Biosynthesis of Sulfur-Containing Small Biomolecules in Plants
Abstract
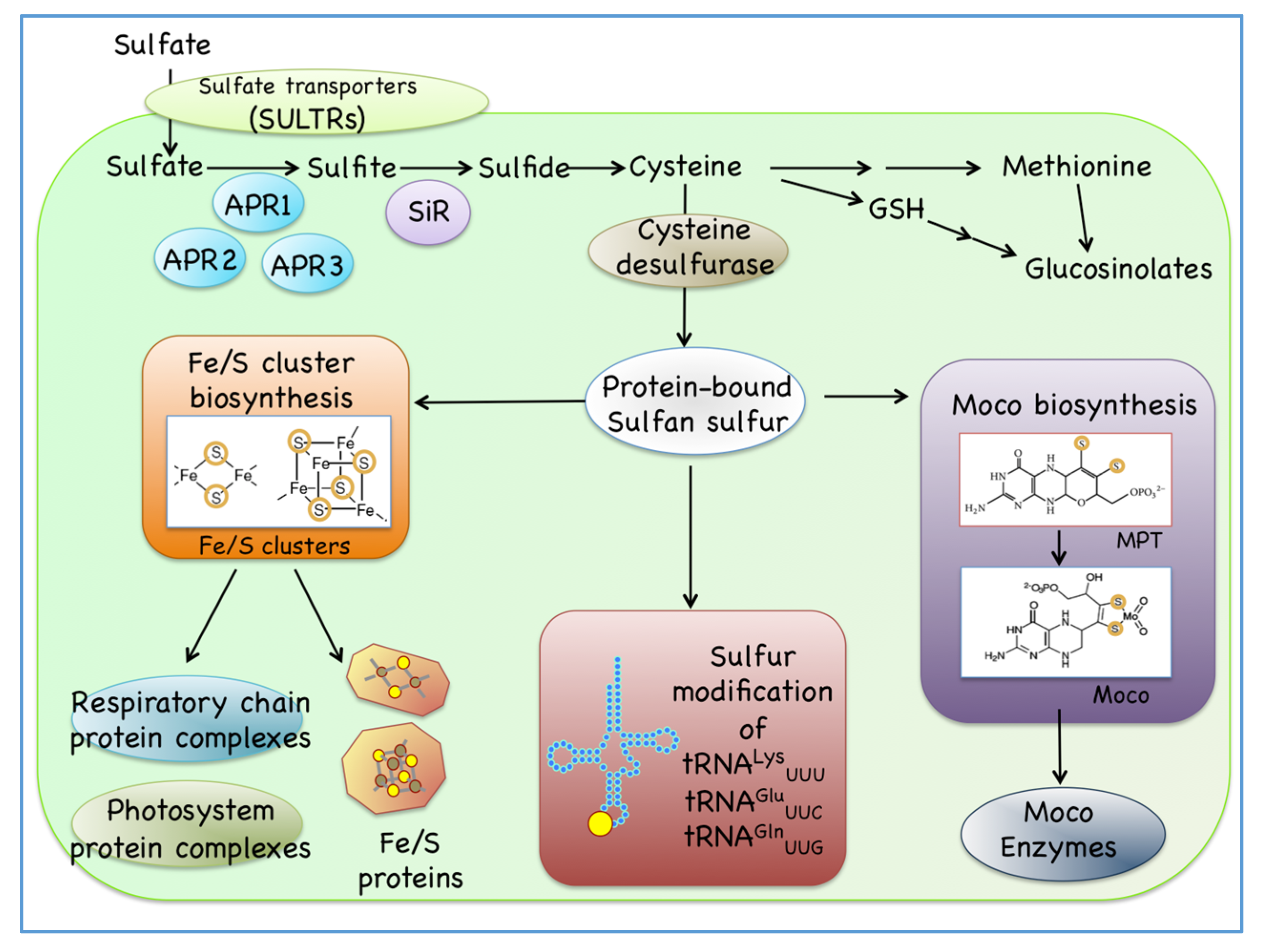
:1. Introduction
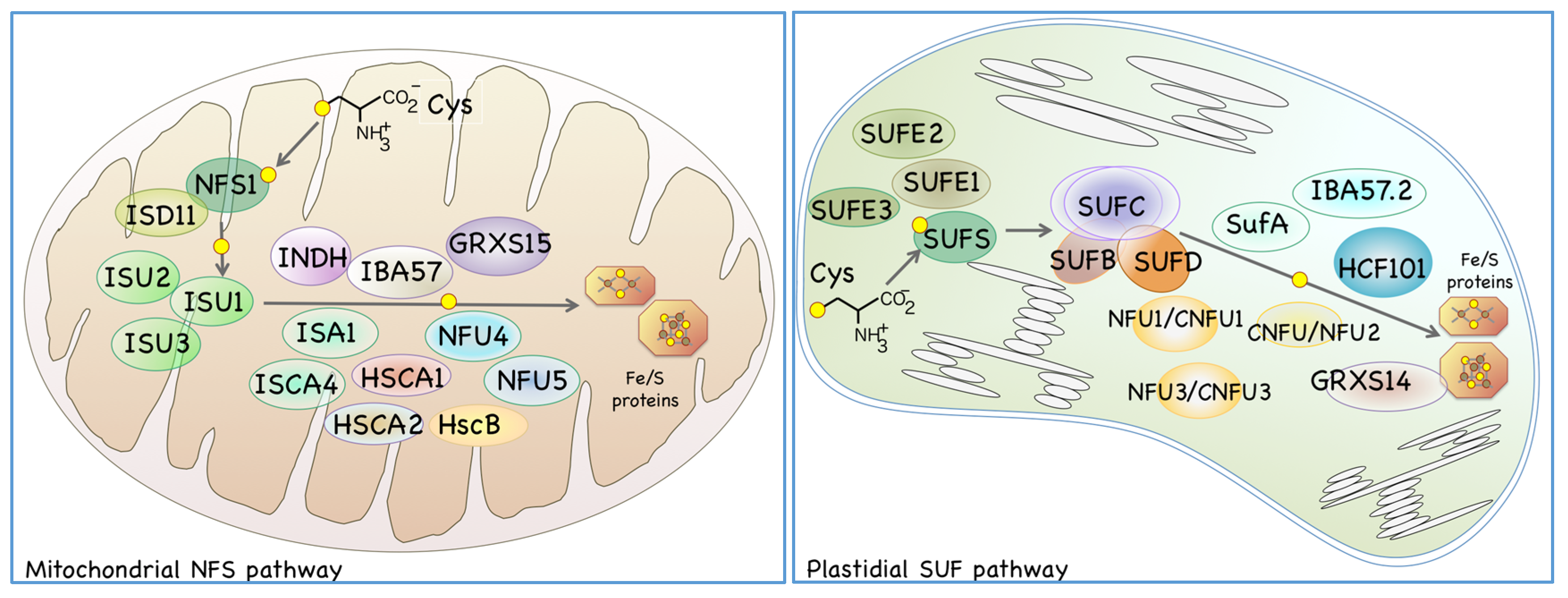
2. Fe/S Cluster Biosynthesis in Plant Organelles
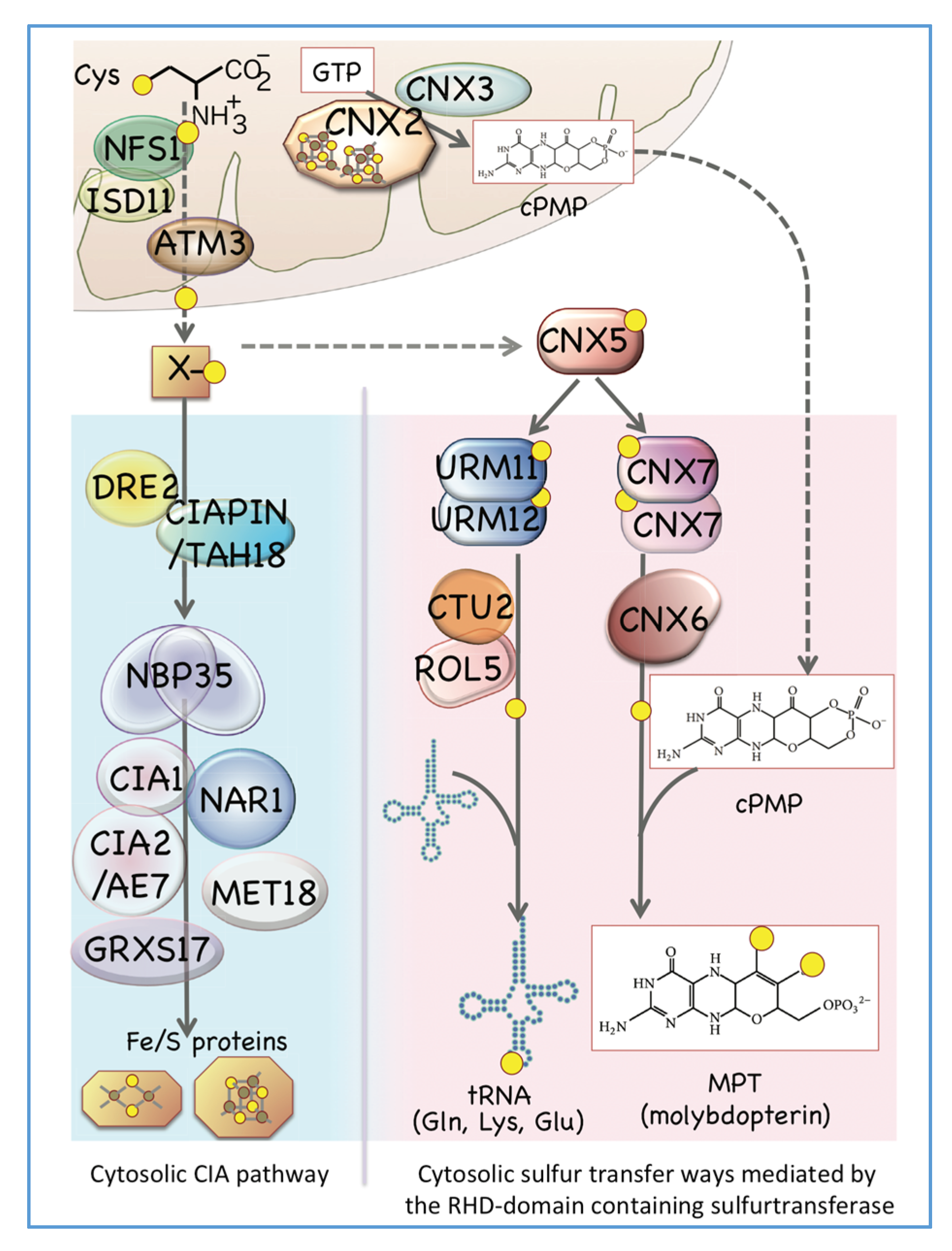
3. Maturation of the Fe/S Cluster in the Cytosol and Related Proteins in Both Mitochondria and the Cytosol
4. Biosynthesis of Cytosolic Sulfur-Containing Small Biomolecules Other Than Fe/S Clusters
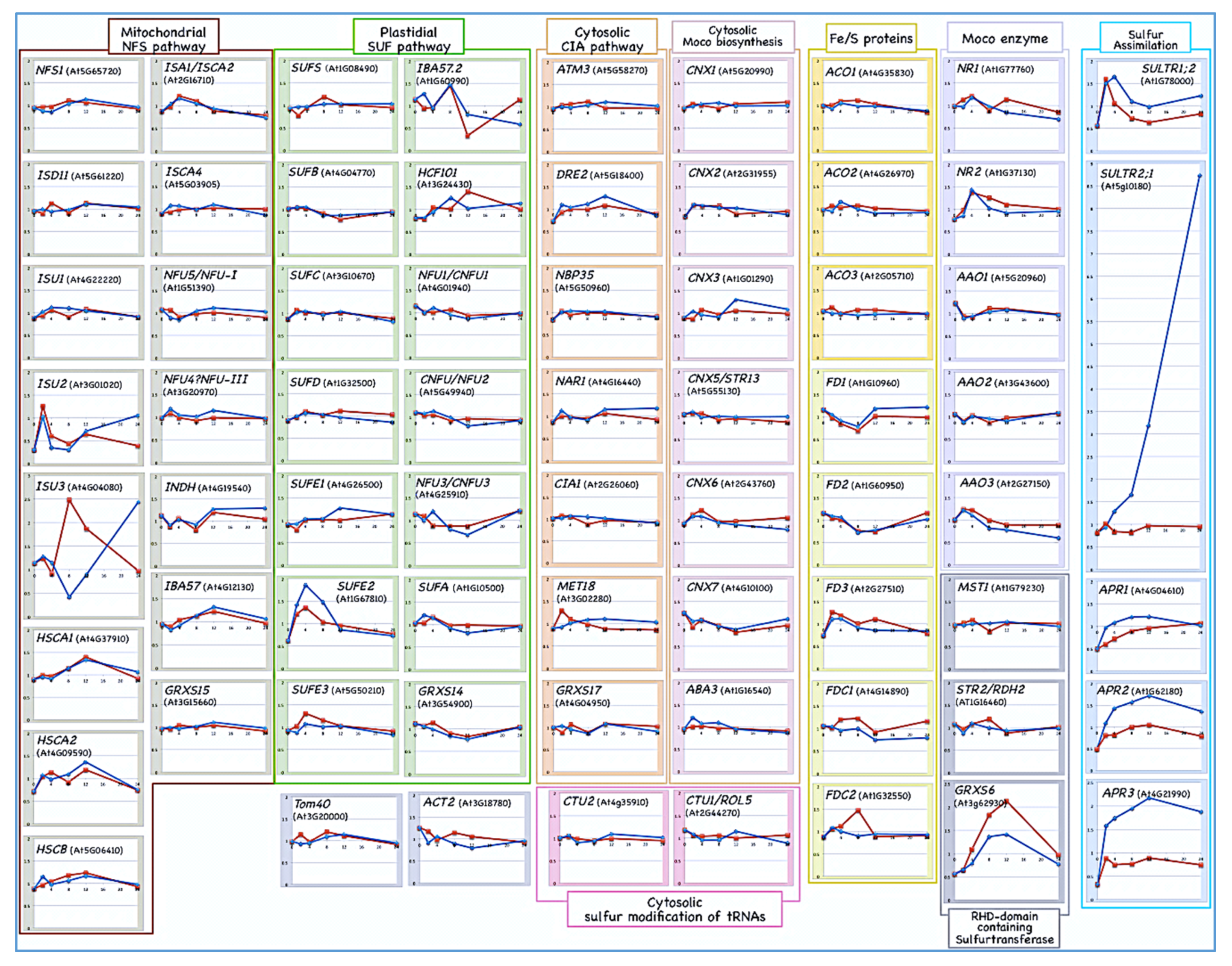
5. Gene Expression Dynamics of the Biosynthesis of Sulfur-Containing Biomolecules in Sulfate-Deprived Roots
6. Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGI | the Arabidopsis Genome Initiative |
| APS | 5’-adenylyl sulfate |
| APR | APS reductase |
| Arabidopsis | Arabidopsis thaliana |
| CIA | cytosolic iron-sulfur cluster assembly |
| Fe/S | iron-sulfur |
| Grx | glutaredoxin |
| Moco | molybdenum cofactor |
| MPT | molybdopterin |
| NFS | nitrogen fixation protein for sulfur transfer |
| RHD | rhodanese |
| S-Moco | sulfur-bound Moco |
| SUF | sulfur utilization factor |
| SULTR | sulfate transporter |
| Trx | thioredoxin |
| URM | ubiquitin-related modifier |
| UBL | ubiquitin-like protein |
| UBA | ubiquitin-activating enzyme-like protein |
References
- Beinert, H. A tribute to sulfur. Eur. J. Biochem. 2000, 267, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.; Kahn, M.; Seefeldt, L.; Tsay, Y.; Kopriva, S. Nitrogen and sulfur. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; WILEY: Blackwell, NJ, USA, 2015; pp. 746–768. [Google Scholar]
- Maruyama-Nakashita, A.; Ohkama-Ohtsu, N. Sulfur assimilation and glutathione metabolism in plants. In Glutathione in Plant Growth, Development, and Stress Tolerance; Hossain, M.A., Ed.; Springer: New York, NY, USA, 2017; pp. 287–308. [Google Scholar]
- Koprivova, A.; North, K.A.; Kopriva, S. Complex signaling network in regulation of adenosine 5’-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol. 2008, 146, 1408–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, M.Y.; Yano, M.; Goodenowe, D.B.; Kanaya, S.; Kimura, T.; Awazuhara, M.; Arita, M.; Fujiwara, T.; Saito, K. Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 10205–10210. [Google Scholar] [CrossRef] [Green Version]
