COVID-19: The Immune Responses and Clinical Therapy Candidates
Abstract
:1. Introduction
2. Virology of SARS-CoV-2
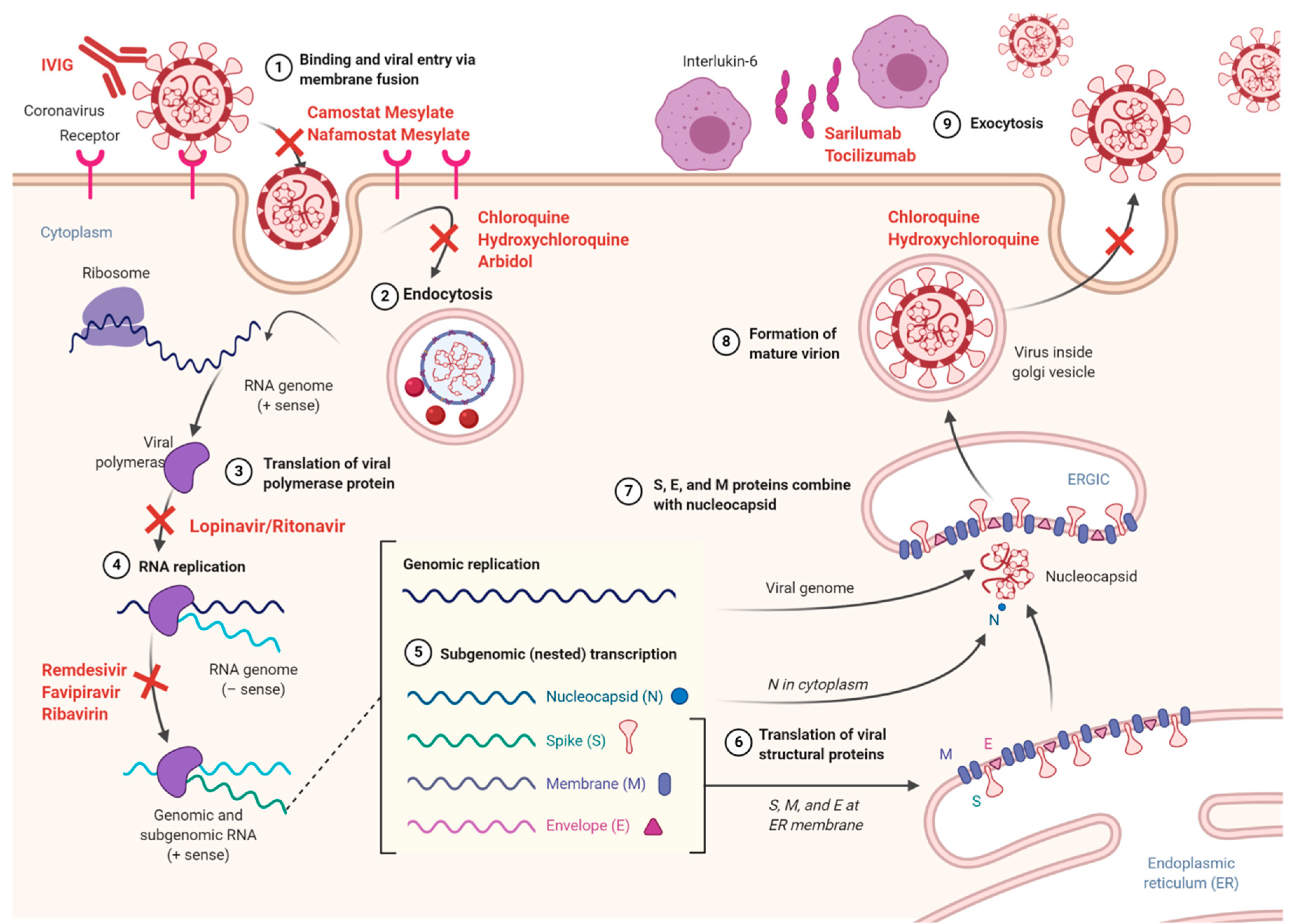
3. Coronavirus Entry and Replication
4. Pathogenesis of COVID-19
5. The Innate Immune Response to SARS-CoV-2 and Virus-Induced Degradation of RNA Sensors
6. Regulation of Adaptive Immune Response in SARS-CoV-2 Infection
6.1. T-Cell Mediated Immune Responses Against SARS-CoV-2 and Mechanisms of Immune Escape
6.2. Humoral Immune and Antibody Responses to Coronavirus Infection
6.3. Cytokine Responses Across COVID-19; the Significance of IL-6 in Cytokine Release Syndrome (CRS)
7. COVID-19 and Comorbidities
8. Available Treatment Options for COVID-19
8.1. Antivirals
8.1.1. Remdesivir
8.1.2. Lopinavir/Ritonavir
8.1.3. Favipiravir
8.1.4. Ribavirin
8.1.5. Arbidol Hydrochloride (Umifenovir)
8.1.6. Camostat Mesylate (FoipanTM)
8.1.7. Nafamostat Mesylate
8.2. Anti-Parasites
8.2.1. Chloroquine/Hydroxychloroquine
8.2.2. Ivermectin
9. Immunotherapy
9.1. Convalescent Plasma Therapy
9.2. Intravenous Immunoglobulin (IVIG)
9.3. Monoclonal Antibodies (mAbs)
10. Adjunctive/Supportive Therapy
10.1. Azithromycin
10.2. Corticosteroids
10.3. Nitric Oxide
11. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| FDA | US Food and Drug Administration |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| ARDS | acute respiratory distress syndrome |
| RSCU | synonymous codon usage |
| NSP | non-structural proteins |
| ORF | open reading frame |
| S | spike glycoprotein |
| E | envelope protein |
| M | matrix protein |
| N | nucleocapsid protein |
| RBD | receptor binding domain |
| ACE2 | angiotensin converting enzyme II |
| Cryo-EM | cryo-electron microscopy |
| HAT | human airway trypsin-like protease |
| ssRNA | single stranded RNA |
| TMPRSS2 | transmembrane protease serine 2 |
| PLpro | papain-like proteases |
| 3CLpro | chymotrypsin-like protease |
| RTC | replication–transcription complex |
| RdRp | RNA-dependent RNA polymerases |
| ER | endoplasmic reticulum |
| TI IF | type I interferons |
| IFN-α | interferon-α |
| Bcl-xL | B-cell lymphoma-extra large |
| PAMPs | pathogen associated molecular patterns |
| TLR | toll-like receptors |
| IRF3 | IFN regulatory factor 3 |
| IL | interleukin |
| TNF | tumor necrosis factor |
| RLRs | RIG-I-like receptors |
| CLRs | C-type lectin-like receptors |
| NLR | NOD-like receptor |
| APCs | antigen presenting cells |
| MHC | major histocompatibility complex |
| HLA | human leukocyte antigen |
| CTLs | cytotoxic T lymphocytes |
| CD | cluster of differentiation |
| DCs | dendritic cells |
| MCP-1 | monocyte chemoattractant protein-1 |
| CRS), | cytokine release syndrome |
| CRP | C-reactive protein |
| sIL-6R | IL-6 receptor |
| JAK-STAT3 | Janus kinase-signal transducer and activator of transcription 3 |
| Tregs | regulatory T cells |
| RDV | Remdesivir |
| EC50 | half-maximal effective concentration |
| CC50 | half-cytotoxic concentration |
| LPV/r | Lopinavir/Ritonavir |
| HIV-1 | human immunodeficiency virus-1 |
| ESICM | The European Society of Intensive Care Medicine |
| SCCM | The Society of Critical Care Medicine |
| NIH | The National Institutes of Health |
| FPV | Favipiravir |
| ARB | Arbidol |
| HCV | hepatitis B virus, hepatitis C virus |
| HA | hemagglutinin |
| HCoV-NL63 | human coronavirus NL63 |
| DENV | dengue virus |
| VEEV | Venezuelan equine encephalitis virus |
| IN | integrase protein |
| IMP | importin |
| mAbs | monoclonal antibodies |
| ERGIC | ER–Golgi intermediate compartment |
| IVIG | intravenous immunoglobulin |
| PE | plasma exchange |
| ICU | intensive care unit |
| PCR | polymerase chain reaction |
| qRT-PCR | quantitative reverse transcriptase PCR |
| WHO | World Health Organization |
| PD-1 | programmed death-1 |
| CDC | left for disease control and prevention |
| NO | nitric oxide |
| cGMP | cyclic guanosine monophosphate |
| GM-CSF | granulocyte-monocyte stimulating factor |
References
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Genet. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- NIH. Potential Antiviral Drugs Under Evaluation for the Treatment of COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/ (accessed on 24 July 2020).
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Administration UFad. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (accessed on 1 May 2020).
- Chaib, F. WHO Welcomes Preliminary Results about Dexamethasone Use in Treating Critically Ill COVID-19 Patients. World Health Organization. Available online: https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients (accessed on 16 June 2020).
