Silver Island Film for Enhancing Light Harvesting in Natural Photosynthetic Proteins
Abstract
:1. Introduction
1.1. Photosynthetic Complexes
1.2. Silver Island Films
2. Methods
3. The Effect of SIF on the Optical Properties of Photosynthetic Complexes
3.1. Simple Photosynthetic Antenna Complexes
3.2. SIF Substrates Obtained with Different Methods of Wet-Chemistry
3.3. Maximum Enhancement Factors
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Hou, H.J.M.; Shen, J.-R.; Allakhverdiev, S.I. Components of Natural Photosynthetic Apparatus in Solar Cells. In Applied Photosynthesis—New Progress; Najafpour, M.M., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2267-8. [Google Scholar]
- Stephens, E.; Ross, I.L.; Mussgnug, J.H.; Wagner, L.D.; Borowitzka, M.A.; Posten, C.; Kruse, O.; Hankamer, B. Future prospects of microalgal biofuel production systems. Trends Plant Sci. 2010, 15, 554–564. [Google Scholar] [CrossRef]
- Cogdell, R. Can photosynthesis provide a “biological blueprint” for the design of novel solar cells? Trends Biotechnol. 1998, 16, 521–527. [Google Scholar] [CrossRef]
- Janna Olmos, J.D.; Kargul, J. Oxygenic photosynthesis: Translation to solar fuel technologies. Acta Soc. Bot. Pol. 2014, 83, 423–440. [Google Scholar] [CrossRef] [Green Version]
- Johansson, T.B. Renewable Energy: Sources for Fuels and Electricity; Island Press: Washington, DC, USA, 1993; ISBN 978-1-55963-138-9. [Google Scholar]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Twidell, J.; Weir, T. Renewable Energy Resources; Routledge: London, UK, 2015; ISBN 978-1-317-66037-8. [Google Scholar]
- Janna Olmos, J.D.; Kargul, J. A quest for the artificial leaf. Int. J. Biochem. Cell Biol. 2015, 66, 37–44. [Google Scholar] [CrossRef]
- Mackowski, S. Hybrid nanostructures for efficient light harvesting. J. Phys. Condens. Matter 2010, 22, 193102. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A. The path to 25% silicon solar cell efficiency: History of silicon cell evolution. Prog. Photovolt. Res. Appl. 2009, 17, 183–189. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Richards, B.S. Enhancing the performance of silicon solar cells via the application of passive luminescence conversion layers. Sol. Energy Mater. Sol. Cells 2006, 90, 2329–2337. