Serum Levels of CXCR4, SDF-1, MCP-1, NF-κB and ERK1/2 in Patients with Skeletal Fluorosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Collection and Processing
2.3. Measurement of Cytokines
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. X-ray Imaging Analysis of SF Patients
3.2.1. Case Analysis of a Patient Diagnosed by X-ray with Mild SF
3.2.2. Case Analysis of a Patient Diagnosed by X-ray with Moderate SF
3.2.3. Case Analysis of a Patient Diagnosed by X-ray with Severe SF
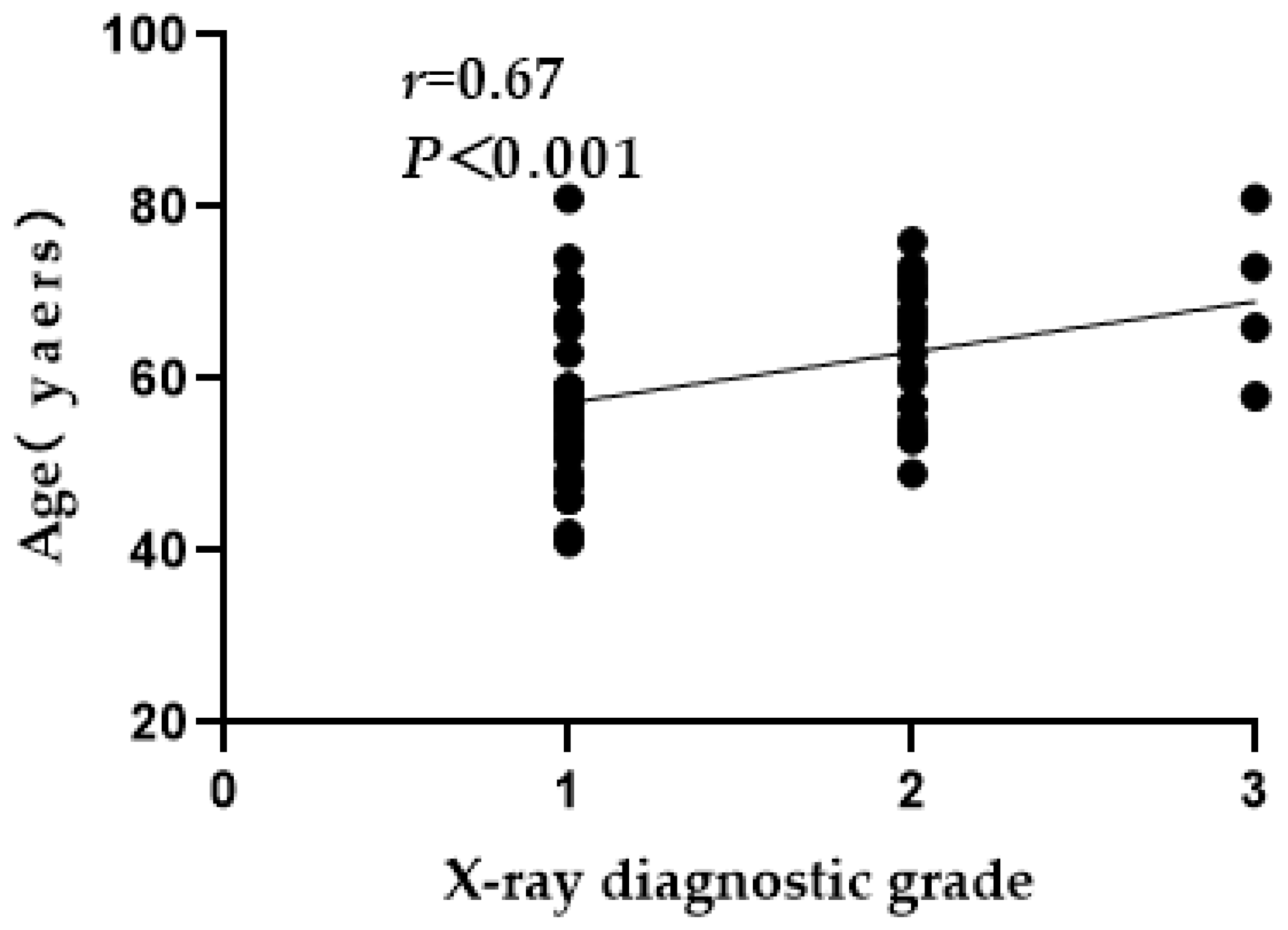
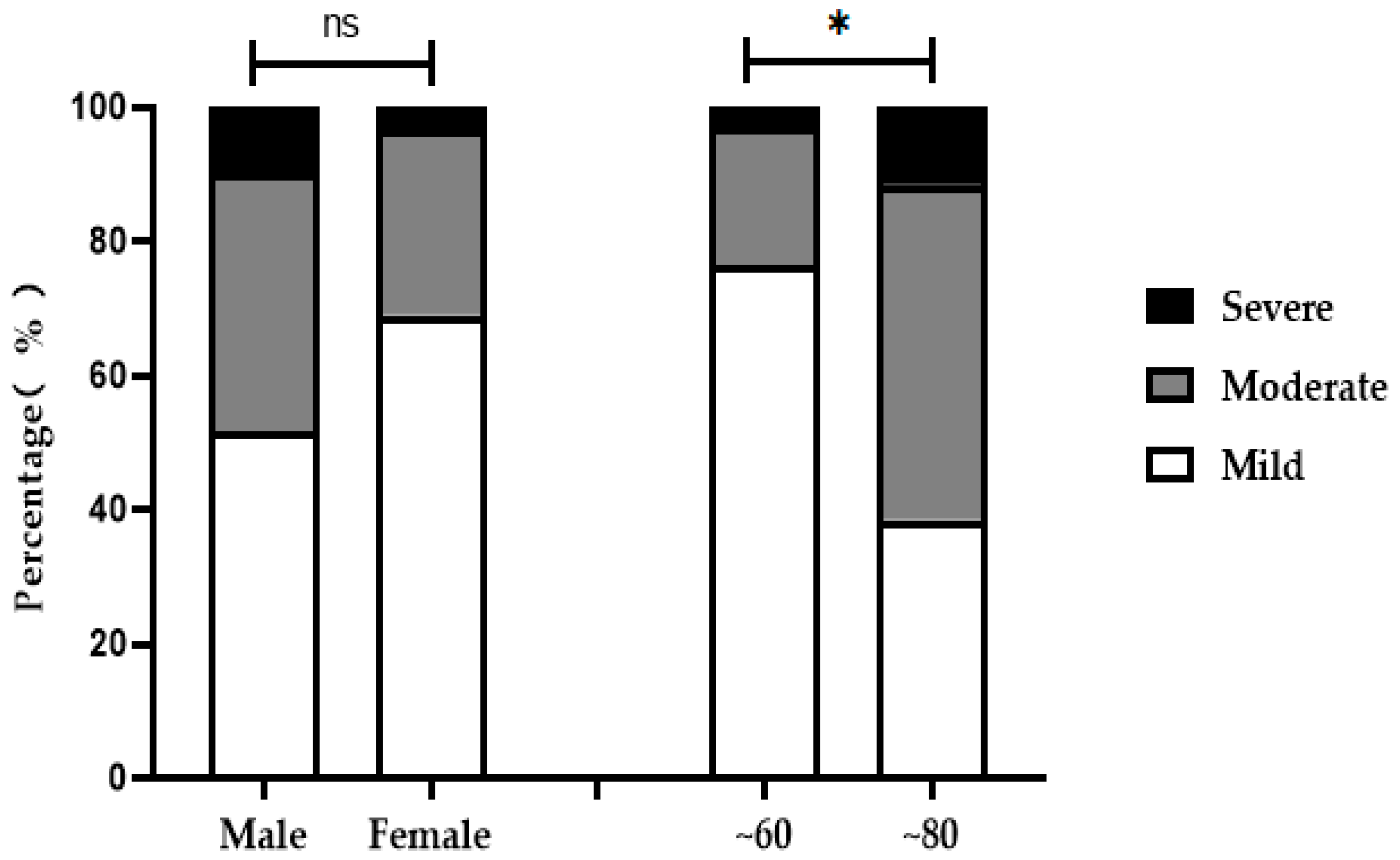
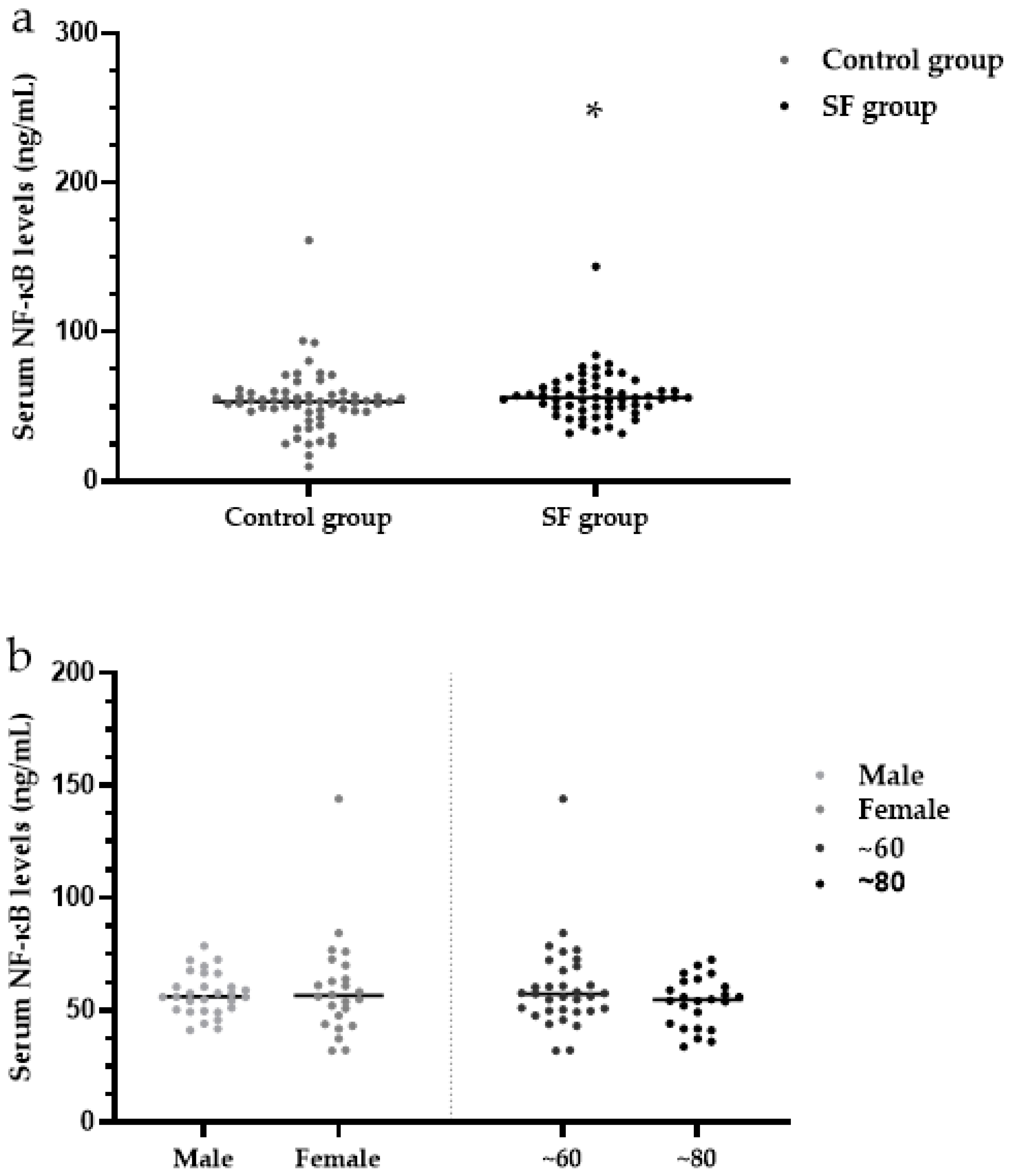
3.3. X-ray Grade Analysis of Ages, Age Groups and Sexes
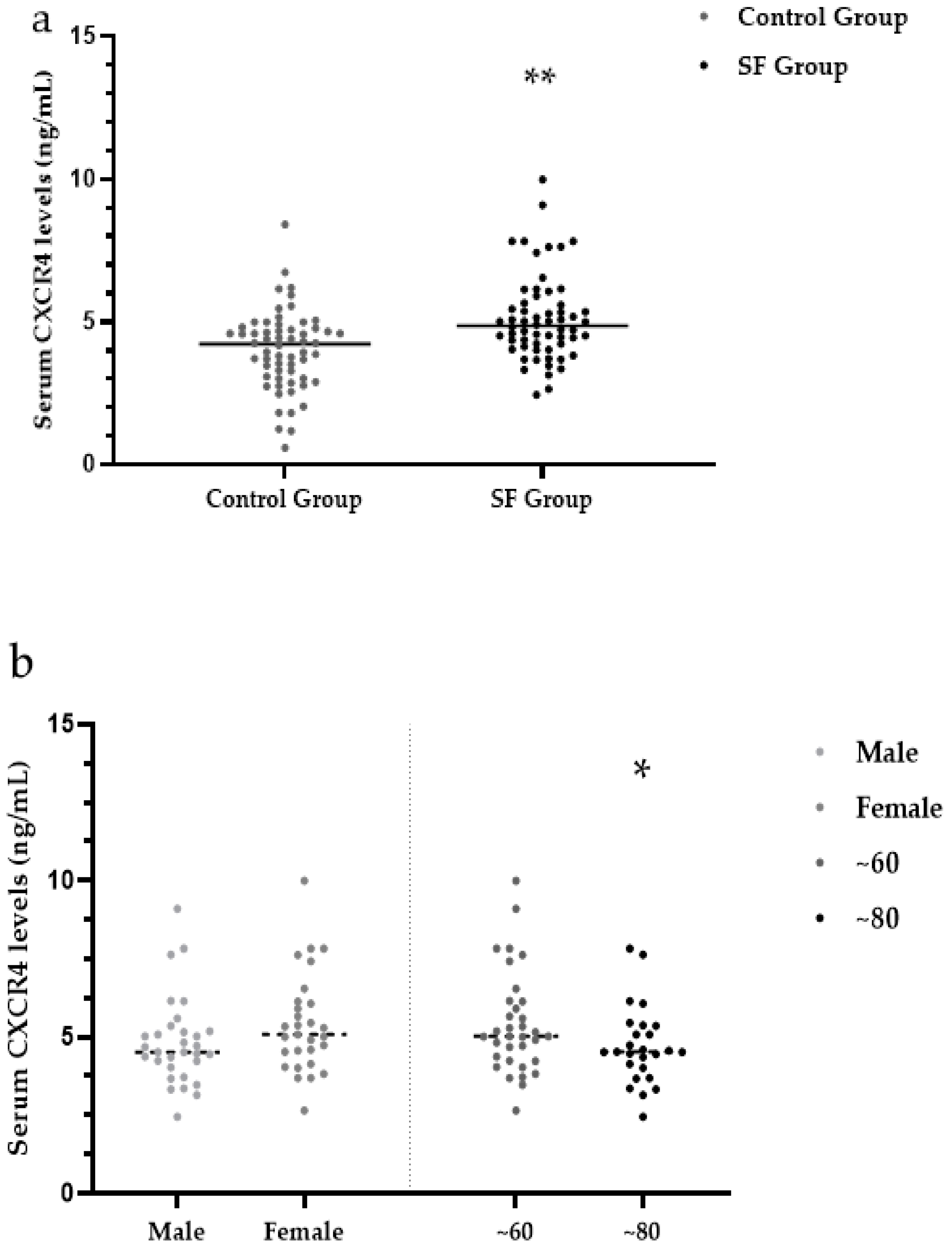
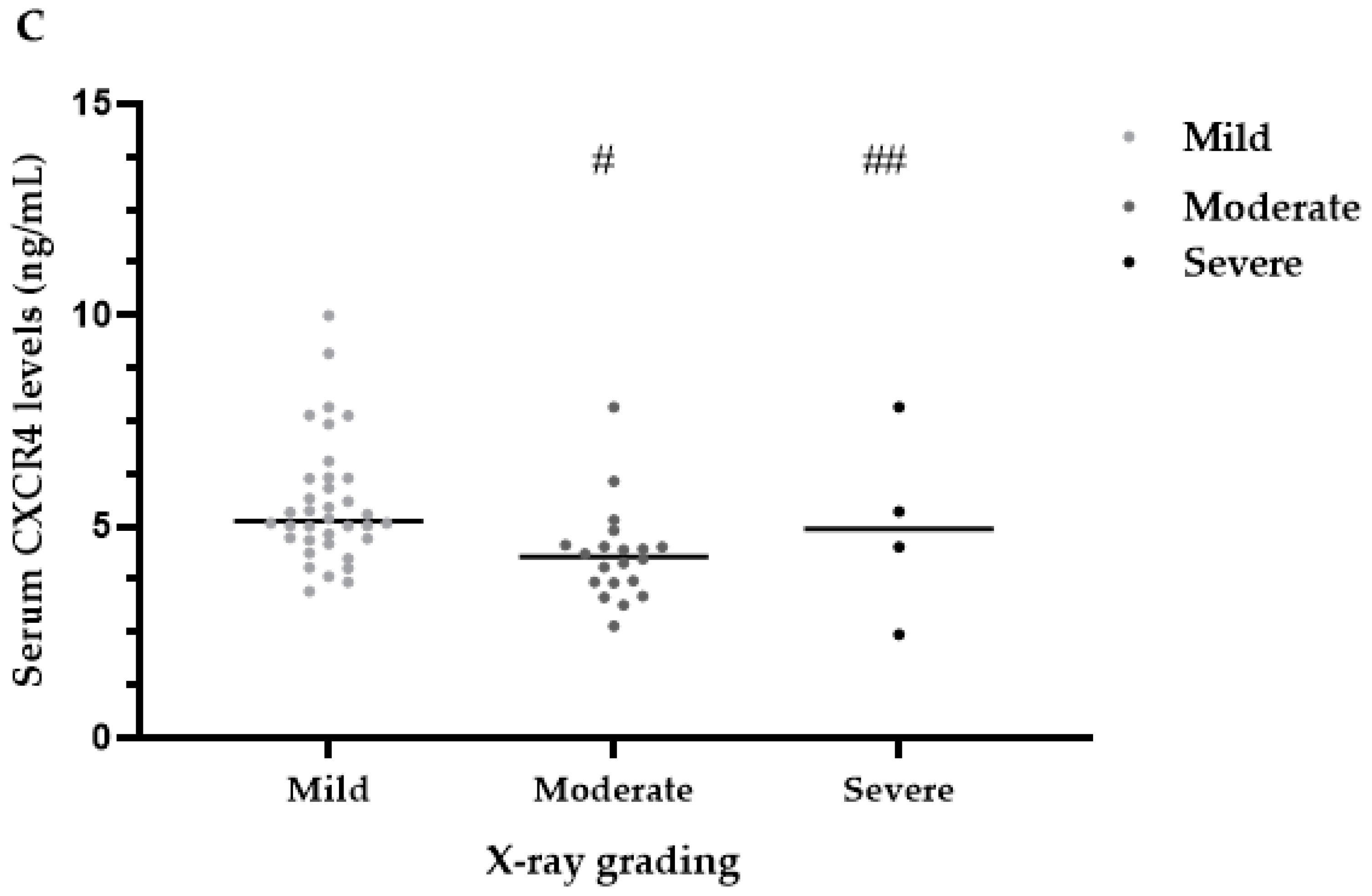
3.4. Serum CXCR4 Levels
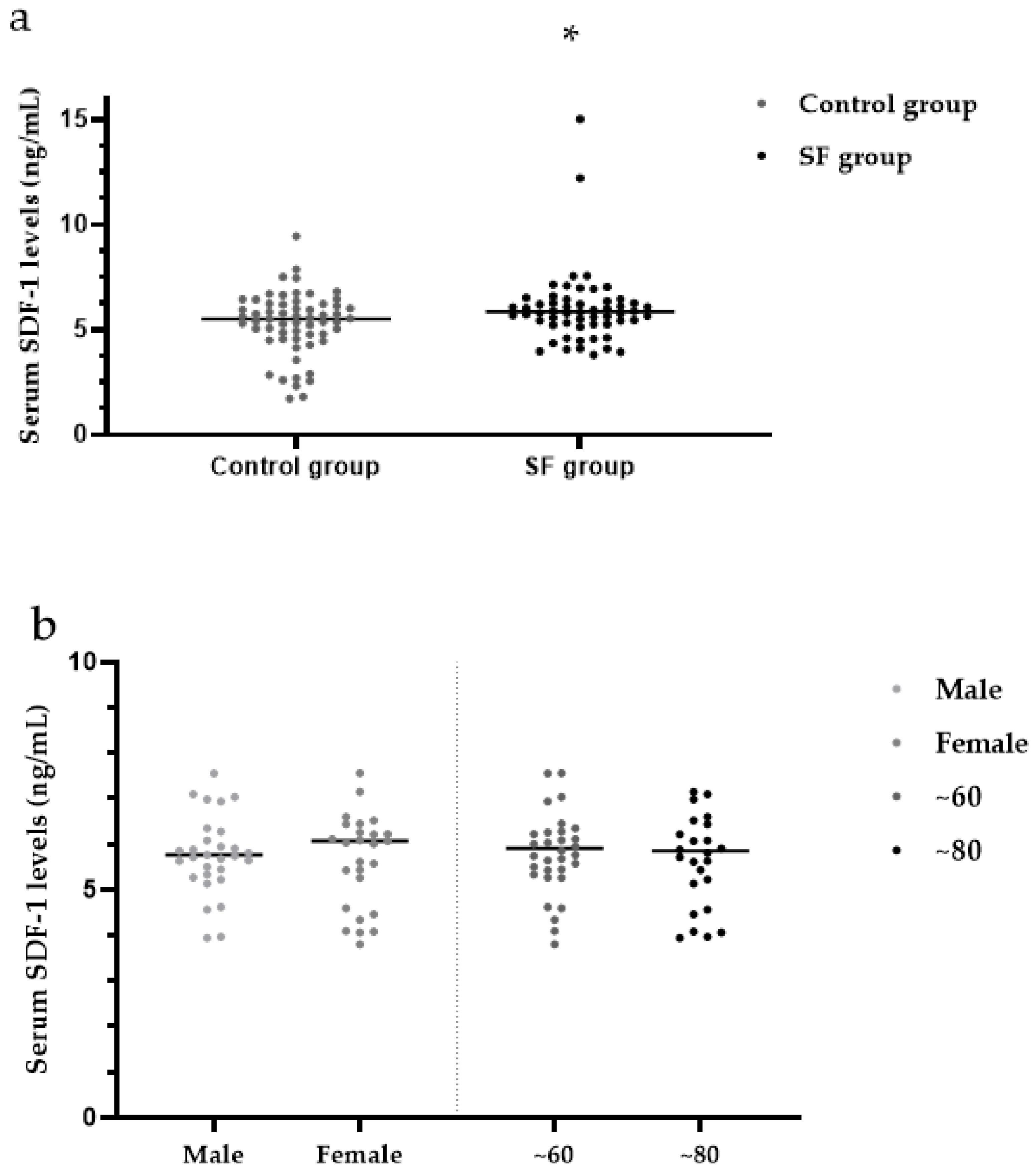
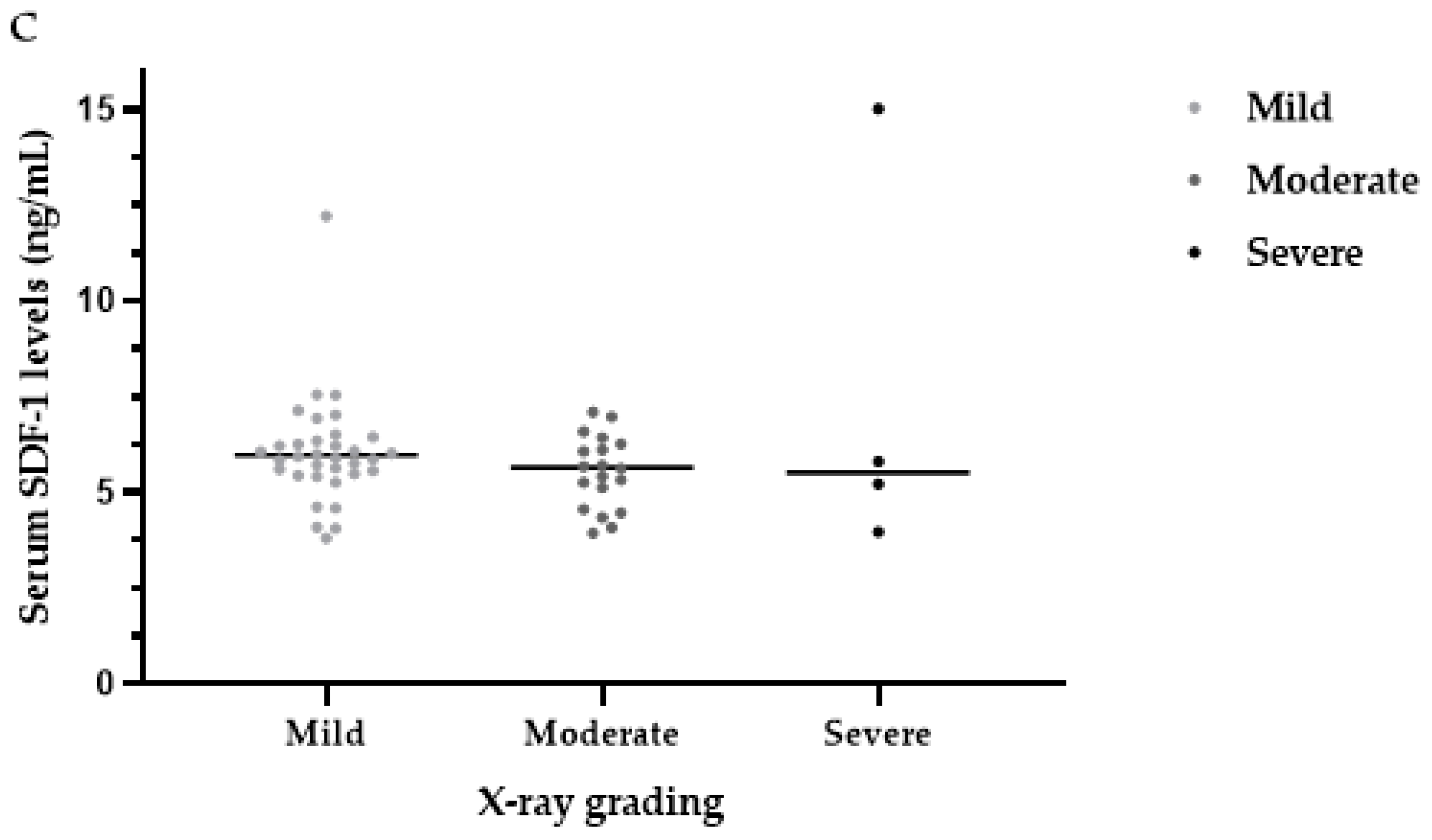
3.5. Serum SDF-1 Levels
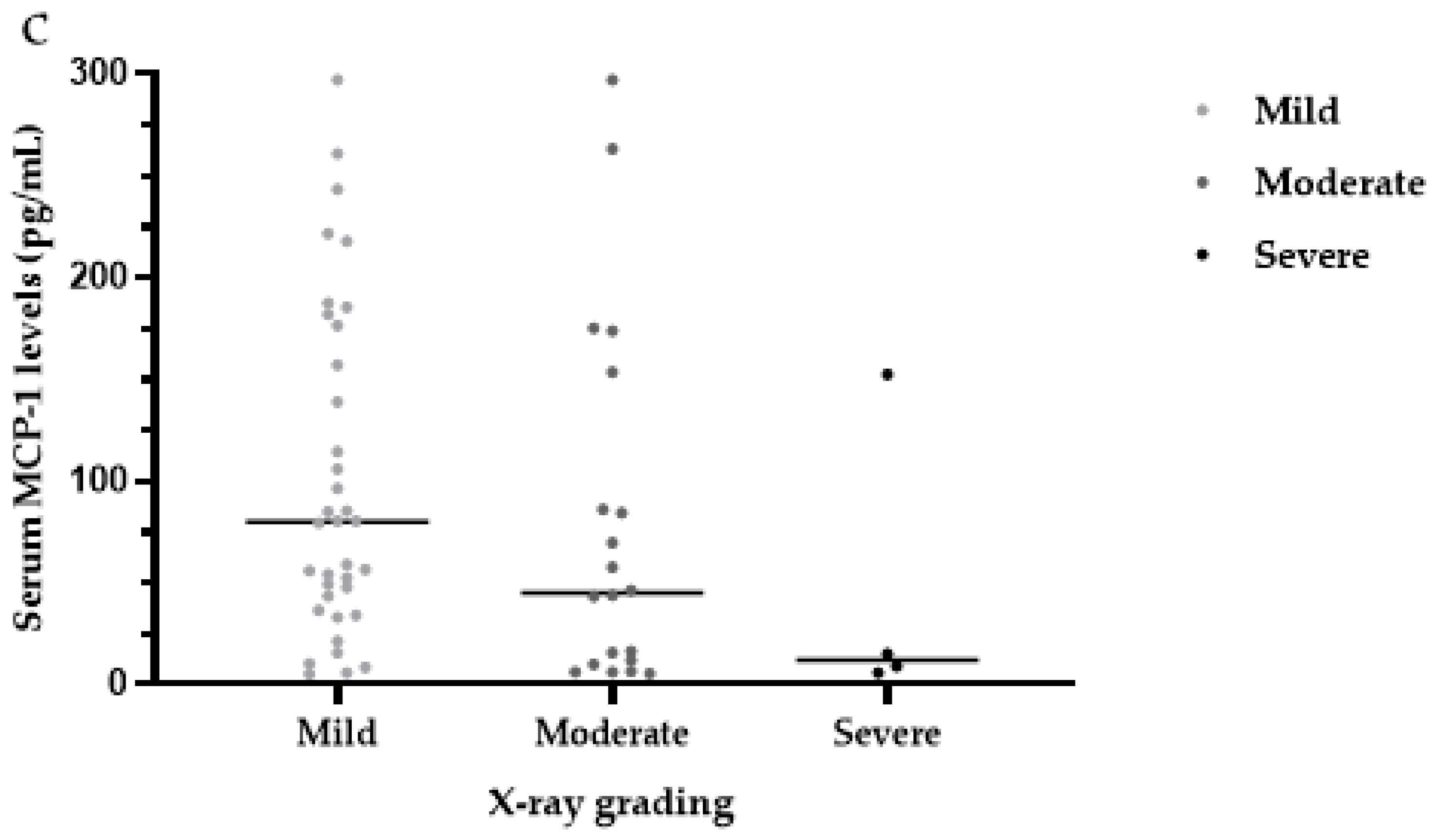
3.6. Serum MCP-1 Levels
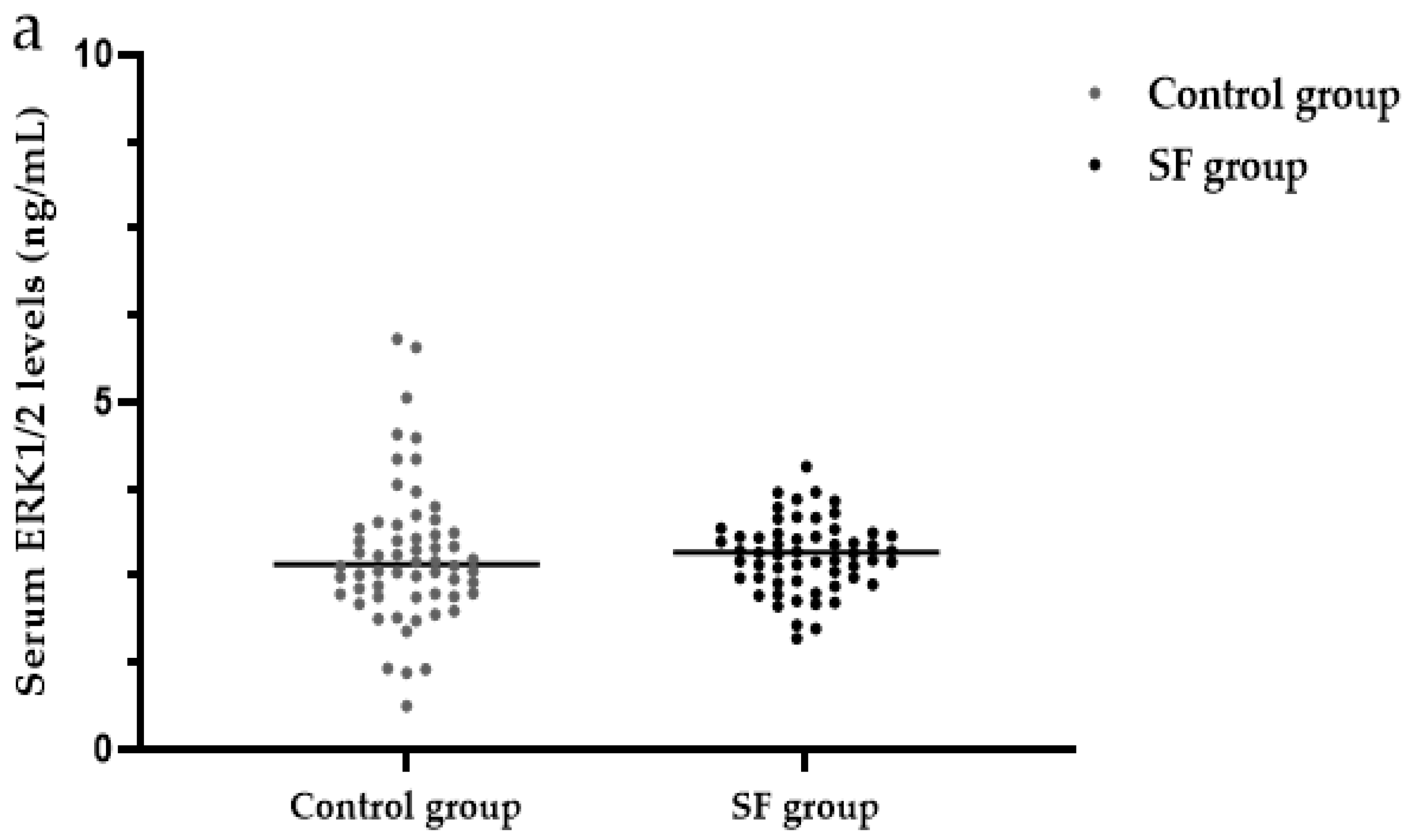
3.7. Serum ERK1/2 Levels
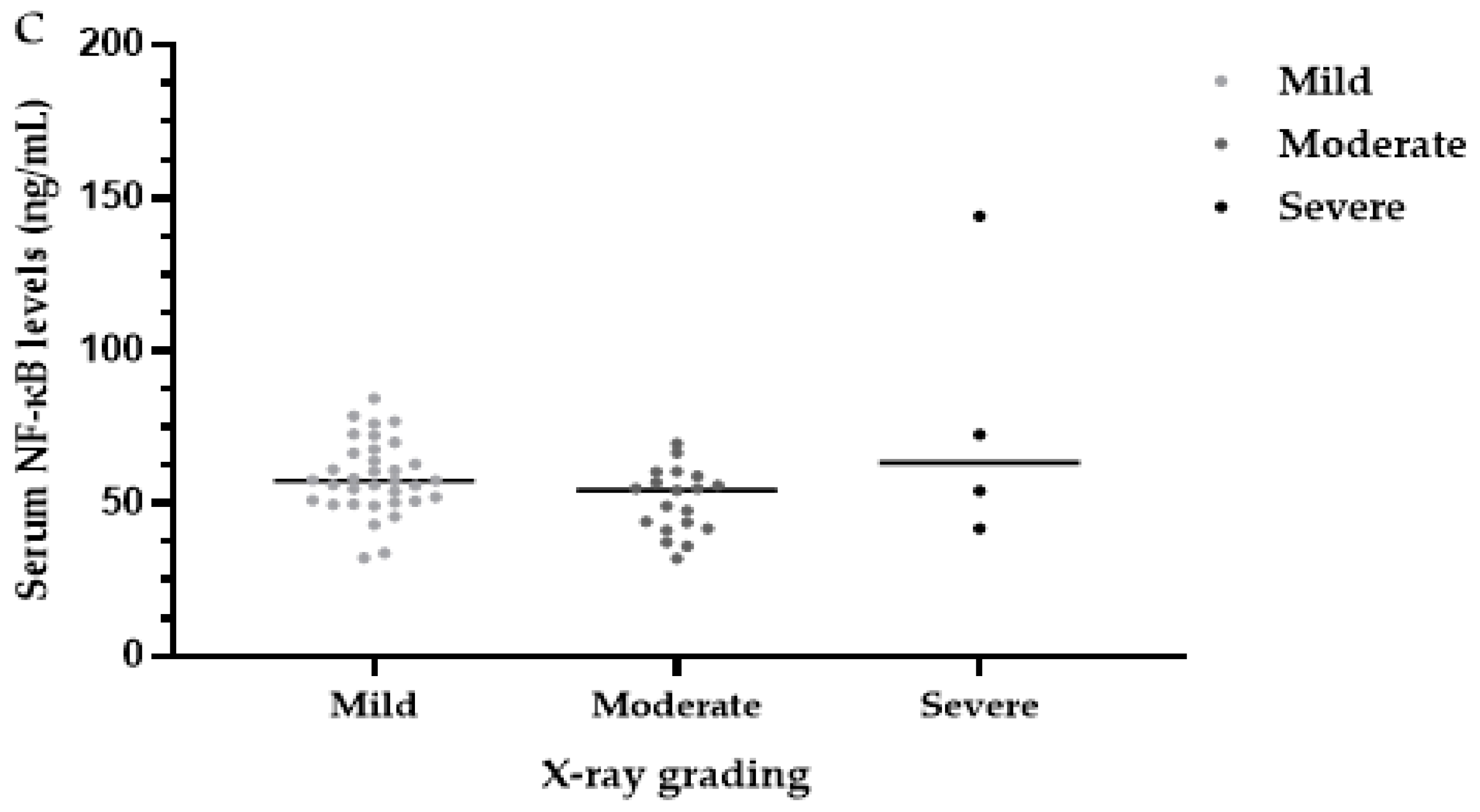
3.8. Serum NF-κB Levels
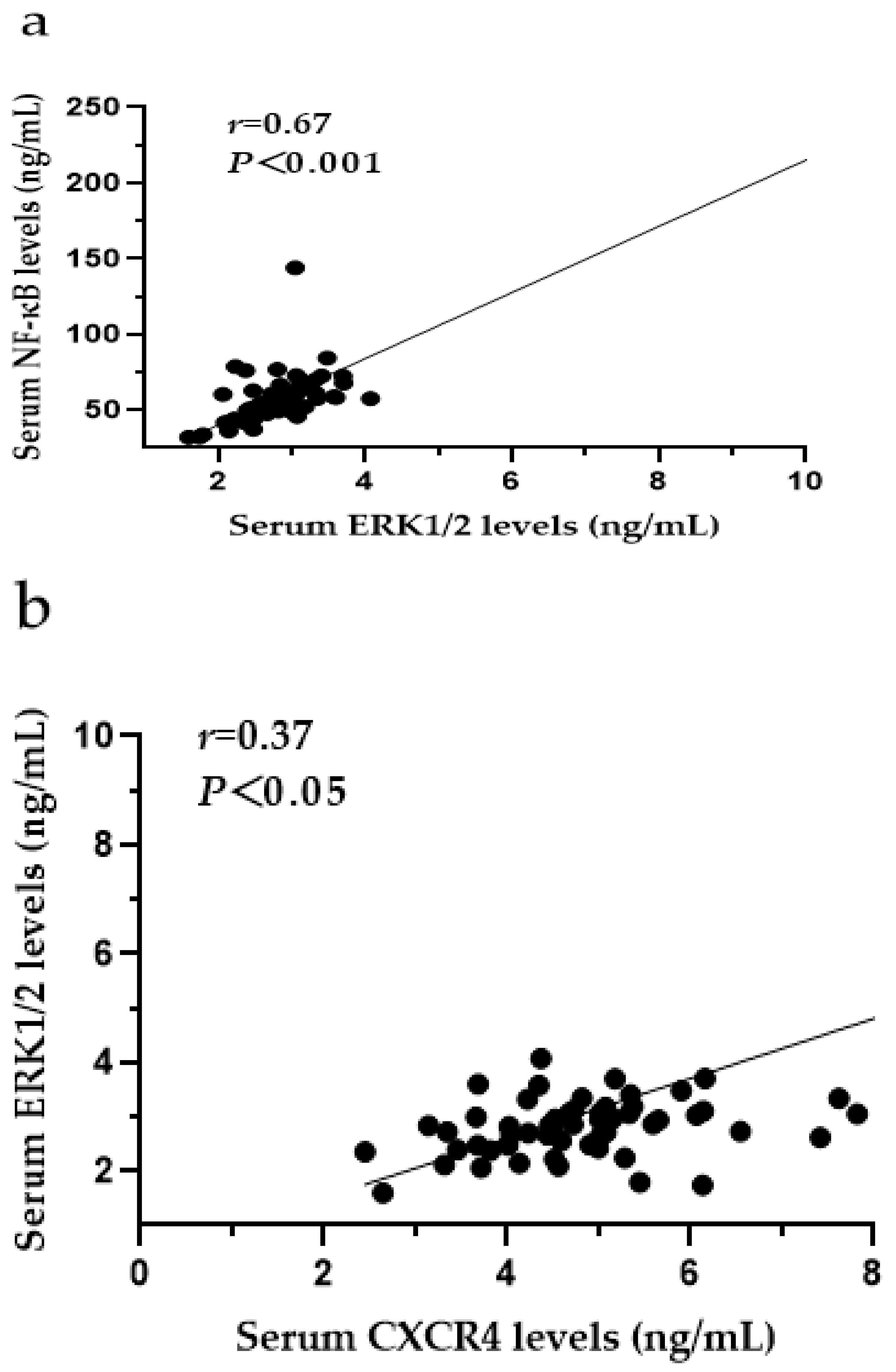
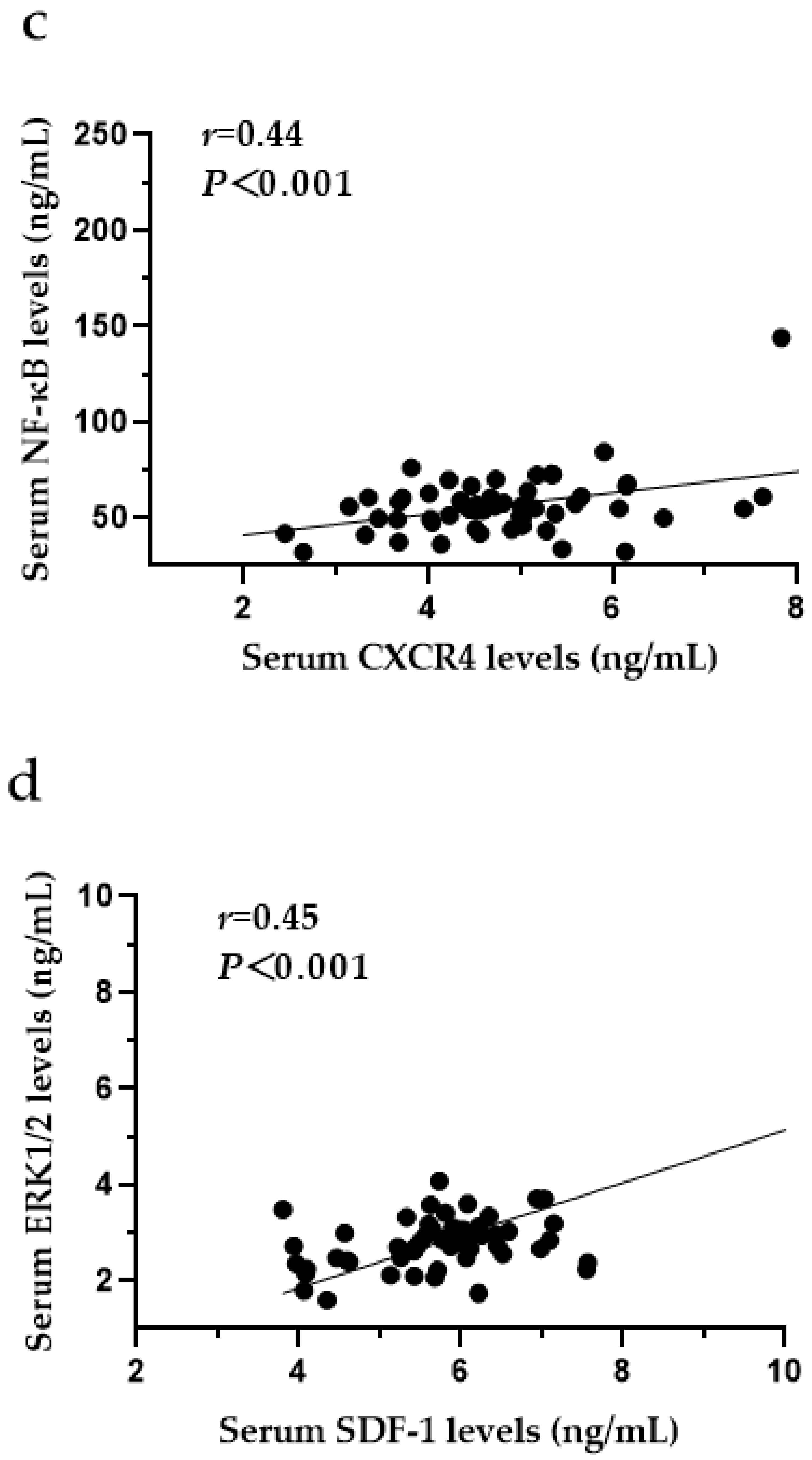
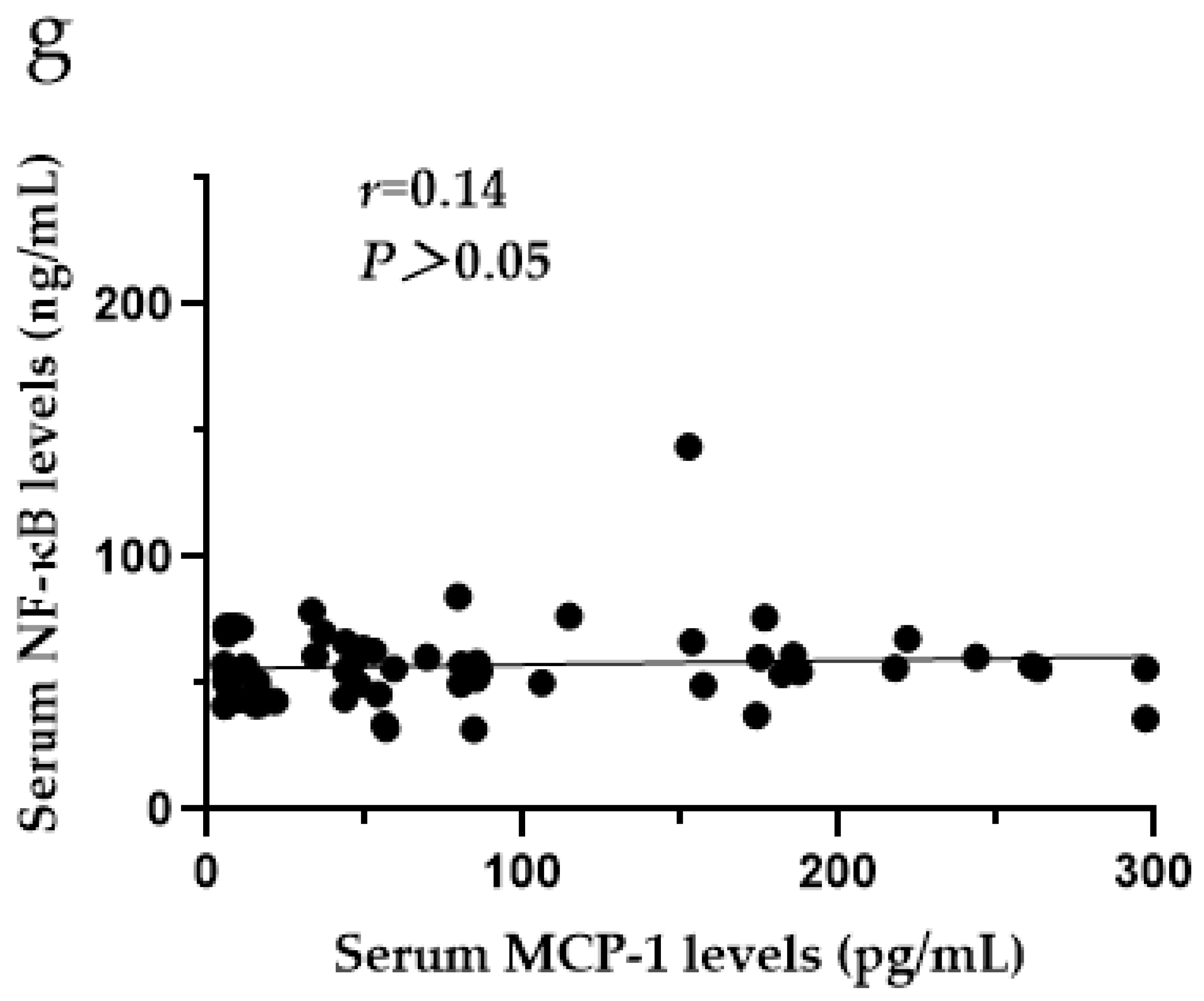
3.9. The Relationships among CXCR4, SDF-1, MCP-1, ERK1/2 and NF-κB in the Serum of SF Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular mechanisms of fluoride toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pei, M.; Luo, K.; Li, L. The State and Trend of Endemic Fluorosis from 1991 to 2012 in China. J. Chongqing Norm. Univ. (Nat. Sci. Ed.) 2016, 33, 142–151. [Google Scholar] [CrossRef]