- Maruyama-Nakashita, A.; Inoue, E.; Watanabe-Takahashi, A.; Yamaya, T.; Takahashi, H. Transcriptome Profiling of Sulfur-Responsive Genes in Arabidopsis Reveals Global Effects of Sulfur Nutrition on Multiple Metabolic Pathways. Plant Physiol. 2003, 132, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Yamazaki, M.; Sasakura, N.; Watanabe, A.; Leustek, T.; Engler, J.A.; Engler, G.; Van Montagu, M.; Saito, K. Regulation of sulfur assimilation in higher plants: A sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 11102–11107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant. Biol. 2017, 39, 144–151. [Google Scholar] [CrossRef]
- Hell, R.; Wirtz, M. Molecular Biology, Biochemistry and Cellular Physiology of Cysteine Metabolism in Arabidopsis thaliana. Arabidopsis Book. 2011, 9, e0154. [Google Scholar] [CrossRef] [Green Version]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A central role for thiols in plant tolerance to abiotic stress. Int J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.J.; Hell, R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth. Res. 2005, 86, 435–457. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant. Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Nakai, M.; Yano, T. Sulfur Modifications of the Wobble U34 in tRNAs and their Intracellular Localization in Eukaryotic Cells. Biomolecules. 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iciek, M.; Bilska-Wilkosz, A.; Górny, M. Sulfane sulfur—New findings on an old topic. Acta Biochim. Pol. 2019, 66, 533–544. [Google Scholar] [CrossRef]
- Mueller, E.G. Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2006, 2, 185–194. [Google Scholar] [CrossRef]
- Subrahmanian, N.; Remacle, C.; Hamel, P.P. Plant mitochondrial Complex I composition and assembly: A review. Biochim. Biophys. Acta 2016, 1857, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Müh, F.; Glöckner, C.; Hellmich, J.; Zouni, A. Light-induced quinone reduction in photosystem II. Biochim. Biophys. Acta 2012, 1817, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Liu, J.; Wen, X.; Lu, C. Molecular mechanism of photosystem I assembly in oxygenic organisms. Biochim. Biophys. Acta 2015, 1847, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Przybyla-Toscano, J.; Roland, M.; Gaymard, F.; Couturier, J.; Rouhier, N. Roles and maturation of iron–sulfur proteins in plastids. J. Biol. Inorg. Chem. 2018, 23, 545–566. [Google Scholar] [CrossRef] [Green Version]
- Balk, J.; Schaedler, T.A. Iron cofactor assembly in plants. Annu Rev. Plant. Biol. 2014, 65, 125–153. [Google Scholar] [CrossRef]
- Frazzon, A.P.; Ramirez, M.V.; Warek, U.; Balk, J.; Frazzon, J.; Dean, D.R.; Winkel, B.S. Functional analysis of Arabidopsis genes involved in mitochondrial iron-sulfur cluster assembly. Plant. Mol. Biol. 2007, 64, 225–240. [Google Scholar] [CrossRef]