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Paraskevis, D.; Kostaki, E.; Magiorkinis, G.; Panayiotakopoulos, G.; Sourvinos, G.; Tsiodras, S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020, 79, 104212. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.T.; Shum, M.H.-H.; Zhu, H.-C.; Tong, Y.-G.; Ni, X.-B.; Liao, Y.-S.; Wei, W.; Cheung, W.Y.-M.; Li, W.-J.; Li, L.-F.; et al. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Wang, W.; Zhao, X.; Zai, J.; Li, X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020, 92, 433–440. [Google Scholar] [CrossRef]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef] [Green Version]
- Van Boheemen, S.; De Graaf, M.; Lauber, C.; Bestebroer, T.M.; Raj, V.S.; Zaki, A.M.; Osterhaus, A.D.; Haagmans, B.L.; Gorbalenya, A.E.; Snijder, E.J.; et al. Genomic Characterization of a Newly Discovered Coronavirus Associated with Acute Respiratory Distress Syndrome in Humans. mBio 2012, 3, e00473-12. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Sigrist, C.J.; Bridge, A.; Le Mercier, P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020, 177, 104759. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Li, T.; Zhang, S.; Wang, L.; Wu, X.; Liu, J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, Y.; Liu, S.; Kou, Z.; Li, W.; Farzan, M.; Jiang, S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004, 324, 773–781. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hong, Y.; Shibayama, K.; Suzuki, Y.; Wakamiya, N.; Kim, Y.U. Functional analysis of the receptor binding domain of SARS coronavirus S1 region and its monoclonal antibody. Genes Genom. 2014, 36, 387–397. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsuyama, S.; Li, X.; Takeda, M.; Kawaguchi, Y.; Inoue, J.-I.; Matsuda, Z. Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob. Agents Chemother. 2016, 60, 6532–6539. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.; Tan, Y.-Y.; Chen, S.; Jin, H.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11–110. [Google Scholar] [CrossRef] [Green Version]
- Astuti, I.J.D. Research MSC, Reviews. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol 2020, 38, 1–9. [Google Scholar]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020. [Google Scholar] [CrossRef]
- Spiegel, M.; Weber, F. Inhibition of cytokine gene expression and induction of chemokine genes in non-lymphatic cells infected with SARS coronavirus. Virol. J. 2006, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Cristina, S.; Concetta, R.; Francesco, R.; Annalisa, C. SARS-Cov-2 infection: Response of human immune system and possible implications for the rapid test and treatment. Int. Immunopharmacol. 2020, 84, 106519. [Google Scholar]
- Merad, M.; Martin, J.C. Author Correction: Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 2020, 335–362. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Lescure, F.-X.; Yazdanpanah, Y.; Mentre, F.; Peiffer-Smadja, N.; Ader, F.; Bouadma, L.; Poissy, J.; Timsit, J.-F.; Lina, B.; et al. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020, 178, 104791. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Greber, U.F. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013, 21, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020, 34, 1. [Google Scholar]
- Moresco, E.M.Y.; LaVine, D.; Beutler, B. Toll-like receptors. Curr. Boil. 2011, 21, R488–R493. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A. Local Immunity Concept in the Context of the Novel Corona Viral Infection: A Consideration. Asian J. Immunol. 2020, 3, 16–25. [Google Scholar]
- Ratajczak, M.Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Ortega, J.T.; Serrano, M.L.; Pujol, F.H.; Rangel, H.R. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. EXCLI J. 2020, 19, 410–417. [Google Scholar]
- Ishii, K.; Hasegawa, H.; Nagata, N.; Mizutani, T.; Morikawa, S.; Suzuki, T.; Taguchi, F.; Tashiro, M.; Takemori, T.; Miyamura, T.; et al. Induction of protective immunity against severe acute respiratory syndrome coronavirus (SARS-CoV) infection using highly attenuated recombinant vaccinia virus DIs. Virology 2006, 351, 368–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, M.; Sharma, A.R.; Patra, P.; Ghosh, P.; Sharma, G.; Patra, B.C.; Lee, S.-S.; Chakraborty, C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J. Med. Virol. 2020, 92, 618–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieman, M.B.; Chen, J.; Morrison, T.E.; Whitmore, A.; Funkhouser, W.; Ward, J.M.; Lamirande, E.W.; Roberts, A.; Heise, M.; Subbarao, K.; et al. SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism. PLoS Pathog. 2010, 6, e1000849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680. [Google Scholar] [CrossRef]
- Rokni, M.; Ghasemi, V.; Tavakoli, Z. Immune responses and pathogenesis of SARS-CoV -2 during an outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 2020, 30. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Nyodu, R.; Maurya, V.K.; Saxena, S.K.; Saxena, S.K. Host Immune Response and Immunobiology of Human SARS-CoV-2 Infection. In Coronavirus Disease 2019 (COVID-19); Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 43–53. [Google Scholar]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; Van Crevel, R.; Van De Veerdonk, F.L.; Bonten, M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef]
