 1.9 mm |
| Epidermal Bladder Cells | thickened basally but grow out to slim trichomes distally | rounded and papillose, large and densely packed |
| Hydrenchyma | mean values of cell diameter and mean aspect ratio of cells considerably higher than in D. cooperi | |
| Biomechanics (Elastic Modulus/Tensile Strength) | ||
| Entire leaf, central vascular strand | considerably stiffer and more rigid than in D. cooperi | |
| Epidermis | considerably stiffer and more rigid than in D. ecklonis | |
| Further Mechanically Important Parameters | ||
| Poisson’s Ratio | significantly higher than in D. cooperi | |
| Turgor | slightly higher than in D. cooperi | |
| Leaf Kinematics | ||
| Absolute and relative contractions (esp. incision region) independent of the given relative humidity (RH) level | more pronounced than in D. ecklonis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesse, L.; Kampowski, T.; Leupold, J.; Caliaro, S.; Speck, T.; Speck, O. Comparative Analyses of the Self-Sealing Mechanisms in Leaves of Delosperma cooperi and Delosperma ecklonis (Aizoaceae). Int. J. Mol. Sci. 2020, 21, 5768. https://doi.org/10.3390/ijms21165768
Hesse L, Kampowski T, Leupold J, Caliaro S, Speck T, Speck O. Comparative Analyses of the Self-Sealing Mechanisms in Leaves of Delosperma cooperi and Delosperma ecklonis (Aizoaceae). International Journal of Molecular Sciences. 2020; 21(16):5768. https://doi.org/10.3390/ijms21165768
Chicago/Turabian StyleHesse, Linnea, Tim Kampowski, Jochen Leupold, Sandra Caliaro, Thomas Speck, and Olga Speck. 2020. "Comparative Analyses of the Self-Sealing Mechanisms in Leaves of Delosperma cooperi and Delosperma ecklonis (Aizoaceae)" International Journal of Molecular Sciences 21, no. 16: 5768. https://doi.org/10.3390/ijms21165768
APA StyleHesse, L., Kampowski, T., Leupold, J., Caliaro, S., Speck, T., & Speck, O. (2020). Comparative Analyses of the Self-Sealing Mechanisms in Leaves of Delosperma cooperi and Delosperma ecklonis (Aizoaceae). International Journal of Molecular Sciences, 21(16), 5768. https://doi.org/10.3390/ijms21165768