- Turowski, V.R.; Busi, M.V.; Gomez-Casati, D.F. Structural and functional studies of the mitochondrial cysteinedesulfurase from Arabidopsis thaliana. Mol. Plant. 2012, 5, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Léon, S.; Touraine, B.; Briat, J.F.; Lobréaux, S. Mitochondrial localization of Arabidopsis thaliana Isu Fe-S scaffold proteins. FEBS Lett. 2005, 579, 1930–1934. [Google Scholar] [CrossRef]
- Uzarska, M.A.; Przybyla-Toscano, J.; Spantgar, F.; Zannini, F.; Lill, R.; Mühlenhoff, U.; Rouhier, N. Conserved functions of Arabidopsis mitochondrial late-acting maturation factors in the trafficking of iron-sulfur clusters. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Lin, H.; Latijnhouwers, M.; Møller, S.G. Dual localized AtHscB involved in iron sulfur protein biogenesis in Arabidopsis. PLoS ONE 2009, 4, e7662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Léon, S.; Touraine, B.; Ribot, C.; Briat, J.F.; Lobréaux, S. Iron-sulphur cluster assembly in plants: Distinct NFU proteins in mitochondria and plastids from Arabidopsis thaliana. Biochem J. 2003, 371, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannini, F.; Roret, T.; Przybyla-Toscano, J.; Dhalleine, T.; Rouhier, N.; Couturier, J. Mitochondrial Arabidopsis thaliana TRXo Isoforms Bind an Iron⁻Sulfur Cluster and Reduce NFU Proteins In Vitro. Antioxidants (Basel) 2018, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Bych, K.; Kerscher, S.; Netz, D.J.; Pierik, A.J.; Zwicker, K.; Huynen, M.A.; Lill, R.; Brandt, U.; Balk, J. The iron-sulphur protein Ind1 is required for effective complex I assembly. EMBO J. 2008, 27, 1736–1746. [Google Scholar] [CrossRef] [Green Version]
- Moseler, A.; Aller, I.; Wagner, S.; Nietzel, T.; Przybyla-Toscano, J.; Mühlenhoff, U.; Lill, R.; Berndt, C.; Rouhier, N.; Schwarzländer, M.; et al. The mitochondrial monothiol glutaredoxin S15 is essential for iron-sulfur protein maturation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 13735–13740. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Tokumoto, U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J. Biol. Chem. 2002, 277, 28380–28383. [Google Scholar] [CrossRef] [Green Version]