- Ganji, A.; Farahani, I.; Khansarinejad, B.; Ghazavi, A.; Mosayebi, G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cell. Mol. Dis 2020, 102437. [Google Scholar] [CrossRef]
- Cai, X. An Insight of Comparison between COVID-19 (2019-nCoV Disease) and SARS in Pathology and Pathogenesis. 2020. Available online: https://osf.io/hw34x/ (accessed on 3 August 2020).
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Hussman, J.P. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafarotti, S. Severe Acute Respiratory Syndrome–Coronavirus-2 Infection and Patients with Lung Cancer: The Potential Role of Interleukin-17 Target Therapy. J. Thorac. Oncol. 2020, 15, e101–e103. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, M.-L.; Chien, C.-S.; Yarmishyn, A.A.; Yang, Y.-P.; Lai, W.-Y.; Luo, Y.-H.; Lin, Y.-T.; Chen, Y.-J.; Chang, P.-C.; et al. Highlight of Immune Pathogenic Response and Hematopathologic Effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lega, S.; Naviglio, S.; Volpi, S.; Tommasini, A. Recent Insight into SARS-CoV2 Immunopathology and Rationale for Potential Treatment and Preventive Strategies in COVID-19. Vaccines 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jia, W.; Wang, P.; Zhang, S.; Shi, X.; Wang, X.; Zhang, L.-Q.; Wang, T.; Golding, H.; Khurana, S. Antibodies and vaccines against Middle East respiratory syndrome coronavirus. Emerg. Microbes Infect. 2019, 8, 841–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, S.; Coyle, E.M.; Klenow, L.; Tang, J.; Grubbs, G.; Liu, S.; Wang, T.; Golding, H.; Khurana, S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci. Trans. Med. 2020, 12, 550–eabc3539. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.J.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.-C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [Green Version]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.-W.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies directed to multiple epitopes on SARS-CoV-2 spike. Nature 2020, 1–10. [Google Scholar] [CrossRef]
- Wilk, C.M. Coronaviruses hijack the complement system. Nat. Rev. Immunol. 2020, 20, 350. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. Coronavirus blood-clot mystery intensifies. Nature 2020, 581, 250. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J.; Srivastava, A. SARS-2 Coronavirus–Associated Hemostatic Lung Abnormality in COVID-19: Is It Pulmonary Thrombosis or Pulmonary Embolism? Semin. Thromb. Hemost. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; Von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J. Antimicrob. Agents. 2020, 105954. [Google Scholar] [CrossRef]
- Fehr, A.R.; Channappanavar, R.; Perlman, S. Middle East respiratory syndrome: Emergence of a pathogenic human coronavirus. Annu. Rev. Med. 2017, 68, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.; Fatima, R.; Assaly, R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Moore, B.J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Rose-John, S. Interleukin-6 and its receptor: From bench to bedside. Med. Microbiol. Immunol. 2006, 195, 173–183. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Bekes, I.; Wulff, C. Controlling Vascular Permeability: How Does It Work and What Is the Impact on Normal and Pathological Angiogenesis. Tumor Angiogenesis A Key Target. Cancer Ther. 2019, 121–132. [Google Scholar]
- Ross, S.H.; Cantrell, A.D. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Maerz, B.; Buckner, M.D.J.H. IL-6: A cytokine at the crossroads of autoimmunity. Curr. Opin. Immunol. 2018, 55, 9–14. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Navarro, G.; Taroumian, S.; Barroso, N.; Duan, L.; Furst, D.E. Tocilizumab in rheumatoid arthritis: A meta-analysis of efficacy and selected clinical conundrums. Semin. Arthritis Rheum. 2014, 43, 458–469. [Google Scholar] [CrossRef]
- Yokota, S.; Miyamae, T.; Imagawa, T.; Iwata, N.; Katakura, S.; Mori, M.; Woo, P.; Nishimoto, N.; Yoshizaki, K.; Kishimoto, T. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005, 52, 818–825. [Google Scholar] [CrossRef]
- Khiali, S.; Khani, E.; Entezari-Maleki, T. A Comprehensive Review on Tocilizumab in COVID-19 Acute Respiratory Distress Syndrome. J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Dong, H.; Xia, S.Q.; Huang, Y.Z.; Wang, D.; Zhao, Y.; Liu, W.; Tu, S.; Zhang, M.; Wang, Q.; et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–1409. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention, Older Adults. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed on 30 July 2020).