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kargul, J.; Janna Olmos, J.D.; Krupnik, T. Structure and function of photosystem I and its application in biomimetic solar-to-fuel systems. J. Plant Physiol. 2012, 169, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Ocakoglu, K.; Krupnik, T.; van den Bosch, B.; Harputlu, E.; Gullo, M.P.; Olmos, J.D.J.; Yildirimcan, S.; Gupta, R.K.; Yakuphanoglu, F.; Barbieri, A.; et al. Photosystem I-based Biophotovoltaics on Nanostructured Hematite. Adv. Funct. Mater. 2014, 24, 7467–7477. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef]
- Sugiyama, M.; Fujii, K.; Nakamura, S. Solar to Chemical Energy Conversion: Theory and Application; Springer International Publishing: Basel, Switzerland, 2016; ISBN 978-3-319-25400-5. [Google Scholar]
- Zhao, F.; Ruff, A.; Rögner, M.; Schuhmann, W.; Conzuelo, F. Extended Operational Lifetime of a Photosystem-Based Bioelectrode. J. Am. Chem. Soc. 2019, 141, 5102–5106. [Google Scholar] [CrossRef]
- Mackowski, S.; Wörmke, S.; Maier, A.J.; Brotosudarmo, T.H.P.; Harutyunyan, H.; Hartschuh, A.; Govorov, A.O.; Scheer, H.; Bräuchle, C. Metal-Enhanced Fluorescence of Chlorophylls in Single Light-Harvesting Complexes. Nano Lett. 2008, 8, 558–564. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Mackowski, S. Theoretical studies of excitation dynamics in a peridinin-chlorophyll-protein coupled to a metallic nanoparticle. Open Phys. 2011, 9, 47–51. [Google Scholar] [CrossRef]
- Czechowski, N.; Lokstein, H.; Kowalska, D.; Ashraf, K.; Cogdell, R.J.; Mackowski, S. Large plasmonic fluorescence enhancement of cyanobacterial photosystem I coupled to silver island films. Appl. Phys. Lett. 2014, 105, 043701. [Google Scholar] [CrossRef]
- Szalkowski, M.; Ashraf, K.U.; Lokstein, H.; Mackowski, S.; Cogdell, R.J.; Kowalska, D. Silver island film substrates for ultrasensitive fluorescence detection of (bio)molecules. Photosynth. Res. 2016, 127, 103–108. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Novotny, L. Spectral dependence of single molecule fluorescence enhancement. Opt. Express 2007, 15, 14266–14274. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R.; Shen, Y.; D’Auria, S.; Malicka, J.; Fang, J.; Gryczynski, Z.; Gryczynski, I. Radiative Decay Engineering: 2. Effects of Silver Island Films on Fluorescence Intensity, Lifetimes, and Resonance Energy Transfer. