Mapping Quantitative Trait Loci for 1000-Grain Weight in a Double Haploid Population of Common Wheat
Abstract
:1. Introduction
2. Results
2.1. Genetic Map Construction
2.2. Phenotypic Statistical Analysis
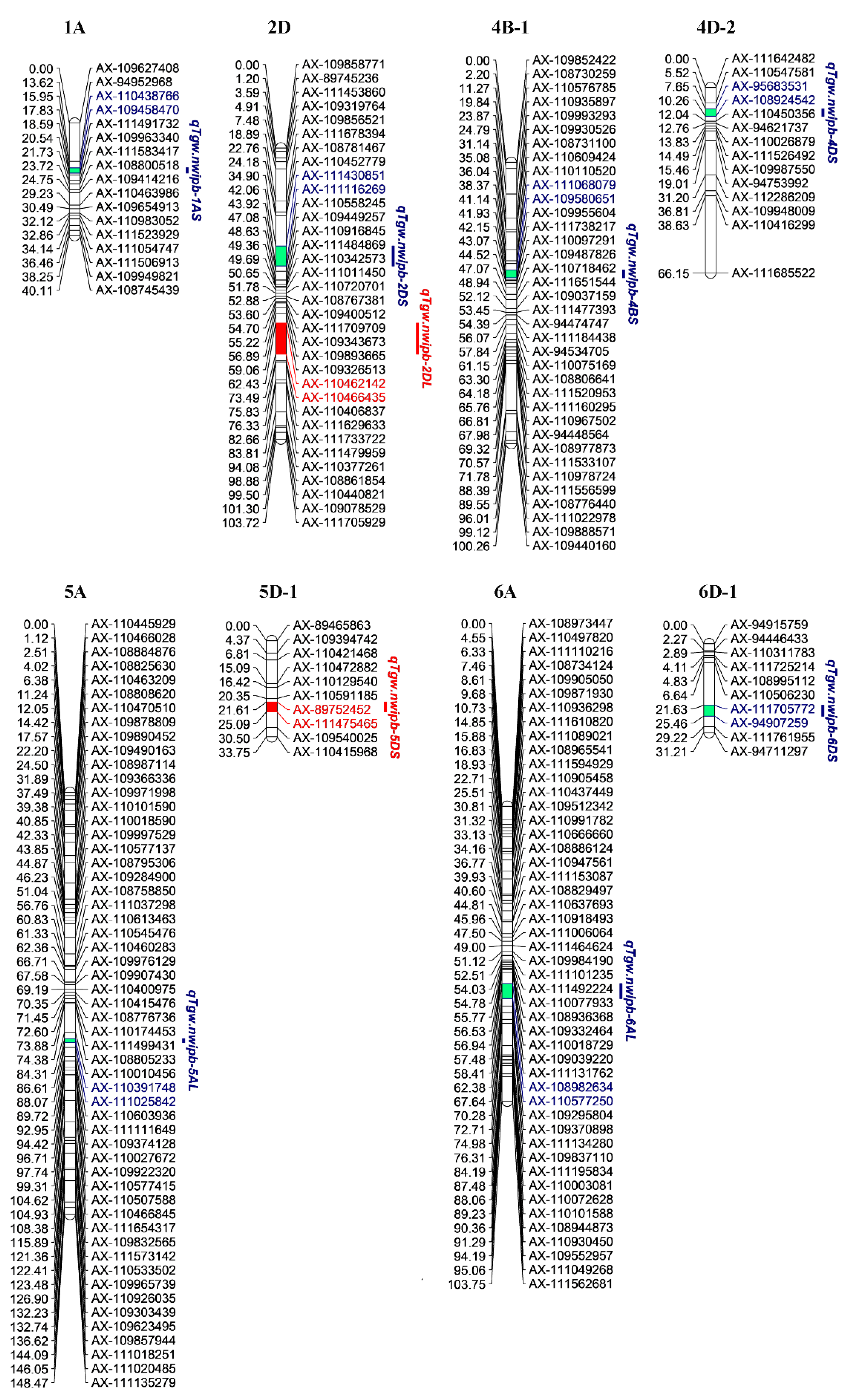
2.3. QTL Analysis for TGW
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Molecular Genotyping and Genetic Linkage Map Construction
4.3. Statistical Analysis and QTL Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TGW | Thousand-grain weight |
| QTL | Quantitative trait locus |
| DH population | Double haploid population |
| SNP | Single-nucleotide polymorphisms |
References
- United Nations, Department of Economic Population Division. Population Division, World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; ESA/P/WP/248; United Nations: Rome, Italy, 2017. [Google Scholar]
- Brinton, J.; Simmonds, J.; Minter, F.; Leverington-Waite, M.; Snape, J.; Uauy, C. Increased pericarp cell length underlies a major quantitative trait locus for grain weight in hexaploid wheat. New Phytol. 2017, 215, 1026–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengistu, N.; Baenziger, P.S.; Eskridge, K.M.; Dweikat, I.; Wegulo, S.N.; Gill, K.S.; Mujeeb-Kazi, A. Validation of QTL for Grain Yield-Related Traits on Wheat Chromosome 3A Using Recombinant Inbred Chromosome Lines. Crop Sci. 2012, 52, 1622–1632. [Google Scholar] [CrossRef]
- Kuchel, H.; Williams, K.J.; Langridge, P.; Eagles, H.A.; Jefferies, S.P. Genetic dissection of grain yield in bread wheat. I. QTL analysis. Theor. Appl. Genet. 2007, 115, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kulwal, P.L.; Gaur, A.; Tyagi, A.K.; Khurana, J.P.; Khurana, P.; Balyan, H.S.; Gupta, P.K. QTL analysis for grain weight in common wheat. Euphytica 2006, 151, 135–144. [Google Scholar] [CrossRef]
- Varshney, R.K.; Prasad, M.; Roy, J.K.; Harjit-Singh, N.K.; Dhaliwal, H.S.; Balyan, H.S.; Gupta, P.K. Identification of eight chromosomes and a microsatellite marker on 1AS associated with QTL for grain weight in bread wheat. Theor. Appl. Genet. 2000, 1000, 1290–1294. [Google Scholar] [CrossRef]
- Narasimhamoorthy, B.; Gill, B.S.; Fritz, A.K.; Nelson, J.C.; Brown-Guedira, G.L. Advanced backcross QTL analysis of a hard winter wheat x synthetic wheat population. Theor. Appl. Genet. 2006, 112, 787–796. [Google Scholar] [CrossRef]