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P.; et al. Clinical Features and Short-term Outcomes of 144 Patients With SARS in the Greater Toronto Area. JAMA 2003, 289, 2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, F.Y.; Aleanizy, F.; Mohamed, R.A.E.H.; Alanazi, M.S.; Mohamed, N.; Alrasheed, M.M.; Abanmy, N.; Alhawassi, T. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: A retrospective study. Epidemiol. Infect. 2018, 147, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Centers for Disease Control and Prevention, People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 17 July 2020).
- Sanyaolu, O.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Bajgain, K.T.; Badal, S.; Bajgain, B.B.; Santana, M.J. Prevalence of Comorbidities Among Individuals With COVID-19: A Rapid Review of current Literature. Am. J. Infect. Control. 2020. [Google Scholar] [CrossRef]
- Paudel, S.S. A Meta-Analysis of 2019 Novel Corona Virus Patient Clinical Characteristics and Comorbidities. 2020. Available online: https://www.researchsquare.com/article/rs-21831/v1 (accessed on 3 August 2020).
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [Green Version]
- Gold, M.S.; Sehayek, D.; Gabrielli, S.; Zhang, X.; McCusker, C.; Ben-Shoshan, M. COVID-19 and comorbidities: A systematic review and meta-analysis. Postgrad. Med. 2020, 1–7. [Google Scholar] [CrossRef]
- Noor, F.M.; Islam, M. Prevalence of Clinical Manifestations and Comorbidities of Coronavirus (COVID-19) Infection: A Meta-Analysis. Fortune J. Health Sci. 2020, 1, 55–97. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052. [Google Scholar] [CrossRef]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H.; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019, 169, 104541. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.E.; Gralinski, L.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hú, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- McCreary, E.K.; Pogue, J.M. COVID-19 Treatment: A Review of Early and Emerging Options. Open Forum Infect. Dis. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Liu, S.; Tan, T.; Huang, W.; Dong, Y.; Chen, L. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest 2020, 158. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Chu, C.; Cheng, V.C.C.; Hung, I.F.N.; Wong, M.M.L.; Chan, K.; Kao, R.Y.T.; Poon, L.L.; Wong, C.L.P.; Guan, Y.; Peiris, J.S.M.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jeon, S.; Shin, H.Y.; Kim, M.J.; Seong, Y.M.; Lee, W.J.; Choe, K.-W.; Kang, Y.M.; Lee, B.; Park, S.-J.; et al. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: The application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020, 35, e79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Lu, Y.; Chen, F.; Zhang, W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends 2020, 14, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.-T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Baden, L.R.; Rubin, E.J. Covid-19—The Search for Effective Therapy. N. Engl. J. Med. 2020, 382, 1851–1852. [Google Scholar] [CrossRef]
- Crotti, L.; Arbelo, E. COVID-19 treatments, QT interval, and arrhythmic risk: The need for an international registry on arrhythmias. Hear. Rhythm. 2020. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef] [Green Version]
- Delang, L.; Abdelnabi, R.; Neyts, J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 2018, 153, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-F.; Chien, C.-S.; Yarmishyn, A.A.; Lin, Y.-Y.; Luo, Y.-H.; Lai, W.-Y.; Yang, D.-M.; Chou, S.-J.; Yang, Y.-P.; Wang, M.-L.; et al. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Huang, J.; Yin, P.; Zhang, Y.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.G.V.; Santos, W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Pública 2020, 44, e40–e13. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Götte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Boil. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef] [Green Version]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.J.T.; Witkowski, R.; Robins, K. Broad-spectrum antiviral activity of virazole: 1-f8-D-ribofuranosyl-1, 2, 4-triazole-3-carboxamide. Science 1972, 177, 705–706. [Google Scholar] [CrossRef]
- Thomas, E.; Ghany, M.G.; Liang, T.J. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir. Chem. Chemother. 2012, 23, 1–12. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2005, 16, 37–48. [Google Scholar] [CrossRef]
- Morgenstern, B.; Michaelis, M.; Baer, P.C.; Doerr, H.W.; Cinatl, J.; Cinatl, J. Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005, 326, 905–908. [Google Scholar] [CrossRef]