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Konrad, A.; Lokstein, H.; Skandary, S.; Metzger, M.; Djouda, J.M.; Maurer, T.; Adam, P.M.; Meixner, A.J.; Brecht, M. Temperature dependence of metal-enhanced fluorescence of photosystem I from Thermosynechococcus elongatus. Nanoscale 2017, 9, 4196–4204. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Kothe, T.; Schuhmann, W.; Rögner, M. Wiring photosynthetic enzymes to electrodes. Energy Environ. Sci. 2011, 4, 3263–3274. [Google Scholar] [CrossRef]
- Ciesielski, P.N.; Faulkner, C.J.; Irwin, M.T.; Gregory, J.M.; Tolk, N.H.; Cliffel, D.E.; Jennings, G.K. Enhanced Photocurrent Production by Photosystem I Multilayer Assemblies. Adv. Funct. Mater. 2010, 20, 4048–4054. [Google Scholar] [CrossRef]
- LeBlanc, G.; Gizzie, E.; Yang, S.; Cliffel, D.E.; Jennings, G.K. Photosystem I Protein Films at Electrode Surfaces for Solar Energy Conversion. Langmuir 2014, 30, 10990–11001. [Google Scholar] [CrossRef]
- Frolov, L.; Wilner, O.; Carmeli, C.; Carmeli, I. Fabrication of Oriented Multilayers of Photosystem I Proteins on Solid Surfaces by Auto-Metallization. Adv. Mater. 2008, 20, 263–266. [Google Scholar] [CrossRef]
- Carmeli, I.; Lieberman, I.; Kraversky, L.; Fan, Z.; Govorov, A.O.; Markovich, G.; Richter, S. Broad Band Enhancement of Light Absorption in Photosystem I by Metal Nanoparticle Antennas. Nano Lett. 2010, 10, 2069–2074. [Google Scholar] [CrossRef]
- Polívka, T.; Hofmann, E. Structure-Function Relationship in Peridinin-Chlorophyll Proteins. In The Structural Basis of Biological Energy Generation; Hohmann-Marriott, M.F., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 39, pp. 39–58. ISBN 978-94-017-8741-3. [Google Scholar]
- Carbonera, D.; Valentin, M.; Spezia, R.; Mezzetti, A. The Unique Photophysical Properties of the Peridinin-Chlorophyll-a-Protein. Curr. Protein Pept. Sci. 2014, 15, 332–350. [Google Scholar] [CrossRef] [Green Version]
- Schulte, T.; Johanning, S.; Hofmann, E. Structure and function of native and refolded peridinin-chlorophyll-proteins from dinoflagellates. Eur. J. Cell Biol. 2010, 89, 990–997. [Google Scholar] [CrossRef]
- Takaichi, S.; Nippon, M.S.; Ohoka, H. Pigment composition in the reaction center complex from the thermophilic green sulfur bacterium, Chlorobium tepidum: Carotenoid glucoside esters, menaquinone [Vitamine K] and chlorophylls. Plant Cell Physiol. Jpn. 1999, 40, 691–694. [Google Scholar] [CrossRef]
