Altered Cell Surface N-Glycosylation of Resting and Activated T Cells in Systemic Lupus Erythematosus
Abstract
1. Introduction
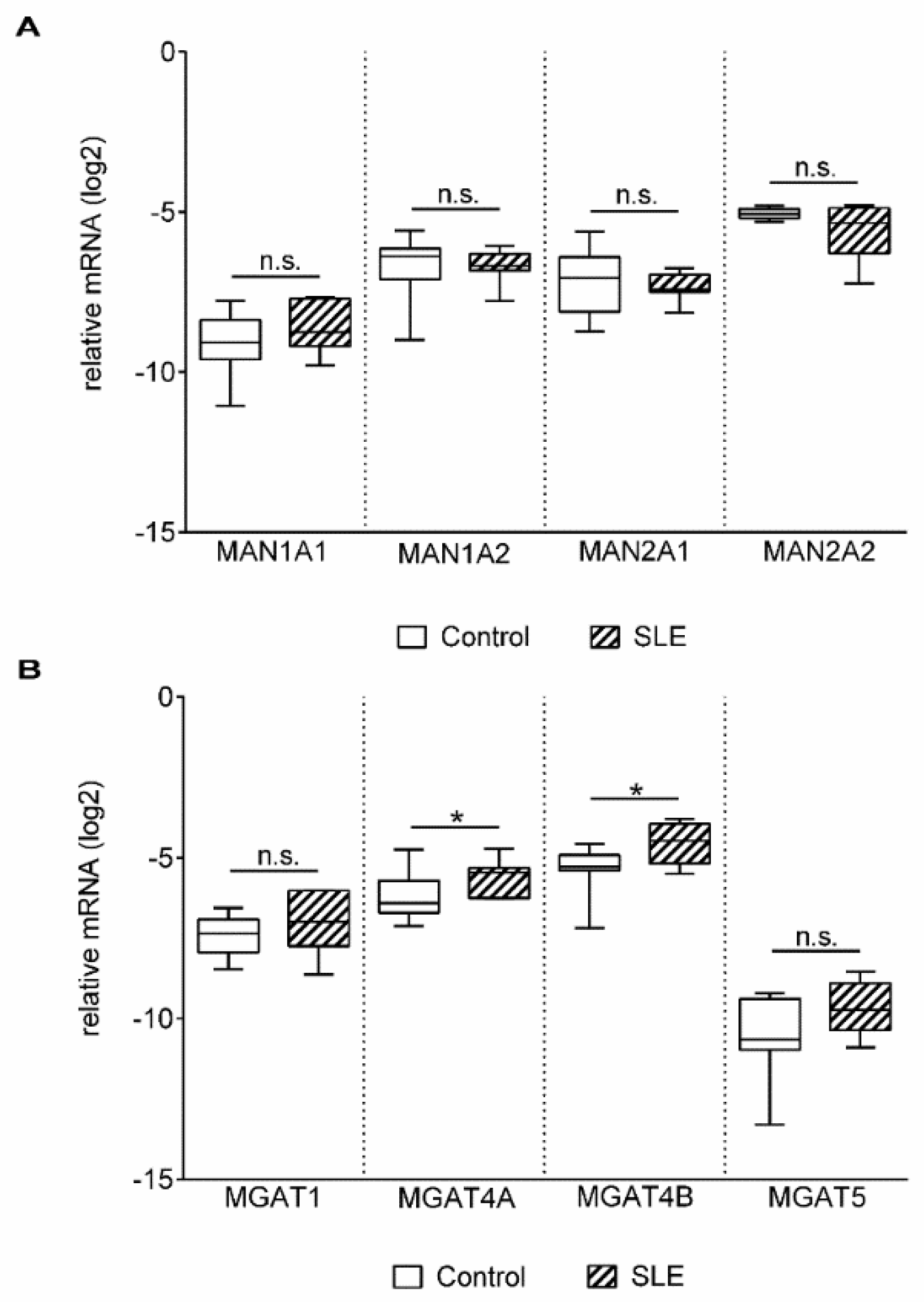
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Patients
4.3. Cells
4.4. Lectin Binding Assay
4.5. Neuraminidase Treatment
4.6. Quantitative Real-Time PCR (qPCR)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SLE | Systemic lupus erythematosus |
| Gal-1 | Galectin-1 |
| NEU | Neuraminidase |
| ConA | Concanavalin-A |
| LCA | Lens culinaris agglutinin |
| WGA | Wheat germ agglutinin |
| PHA | phytohaemagglutinin |
| PHA-L | Phaseolus vulgaris leukoagglutinin |
| SNA | Sambucus nigra agglutinin |
| MFI | Median fluorescence intensity |
| MGAT1–5- | Beta-N acetylglucosaminyltransferases |
| MAN | Mannosidase |
| MGAT | N-Acetyl glucosaminyltransferase |
| ST | Sialyltransferase |
| SLEDAI-2K | SLE disease activity index-2000 |
| Anti-dsDNA | Antibody to double-stranded DNA |
| PBMC | Peripheral blood mononuclear cell |
| PBS | Phosphate buffered saline |
| FACS | Fluorescence-activated cell sorting |
References
- Chachadi, V.B.; Cheng, H.; Klinkebiel, D.; Christman, J.K.; Cheng, P.W. 5-Aza-2′-deoxycytidine increases sialyl Lewis X on MUC1 by stimulating β-galactoside:α2,3-sialyltransferase 6 gene. Int. J. Biochem. Cell Biol. 2011, 43, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed]
- Axford, J.S. Glycosylation and rheumatic disease. Biochim. Biophys. Acta-Mol. Basis Dis. 1999, 1455, 219–229. [Google Scholar] [CrossRef][Green Version]
- Mackiewicz, A.; Mackiewicz, K. Glycoforms of serum alpha 1-acid glycoprotein as markers of inflammation and cancer. Glycoconj. J. 1995, 12, 241–247. [Google Scholar] [CrossRef]
- Sell, S. Progress in pathology cancer-associated carbohydrates identified by monoclonal antibodies. Hum. Pathol. 1990, 21, 1003–1019. [Google Scholar] [CrossRef]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and the altered glycan theory of autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef]
- Hauser, M.A.; Kindinger, I.; Laufer, J.M.; Späte, A.-K.; Bucher, D.; Vanes, S.L.; Krueger, W.A.; Wittmann, V.; Legler, D.F. Distinct CCR7 glycosylation pattern shapes receptor signaling and endocytosis to modulate chemotactic responses. J. Leukoc. Biol. 2016, 99, 993–1007. [Google Scholar] [CrossRef]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef]