- Falzarano, D.; De Wit, E.; Martellaro, C.; Callison, J.; Munster, V.J.; Feldmann, H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013, 3, srep01686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, S.R.; Phillips, E.J.; Dresser, L.; Matukas, L. Common Adverse Events Associated with the Use of Ribavirin for Severe Acute Respiratory Syndrome in Canada. Clin. Infect. Dis. 2003, 37, 1139–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altınbas, S.; Holmes, J.A.; Altınbas, A. Hepatitis C Virus Infection in Pregnancy. Gastroenterol. Nurs. 2020, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Qi, C.; Shen, L.; Li, J. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020, 92, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Boriskin, Y.; Leneva, I.; Pecheur, E.-I.; Polyák, S. Arbidol: A Broad-Spectrum Antiviral Compound that Blocks Viral Fusion. Curr. Med. Chem. 2008, 15, 997–1005. [Google Scholar] [CrossRef]
- Blaising, J.; Polyak, S.J.; Pécheur, E.-I. Arbidol as a broad-spectrum antiviral: An update. Antivir. Res. 2014, 107, 84–94. [Google Scholar] [CrossRef]
- Kadam, R.U.; Wilson, I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. USA 2016, 114, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Khamitov, A.; Loginova, R.; Shchukina, S.I.; Borisevich, V.N.S.V.; Maksimov, V.A.; Shuster, A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr. Virusol. 2008, 53, 9–13. [Google Scholar]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- McKee, D.L.; Sternberg, A.; Stange, U.; Laufer, S.; Naujokat, C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020, 157, 104859. [Google Scholar] [CrossRef]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pöhlmann, S.; Soilleux, E. Influenza and SARS-Coronavirus Activating Proteases TMPRSS2 and HAT Are Expressed at Multiple Sites in Human Respiratory and Gastrointestinal Tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by the Transmembrane Protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Shirato, K.; Van Der Hoek, L.; Taguchi, F.; Matsuyama, S. Simultaneous Treatment of Human Bronchial Epithelial Cells with Serine and Cysteine Protease Inhibitors Prevents Severe Acute Respiratory Syndrome Coronavirus Entry. J. Virol. 2012, 86, 6537–6545. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Ino, Y.; Motoyoshi, A.; Ozeki, M.; Sato, T.; Kurumi, M.; Aoyama, T. Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn. J. Pharmacol. 1986, 41, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, Z.; Zeng, S.; Wang, X.; Liu, W.; Qian, L.; Wei, J.; Yang, X.; Shen, Q.; Gong, Z.; et al. The Molecular Aspect of Antitumor Effects of Protease Inhibitor Nafamostat Mesylate and Its Role in Potential Clinical Applications. Front. Oncol. 2019, 9, 852. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- Devaux, C.A.; Rolain, J.-M.; Colson, P.; Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55, 105938. [Google Scholar] [CrossRef]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Singh, A.; Shaikh, A.; Singh, R.; Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Colson, P.; Rolain, J.-M.; Lagier, J.-C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.N. Ocular safety of hydroxychloroquine. Ann. Ophthalmol. 1991, 23, 292–296. [Google Scholar]
- Ratliff, N.B.; Estes, M.L.; Myles, J.L.; Shirey, E.K.; McMahon, J.T. Diagnosis of Chloroquine Cardiomyopathy by Endomyocardial Biopsy. N. Engl. J. Med. 1987, 316, 191–193. [Google Scholar] [CrossRef]
- McChesney, E.W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983, 75, 11–18. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017, 5, e00293. [Google Scholar] [CrossRef]
- Biot, C.; Daher, W.; Chavain, N.; Fandeur, T.; Khalife, J.; Dive, D.; De Clercq, E. Design and Synthesis of Hydroxyferroquine Derivatives with Antimalarial and Antiviral Activities. J. Med. Chem. 2006, 49, 2845–2849. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 2020, 105949. [Google Scholar] [CrossRef]
- Ferner, R.E.; Aronson, J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ 2020, 369, m1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, D.; Roberts, D.; Hill, C. Hydroxychloroquine for COVID-19: A Cautionary Tale. InSight+. Available online: https://insightplus.mja.com.au/2020/21/hydroxychloroquine-for-covid-19-a-cautionary-tale/ (accessed on 1 June 2020).
- Chorin, E.; Wadhwani, L.; Magnani, S.; Dai, M.; Shulman, E.; Nadeau-Routhier, C.; Knotts, R.; Bar-Cohen, R.; Kogan, E.; Barbhaiya, C.; et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Hear. Rhythm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Administration USFaD. Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-newly-discovered-potential-drug-interaction-may-reduce (accessed on 15 June 2020).
- Administration FaD. FDA Cautions Against Use of Hydroxychloroquine or Chloroquine for COVID-19 Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems. 1 July 2020. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or (accessed on 1 July 2020).
- No Clinical Benefit from Use of Hydroxychloroquine in Hospitalised Patients with COVID-19. 5 June 2020. Available online: https://www.ox.ac.uk/news/2020-06-05-no-clinical-benefit-use-hydroxychloroquine-hospitalised-patients-covid-19 (accessed on 5 June 2020).
- Health. NIo. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 16 June 2020. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 30 July 2020).