- McCartney, C.A.; Somers, D.J.; Humphreys, D.G.; Lukow, O.; Ames, N.; Noll, J.; Cloutier, S.; McCallum, B.D. Mapping quantitative trait loci controlling agronomic traits in the spring wheat cross RL4452x ‘AC Domain’. Genome 2005, 48, 870–883. [Google Scholar] [CrossRef]
- Quarrie, S.A.; Steed, A.; Calestani, C.; Semikhodskii, A.; Lebreton, C.; Chinoy, C.; Steele, N.; Pljevljakusic, D.; Waterman, E.; Weyen, J.; et al. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring x SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor. Appl. Genet. 2005, 110, 865–880. [Google Scholar] [CrossRef]
- Campbell, K.G.; Bergman, C.J.; Gualberto, D.G.; Anderson, J.A.; Giroux, M.J.; Hareland, G.; Fulcher, R.G.; Sorrells, M.E.; Finney, P.L. Quantitative Trait Loci Associated with Kernel Traits in a Soft Hard Wheat Cross. Crop Sci. 1999, 39, 1184–1195. [Google Scholar] [CrossRef]
- Huang, X.Q.; Coster, H.; Ganal, M.W.; Roder, M.S. Advanced backcross QTL analysis for the identification of quantitative trait loci alleles from wild relatives of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2003, 106, 1379–1389. [Google Scholar] [CrossRef]
- Ramya, P.; Chaubal, A.; Kulkarni, K.; Gupta, L.; Kadoo, N.; Dhaliwal, H.S.; Chhuneja, P.; Lagu, M.; Gupt, V. QTL mapping of 1000-kernel weight, kernel length, and kernel width in bread wheat (Triticum aestivum L.). J. Appl. Genet. 2010, 51, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jia, J.; Wei, X. y.; Zhang, X.; Li, L.; Chen, H.; Fan, Y.; Sun, H.; Zhao, X.; Lei, T.; et al. A intervarietal genetic map and QTL analysis for yield traits in wheat. Mol. Breed. 2007, 20, 167–178. [Google Scholar] [CrossRef]
- Marza, F.; Bai, G.H.; Carver, B.F.; Zhou, W.C. Quantitative trait loci for yield and related traits in the wheat population Ning7840 x Clark. Theor. Appl. Genet. 2006, 112, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jia, L.; Lu, L.; Qin, D.; Zhang, J.; Guan, P.; Ni, Z.; Yao, Y.; Sun, Q.; Peng, H. Mapping QTLs of yield-related traits using RIL population derived from common wheat and Tibetan semi-wild wheat. Theor. Appl. Genet. 2014, 127, 2415–2432. [Google Scholar] [CrossRef]