- Tsukatani, Y.; Miyamoto, R.; Itoh, S.; Oh-Oka, H. Function of a PscD subunit in a homodimeric reaction center complex of the photosynthetic green sulfur bacterium Chlorobium tepidum studied by insertional gene inactivation. Regulation of energy transfer and ferredoxin-mediated NADP+ reduction on the cytoplasmic side. J. Biol. Chem. 2004, 279, 51122–51130. [Google Scholar] [PubMed] [Green Version]
- Frigaard, N.-U.; Chew, A.G.M.; Li, H.; Maresca, J.A.; Bryant, D.A. Chlorobium tepidum: Insights into the structure, physiology, and metabolism of a green sulfur bacterium derived from the complete genome sequence. Photosynth. Res. 2003, 78, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Azai, C.; Kim, K.; Kondo, T.; Harada, J.; Itoh, S.; Oh-oka, H. A heterogeneous tag-attachment to the homodimeric type 1 photosynthetic reaction center core protein in the green sulfur bacterium Chlorobaculum tepidum. Biochim. Biophys. Acta 2011, 1807, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.; Niedzwiedzki, D.M.; Orf, G.S.; Zhang, H.; Blankenship, R.E. Dynamics of Energy and Electron Transfer in the FMO-Reaction Center Core Complex from the Phototrophic Green Sulfur Bacterium Chlorobaculum tepidum. J. Phys. Chem. B 2015, 119, 8321–8329. [Google Scholar] [CrossRef]
- Rémigy, H.W.; Stahlberg, H.; Fotiadis, D.; Müller, S.A.; Wolpensinger, B.; Engel, A.; Hauska, G.; Tsiotis, G. The reaction center complex from the green sulfur bacterium Chlorobium tepidum: A structural analysis by scanning transmission electron microscopy. J. Mol. Biol. 1999, 290, 851–858. [Google Scholar] [CrossRef]
- Maćkowski, S.; Czechowski, N.; Ashraf, K.U.; Szalkowski, M.; Lokstein, H.; Cogdell, R.J.; Kowalska, D. Origin of bimodal fluorescence enhancement factors of Chlorobaculum tepidum reaction centers on silver island films. FEBS Lett. 2016, 590, 2558–2565. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.M. The FMO protein. In Discoveries in Photosynthesis; Govindjee, B.J.T., Gest, H., Allen, J.F., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2005; pp. 421–427. ISBN 978-1-4020-3323-0. [Google Scholar]