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An AlphaScreen(R)-Based Assay for High-Throughput Screening for Specific Inhibitors of Nuclear Import. J. Biomol. Screen. 2011, 16, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.N.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Momekov, G.; Momekova, D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: Antiviral levels are not likely attainable with known dosing regimens. Biotechnol. Biotechnol. Equip. 2020, 34, 469–474. [Google Scholar] [CrossRef]
- Beigel, J.H. Polyclonal and monoclonal antibodies for the treatment of influenza. Curr. Opin. Infect. Dis. 2018, 31, 527–534. [Google Scholar] [CrossRef]
- Marano, G.; Vaglio, S.; Pupella, S.; Facco, G.; Catalano, L.; Liumbruno, G.M.; Grazzini, G. Convalescent plasma: New evidence for an old therapeutic tool? High Speed Blood Transfus. Equip. 2015, 14, 152–157. [Google Scholar]
- Chaudhuri, S.; Symons, J.A.; Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir. Res. 2018, 155, 76–88. [Google Scholar] [CrossRef]
- Rosenke, K.E.; Bounds, C.; Hanley, P.W.; Saturday, G.; Sullivan, E.; Wu, H.; Jiao, J.-A.; Feldmann, H.; Schmaljohn, C.; Safronetz, D. Human Polyclonal Antibodies Produced by Transchromosomal Cattle Provide Partial Protection Against Lethal Zaire Ebolavirus Challenge in Rhesus Macaques. J. Infect. Dis. 2018, 218, S658–S661. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Chen, J.-T.; Liu, Y.-X.; Zhang, Z.-S.; Gao, H.; Liu, Y.; Wang, X.; Ning, Y.; Liu, Y.-F.; Gao, Q.; et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J. Med. Virol. 2005, 77, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Van Griensven, J.; Edwards, T.; De Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; Horby, P.; Raoul, H.; Magassouba, N.; Antierens, A.; et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N. Engl. J. Med. 2016, 374, 33–42. [Google Scholar] [CrossRef]
- Anudeep, T.; Jeyaraman, M.; Shetty, D.U.; Raj, H.; Ajay, S.; Rajeswari Somasundaram, V.K.V.; Kumar, V.; Jain, R.; Shirodkar, J. Convalescent Plasma as a plausible therapeutic option in nCOVID-19—A Review. J. Clin. Trials 2020, 10, 409. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. The convalescent sera option for containing COVID-19. J. Clin. Investig. 2020, 130, 1545–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Wong, R.S.-M.; Soo, Y.O.Y.; Wong, W.S.; Lee, C.K.; Ng, M.H.L.; Chan, P.; Wong, K.C.; Leung, C.B.; Cheng, G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 24, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.-M.; Chiueh, T.-S.; Siu, L.K.; Lin, J.-C.; Chan, P.K.; Peng, M.-Y.; Wan, H.-L.; Chen, J.-H.; Hu, B.-S.; Perng, C.-L.; et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J. Antimicrob. Chemother. 2005, 56, 919–922. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.-H.; Seok, H.; Cho, S.Y.; Ha, Y.E.; Baek, J.Y.; Kim, S.H.; Kim, Y.-J.; Park, J.K.; Chung, C.R.; Kang, E.-S.; et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience. Antivir. Ther. 2018, 23, 617–622. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, R.; Xue, X.; Bao, J.; Ye, S.; Dai, Y.; Zheng, Y.; Fu, Q.; Hu, Z.; Yi, Y. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging 2020, 12, 6536–6542. [Google Scholar] [CrossRef]
- The Efficacy of Intravenous Immunoglobulin Therapy for Severe 2019-nCoV Infected Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04261426 (accessed on 3 August 2020).
- Anti-SARS-CoV-2 Inactivated Convalescent Plasma in the Treatment of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04292340 (accessed on 3 August 2020).
- Treatment of Acute Severe 2019-nCoV Pneumonia with Immunoglobulin from Cured Patients. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04264858 (accessed on 3 August 2020).
- Efficacy and Safety Human Coronavirus Immune Plasma (HCIP) vs. Control (SARS-CoV-2 Non-Immune Plasma) Among Adults Exposed to COVID-19. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04323800 (accessed on 3 August 2020).
- Convalescent Plasma for Patients with COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04332380 (accessed on 3 August 2020).
- Investigating Effect of Convalescent Plasma on COVID-19 Patients Outcome: A Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT04327349 (accessed on 3 August 2020).
- Study Testing Convalescent Plasma vs Best Supportive Care. Available online: https://clinicaltrials.gov/ct2/show/NCT04333251 (accessed on 3 August 2020).
- Safety in Convalescent Plasma Transfusion to COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04333355 (accessed on 3 August 2020).
- Hartung, H.-P.; Mouthon, L.; Ahmed, R.; Jordan, S.; Laupland, K.B.; Jolles, S. Clinical applications of intravenous immunoglobulins (IVIg)—Beyond immunodeficiencies and neurology. Clin. Exp. Immunol. 2009, 158, 23–33. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Kaveri, S. Immunomodulation of Autoimmune and Inflammatory Diseases with Intravenous Immune Globulin. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef]
- Ferrara, G.; Zumla, A.; Maeurer, M. Intravenous Immunoglobulin (IVIg) for Refractory and Difficult-to-treat Infections. Am. J. Med. 2012, 125, 1036.e1–1036.e8. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic Review of Treatment Effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, S.; Balkhy, H.; Gabere, M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): A review. J. Infect. Public Health 2018, 11, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.P.; Song, J.Y.; Bin Seo, Y.; Choi, J.-P.; Shin, H.-S.; Team, R.R. Antiviral Treatment Guidelines for Middle East Respiratory Syndrome. Infect. Chemother. 2015, 47, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, C.; He, P.; Huang, S.; Duan, Y.; Wang, X.; Lin, K.; Zhou, C.; Zhang, X.; Zha, Y. Successful treatment of plasma exchange followed by intravenous immunogloblin in a critically ill patient with 2019 novel coronavirus infection. Int. J. Antimicrob. Agents 2020, 105974. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Feng, Y.; Zhong, L.; Xie, Q.; Lei, M.; Liu, Z.; Wang, C.; Ji, J.; Li, W.; Liu, H.; et al. Clinical Efficacy of Intravenous Immunoglobulin Therapy in Critical Patients with COVID-19: A Multicenter Retrospective Cohort Study. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, S.; Dong, H.; Li, Q.; Chen, E.; Zhang, W.; Yang, L.; Fu, S.; Wang, R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar] [CrossRef]
- Fu, B.; Xu, X.; Wei, H. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020, 18, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.-X.; Chen, X.-P. Favipiravir: Pharmacokinetics and Concerns about Clinical Trials for 2019-nCoV Infection. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef] [Green Version]