- Roder, M.S.; Huang, X.Q.; Borner, A. Fine mapping of the region on wheat chromosome 7D controlling grain weight. Funct. Integr. Genom. 2008, 8, 79–86. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Wu, K.; Zhao, Y.; Kong, F.-M.; Han, G.-Z.; Jiang, H.-M.; Huang, X.-J.; Li, R.-J.; Wang, H.-G.; Li, S.-S. QTL analysis of kernel shape and weight using recombinant inbred lines in wheat. Euphytica 2009, 165, 165:615. [Google Scholar] [CrossRef]
- Simmonds, J.; Scott, P.; Leverington-Waite, M.; Turner, A.S.; Brinton, J.; Korzun, V.; Snape, J.; Uauy, C. Identification and independent validation of a stable yield and thousand grain weight QTL on chromosome 6A of hexaploid wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 191. [Google Scholar] [CrossRef] [Green Version]
- Zanke, C.D.; Ling, J.; Plieske, J.; Kollers, S.; Ebmeyer, E.; Korzun, V.; Argillier, O.; Stiewe, G.; Hinze, M.; Neumann, F.; et al. Analysis of main effect QTL for thousand grain weight in European winter wheat (Triticum aestivum L.) by genome-wide association mapping. Front. Plant Sci. 2015, 6, 644. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Luo, W.; Qin, N.; Ding, P.; Zhang, H.; Yang, C.; Mu, Y.; Tang, H.; Liu, Y.; Li, W.; et al. A 55 K SNP array-based genetic map and its utilization in QTL mapping for productive tiller number in common wheat. Theor. Appl. Genet. 2018, 131, 2439–2450. [Google Scholar] [CrossRef]
- Su, Z.; Hao, C.; Wang, L.; Dong, Y.; Zhang, X. Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 122, 211–223. [Google Scholar] [CrossRef]
- Sajjad, M.; Ma, X.; Habibullah Khan, S.; Shoaib, M.; Song, Y.; Yang, W.; Zhang, A.; Liu, D. TaFlo2-A1, an ortholog of rice Flo2, is associated with thousand grain weight in bread wheat (Triticum aestivum L.). BMC Plant Biol. 2017, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhang, N.; Fan, X.L.; Zhang, W.; Zhao, C.H.; Yang, L.J.; Pan, R.Q.; Chen, M.; Han, J.; Zhao, X.Q.; et al. Utilization of a Wheat660K SNP array-derived high-density genetic map for high-resolution mapping of a major QTL for kernel number. Sci. Rep. 2017, 7, 3788. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.J.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Seeb, J.E.; Carvalho, G.; Hauser, L.; Naish, K.; Roberts, S.; Seeb, L.W. Single-nucleotide polymorphism (SNP) discovery and applications of SNP genotyping in nonmodel organisms. Mol. Ecol. Resour. 2011, 11 (Suppl. 1), 1–8. [Google Scholar] [CrossRef]