- Olson, J.M. Chlorophyll Organization and Function in Green Photosynthetic Bacteria*. Photochem. Photobiol. 1998, 67, 61–75. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Olson, J.M.; Miller, M. Antenna Complexes from Green Photosynthetic Bacteria. In Anoxygenic Photosynthetic Bacteria; Blankenship, R.E., Madigan, M.T., Bauer, C.E., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 1995; pp. 399–435. ISBN 978-0-7923-3681-5. [Google Scholar]
- Matthews, B.W.; Fenna, R.E. Structure of a green bacteriochlorophyll protein. Acc. Chem. Res. 1980, 13, 309–317. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Wiley-Blackwell: Hoboken, NJ, USA, 2014; ISBN 978-1-4051-8976-7. [Google Scholar]
- Haniewicz, P.; Abram, M.; Nosek, L.; Kirkpatrick, J.; El-Mohsnawy, E.; Olmos, J.D.J.; Kouřil, R.; Kargul, J.M. Molecular Mechanisms of Photoadaptation of Photosystem I Supercomplex from an Evolutionary Cyanobacterial/Algal Intermediate. Plant Physiol. 2018, 176, 1433–1451. [Google Scholar] [CrossRef] [Green Version]
- Antoshvili, M.; Caspy, I.; Hippler, M.; Nelson, N. Structure and function of photosystem I in Cyanidioschyzon merolae. Photosynth. Res. 2018, 139, 499–508. [Google Scholar] [CrossRef]
- Pi, X.; Tian, L.; Dai, H.-E.; Qin, X.; Cheng, L.; Kuang, T.; Sui, S.-F.; Shen, J.-R. Unique organization of photosystem I-light-harvesting supercomplex revealed by cryo-EM from a red alga. Proc. Natl. Acad. Sci. USA 2018, 115, 4423–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauss, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Fromme, P.; Melkozernov, A.; Jordan, P.; Krauss, N. Structure and function of photosystem I: Interaction with its soluble electron carriers and external antenna systems. FEBS Lett. 2003, 555, 40–44. [Google Scholar] [CrossRef]
- Grotjohann, I.; Fromme, P. Structure of cyanobacterial photosystem I. Photosynth. Res. 2005, 85, 51–72. [Google Scholar] [CrossRef]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Annealed Silver-Island Films for Applications in Metal-Enhanced Fluorescence: Interpretation in Terms of Radiating Plasmons. J. Fluoresc. 2005, 15, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Zhang, J.; Oyama, M.; Hirao, K. Silver-Nanoparticle-Attached Indium Tin Oxide Surfaces Fabricated by a Seed-Mediated Growth Approach. J. Phys. Chem. B 2005, 109, 1204–1209. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowiresof controllable aspect ratio. Chem. Commun. 2001, 617–618. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef]