- Choy, E.; Freemantle, N.; Proudfoot, C.; Chen, C.-I.; Pollissard, L.; Kuznik, A.; Van Hoogstraten, H.; Mangan, E.; Carita, P.; Huynh, T.-M.-T. Evaluation of the efficacy and safety of sarilumab combination therapy in patients with rheumatoid arthritis with inadequate response to conventional disease-modifying antirheumatic drugs or tumour necrosis factor α inhibitors: Systematic literature review and network meta-analyses. RMD Open 2019, 5, e000798. [Google Scholar] [CrossRef]
- Berkhout, L.C.; L’Ami, M.J.; Ruwaard, J.; Hart, M.H.; Heer, P.O.-D.; Bloem, K.; Nurmohamed, M.T.; Van Vollenhoven, R.F.; Boers, M.; Alvarez, D.F.; et al. Dynamics of circulating TNF during adalimumab treatment using a drug-tolerant TNF assay. Sci. Transl. Med. 2019, 11, eaat3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, A. Baricitinib: First Global Approval. Drugs 2017, 184, 5298–5704. [Google Scholar] [CrossRef] [PubMed]
- Praveen, D.; Chowdary, P.R.; Aanandhi, M. Baricitinib-a januase kinase inhibitor-not an ideal option for management of covid 19. Int. J. Antimicrobial Agents 2020. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Wang, C.; Liu, Y.; Yang, Q.; Mei, Q.; Dong, L.; Li, X.; Liu, J.; Ku, W.; Zhang, Y.; et al. Addition of Low-Dose Decitabine to Anti–PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma. J. Clin. Oncol. 2019, 37, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection. Drugs 2020, 80, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.; Velcheti, V.; Mukhopadhyay, S.; Stoller, J.K. Focal lung infiltrate complicating PD-1 inhibitor use: A new pattern of drug-associated lung toxicity? Respirat. Med. Rep. 2016, 19, 118–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, T.; Lin, W.; Zheng, W.; Chen, J.; Huang, F.; Xie, X. Functional tumor specific CD8 + T cells in spleen express a high level of PD-1. Int. Immunopharmacol. 2020, 80, 106242. [Google Scholar] [CrossRef]
- Wang, C.; Rademaker, M.; Baker, C.; Foley, P. COVID-19 and the use of immunomodulatory and biologic agents for severe cutaneous disease: An Australia/New Zealand consensus statement. Austral. J. Dermatol. 2020. [Google Scholar] [CrossRef] [Green Version]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Della-Torre, E.; Angelillo, P.; Tomelleri, A.; Boffini, N.; Tentori, S.; Mette, F.; Farina, N.; et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: A single-centre, prospective cohort study. Lancet Rheumatol. 2020. [Google Scholar] [CrossRef]
- Jiang, S.; Hillyer, C.; Du, L.J.T. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.; Van Haperen, R.; Osterhaus, A.D.M.E.; Van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Sweiti, H.; Ekwunife, O.; Jaschinski, T.; Lhachimi, S.K. Repurposed Therapeutic Agents Targeting the Ebola Virus: A Systematic Review. Curr. Ther. Res. 2017, 84, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gielen, V.; Johnston, S.L.; Edwards, M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010, 36, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zu, S.; Deng, Y.-Q.; Li, D.; Parvatiyar, K.; Quanquin, N.; Shang, J.; Sun, N.; Su, J.; Liu, Z.; et al. Azithromycin Protects against Zika Virus Infection by Upregulating Virus-Induced Type I and III Interferon Responses. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Tran, D.H.; Sugamata, R.; Hirose, T.; Suzuki, S.; Noguchi, Y.; Sugawara, A.; Ito, F.; Yamamoto, T.; Kawachi, S.; Akagawa, K.S.; et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J. Antibiot. 2019, 72, 759–768. [Google Scholar] [CrossRef]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2009, 148, 245–254. [Google Scholar] [CrossRef]
- Stern, A.; Skalsky, K.; Avni, T.; Carrara, E.; Leibovici, L.; Paul, M. Corticosteroids for pneumonia. Cochrane Database Syst. Rev. 2017, 2017, CD007720. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, C.; Leonardi-Bee, J.; Nguyen-Van-Tam, J.; Lim, W.S. Effect of Corticosteroid Therapy on Influenza-Related Mortality: A Systematic Review and Meta-analysis. J. Infect. Dis. 2014, 212, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, Y.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Zhou, Y.; Zhao, X.; Zhao, Q.; Liu, J. The effect of corticosteroid treatment on patients with coronavirus infection: A systematic review and meta-analysis. J. Infect. 2020, 81, e13–e20. [Google Scholar] [CrossRef]
- Zha, L.; Li, S.; Pan, L.; Tefsen, B.; Li, Y.; French, N.; Chen, L.; Yang, G.; Villanueva, E.V. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID -19). Med. J. Aust. 2020, 212, 416–420. [Google Scholar] [CrossRef]
- Fang, X.; Mei, Q.; Yang, T.; Li, L.; Wang, Y.; Tong, F.; Geng, S.; Pan, A. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J. Infect. 2020. [Google Scholar] [CrossRef]
- World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. Pediatr. Med. Rodz. 2020, 16, 9–26. [CrossRef]
- Ledford, H. Coronavirus breakthrough: Dexamethasone is first drug shown to save lives. Nature 2020, 582, 469. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv 2020. [Google Scholar] [CrossRef]
- Gewaltig, M.T.; Kojda, G. Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc. Res. 2002, 55, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Åkerstroöm, S.; Mousavi-Jazi, M.; Klingstrom, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellinger, R.P.; Trzeciak, S.; Criner, G.; Zimmerman, J.L.; Taylor, R.W.; Usansky, H.; Young, J.; Goldstein, B. Association between inhaled nitric oxide treatment and long-term pulmonary function in survivors of acute respiratory distress syndrome. Crit. Care 2012, 16, R36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.-J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, P.; Gao, H.; Sun, B.; Chao, D.; Wang, F.; Zhu, Y.; Hedenstierna, G.; Wang, C.G. Inhalation of Nitric Oxide in the Treatment of Severe Acute Respiratory Syndrome: A Rescue Trial in Beijing. Clin. Infect. Dis. 2004, 39, 1531–1535. [Google Scholar] [CrossRef] [Green Version]
- Kakodkar, P.; Kaka, N.; Baig, M. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19). Cureus 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020. [Google Scholar] [CrossRef]
- Uno, Y. Camostat mesilate therapy for COVID-19. Intern. Emerg. Med. 2020, 2020, 1–2. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Yang, N.; Ma, Y.; Zhou, Q.; Li, W.; Wang, X.; Huang, L.; Luo, X.; Fukuoka, T.; et al. Effectiveness of intravenous immunoglobulin for children with severe COVID-19: A rapid review. Ann. Transl. Med. 2020, 8, 625. [Google Scholar] [CrossRef]
- Bray, M.; Rayner, C.; Noël, F.; Jans, D.; Wagstaff, K. Ivermectin and COVID-19: A report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses. Antivir. Res. 2020, 178, 104805. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines, Corticosteroids (Including Dexamethasone) [Internet]. 17 July 2020. Available online: https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/<monospace></monospace> (accessed on 30 July 2020).