- Pei, J.; Li, B.; Gao, Y.; Wei, Y.; Zhou, L.; Yao, H.; Wang, J.; Sun, D. Fluoride decreased osteoclastic bone resorption through the inhibition of NFATc1 gene expression. Environ. Toxicol. 2014, 29, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jiang, N.; Yu, X.; Zhao, Z.; Zhang, X.; Xu, H. The role of TGFbeta receptor 1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 2018, 393, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Huang, J.Z. Outline of control practice of endemic fluorosis in China. Soc. Sci. Med. 1995, 41, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kong, L.; Zhao, H.; Dong, R.; Li, J.; Jia, Z.; Ji, N.; Deng, S.; Sun, Z.; Zhou, J. Thoracic ossification of ligamentum flavum caused by skeletal fluorosis. Eur. Spine J. 2007, 16, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Pang, S.; Sun, D. The pathogenesis of endemic fluorosis: Research progress in the last 5 years. J. Cell Mol. Med. 2019, 23, 2333–2342. [Google Scholar] [CrossRef]
- Mou, W.; Yan, H.; Zhang, L. The role of SDF-1/CXCR4 and downstream signaling pathways in the course. J. Pract. Med. 2011, 9, 158–160. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, F.; Li, Y.; Lu, S.; Zhu, M.; Qiu, M. Expression of Fas, FasL, and NF-κB in the Process of Osteoclast-like Cell Apoptosis Effected by Sodium Fluoride. J. Chin. Acad. Med. Sci. 2002, 24, 491–494. [Google Scholar]
- Zhang, M.; Wang, A.; Xia, T.; He, P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons. Toxicol. Lett. 2008, 179, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Chi, H.S.; Chen, J.; Denbesten, P.K. JNK/c-Jun signaling pathway mediates the fluoride-induced down-regulation of MMP-20 in vitro. Matrix Biol. 2007, 26, 633–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karube, H.; Nishitai, G.; Inageda, K.; Kurosu, H.; Matsuoka, M. NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J. Dent. Res. 2009, 88, 461–465. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Xiang, Y.; Chen, Y.; Yang, X. The role of SDF-1/CXCR4 and downstream signaling pathways in the course of OA. J. Pract. Med. 2019, 35, 3563–3567. [Google Scholar] [CrossRef]
- Smith, J.T.; Schneider, A.D.; Katchko, K.M.; Yun, C.; Hsu, E.L. Environmental Factors Impacting Bone-Relevant Chemokines. Front. Endocrinol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, A. Chemokines and Bone. Handb. Exp. Pharmacol. 2020, 262, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. The role of C-C motif chemokine ligand 2 in respiratory diseases. Int. J. Pediatr. 2018, 45, 204–207. [Google Scholar] [CrossRef]
- Nagasawa, T. CXCL12/SDF-1 and CXCR4. Front. Immunol. 2015, 6, 301. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Jankowski, K.; Reca, R.; Wysoczynski, M.; Bandura, L.; Allendorf, D.J.; Zhang, J.; Ratajczak, J.; Ratajczak, M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004, 35, 233–245. [Google Scholar] [CrossRef]
- Horuk, R. Chemokine receptors. Cytokine Growth Factor Rev. 2001, 12, 313–335. [Google Scholar] [CrossRef]
- Er, Z.J.; Yin, C.F.; Wang, W.J.; Chen, X.J. Serum CXCL12/SDF-1 level is positively related with lumbar intervertebral disc degeneration and clinical severity. Innate Immun. 2020, 26, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.; Bragg, R.; Elmansi, A.M.; McGee-Lawrence, M.E.; Isales, C.M.; Hamrick, M.W.; Hill, W.D.; Fulzele, S. Stromal cell-derived factor-1 (CXCL12) and its role in bone and muscle biology. Cytokine 2019, 123, 154783. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Zhang, D.; Xie, J.; Zhou, X. The role of stromal cell-derived factor 1 on cartilage development and disease. Osteoarthr. Cartil. 