- Chowdhury, M.H.; Ray, K.; Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Metal-Enhanced Fluorescence of Phycobiliproteins from Heterogeneous Plasmonic Nanostructures. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 18856–18863. [Google Scholar] [CrossRef] [Green Version]
- Mackowski, S.; Wörmke, S.; Brotosudarmo, T.H.P.; Jung, C.; Hiller, R.G.; Scheer, H.; Bräuchle, C. Energy Transfer in Reconstituted Peridinin-Chlorophyll-Protein Complexes: Ensemble and Single-Molecule Spectroscopy Studies. Biophys. J. 2007, 93, 3249–3258. [Google Scholar] [CrossRef] [Green Version]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Metal-Enhanced Fluorescence from CdTe Nanocrystals: A Single-Molecule Fluorescence Study. J. Am. Chem. Soc. 2006, 128, 8998–8999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.-R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 2015, 348, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Brecht, M.; Hussels, M.; Nieder, J.B.; Fang, H.; Elsässer, C. Plasmonic interactions of photosystem I with Fischer patterns made of Gold and Silver. Chem. Phys. 2012, 406, 15–20. [Google Scholar] [CrossRef]
- Nabiev, I.; Rakovich, A.; Sukhanova, A.; Lukashev, E.; Zagidullin, V.; Pachenko, V.; Rakovich, Y.P.; Donegan, J.F.; Rubin, A.B.; Govorov, A.O. Fluorescent Quantum Dots as Artificial Antennas for Enhanced Light Harvesting and Energy Transfer to Photosynthetic Reaction Centers. Angew. Chem. Int. Ed. 2010, 49, 7217–7221. [Google Scholar] [CrossRef] [PubMed]
- Feifel, S.C.; Stieger, K.R.; Lokstein, H.; Lux, H.; Lisdat, F. High photocurrent generation by photosystem I on artificial interfaces composed of π-system-modified graphene. J. Mater. Chem. A 2015, 3, 12188–12196. [Google Scholar] [CrossRef]
- Stieger, K.R.; Feifel, S.C.; Lokstein, H.; Lisdat, F. Advanced unidirectional photocurrent generation via cytochrome c as reaction partner for directed assembly of photosystem I. Phys. Chem. Chem. Phys. 2014, 16, 15667. [Google Scholar] [CrossRef]
- Carmeli, I.; Mangold, M.; Frolov, L.; Zebli, B.; Carmeli, C.; Richter, S.; Holleitner, A.W. A Photosynthetic Reaction Center Covalently Bound to Carbon Nanotubes. Adv. Mater. 2007, 19, 3901–3905. [Google Scholar] [CrossRef]