- Bieberich, E. Synthesis, Processing, and Function of N-glycans in N-glycoproteins. Adv. Neurobiol. 2014, 9, 47–70. [Google Scholar] [PubMed]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef] [PubMed]
- Garin, M.I.; Chu, C.-C.; Golshayan, D.; Cernuda-Morollon, E.; Wait, R.; Lechler, R.I. Galectin-1: A key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007, 109, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Motran, C.C.; Molinder, K.M.; Liu, S.D.; Poirier, F.; Miceli, M.C. Galectin-1 functions as a {Th}2 cytokine that selectively induces {Th}1 apoptosis and promotes {Th}2 function. Eur. J. Immunol. 2008, 38, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- Ion, G.; Fajka-Boja, R.; Tóth, G.K.; Caron, M.; Monostori, É. Role of p56lck and ZAP70-mediated tyrosine phosphorylation in galectin-1-induced cell death. Cell Death Differ. 2005, 12, 1145–1147. [Google Scholar] [CrossRef]
- Ion, G.; Fajka-Boja, R.; Kovács, F.; Szebeni, G.; Gombos, I.; Czibula, Á.; Matkó, J.; Monostori, É. Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cell Signal. 2006, 18, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Sólyom, F.; Blaskó, A.; Fajka-Boja, R.; Katona, R.L.; Végh, L.; Novák, J.; Szebeni, G.J.; Krenács, L.; Uher, F.; Tubak, V.; et al. Mechanism of tumor cell-induced T-cell apoptosis mediated by galectin-1. Immunol. Lett. 2010, 127, 108–118. [Google Scholar] [CrossRef]
- Blaskó, A.; Fajka-Boja, R.; Ion, G.; Monostori, É. How does it act when soluble? Critical evaluation of mechanism of galectin-1 induced T-cell apoptosis. Acta Biol. Hung. 2011, 62, 106–111. [Google Scholar] [CrossRef]
- Novák, J.; Kriston-Pál, É.; Czibula, Á.; Deák, M.; Kovács, L.; Monostori, É.; Fajka-Boja, R. GM1 controlled lateral segregation of tyrosine kinase Lck predispose T-cells to cell-derived galectin-1-induced apoptosis. Mol. Immunol. 2014, 57, 302–309. [Google Scholar] [CrossRef][Green Version]
- Cabrera, P.V.; Amano, M.; Mitoma, J.; Chan, J.; Said, J.; Fukuda, M.; Baum, L.G. Haploinsufficiency of C2GnT-I glycosyltransferase renders T lymphoma cells resistant to cell death. Blood 2006, 108, 2399–2406. [Google Scholar] [CrossRef]