- Huang, X.; Wei, X.; Sang, T.; Zhao, Q.; Feng, Q.; Zhao, Y.; Li, C.; Zhu, C.; Lu, T.; Zhang, Z.; et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010, 42, 961–967. [Google Scholar] [CrossRef]
- Palaisa, K.A.; Morgante, M.; Williams, M.; Rafalski, A. Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. Plant Cell 2003, 15, 1795–1806. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A.; et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Winfield, M.O.; Allen, A.M.; Burridge, A.J.; Barker, G.L.; Benbow, H.R.; Wilkinson, P.A.; Coghill, J.; Waterfall, C.; Davassi, A.; Scopes, G.; et al. High-density SNP genotyping array for hexaploid wheat and its secondary and tertiary gene pool. Plant Biotechnol. J. 2016, 14, 1195–1206. [Google Scholar] [CrossRef]
- Ren, T.; Hu, Y.; Tang, Y.; Li, C.; Yan, B.; Ren, Z.; Tan, F.; Tang, Z.; Fu, S.; Li, Z. Utilization of a Wheat55K SNP Array for Mapping of Major QTL for Temporal Expression of the Tiller Number. Front. Plant Sci. 2018, 9, 333. [Google Scholar] [CrossRef]
- Li, F.; Wen, W.; He, Z.; Liu, J.; Jin, H.; Cao, S.; Geng, H.; Yan, J.; Zhang, P.; Wan, Y.; et al. Genome-wide linkage mapping of yield-related traits in three Chinese bread wheat populations using high-density SNP markers. Theor. Appl. Genet. 2018, 131, 1903–1924. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Sayed-Tabatabaei, B.E.; Saeidi, G.; Kearsey, M.; Suenaga, K. Mapping QTL for grain yield, yield components, and spike features in a doubled haploid population of bread wheat. Genome 2011, 54, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Leiser, W.L.; Reif, J.C.; Tucker, M.R.; Losert, D.; Weissmann, S.; Hahn, V.; Maurer, H.P.; Würschum, T. Multiple-line cross QTL mapping for grain yield and thousand kernel weight in triticale. Plant Breed. 2016. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, J.-U.; Kang, C.-S.; Heo, H.-Y.; Park, C.S. Mapping of QTL for yield and its related traits in a doubled haploid population of Korean wheat. Plant Biotechnol. Rep. 2014, 8, 443–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. Cytological Studies on Interspecific Hybrids of Different Ploidy Wheat. J. Henan Agric. Sci. 2011, 40, 14–17. [Google Scholar]