- Chahal, H.K.; Dai, Y.; Saini, A.; Ayala-Castro, C.; Outten, F.W. The SufBCD Fe-S scaffold complex interacts with SufA for Fe-S cluster transfer. Biochem. 2009, 48, 10644–10653. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Kato, Y.; Sumida, A.; Tanaka, A.; Tanaka, R. The SUFBC2 D complex is required for the biogenesis of all major classes of plastid Fe-S proteins. Plant. J. 2017, 90, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Abdel-Ghany, S.E.; Anderson, T.D.; Pilon-Smits, E.A.; Pilon, M. CpSufE activates the cysteine desulfurase CpNifS for chloroplastic Fe-S cluster formation. J. Biol. Chem. 2006, 281, 8958–8969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.M.; Møller, S.G. AtSufE is an essential activator of plastidic and mitochondrial desulfurases in Arabidopsis. EMBO J. 2006, 25, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayana Murthy, U.M.; Ollagnier-de-Choudens, S.; Sanakis, Y.; Abdel-Ghany, S.E.; Rousset, C.; Ye, H.; Fontecave, M.; Pilon-Smits, E.A.; Pilon, M. Characterization of Arabidopsis thaliana SufE2 and SufE3: Functions in chloroplast iron-sulfur cluster assembly and NAD synthesis. J. Biol. Chem. 2007, 282, 18254–18264. [Google Scholar]
- Chahal, H.K.; Outten, F.W. Separate FeS scaffold and carrier functions for SufB₂C₂ and SufA during in vitro maturation of [2Fe2S] Fdx. J. Inorg Biochem. 2012, 116, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Ghany, S.E.; Ye, H.; Garifullina, G.F.; Zhang, L.; Pilon-Smits, E.A.; Pilon, M. Iron-sulfur cluster biogenesis in chloroplasts. Involvement of the scaffold protein CpIscA. Plant. Physiol. 2005, 138, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Yabe, T.; Nakai, M. Arabidopsis AtIscA-I is affected by deficiency of Fe-S cluster biosynthetic scaffold AtCnfU-V. Biochem. Biophys. Res. Commun. 2006, 340, 1047–1052. [Google Scholar] [CrossRef]
- Py, B.; Gerez, C.; Angelini, S.; Planel, R.; Vinella, D.; Loiseau, L.; Talla, E.; Brochier-Armanet, C.; Garcia Serres, R.; Latour, J.M.; et al. Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol. Microbiol. 2012, 86, 155–171. [Google Scholar] [CrossRef]
- Gao, H.; Subramanian, S.; Couturier, J.; Naik, S.G.; Kim, S.K.; Leustek, T.; Knaff, D.B.; Wu, H.C.; Vignols, F.; Huynh, B.H.; et al. Arabidopsis thaliana Nfu2 accommodates [2Fe-2S] or [4Fe-4S] clusters and is competent for in vitro maturation of chloroplast [2Fe-2S] and [4Fe-4S] cluster-containing proteins. Biochemistry 2013, 52, 6633–6645. [Google Scholar] [CrossRef] [Green Version]
- Nath, K.; O’Donnell, J.P.; Lu, Y. Chloroplastic iron-sulfur scaffold protein NFU3 is essential to overall plant fitness. Plant. Signal. Behav. 2017, 12, e1282023. [Google Scholar] [CrossRef] [Green Version]