- Deák, M.; Hornung, Á.; Novák, J.; Demydenko, D.; Szabó, E.; Czibula, Á.; Fajka-Boja, R.; Kriston-Pál, É.; Monostori, É.; Kovács, L. Novel role for galectin-1 in T-cells under physiological and pathological conditions. Immunobiology 2015, 220, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Etzler, M.E. Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Maupin, K.A.; Liden, D.; Haab, B.B. The fine specificity of mannose-binding and galactose-binding lectins revealed using outlier motif analysis of glycan array data. Glycobiology 2012, 22, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisumsativum using frontal affinity chromatography. Glycobiology 2009, 19, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.P.; Goldstein, I.J.; Flashner, M.; Ebisu, S. Interaction of Wheat Germ Agglutinin with Sialic Acid. Biochemistry 1979, 18, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Ibanez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar] [PubMed]
- Schwarz, R.E.; Wojciechowicz, D.C.; Park, P.Y.; Paty, P.B. Phytohemagglutinin-L (PHA-L) lectin surface binding of N-linked β1-6 carbohydrate and its relationship to activated mutant ras in human pancreatic cancer cell-lines. Cancer Lett. 1996, 107, 285–291. [Google Scholar] [CrossRef]
- Fischer, E.; Brossmer, R. Sialic acid-binding lectins: Submolecular specificity and interaction with sialoglycoproteins and tumour cells. Glycoconj. J. 1995, 12, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Itakura, Y.; Nakamura-Tsuruta, S.; Kominami, J.; Tateno, H.; Hirabayashi, J. Sugar-binding profiles of chitin-binding lectins from the hevein family: A comprehensive study. Int. J. Mol. Sci. 2017, 18, 1160. [Google Scholar] [CrossRef] [PubMed]
- Pothukuchi, P.; Agliarulo, I.; Russo, D.; Rizzo, R.; Russo, F.; Parashuraman, S. Translation of genome to glycome: Role of the Golgi apparatus. FEBS Lett. 2019. [Google Scholar] [CrossRef]
- Comelli, E.M.; Head, S.R.; Gilmartin, T.; Whisenant, T.; Haslam, S.M.; North, S.J.; Wong, N.K.; Kudo, T.; Narimatsu, H.; Esko, J.D.; et al. A focused microarray approach to functional glycomics: Transcriptional regulation of the glycome. Glycobiology 2006, 16, 117–131. [Google Scholar] [CrossRef]
- Nairn, A.V.; York, W.S.; Harris, K.; Hall, E.M.; Pierce, J.M.; Moremen, K.W. Regulation of glycan structures in animal tissues. J. Biol. Chem. 2008, 283, 17298–17313. [Google Scholar] [CrossRef] [PubMed]