- Randhawa, H.; Puchalski, B.J.; Frick, M.; Goyal, A.; Despins, T.; Graf, R.J.; Laroche, A.; Gaudet, D.A. Stripe rust resistance among western Canadian spring wheat and triticale varieties. Can. J. Plant Sci. 2012, 92, 713–722. [Google Scholar] [CrossRef] [Green Version]
- DePauw, R.M.; Knox, R.E.; McCaig, T.N.; Clarke, F.R.; Clarke, J.M. Carberry hard red spring wheat. Can. J. Plant Sci. 2011, 91, 529–534. [Google Scholar] [CrossRef]
- DePauw, R.M.; Townley-Smith, T.F.; Humphreys, G.; Knox, R.E.; Clarke, F.R.; Clarke, J.M. Lillian hard red spring wheat. Can. J. Plant Sci. 2005, 85, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Oh, E.U.; Lee, M.S.; Kim, H.B.; Moon, D.-G.; Song, K.J. Construction of a genetic linkage map based on RAPD, AFLP, and SSR markers for tea plant (Camellia sinensis). Euphytica 2017, 213, 190. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.S.; Ren, T.H.; Li, Z.; Tang, Y.Z.; Ren, Z.L.; Yan, B.J. Molecular mapping and genetic analysis of a QTL controlling spike formation rate and tiller number in wheat. Gene 2017, 634, 15–21. [Google Scholar] [CrossRef] [PubMed]
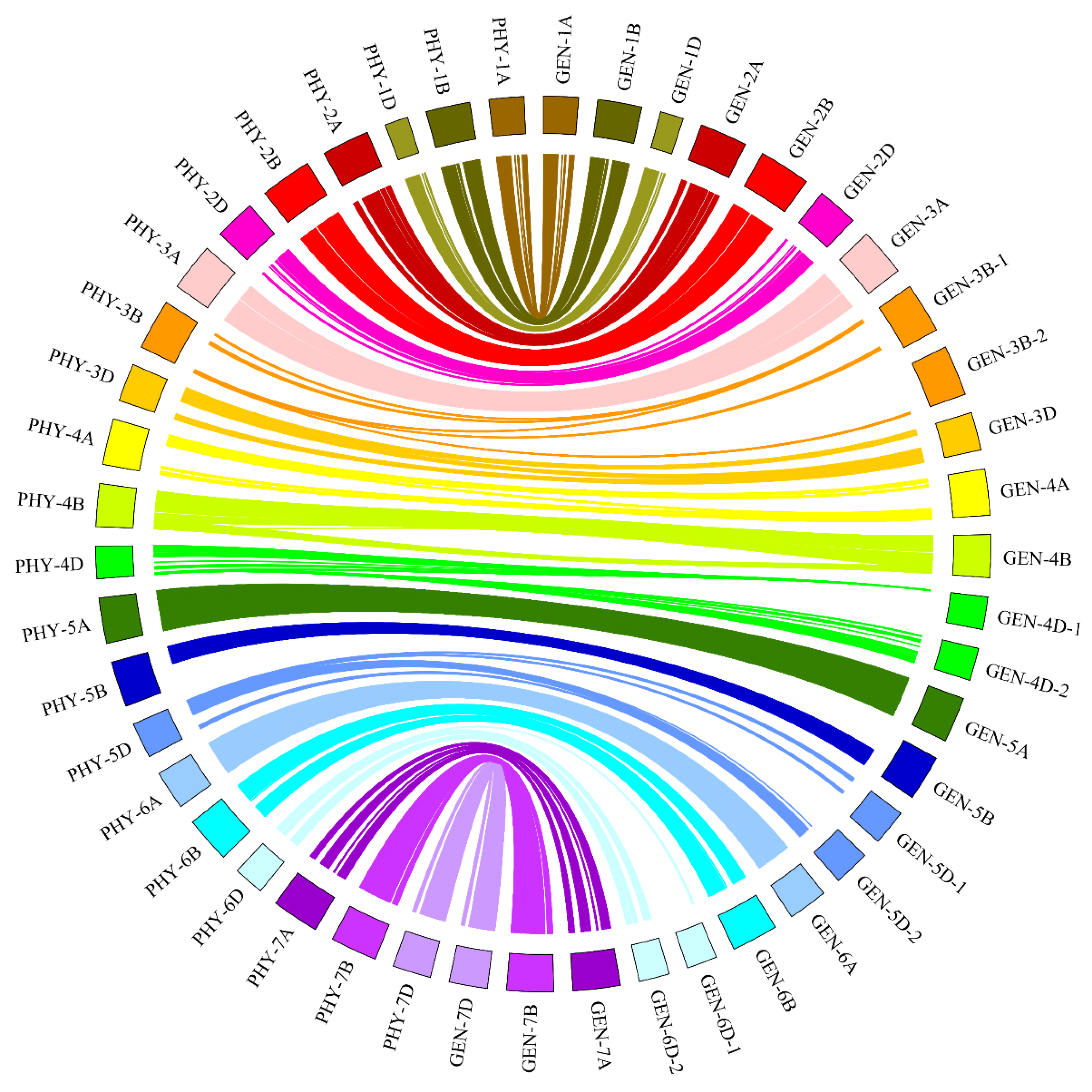
| Chr. | Group | Length (cM) | SNP Markers | cM per | Number of | cM per |
|---|---|---|---|---|---|---|
| SNP Marker | Bin Markers | Bin Marker | ||||
| 1A | 1 | 40.11 | 535 | 0.07 | 17 | 2.36 |
| 1B | 1 | 95.80 | 726 | 0.13 | 31 | 3.09 |
| 1D | 1 | 60.99 | 527 | 0.12 | 16 | 3.81 |
| 2A | 1 | 86.71 | 1613 | 0.05 | 32 | 2.71 |
| 2B | 1 | 146.21 | 1116 | 0.13 | 52 | 2.81 |
| 2D | 1 | 103.72 | 194 | 0.53 | 34 | 3.05 |
| 3A | 1 | 158.98 | 897 | 0.18 | 53 | 3.00 |
| 3B | 1 | 67.69 | 146 | 0.46 | 33 | 2.05 |
| 2 | 13.11 | 31 | 0.42 | 10 | 1.31 | |
| 3D | 1 | 112.02 | 93 | 1.20 | 27 | 4.15 |
| 4A | 1 | 110.26 | 650 | 0.17 | 41 | 2.69 |
| 4B | 1 | 100.26 | 1496 | 0.07 | 36 | 2.79 |
| 4D | 1 | 21.17 | 56 | 0.38 | 10 | 2.12 |
| 2 | 66.15 | 48 | 1.38 | 14 | 4.72 | |
| 5A | 1 | 148.47 | 896 | 0.17 | 55 | 2.70 |
| 5B | 1 | 92.68 | 549 | 0.17 | 44 | 2.11 |
| 5D | 1 | 33.75 | 26 | 1.30 | 10 | 3.38 |
| 2 | 90.25 | 75 | 1.20 | 33 | 2.73 | |
| 6A | 1 | 103.75 | 1678 | 0.06 | 48 | 2.16 |
| 6B | 1 | 119.78 | 955 | 0.13 | 42 | 2.85 |
| 6D | 1 | 31.21 | 14 | 2.23 | 10 | 3.12 |
| 2 | 67.26 | 89 | 0.76 | 18 | 3.74 | |
| 7A | 1 | 143.21 | 607 | 0.24 | 43 | 3.33 |