2021, 29, 313–322. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J. Bone Min. Res. 2003, 18, 1404–1418. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Mao, Z.; Zhou, R.; Zhao, X.; Wang, B. Research Developments of Monocyte Chemoattractant Protein-1 in Inflammatory Reaction. Med. Recapitul. 2013, 19, 964–966. [Google Scholar] [CrossRef]
- Binder, N.B.; Niederreiter, B.; Hoffmann, O.; Stange, R.; Pap, T.; Stulnig, T.M.; Mack, M.; Erben, R.G.; Smolen, J.S.; Redlich, K. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat. Med. 2009, 15, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Ke, K.; Kim, W.K.; Kim, S.H.; Lee, S.C.; Kim, H.J.; Kim, S.Y.; Suh, J.H.; Choi, H.S. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. J. Cell Physiol. 2012, 227, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. CCL2/Monocyte Chemoattractant Protein 1 and Parathyroid Hormone Action on Bone. Front. Endocrinol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Shaul, Y.D.; Seger, R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Malemud, C.J. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int. J. Mol. Sci. 2019, 20, 3792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Dong, Z.; Wang, J.; Lou, Y. Thyrocalcitonin regulates the cbfa1mRNA expression of osteoblasts via signal pathway of ERK-MAPK. J. Clin. Med. Pract. 2009, 13, 35–38. [Google Scholar] [CrossRef]
- Kim, G.W.; Han, M.S.; Park, H.R.; Lee, E.J.; Jung, Y.K.; Usmani, S.E.; Ulici, V.; Han, S.W.; Beier, F. CXC chemokine ligand 12a enhances chondrocyte proliferation and maturation during endochondral bone formation. Osteoarthr. Cartil. 2015, 23, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, X.; Niu, F.; Chen, M.; Song, N. Expression of stromal cell-derived factor 1, CXCR4 and ERK1/2 in pathologic scar tissue. Chin. J. Aesthetic Med. 2009, 18, 1638–1639. [Google Scholar] [CrossRef]