- Hatazaki, S.; Sharma, D.K.; Hirata, S.; Nose, K.; Iyoda, T.; Kölsch, A.; Lokstein, H.; Vacha, M. Identification of Short- and Long-Wavelength Emitting Chlorophylls in Cyanobacterial Photosystem I by Plasmon-Enhanced Single-Particle Spectroscopy at Room Temperature. J. Phys. Chem. Lett. 2018, 9, 6669–6675. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [Green Version]
- Szalkowski, M.; Olmos, J.D.J.; Buczyńska, D.; Maćkowski, S.; Kowalska, D.; Kargul, J. Plasmon-induced absorption of blind chlorophylls in photosynthetic proteins assembled on silver nanowires. Nanoscale 2017, 9, 10475–10486. [Google Scholar] [CrossRef]
- LeBlanc, G.; Winter, K.M.; Crosby, W.B.; Jennings, G.K.; Cliffel, D.E. Integration of Photosystem I with Graphene Oxide for Photocurrent Enhancement. Adv. Energy Mater. 2014, 4, 1301953. [Google Scholar] [CrossRef]
- Mershin, A.; Matsumoto, K.; Kaiser, L.; Yu, D.; Vaughn, M.; Nazeeruddin, M.K.; Bruce, B.D.; Graetzel, M.; Zhang, S. Self-assembled photosystem-I biophotovoltaics on nanostructured TiO2 and ZnO. Sci. Rep. 2012, 2, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciornii, D.; Riedel, M.; Stieger, K.R.; Feifel, S.C.; Hejazi, M.; Lokstein, H.; Zouni, A.; Lisdat, F. Bioelectronic Circuit on a 3D Electrode Architecture: Enzymatic Catalysis Interconnected with Photosystem I. J. Am. Chem. Soc. 2017, 139, 16478–16481. [Google Scholar] [CrossRef] [PubMed]
- Stieger, K.R.; Feifel, S.C.; Lokstein, H.; Hejazi, M.; Zouni, A.; Lisdat, F. Biohybrid architectures for efficient light-to-current conversion based on photosystem I within scalable 3D mesoporous electrodes. J. Mater. Chem. A 2016, 4, 17009–17017. [Google Scholar] [CrossRef]
- Kondo, M.; Iida, K.; Dewa, T.; Tanaka, H.; Ogawa, T.; Nagashima, S.; Nagashima, K.V.P.; Shimada, K.; Hashimoto, H.; Gardiner, A.T.; et al. Photocurrent and electronic activities of oriented-His-tagged photosynthetic light-harvesting/reaction center core complexes assembled onto a gold electrode. Biomacromolecules 2012, 13, 432–438. [Google Scholar] [CrossRef]
- Friebe, V.M.; Delgado, J.D.; Swainsbury, D.J.K.; Gruber, J.M.; Chanaewa, A.; van Grondelle, R.; von Hauff, E.; Millo, D.; Jones, M.R.; Frese, R.N. Plasmon-Enhanced Photocurrent of Photosynthetic Pigment Proteins on Nanoporous Silver. Adv. Funct. Mater. 2016, 26, 285–292. [Google Scholar] [CrossRef] [Green Version]