| 7B | 1 | 112.93 | 1355 | 0.08 | 32 | 3.53 |
| 7D | 1 | 83.17 | 629 | 0.13 | 32 | 2.60 |
| Total | 25 | 2209.64 | 15,001 | 0.15 | 773 | 2.86 |
| Environment | Parents | DH Lines | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | Range | Min. | Max. | Mean | SD | CV (%) | Sk. | Ku. | h2 | |
| HX2015 | 48.23 | 39.18 | 19.72 | 32.20 | 51.95 | 40.59 | 4.46 | 19.93 | 0.056 | −0.631 | 0.59 |
| HD2016 | 48.39 | 39.90 | 21.54 | 30.90 | 52.44 | 43.71 | 4.33 | 18.78 | −0.178 | −0.175 | |
| XN2016 | 46.64 | 38.46 | 20.37 | 30.11 | 50.48 | 41.54 | 4.18 | 17.45 | −0.461 | −0.026 | |
| HD2017 | 48.30 | 40.07 | 20.88 | 30.00 | 50.88 | 42.04 | 4.17 | 17.35 | −0.150 | −0.112 | |
| XN2017 | 44.78 | 36.49 | 20.02 | 31.08 | 51.10 | 41.09 | 5.04 | 25.44 | −0.070 | −0.785 | |
| HX2017 | 48.08 | 38.46 | 18.78 | 31.70 | 50.48 | 41.85 | 4.28 | 18.28 | −0.345 | −0.466 | |
| QTL | Pos.(cM) | Environment | Chr. | Interval (cM) | Flanking markers | LOD | Add | PVE (%) | Physical pos. (Mb) | Near Locus in Previous Studies |
|---|---|---|---|---|---|---|---|---|---|---|
| qTgw.nwipb-1AS | 16.890 | HD2017 | 1A | 15.954–17.833 | AX-110438766--AX-109458470 | 3.68 | −1.204 | 8.09 | 20.02–25.31 | QGw.ccsu-1A.1 [5] |
| qTgw.nwipb-2DS | 40.270 | DLH2015 | 2D | 34.900–42.060 | AX-111430851--AX-111116269 | 4.98 | −1.698 | 14.54 | 243.79–345.97 | |
| qTgw.nwipb-2DL | 70.414 | XN2017 | 2D | 62.425–73.487 | AX-110462142--AX-110466435 | 4.44 | −1.449 | 9.81 | 585.69–601.05 | |
| qTgw.nwipb-4BS | 40.678 | DLH2017 | 4B | 38.368–41.140 | AX-111068079--AX-109580651 | 4.24 | 1.692 | 12.75 | 25.35–29.20 | QTKW.caas-4BS [32] |
| qTgw.nwipb-4DS | 8.956 | DLH2015 | 4D | 7.652–10.261 | AX-95683531--AX-108924542 | 5.16 | 1.744 | 15.31 | 28.44–94.25 | |
| XN2016 | 4D | 7.652–10.261 | AX-95683531--AX-108924542 | 5.12 | 1.789 | 17.17 | ||||
| HD2017 | 4D | 7.652–10.261 | AX-95683531--AX-108924542 | 9.44 | 2.103 | 24.65 | ||||
| XN2017 | 4D | 7.652–10.261 | AX-95683531--AX-108924542 | 12.19 | 2.605 | 32.43 | ||||
| qTgw.nwipb-5AL | 87.339 | XN2017 | 5A | 86.608–88.069 | AX-110391748--AX-111025842 | 3.38 | 1.235 | 6.97 | 594.37–596.03 | QTKW.caas-5AL [32] |
| qTgw.nwipb-5DS | 22.651 | HD2016 | 5D | 21.606–25.089 | AX-89752452--AX-111475465 | 3.59 | 1.415 | 13.16 | 41.82–44.93 | |