- Schwenkert, S.; Netz, D.J.; Frazzon, J.; Pierik, A.J.; Bill, E.; Gross, J.; Lill, R.; Meurer, J. Chloroplast HCF101 is a scaffold protein for [4Fe-4S] cluster assembly. Biochem. J. 2009, 425, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, J.C.; Ellens, K.W.; Alvarez, S.; Loizeau, K.; Ravanel, S.; Hanson, A.D. Mitochondrial and plastidial COG0354 proteins have folate-dependent functions in iron-sulphur cluster metabolism. J. Exp. Bot. 2012, 63, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Gama, F.; Molina-Navarro, M.M.; Gualberto, J.M.; Claxton, R.; Naik, S.G.; Huynh, B.H.; Herrero, E.; Jacquot, J.P.; Johnson, M.K.; et al. Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe-2S] clusters. EMBO J. 2008, 27, 1122–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biederbick, A.; Stehling, O.; Rösser, R.; Niggemeyer, B.; Nakai, Y.; Elsässer, H.P.; Lill, R. Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation. Mol. Cell Biol. 2006, 26, 5675–5687. [Google Scholar] [CrossRef] [Green Version]
- Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Kispal, G.; Csere, P.; Prohl, C.; Lill, R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999, 18, 3981–3989. [Google Scholar] [CrossRef] [Green Version]
- Pondarré, C. , Antiochos, B.B.; Campagna, D.R.; Clarke, S.L.; Greer, E.L.; Deck, K.M.; McDonald, A.; Han, A.P.; Medlock, A.; Kutok, J.L.; et al. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron-sulfur cluster biogenesis. Hum. Mol. Genet. 2006, 15, 953–964. [Google Scholar]
- Chen, S.; Sánchez-Fernández, R.; Lyver, E.R.; Dancis, A.; Rea, P.A. Functional characterization of AtATM1, AtATM2, and AtATM3, a subfamily of Arabidopsis half-molecule ATP-binding cassette transporters implicated in iron homeostasis. J. Biol. Chem. 2007, 282, 21561–21571. [Google Scholar] [CrossRef] [Green Version]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, U.; Lee, Y.; Martinoia, E.; et al. Plant ABC proteins--a unified nomenclature and updated inventory. Trends Plant. Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Bernard, D.G.; Netz, D.J.; Lagny, T.J.; Pierik, A.J.; Balk, J. Requirements of the cytosolic iron-sulfur cluster assembly pathway in Arabidopsis. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20120259. [Google Scholar] [CrossRef] [Green Version]
- Bych, K.; Netz, D.J.; Vigani, G.; Bill, E.; Lill, R.; Pierik, A.J.; Balk, J. The essential cytosolic iron-sulfur protein Nbp35 acts without Cfd1partner in the green lineage. J. Biol. Chem. 2008, 283, 35797–35804. [Google Scholar] [CrossRef] [Green Version]
- Kohbushi, H.; Nakai, Y.; Kikuchi, S.; Yabe, T.; Hori, H.; Nakai, M. Arabidopsis cytosolic Nbp35 homodimer can assemble both [2Fe-2S] and [4Fe-4S] clusters in two distinct domains. Biochem Biophys Res. Commun. 2009, 378, 810–815. [Google Scholar] [CrossRef]