- Altheide, T.K.; Hayakawa, T.; Mikkelsen, T.S.; Diaz, S.; Varki, N.; Varki, A. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents. J. Biol. Chem. 2006, 281, 25689–25702. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Carubelli, I.; Stamatos, N.M. Sialidase expression in activated human T lymphocytes influences production of IFN-γ. J. Leukoc. Biol. 2007, 81, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Katsiari, C.G.; Liossis, S.N.C.; Dimopoulos, A.M.; Charalambopoulos, D.V.; Mavrikakis, M.; Sfikakis, P.P. CD40L overexpression on T cells and monocytes from patients with systemic lupus erythematosus is resistant to calcineurin inhibition. Lupus 2002, 11, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Lesley, J. CD44 structure and function. Front. Biosci. 2016, 3, 616–630. [Google Scholar] [CrossRef]
- Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Role of CD44 in the differentiation of Th1 and Th2 cells: CD44-deficiency enhances the development of Th2 effectors in response to sheep RBC and chicken ovalbumin. J. Immunol. 2009, 183, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Harada, T.; Juang, Y.-T.; Kyttaris, V.C.; Wang, Y.; Zidanic, M.; Tung, K.; Tsokos, G.C. Phosphorylated ERM Is Responsible for Increased T Cell Polarization, Adhesion, and Migration in Patients with Systemic Lupus Erythematosus. J. Immunol. 2007, 178, 1938–1947. [Google Scholar] [CrossRef]
- Jury, E.C.; Flores-Borja, F.; Kabouridis, P.S. Lipid rafts in T cell signalling and disease. Semin. Cell Dev. Biol. 2007, 18, 608–615. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef]
- Polley, A.; Orłowski, A.; Danne, R.; Gurtovenko, A.A.; Bernardino de la Serna, J.; Eggeling, C.; Davis, S.J.; Róg, T.; Vattulainen, I. Glycosylation and Lipids Working in Concert Direct CD2 Ectodomain Orientation and Presentation. J. Phys. Chem. Lett. 2017, 8, 1060–1066. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, C.F.; Grigorian, A.; Tian, W.; Demetriou, M. T cell receptor signaling co-regulates multiple Golgi genes to enhance N-glycan branching. J. Biol. Chem. 2009, 284, 32454–32461. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.D.; Klein, J.; Van Dyken, S.J.; Marth, J.D.; Baum, L.G. T-cell activation results in microheterogeneous changes in glycosylation of CD45. Int. Immunol. 2007, 19, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Comelli, E.M.; Sutton-Smith, M.; Yan, Q.; Amado, M.; Panico, M.; Gilmartin, T.; Whisenant, T.; Lanigan, C.M.; Head, S.R.; Goldberg, D.; et al. Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J. Immunol. 2006, 177, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, S.; Regola, F.; Zanola, A.; Andreoli, L.; Dall’Ara, F.; Tincani, A.; Airo’, P. Effector T-cells are expanded in systemic lupus erythematosus patients with highdisease activity and damage indexes. Lupus 2018, 27, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, R.D.; Shen, X.; Illei, G.G.; Yarboro, C.H.; Prussin, C.; Hathcock, K.S.; Hodes, R.J.; Lipsky, P.E. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2184–2197. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 exhibit differential recognition of sialylatedglycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef] [PubMed]
- Dotan, N.; Altstock, R.T.; Schwarz, M.; Dukler, A. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus 2006, 15, 442–450. [Google Scholar] [CrossRef]
- Harding, C.V.; Kihlberg, J.; Elofsson, M.; Magnusson, G.; Unanue, E.R. Glycopeptides bind MHC molecules and elicit specific T cell responses. J. Immunol. 1993, 151, 2419. [Google Scholar]
- Jensen, T.; Hansen, P.; Galli-Stampino, L.; Mouritsen, S.; Frische, K.; Meinjohanns, E.; Meldal, M.; Werdelin, O. Glycopeptide specific T cell hybridomas raised against an αGalNAc O-glycosylated self peptide are discriminating between highly related carbohydrate groups. Immunol. Lett. 1997, 56, 449. [Google Scholar] [CrossRef]
- Green, R.S.; Stone, E.L.; Tenno, M.; Lehtonen, E.; Farquhar, M.G.; Marth, J.D. Mammalian N-Glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity 2007, 27, 308–320. [Google Scholar] [CrossRef]
- Moremen, K.W. Golgi alpha-mannosidase II deficiency in vertebrate systems: Implications for asparagine-linked oligosaccharide processing in mammals. Biochim. Biophys. Acta 2002, 1573, 225–235. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.-M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Ramhorst, R.E.; Rubinstein, N.; Corigliano, A.; Daroqui, M.C.; Kier-Joffé, E.B.; Fainboim, L. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 2002, 9, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, H.; Song, X.; Shi, S.; Zhang, J.; Jia, L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene 2015, 34, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Lupo, B.; Cirillo, F.; Papini, N.; Anastasia, L.; Lamorte, G.; Colombi, P.; Bresciani, R.; Monti, E.; Tettamanti, G.; et al. Silencing of membrane-associated sialidase NEU3 diminishes apoptosis resistance and triggers megakaryocytic differentiation of chronic myeloid leukemic cells K562 through the increase of ganglioside GM3. Cell Death Differ. 2009, 16, 164–174. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, H.; Wei, W.; Ji, D.; Song, X.; Sun, J.; Zhang, J.; Jia, L. B4GALT family mediates the multidrug resistance of human leukemia cells by regulating the hedgehog pathway and the expression of p-glycoprotein and multidrug resistance-associated protein 1. Cell Death Dis. 2013, 4, e654. [Google Scholar] [CrossRef]
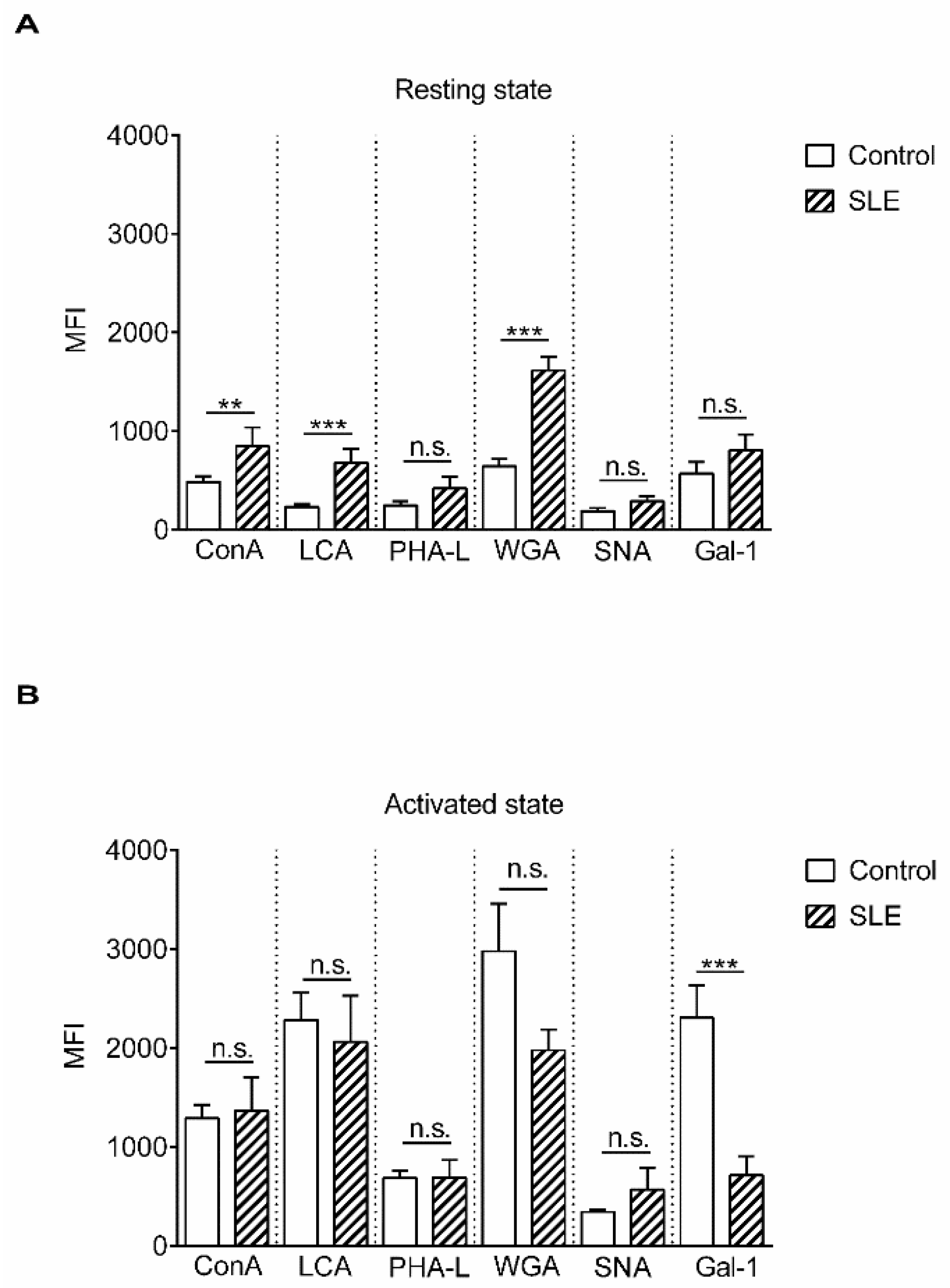


| Lectins | Abbreviation | Specificity | Reference |
|---|---|---|---|
| Concanavalin A | ConA | mannose, glucose (low affinity) | [21,22] |
| Lens culinaris agglutinin | LCA | core-fucosylated bi-antennary N-glycan | [22,23] |
| Wheat germ agglutinin | WGA | GlcNAc, sialic acid | [24,25] |
| Phaseolus vulgaris leucoagglutinin | PHA-L | β-1,6-branched tri- and tetra-antennary N-glycans | [26] |
| Sambucus nigra agglutinin | SNA | α-2,6-linked sialic acid | [27] |
| Galectin-1 | Gal-1 | LAcNAc | [28] |
| Enzyme Genes | Gene Symbol | Full Gene Name |
|---|---|---|
| Mannosidases | MAN1A1 | Mannosidase alpha class 1A member 1 |
| MAN1A2 | Mannosidase alpha class 1A member 2 | |
| MAN2A1 | Mannosidase alpha class 2A member 1 | |
| MAN2A2 | Mannosidase alpha class 2A member 2 | |
| N-Acetylglucosaminyltransferase | MGAT1 | Mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyl-transferase |
| MGAT4A | Mannosyl (alpha-1,3-)-glycoprotein beta-1,4-N-acetylglucosaminyl-transferase isozyme A | |
| MGAT4B | Mannosyl (alpha-1,3-)-glycoprotein beta-1,4-N-acetylglucosaminyl-transferase isozyme B | |
| MGAT5 | Mannosyl (alpha-1,6-)-glycoprotein beta-1,6-N-Acetyl-glucosaminyltransferase | |
| Sialyltransferases | ST3GAL3 | ST3 beta-galactosidealpha-2,3-sialyltransferase 3 |
| ST3GAL4 | ST3 beta-galactosidealpha-2,3-sialyltransferase 4 | |
| ST3GAL6 | ST3 beta-galactosidealpha-2,3-sialyltransferase 6 | |
| ST6GAL1 | ST6 beta-galactosamidealpha-2,6-sialyltranferase 1 | |
| Neuraminidases | NEU1 | Neuraminidase 1 |
| Subject Characteristics | Age | Female/Male | Disease Activity Parameter |