- Cao, X. Function of NF-κB Signaling Pathway in Bone and Bone Diseases. Sichuan J. Anat. 2015, 23, 30–35. [Google Scholar] [CrossRef]
- Novack, D.V. Role of NF-kappaB in the skeleton. Cell Res. 2011, 21, 169–182. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L.; Franzoso, G.; Siebenlist, U. Required and nonessential functions of nuclear factor-kappa B in bone cells. Bone 1999, 25, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y. NF-kappaB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Chen, P. To Investigate the Tea-Based Fluorosis in Qinghai Province. Master’s Thesis, Shanxi Medical University, Taiyuan, China, 2012. [Google Scholar] [CrossRef]
- Chen, T.L. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone 2004, 35, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Huang, H.X.; Wang, F.; Zhou, Q.L.; Huang, Y.Q.; Qin, R.Z. Elevated plasma CXCL12/SDF-1 levels are linked with disease severity of postmenopausal osteoporosis. Innate Immun. 2020, 26, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Chen, L.; Li, W.; Li, F.; Chen, Q. Stromal cell-derived factor-1 and its receptor CXCR4 are upregulated expression in degenerated intervertebral discs. Int. J. Med. Sci. 2014, 11, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Bragg, R.; Gilbert, W.; Elmansi, A.M.; Isales, C.M.; Hamrick, M.W.; Hill, W.D.; Fulzele, S. Stromal cell-derived factor-1 as a potential therapeutic target for osteoarthritis and rheumatoid arthritis. Ther. Adv. Chronic. Dis. 2019, 10, 2040622319882531. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhu, N.; Mao, J.; Huang, L.; Yang, Y.; Zhou, Z.; Wang, L.; Wu, B. Expression Levels of CXCR4 and CXCL12 in Patients with Rheumatoid Arthritis and its Correlation with Disease Activity. Exp. Ther. Med. 2020, 20, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, Y.; Yang, L.; He, Y.; Jia, D.; Hu, X. miR-142-5p as a CXCR4-Targeted MicroRNA Attenuates SDF-1-Induced Chondrocyte Apoptosis and Cartilage Degradation via Inactivating MAPK Signaling Pathway. Biochem. Res. Int. 2020, 2020, 4508108. [Google Scholar] [CrossRef] [PubMed]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. Effect of CASR/MAPK Signaling Pathway on the Pathogenesis of Fluorosis. Master’s Thesis, Jilin University, Jilin, China, 2020. [Google Scholar]
- Chen, H.; Shao, X.; Li, L.; Zheng, C.; Xu, X.; Hong, X.; Li, X.; Wu, M. Electroacupuncture serum inhibits TNF-α-mediated chondrocyte inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling pathway. Mol. Med. Rep. 2017, 16, 5807–5814. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Wang, C.F.; Han, L.; Cao, G.P.; Shen, Q.R. Chondroprotective Effect of Wufu Decoction on Tumor Necrosis Factor-alpha-Induced Chondrocytes via the Extracellular Signal Regulated Kinase 1/2 Signaling Pathway. Orthop. Surg. 2020, 12, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.; Faddaa, L.; Ali, H.M.; Hassan, I.; El Orabi, N.F.; Bassiouni, Y. Amelioration of the Protein Expression of Cox2, NFkappaB, and STAT-3 by Some Antioxidants in the Liver of Sodium Fluoride-Intoxicated Rats. Dose Response 2018, 16, 1559325818800153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Research Progress on the Role of CCL2 and Its Signal Pathway in the Pathogenesis of Rheumatoid Arthritis and Periodontitis. Shandong Med. J. 2020, 60, 98–102. [Google Scholar] [CrossRef]