- Varadarajan, J.; Guilleminot, J.; Saint-Jore-Dupas, C.; Piégu, B.; Chabouté, M.E.; Gomord, V.; Coolbaugh, R.C.; Devic, M.; Delorme, V. ATR3 encodes a diflavin reductase essential for Arabidopsis embryo development. New Phytol. 2010, 187, 67–82. [Google Scholar] [CrossRef]
- Bastow, E.L.; Bych, K.; Crack, J.C.; Le Brun, N.E.; Balk, J. NBP35 interacts with DRE2 in the maturation of cytosolic iron-sulphur proteins in Arabidopsis thaliana. Plant J. 2017, 89, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Song, L.; Sengupta, S.; McInturf, S.A.; Grant, D.G.; Marjault, H.B.; Castro-Guerrero, N.A.; Burks, D.; Azad, R.K.; Mendoza-Cozatl, D.G.; et al. Expression of a dominant-negative AtNEET-H89C protein disrupts iron-sulfur metabolism and iron homeostasis in Arabidopsis. Plant J. 2020, 101, 1152–1169. [Google Scholar] [CrossRef] [PubMed]
- Su, L.W.; Chang, S.H.; Li, M.Y.; Huang, H.Y.; Jane, W.N.; Yang, J.Y. Purification and biochemical characterization of Arabidopsis At-NEET, an ancient iron-sulfur protein, reveals a conserved cleavage motif for subcellular localization. Plant. Sci. 2013, 213, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Nechushtai, R.; Conlan, A.R.; Harir, Y.; Song, L.; Yogev, O.; Eisenberg-Domovich, Y.; Livnah, O.; Michaeli, D.; Rosen, R.; Ma, V.; et al. Characterization of Arabidopsis NEET reveals an ancient role for NEET proteins in iron metabolism. Plant. Cell. 2012, 24, 2139–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, D.; Bernard, D.G.; Balk, J.; Hai, H.; Cui, X. The DUF59 family gene AE7 acts in the cytosolic iron-sulfur cluster assembly pathway to maintain nuclear genome integrity in Arabidopsis. Plant Cell. 2012, 24, 4135–4148. [Google Scholar] [CrossRef] [Green Version]
- Couturier, J.; Jacquot, J.P.; Rouhier, N. Evolution and diversity of glutaredoxins in photosynthetic organisms. Cell Mol. Life Sci. 2009, 66, 2539–2557. [Google Scholar] [CrossRef]
- Iñigo, S.; Durand, A.N.; Ritter, A.; Le Gall, S.; Termathe, M.; Klassen, R.; Tohge, T.; De Coninck, B.; Van Leene, J.; De Clercq, R.; et al. Glutaredoxin GRXS17 Associates with the Cytosolic Iron-Sulfur Cluster Assembly Pathway. Plant. Physiol. 2016, 172, 858–873. [Google Scholar]
- Cheng, N.H.; Liu, J.Z.; Liu, X.; Wu, Q.; Thompson, S.M.; Lin, J.; Chang, J.; Whitham, S.A.; Park, S.; Cohen, J.D.; et al. Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J. Biol. Chem. 2011, 286, 20398–20406. [Google Scholar] [PubMed] [Green Version]