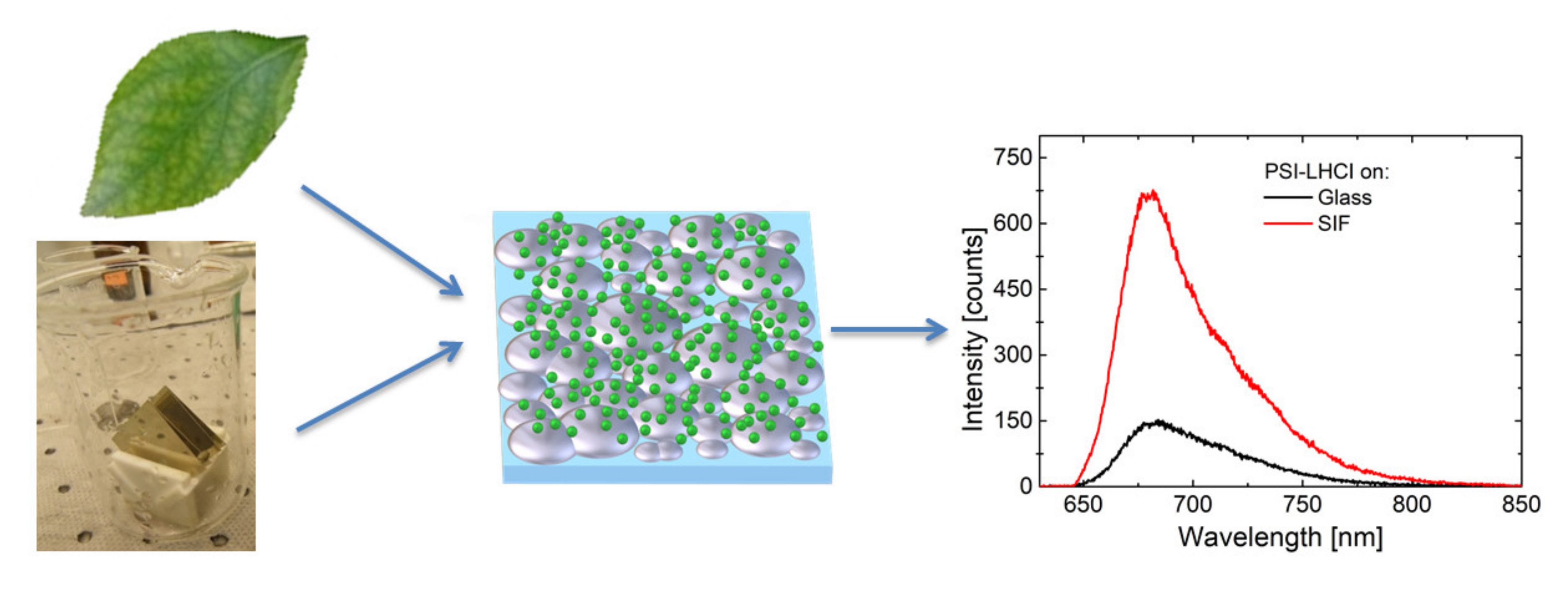


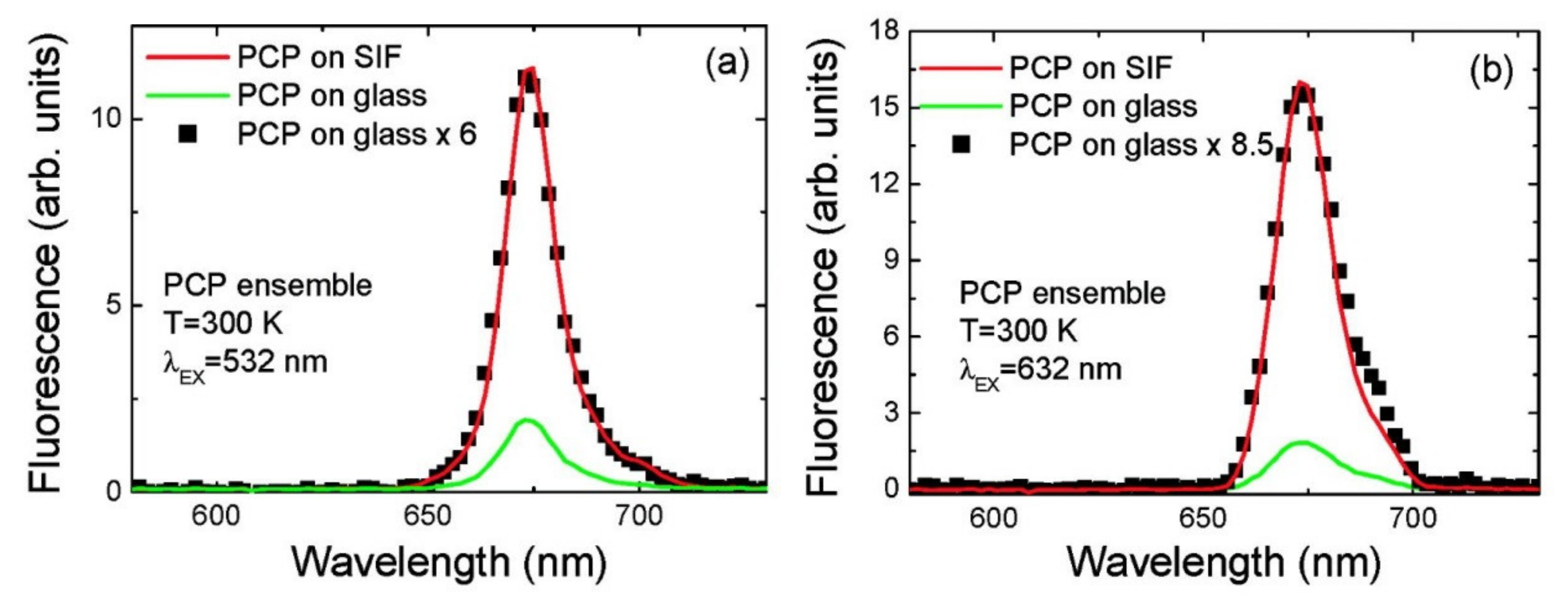
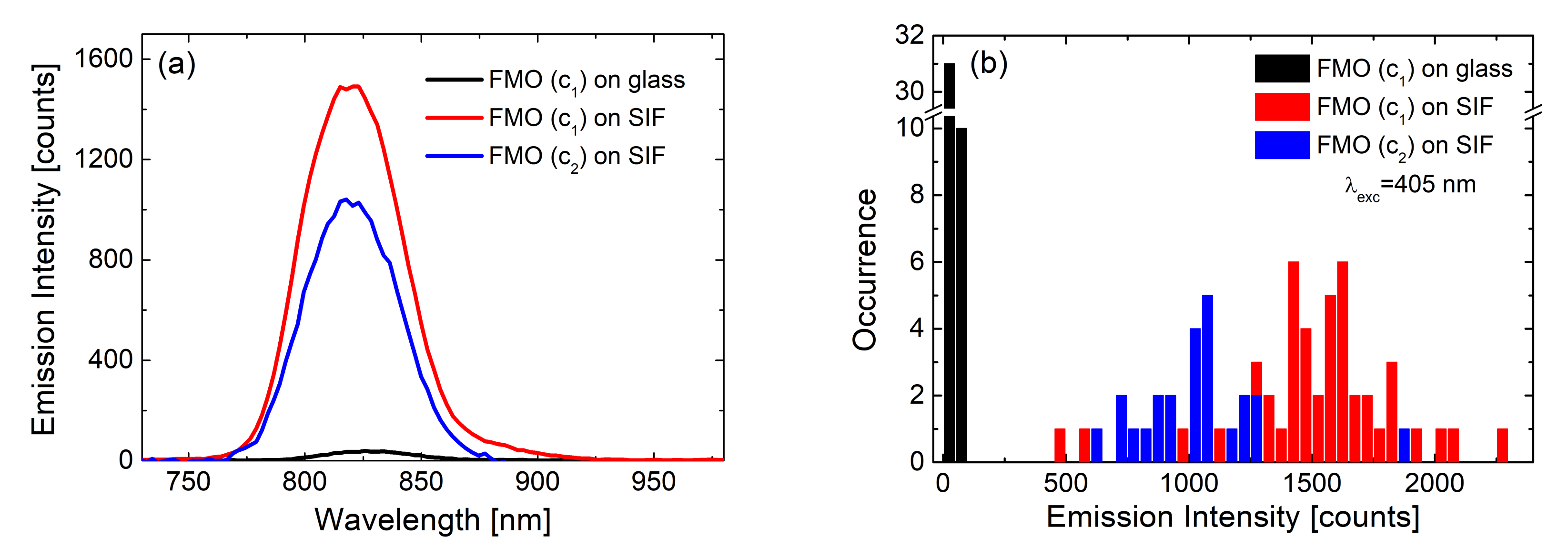
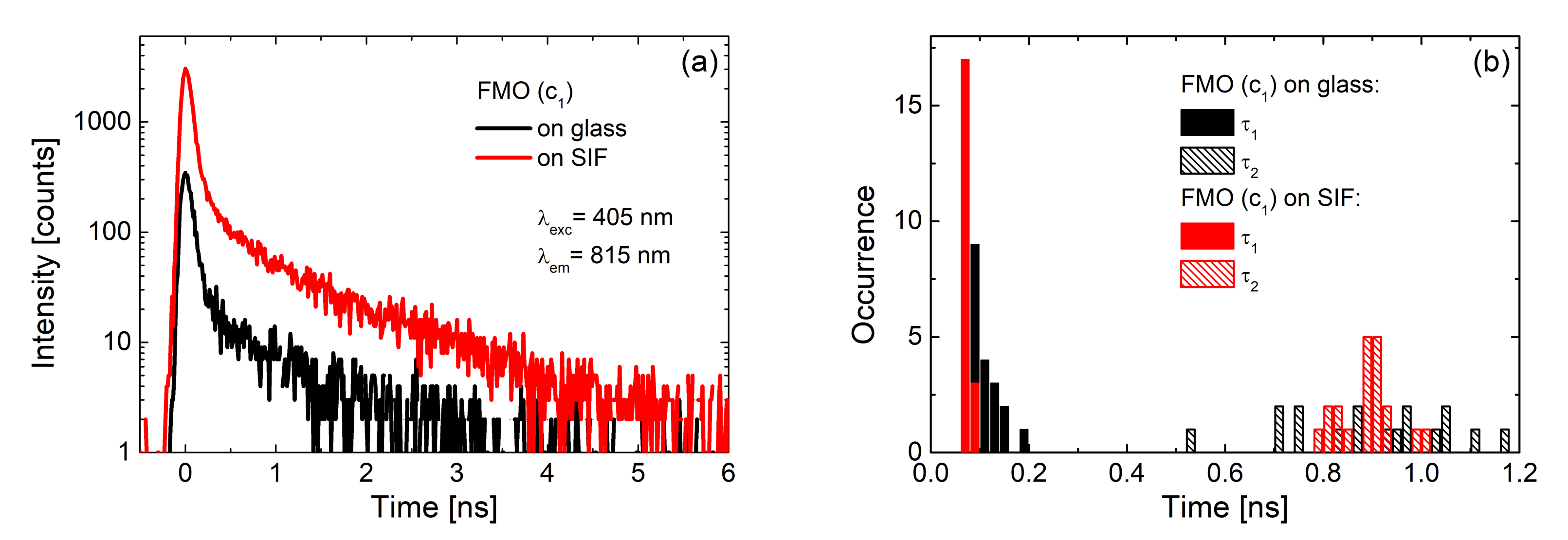
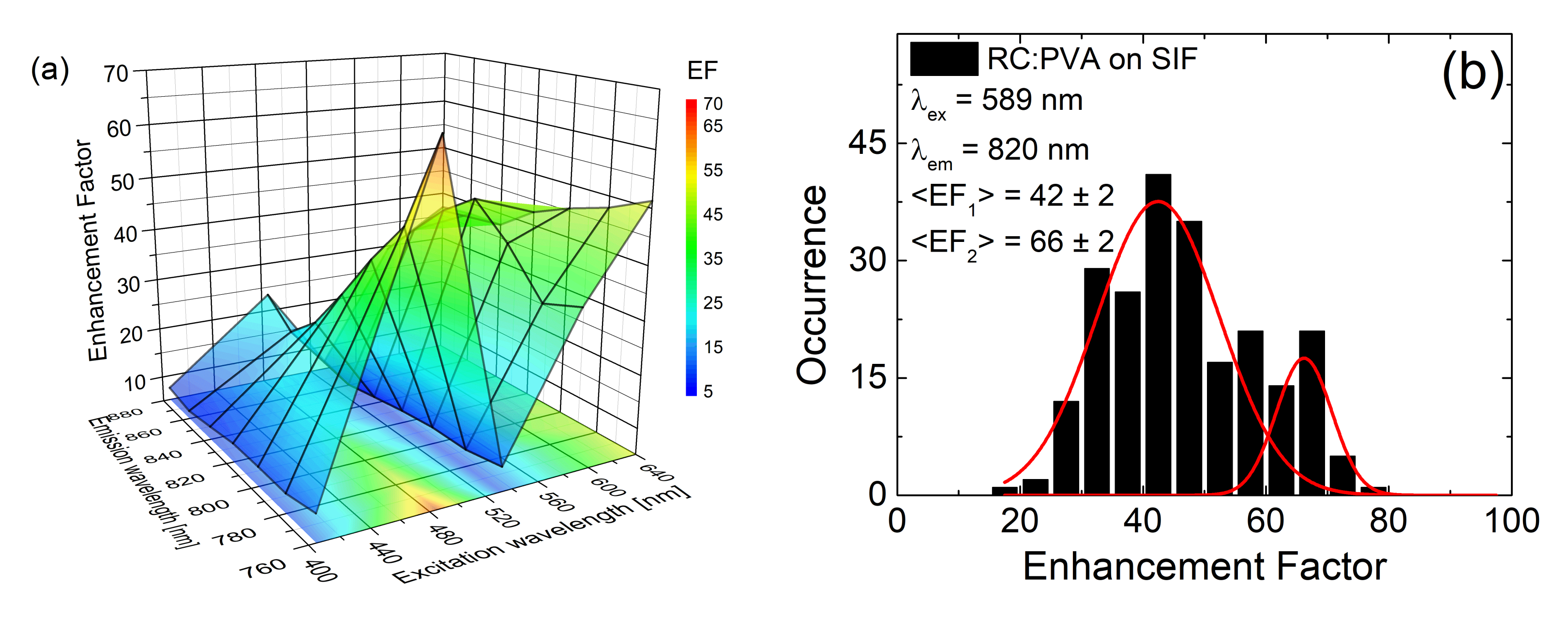
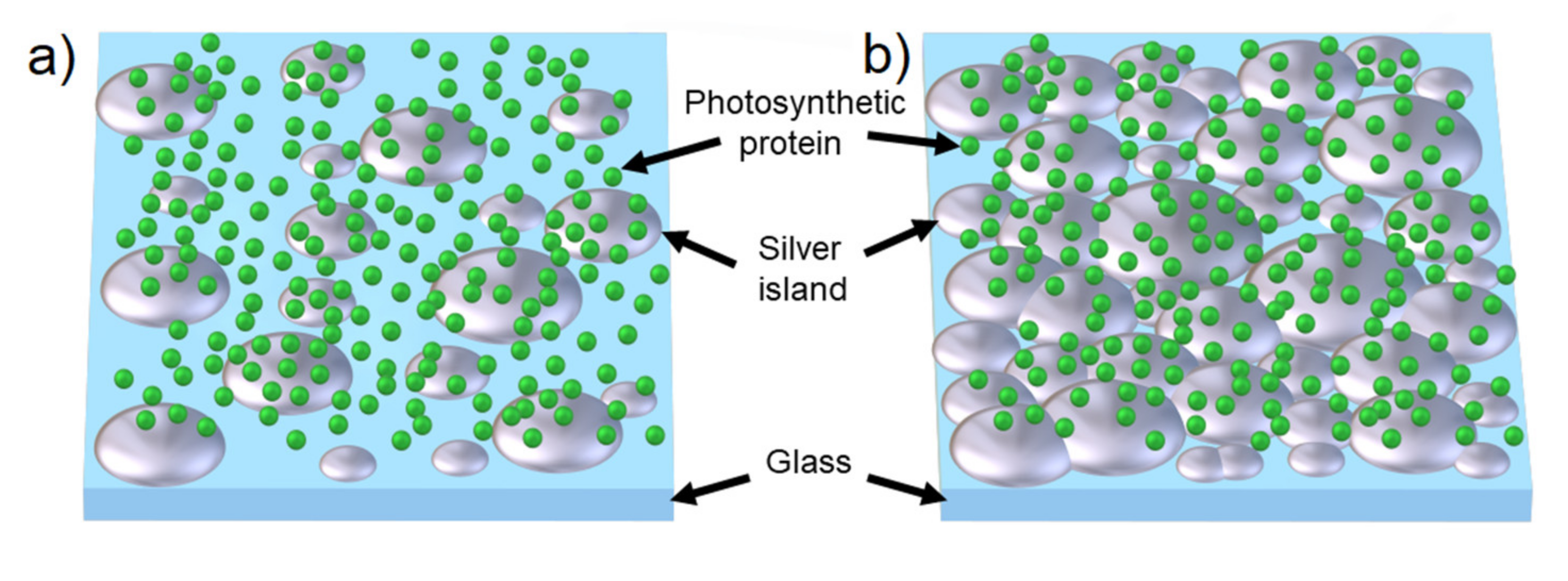
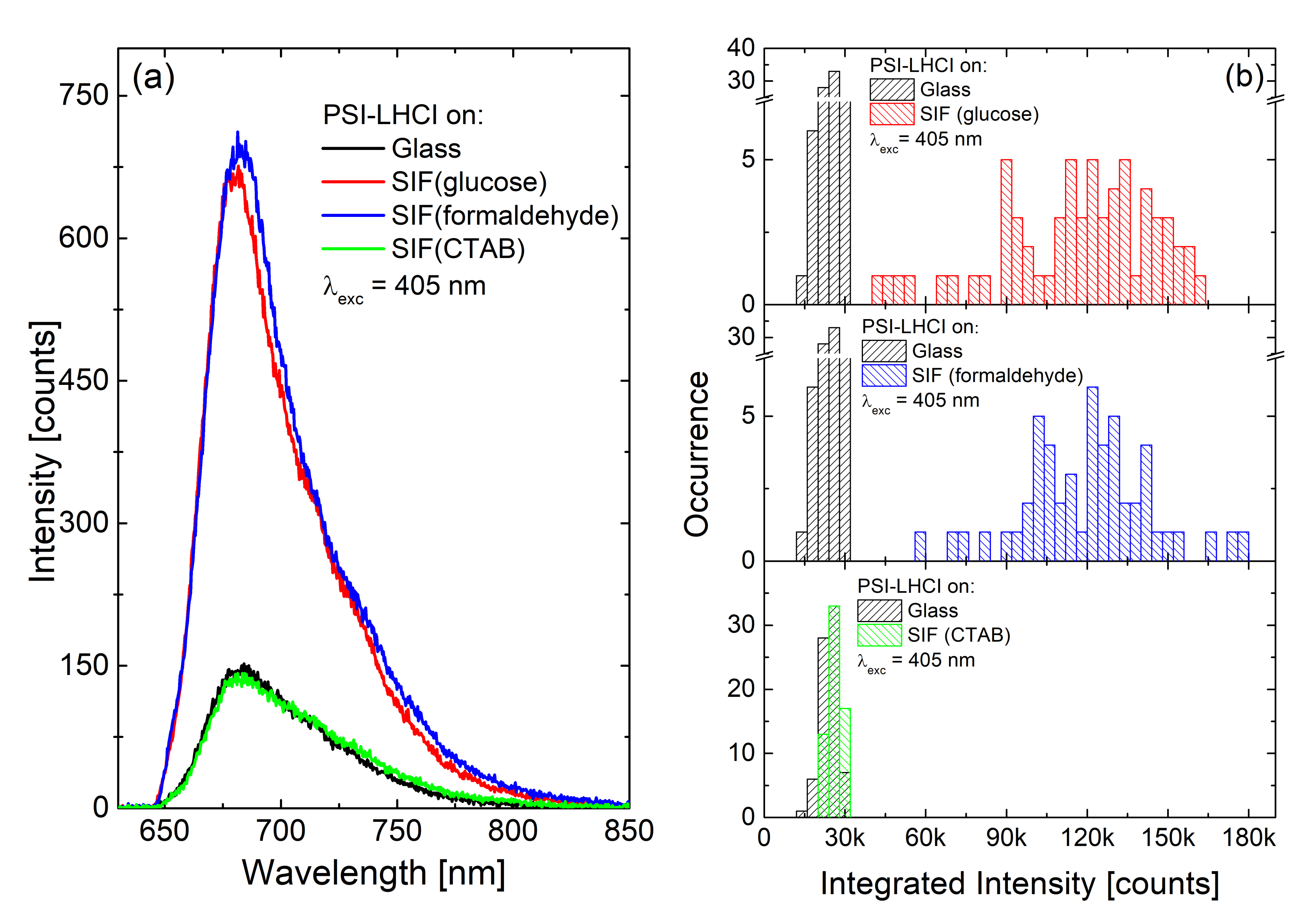
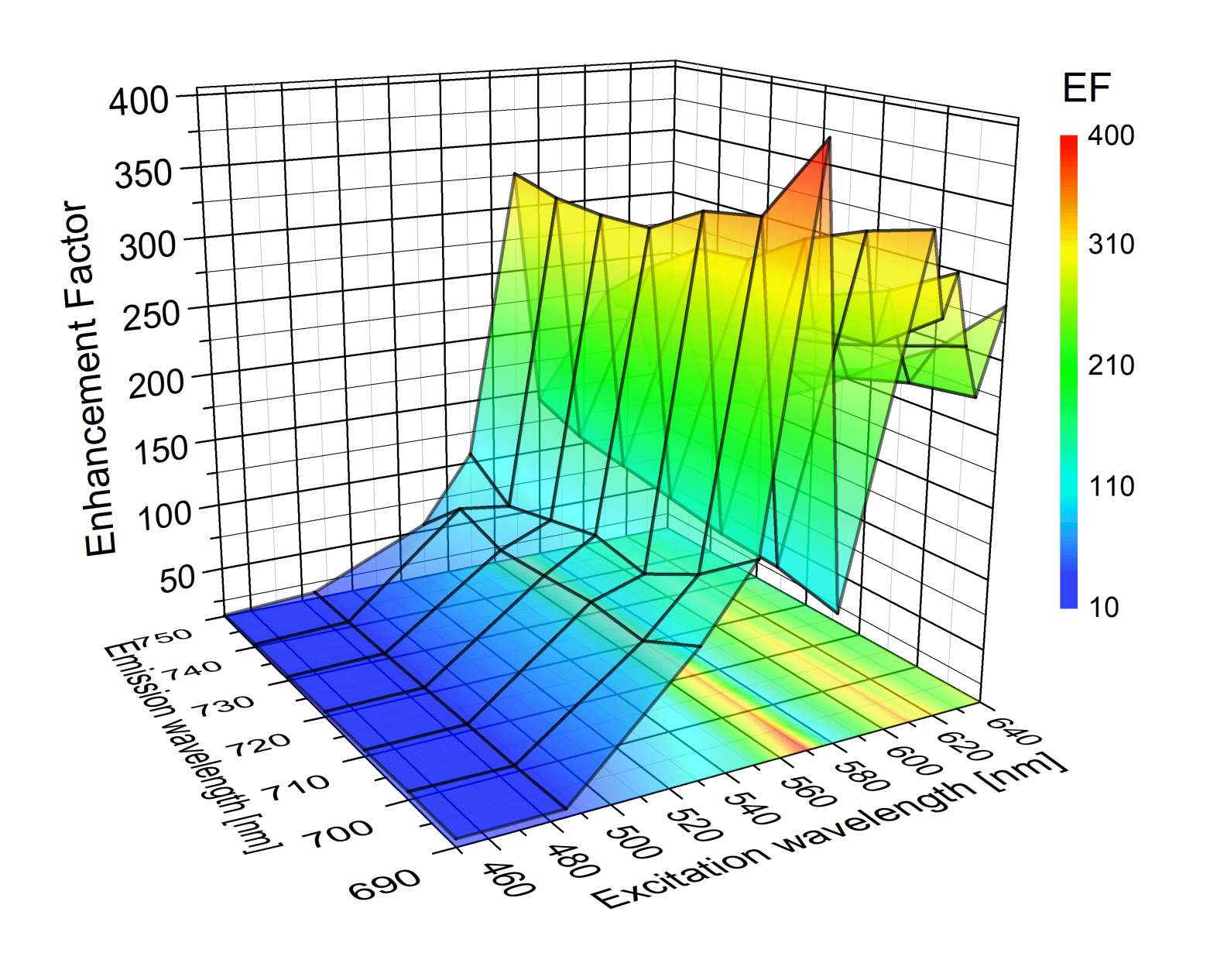
| Complex | SIF | λexc [nm] | EF |
|---|---|---|---|
| PCP [20] | (glucose) semi-transparent | 532 (632) | 6 (8.5) |
| FMO [23] | (glucose) dense | 405 | 40 |
| RC [41] | (glucose) dense | 405–640 | up to 60 |
| PSI-LHCI | (glucose) semi-transparent | 405 (570) | 5 (7) |
| (formaldehyde) semi-transparent | 5 (1) | ||
| (CTAB) semi-transparent | 1 (0.2) | ||
| PSI | (glucose) semi-transparent | 405 (570) | 5 (8) |
| SIF (formaldehyde) semi-transparent | 3 (12) | ||
| (CTAB) semi-transparent | 1 (0.8) | ||
| PSI [22] | (glucose) dense | 450–640 | up to 400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, D.; Szalkowski, M.; Sulowska, K.; Buczynska, D.; Niedziolka-Jonsson, J.; Jonsson-Niedziolka, M.; Kargul, J.; Lokstein, H.; Mackowski, S. Silver Island Film for Enhancing Light Harvesting in Natural Photosynthetic Proteins. Int. J. Mol. Sci. 2020, 21, 2451. https://doi.org/10.3390/ijms21072451
Kowalska D, Szalkowski M, Sulowska K, Buczynska D, Niedziolka-Jonsson J, Jonsson-Niedziolka M, Kargul J, Lokstein H, Mackowski S. Silver Island Film for Enhancing Light Harvesting in Natural Photosynthetic Proteins. International Journal of Molecular Sciences. 2020; 21(7):2451. https://doi.org/10.3390/ijms21072451
Chicago/Turabian StyleKowalska, Dorota, Marcin Szalkowski, Karolina Sulowska, Dorota Buczynska, Joanna Niedziolka-Jonsson, Martin Jonsson-Niedziolka, Joanna Kargul, Heiko Lokstein, and Sebastian Mackowski. 2020. "Silver Island Film for Enhancing Light Harvesting in Natural Photosynthetic Proteins" International Journal of Molecular Sciences 21, no. 7: 2451. https://doi.org/10.3390/ijms21072451
APA StyleKowalska, D., Szalkowski, M., Sulowska, K., Buczynska, D., Niedziolka-Jonsson, J., Jonsson-Niedziolka, M., Kargul, J., Lokstein, H., & Mackowski, S. (2020). Silver Island Film for Enhancing Light Harvesting in Natural Photosynthetic Proteins. International Journal of Molecular Sciences, 21(7), 2451. https://doi.org/10.3390/ijms21072451