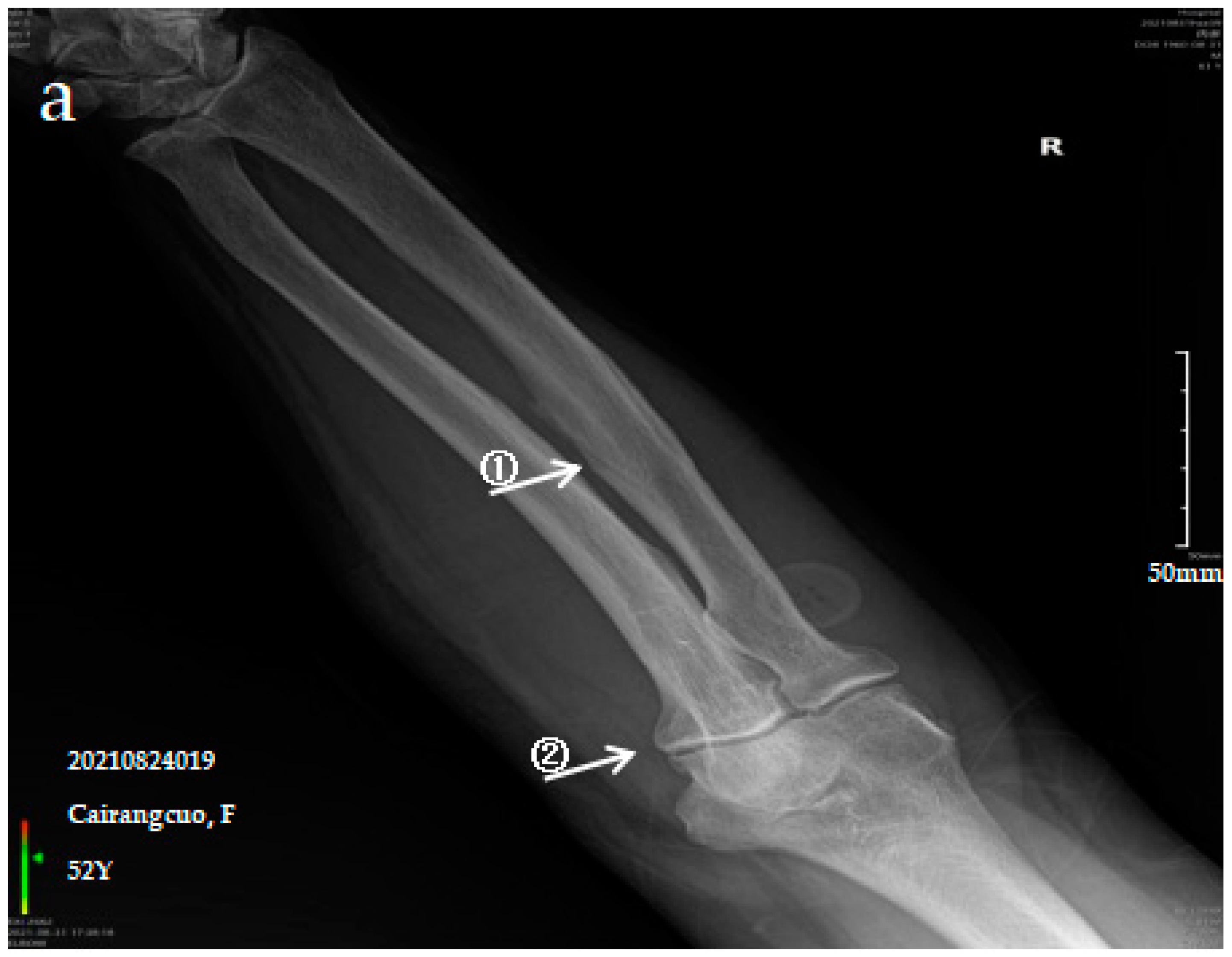
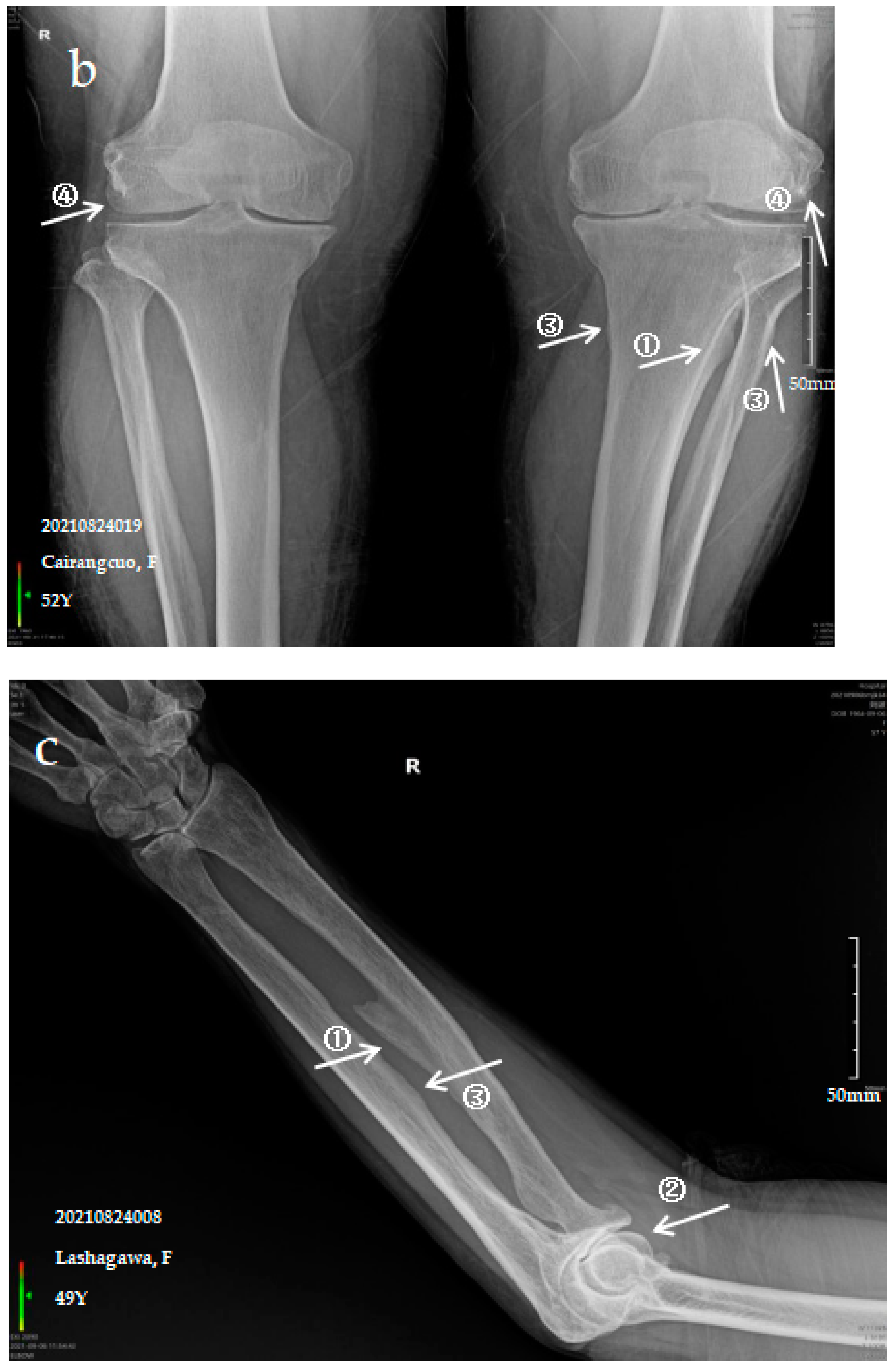

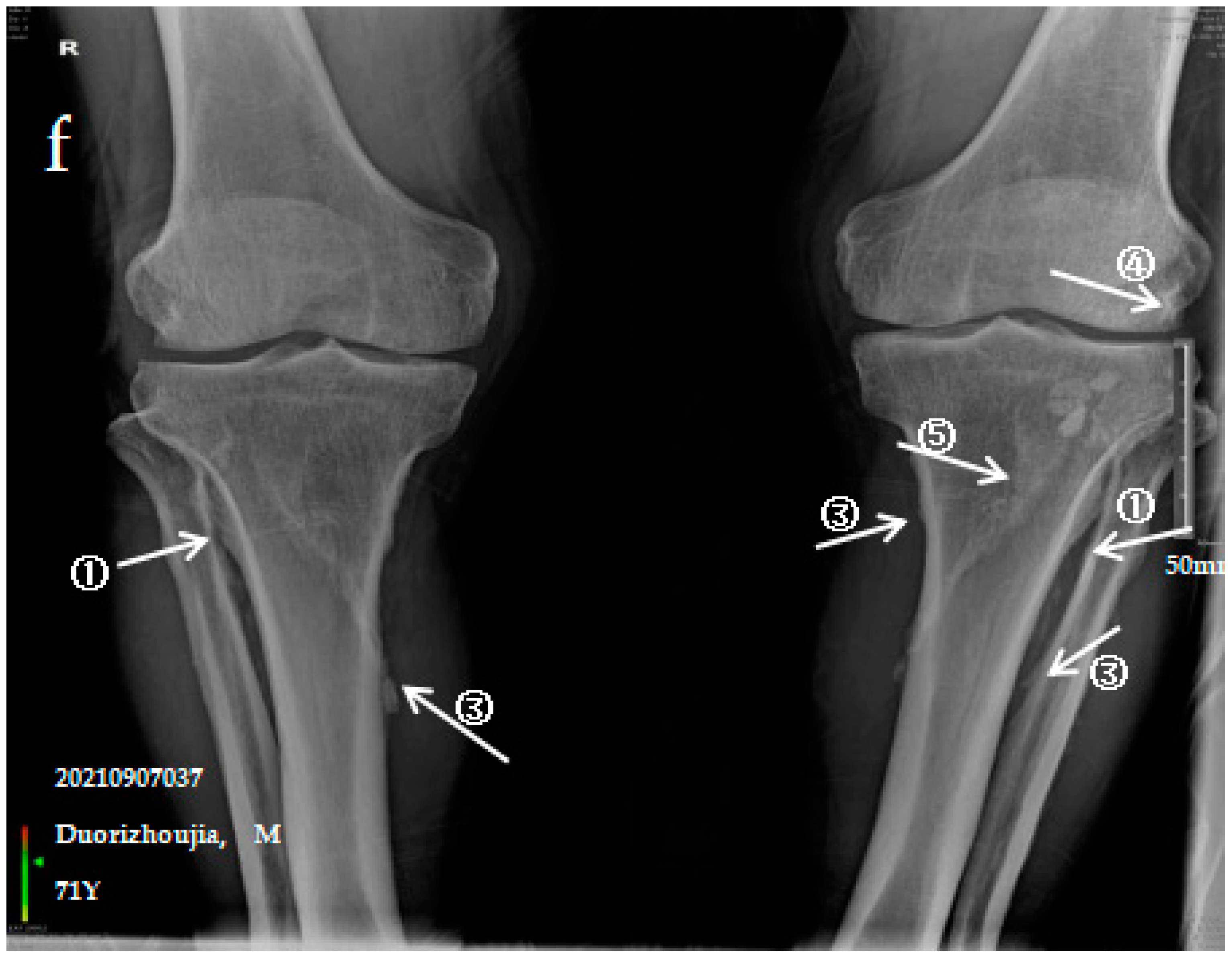


| SF | Control | Z/χ* Value | p-Value | |
|---|---|---|---|---|
| Age (years) | 60.03 ± 9.67 | 57.42 ± 6.88 | 1.71 | >0.05 |
| Sex (Male/Female) | 31/29 | 28/32 | 3.00 | >0.05 |
| X-ray grade | ||||
| Mild | 36 | |||
| Moderate | 20 | |||
| Severe | 4 |
| Characteristic | SF Group N (%) | X-ray Grade | Χ* | p-Value | ||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| Sex | ||||||
| Male | 31 (51.67) | 16 | 12 | 3 | 2.18 | > 0.05 |
| Female | 29 (48.33) | 20 | 8 | 1 | ||
| Age (years) | ||||||
| ~60 | 34 (56.67) | 26 | 7 | 1 | 9.01 | < 0.05 |
| ~80 | 26 (43.33) | 10 | 13 | 3 | ||
| Group | N | CXCR4 (ng/mL) | SDF-1 (ng/mL) | MCP-1 (pg/mL) | ERK1/2 (ng/mL) | NF-κB (ng/mL) |
|---|---|---|---|---|---|---|
| Control group | 60 | 4.22 (3.03, 4.76) | 5.51 (4.62, 6.22) | 48.45 (11.07, 102.33) | 2.67 (2.24, 3.22) | 53.25 (46.68, 58.90) |
| SF group | 60 | 4.86 (4.16, 5.64) ** | 5.86 (5.28, 6.45) * | 57.11 (15.97, 153.30) * | 2.84 (2.47, 3.16) ** | 56.08 (49.29, 66.53) * |
| Z value | 3.74 | 2.12 | 1.99 | 1.07 | 2.05 | |
| p-value | <0.001 | <0.05 | <0.05 | >0.05 | <0.05 |
| Characteristic | SF Group N (%) | CXCR4 (ng/mL) | SDF-1 (ng/mL) | MCP-1 (pg/mL) | NF-κB (ng/mL) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 31 (51.67) | 4.52 (4.03, 5.18) | 5.77 (5.33, 6.35) | 54.27 (9.03, 153.60) | 57.25 (50.30, 66.55) |
| Female | 29 (48.33) | 5.08 (4.33, 6.10) | 6.07 (4.93, 6.49) | 79.34 (27.73, 163.15) | 54.85 (43.47, 66.98) |
| Age (years) | |||||
| ~60 | 34 (56.67) | 5.02 (4.34, 6.14) | 5.90 (5.40, 6.38) | 80.39 (20.01, 175.48) | 57.38 (49.81, 70.30) |