- Yu, H.; Yang, J.; Shi, Y.; Donelson, J.; Thompson, S.M.; Sprague, S.; Roshan, T.; Wang, D.L.; Liu, J.; Park, S.; et al. Arabidopsis Glutaredoxin S17 Contributes to Vegetative Growth, Mineral Accumulation, and Redox Balance during Iron Deficiency. Front. Plant Sci. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, P.; Taupin-Broggini, M.; Couturier, J.; Vignols, F.; Rouhier, N. Is There a Role for Glutaredoxins and BOLAs in the Perception of the Cellular Iron Status in Plants? Front. Plant Sci. 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, G. Molybdenum cofactor biosynthesis and deficiency. Cell Mol. Life Sci. 2005, 62, 2792–2810. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Harada, A.; Hashiguchi, Y.; Nakai, M.; Hayashi, H. Arabidopsis molybdopterin biosynthesis protein Cnx5 collaborateswith the ubiquitin-like protein Urm11 in the thio-modification of tRNA. J. Biol. Chem. 2012, 287, 30874–30884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gladyshev, V.N. Molybdoproteomes and evolution of molybdenum utilization. J. Mol. Biol. 2008, 379, 881–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendel, R.R.; Bittner, F. Cell biology of molybdenum. Biochim. Biophys. Acta 2006, 1763, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.; Papenbrock, J. Identification and characterization of single-domain thiosulfate sulfurtransferases from Arabidopsis thaliana. FEBS Lett. 2002, 532, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Bartels, A.; Mock, H.P.; Papenbrock, J. Differential expression of Arabidopsis sulfurtransferases under various growth conditions. Plant. Physiol. Biochem. 2007, 45, 178–187. [Google Scholar] [CrossRef]
- Krepinsky, K.; Leimkühler, S. Site-directed mutagenesis of the active site loop of the rhodanese-likedomain of the human molybdopterin synthase sulfurase MOCS3. Major differences in substrate specificity between eukaryotic and bacterial homologs. FEBS J. 2007, 274, 2778–2787. [Google Scholar] [CrossRef]
- Leimkühler, S.; Rajagopalan, K.V. A sulfurtransferase is required in the transfer of cysteine sulfur in the in vitro synthesis of molybdopterin from precursor Z in Escherichia coli. J. Biol. Chem. 2001, 276, 22024–22031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthies, A.; Rajagopalan, K.V.; Mendel, R.R.; Leimkühler, S. Evidence for the physiological role of a rhodanese-like protein for the biosynthesis of the molybdenum cofactor in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 5946–5951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phizicky, E.M.; Hopper, A.K. tRNA biology charges to the front. Genes Dev. 2010, 24, 1832–1860. [Google Scholar] [CrossRef] [Green Version]