| qTgw.nwipb-6AL | 64.353 | DLH2015 | 6A | 62.382–67.638 | AX-108982634--AX-110577250 | 7.18 | −2.113 | 22.43 | 573.48–579.45 | |
| XN2016 | 6A | 62.382–67.638 | AX-108982634--AX-110577250 | 6.91 | −2.139 | 24.44 | ||||
| HD2016 | 6A | 62.382–67.638 | AX-108982634--AX-110577250 | 7.38 | −2.119 | 29.46 | ||||
| DLH2017 | 6A | 62.382–67.638 | AX-108982634--AX-110577250 | 7.61 | −2.404 | 25.97 | ||||
| HD2017 | 6A | 62.382–67.638 | AX-108982634--AX-110577250 | 8.41 | −1.956 | 21.34 | ||||
| qTgw.nwipb-6DS | 23.301 | XN2017 | 6D | 21.626–25.455 | AX-111705772--AX-94907259 | 3.29 | −1.195 | 6.81 | 10.91–24.55 |
| QTL | Chr. | Interval (cM) | LOD | LOD (A) | LOD (AE) | PVE | PVE (A) | PVE (AE) | Add |
|---|---|---|---|---|---|---|---|---|---|
| qTgw.nwipb-1AS | 1A | 15.954–17.833 | 9.06 | 7.97 | 1.09 | 5.02 | 4.62 | 0.40 | −0.792 |
| qTgw.nwipb-2DS | 2D | 34.900–42.060 | 9.11 | 7.01 | 2.11 | 6.06 | 4.15 | 1.90 | −0.753 |
| qTgw.nwipb-4BS | 4B | 38.368–41.140 | 6.69 | 3.66 | 3.04 | 4.82 | 2.14 | 2.68 | 0.545 |
| qTgw.nwipb-4DS | 4D | 7.652–10.261 | 33.73 | 26.70 | 7.03 | 22.24 | 17.66 | 4.57 | 1.551 |
| qTgw.nwipb-5AL | 5A | 86.608–88.069 | 10.92 | 10.20 | 0.71 | 6.06 | 5.86 | 0.20 | 0.914 |
| qTgw.nwipb-6AL | 6A | 62.382–67.638 | 36.99 | 34.29 | 2.70 | 26.96 | 23.55 | 3.41 | −1.795 |
| qTgw.nwipb-6DS | 6D | 21.626–25.455 | 6.67 | 5.67 | 1.00 | 3.81 | 3.28 | 0.53 | −0.669 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wu, L.; Gan, X.; Chen, W.; Liu, B.; Fedak, G.; Cao, W.; Chi, D.; Liu, D.; Zhang, H.; et al. Mapping Quantitative Trait Loci for 1000-Grain Weight in a Double Haploid Population of Common Wheat. Int. J. Mol. Sci. 2020, 21, 3960. https://doi.org/10.3390/ijms21113960
Liu T, Wu L, Gan X, Chen W, Liu B, Fedak G, Cao W, Chi D, Liu D, Zhang H, et al. Mapping Quantitative Trait Loci for 1000-Grain Weight in a Double Haploid Population of Common Wheat. International Journal of Molecular Sciences. 2020; 21(11):3960. https://doi.org/10.3390/ijms21113960
Chicago/Turabian StyleLiu, Tao, Lijun Wu, Xiaolong Gan, Wenjie Chen, Baolong Liu, George Fedak, Wenguang Cao, Dawn Chi, Dengcai Liu, Huaigang Zhang, and et al. 2020. "Mapping Quantitative Trait Loci for 1000-Grain Weight in a Double Haploid Population of Common Wheat" International Journal of Molecular Sciences 21, no. 11: 3960. https://doi.org/10.3390/ijms21113960