| ~80 | 26 (43.33) | 4.52 (3.93, 5.36) | 5.84 (4.99, 6.54) | 47.84 (14.29, 107.12) | 55.37 (43.67, 64.54) |
| X-ray grade | |||||
| Mild | 36 (60.00) | 5.13 (4.69, 6.14) | 5.98 (5.52, 6.50) | 79.82 (38.35, 171.71) | 57.58 (50.88, 69.48) |
| Moderate | 20 (33.33) | 4.29 (3.67, 4.55) | 5.66 (4.71, 6.40) | 45.11 (10.49, 136.71) | 54.55 (42.28, 59.95) |
| Severe | 4 (6.67) | 4.93 (2.96, 7.21) | 5.52 (4.28, 12.72) | 12.06 (6.66, 118.07) | 63.30 (44.89, 126.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Pu, G.; Li, Y.; Jiang, H.; Zhang, Q.; Chen, P.; Lu, Q.; Wang, M.; Yang, R. Serum Levels of CXCR4, SDF-1, MCP-1, NF-κB and ERK1/2 in Patients with Skeletal Fluorosis. Int. J. Environ. Res. Public Health 2022, 19, 16555. https://doi.org/10.3390/ijerph192416555
Zhao Y, Pu G, Li Y, Jiang H, Zhang Q, Chen P, Lu Q, Wang M, Yang R. Serum Levels of CXCR4, SDF-1, MCP-1, NF-κB and ERK1/2 in Patients with Skeletal Fluorosis. International Journal of Environmental Research and Public Health. 2022; 19(24):16555. https://doi.org/10.3390/ijerph192416555
Chicago/Turabian StyleZhao, Yaqian, Guanglan Pu, Yanan Li, Hong Jiang, Qiang Zhang, Ping Chen, Qing Lu, Mingjun Wang, and Rui Yang. 2022. "Serum Levels of CXCR4, SDF-1, MCP-1, NF-κB and ERK1/2 in Patients with Skeletal Fluorosis" International Journal of Environmental Research and Public Health 19, no. 24: 16555. https://doi.org/10.3390/ijerph192416555
APA StyleZhao, Y., Pu, G., Li, Y., Jiang, H., Zhang, Q., Chen, P., Lu, Q., Wang, M., & Yang, R. (2022). Serum Levels of CXCR4, SDF-1, MCP-1, NF-κB and ERK1/2 in Patients with Skeletal Fluorosis. International Journal of Environmental Research and Public Health, 19(24), 16555. https://doi.org/10.3390/ijerph192416555