|---|---|---|---|
| SLE | 42 (23–54) | 17/1 | |
| SLEDAI-2K | 14 (6–30) | ||
| anti-dsDNA (IU/mL) | 88 (2–220) | ||
| Control | 54 (31–75) | 17/2 |
| Name | Forward Primer | Reverse Primer |
|---|---|---|
| RPL27 | 5′-CGCAAAGCTGTCATCGTG-3′ | 5′-GTCACTTTGCGGGGGTAG-3′ |
| MAN1A1 | 5′-TTGGGCATTGCTGAATATGA-3′ | 5′-CAGAATACTGCTGCCTCCAGA-3′ |
| MAN1A2 | 5′-GGAGGCCTACTTGCAGCATA-3′ | 5′-GAGTTTCTCAGCCAATTGCAC-3′ |
| MAN2A1 | 5′-CCTGGAAATGTCCAAAGCA-3′ | 5′-GCGGAAATCATCTCCTAGTGG-3′ |
| MAN2A2 | 5′-TCCACCTGCTCAACCTACG-3′ | 5′-TGTAAGATGAGTGCGGTCTCC-3′ |
| MGAT1 | 5′-CGGAGCAGGCCAAGTTC-3′ | 5′-CCTTGCCCGCAGTCCTA-3′ |
| MGAT4A | 5′-CATAGCGGCAACCAAGAAC-3′ | 5′-TGCTTATTTCCAAACCTTCACTC-3′ |
| MGAT4B | 5′-CACTCTGCACTCGCTCATCT-3′ | 5′-CACTGCCGAAGTGTACTGTGA-3′ |
| MGAT5 | 5′-GCTCATCTGCGAGCCTTCT-3′ | 5′-TTGGCAGGTCACCTTGTACTT-3′ |
| ST3GAL3 | 5′-TATGCTTCAGCCTTGATG-3′ | 5′-TTGGTGACTGACAAGATGG-3′ |
| ST3GAL4 | 5′-ATGTTGGCTCTGGTCCTG-3′ | 5′-AGGAAGATGGGCTGATCC-3′ |
| ST3GAL6 | 5′-TCTATTGGGTGGCACCTGTGGAAA-3 | 5′-TGATGAAACCTCAGCAGAGAGGCA-3′ |
| ST6GAL1 | 5′-TGGGACCCATCTGTATACCACT-3′ | 5′-ATTGGGGTGCAGCTTACGAT-3′ |
| NEU1 | 5′-CCTGGATATTGGCACTGAA-3′ | 5′-CATCGCTGAGGAGACAGAAG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, E.; Hornung, Á.; Monostori, É.; Bocskai, M.; Czibula, Á.; Kovács, L. Altered Cell Surface N-Glycosylation of Resting and Activated T Cells in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 4455. https://doi.org/10.3390/ijms20184455
Szabó E, Hornung Á, Monostori É, Bocskai M, Czibula Á, Kovács L. Altered Cell Surface N-Glycosylation of Resting and Activated T Cells in Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2019; 20(18):4455. https://doi.org/10.3390/ijms20184455
Chicago/Turabian StyleSzabó, Enikő, Ákos Hornung, Éva Monostori, Márta Bocskai, Ágnes Czibula, and László Kovács. 2019. "Altered Cell Surface N-Glycosylation of Resting and Activated T Cells in Systemic Lupus Erythematosus" International Journal of Molecular Sciences 20, no. 18: 4455. https://doi.org/10.3390/ijms20184455
APA StyleSzabó, E., Hornung, Á., Monostori, É., Bocskai, M., Czibula, Á., & Kovács, L. (2019). Altered Cell Surface N-Glycosylation of Resting and Activated T Cells in Systemic Lupus Erythematosus. International Journal of Molecular Sciences, 20(18), 4455. https://doi.org/10.3390/ijms20184455