| Name of Medicine | Target | Classification | Effective Dosage | Registered Clinical Trial | Reference |
|---|---|---|---|---|---|
| Remdesivir | Viral RNA polymerase | Anti-viral | IV injection 200 mg at day first, 100 mg for 9 days | NCT04257656, NCT04252664, NCT04292730, NCT04315948, NCT04321616 | [237] |
| Lopinavir/Ritonavir | Viral protease | Anti-viral | Oral administration 400 mg lopinavir and 100 mg ritonavir twice a day for 14 days, peroral | ACTRN12620000445976, NCT02735707, ISRCTN83971151, NCT04321174, NCT04350684, ISRCTN50189673, NCT04315948, NCT04328012, NCT04276688, ISRCTN50189673, NCT04321993 | [114] |
| Favipiravir | Viral RdRP | Anti-viral | Oral administration 1600 mg twice daily on first day and 600 mg twice a day on day 2−14. | 2020-001435-27, NCT04359615, NCT04303299, NCT04402203 | [120] |
| Ribavirin | viral RNA | Anti-viral | 500 mg each time, 2 to 3 times/day in combination with IFN-α or lopinavir/ritonavir | NCT04276688, NCT04392427, ChiCTR2000029387 | [238] |
| Arbidol | Viral RNA polymerase | Anti-viral | 200 mg three times a day for a duration of 7–14 days | NCT04260594, NCT04350684, NCT04255017 | [239] |
| Camostat | TMPRSS2 | Anti-viral | 600 mg (200 mg, three times) | NCT04353284 | [240] |
| Nafamostat | TMPRSS2 | Anti-viral | 240 mg daily, for 5 days, peroral | NCT04418128 | [138] |
| Chloroquine Phosphate | ACE2 | Anti-parasite | 500 mg (300 mg for chloroquine) each time, 2 times/day | NCT04303507, NCT04324463, NCT04353336, NCT04328493 | [238] |
| Hydroxychloroquine | Endosome, pH elevation | Anti-parasite | 200 mg, three times per day for ten days | NCT04261517, NCT04308668, NCT02735707, ISRCTN83971151, NCT04315948, NCT04321616, NCT04350684 | [158,239] |
| IVIG | immune modulation | Immunoglobulin | 400 mg/kg for a duration of five days in children | NCT04411667, NCT04261426 | [241] |
| Ivermectin | Inhibition of nuclear transport | Anti-parasite | Oral administration 600 μg/kg) daily for 3 days | NCT04343092, NCT04392427 | [242] |
| Tocilizumab | IL-6 receptor subunit alpha | Monoclonal antibody | 400 mg intravenous or 8 mg/kg × 1–2 doses. Second dose 8–12 h after first dose if inadequate response. | NCT04335071, ChiCTR2000030894 | [239] |
| Azitromycin | 23S rRNA | Anti-microbial | 500 mg on day 1 followed by 250 mg/day for the next four days (in combination with hydroxychloroquine | NCT04359316, NCT04332107, NCT04336332, NCT04329832 | [158] |
| Corticosteroides | Binds glucocorticoid receptor and suppress inflammation | Anti-Inflammation | 40 mg methylprednisolone once or twice daily | NCT04273321 | [226] |
| Dexamethasone | Binds glucocorticoid receptor and suppress inflammation | Anti-Inflammation | 6 milligrams/day for 10 days | ISRCTN50189673, NCT04381936 | [243] |
| Nitricoxide | Activates cGMP | vasodilator | For SARS patients; Inhalation for ≥3 days (30 ppm on day 1, 20 and 10 ppm on days 2 & 3) | NCT04383002, NCT04338828, NCT04358588, NCT04305457 | [236] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhand, S.; Saghaeian Jazi, M.; Mohammadi, S.; Tarighati Rasekhi, R.; Rostamian, G.; Kalani, M.R.; Rostamian, A.; George, J.; Douglas, M.W. COVID-19: The Immune Responses and Clinical Therapy Candidates. Int. J. Mol. Sci. 2020, 21, 5559. https://doi.org/10.3390/ijms21155559
Zhand S, Saghaeian Jazi M, Mohammadi S, Tarighati Rasekhi R, Rostamian G, Kalani MR, Rostamian A, George J, Douglas MW. COVID-19: The Immune Responses and Clinical Therapy Candidates. International Journal of Molecular Sciences. 2020; 21(15):5559. https://doi.org/10.3390/ijms21155559
Chicago/Turabian StyleZhand, Sareh, Marie Saghaeian Jazi, Saeed Mohammadi, Roozbeh Tarighati Rasekhi, Ghassem Rostamian, Mohammad Reza Kalani, Aida Rostamian, Jacob George, and Mark W Douglas. 2020. "COVID-19: The Immune Responses and Clinical Therapy Candidates" International Journal of Molecular Sciences 21, no. 15: 5559. https://doi.org/10.3390/ijms21155559
APA StyleZhand, S., Saghaeian Jazi, M., Mohammadi, S., Tarighati Rasekhi, R., Rostamian, G., Kalani, M. R., Rostamian, A., George, J., & Douglas, M. W. (2020). COVID-19: The Immune Responses and Clinical Therapy Candidates. International Journal of Molecular Sciences, 21(15), 5559. https://doi.org/10.3390/ijms21155559