- Nakai, Y.; Nakai, M.; Hayashi, H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J. Biol. Chem. 2008, 283, 27469–27476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, M.M.; Dosche, C.; Löhmannsröben, H.-G.; Leimkühler, S. Dual Role of the Molybdenum Cofactor Biosynthesis Protein MOCS3 in tRNA Thiolation and Molybdenum Cofactor Biosynthesis in Humans. J. Biol. Chem. 2012, 287, 17297–17307. [Google Scholar] [CrossRef] [Green Version]
- Wollers, S.; Heidenreich, T.; Zarepour, M.; Zachmann, D.; Kraft, C.; Zhao, Y.; Mendel, R.R.; Bittner, F. Binding of sulfurated molybdenum cofactor to the C-terminal domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J. Biol. Chem. 2008, 283, 9642–9650. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Nakamura, Y.; Watanabe-Takahashi, A.; Inoue, E.; Yamaya, T.; Takahashi, H. Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J. 2005, 42, 305–314. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Iyer, L.M.; Aravind, L. Natural history of the E1-like superfamily: Implication for adenylation, sulfur transfer, and ubiquitin conjugation. Proteins 2009, 75, 895–910. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, J.; Wang, L.; Zhou, J.; Huang, H.; Wu, J.; Zhong, Y.; Shi, Y. Solution structure of Urm1 and its implications for the origin of protein modifiers. Proc. Natl. Acad. Sci. USA 2006, 103, 11625–11630. [Google Scholar] [CrossRef] [Green Version]
- Schulman, B.A.; Harper, J.W. Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 2009, 10, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selles, B.; Moseler, A.; Rouhier, N.; Couturier, J. Rhodanese domain-containing sulfurtransferases: Multifacetedproteins involved in sulfur trafficking in plants. J. Exp. Bot. 2019, 70, 4139–4154. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakai, Y.; Maruyama-Nakashita, A. Biosynthesis of Sulfur-Containing Small Biomolecules in Plants. Int. J. Mol. Sci. 2020, 21, 3470. https://doi.org/10.3390/ijms21103470
Nakai Y, Maruyama-Nakashita A. Biosynthesis of Sulfur-Containing Small Biomolecules in Plants. International Journal of Molecular Sciences. 2020; 21(10):3470. https://doi.org/10.3390/ijms21103470
Chicago/Turabian StyleNakai, Yumi, and Akiko Maruyama-Nakashita. 2020. "Biosynthesis of Sulfur-Containing Small Biomolecules in Plants" International Journal of Molecular Sciences 21, no. 10: 3470. https://doi.org/10.3390/ijms21103470




