Association between Breastfeeding and Restrictive Spirometric Pattern in Women Aged over 40 Years: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
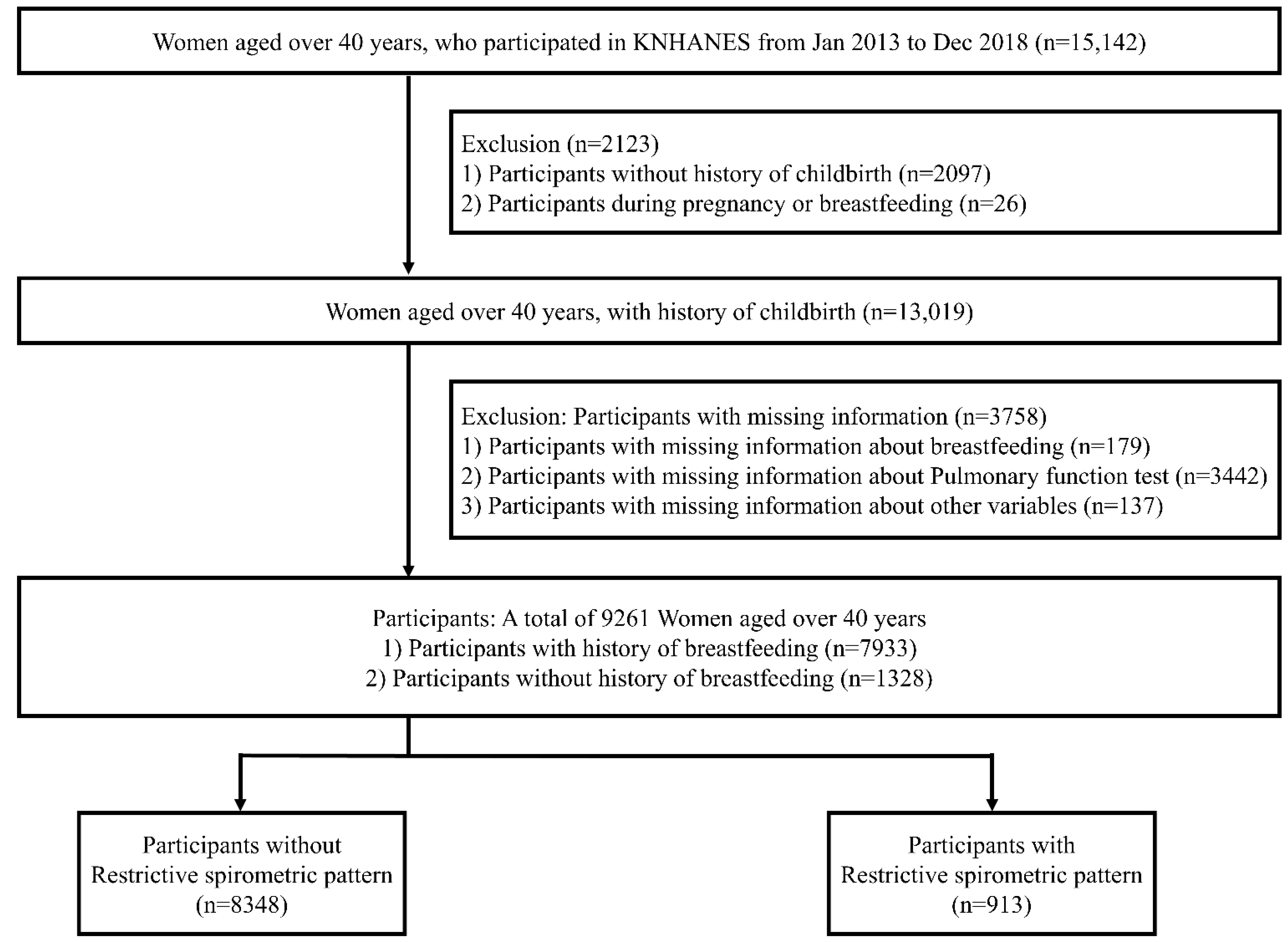
2.1. Data Source, Study Design, and Population
2.2. Study’s Main Variables
2.3. Covariates and Measurements
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Participants
3.2. Association between RSP and Breastfeeding
3.3. Correlation between Breastfeeding and the Results of the Respiratory Function Test
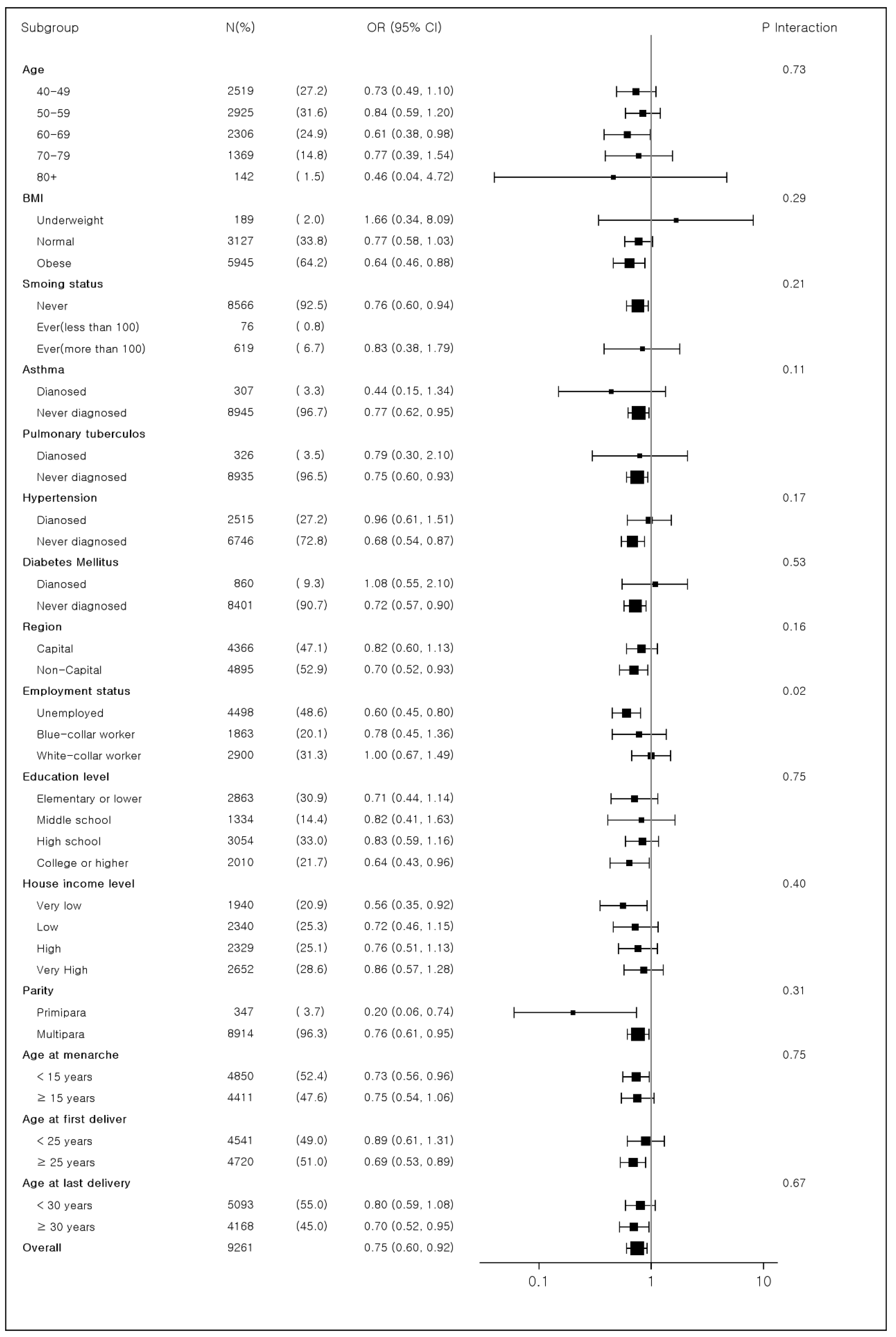
3.4. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| KNHANES | Korean National Health and Nutrition Examination Survey |
| KCDA | Korea Disease Control and Prevention Agency |
| PFT | pulmonary function test |
| RSP | restrictive spirometric pattern |
| TLC | total lung capacity |
References
- Kasper, D.; Fauci, A.; Hauser, S.; Longo, D.; Jameson, J.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 11th ed.; Mcgraw-Hill: New York, NY, USA, 2022; Volume 1. [Google Scholar]
- Meyer, K.B.; Wilbrey-Clark, A.; Nawijn, M.; Teichmann, S.A. The Human Lung Cell Atlas: A Transformational Resource for Cells of the Respiratory System. In Lung Stem Cells in Development, Health and Disease (ERS Monograph); European Respiratory Society: Sheffield, UK, 2021; pp. 158–174. [Google Scholar]
- Bartels, M.N.; Prince, D.Z. Acute Medical Conditions: Cardiopulmonary Disease, Medical Frailty, and Renal Failure. Braddom’s Phys. Med. Rehabil. 2021, 511–534.e515. [Google Scholar] [CrossRef]
- Brack, T.; Jubran, A.; Tobin, M.J. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am. J. Respir. Crit. Care Med. 2002, 165, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pitre, P.J.; Sabbula, B.R.; Cascella, M. Restrictive Lung Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mannino, D.M.; Ford, E.S.; Redd, S.C. Obstructive and restrictive lung disease and markers of inflammation: Data from the Third National Health and Nutrition Examination. Am. J. Med. 2003, 114, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Holguin, F.; Pavlin, B.I.; Ferdinands, J.M. Risk factors for prevalence of and mortality related to restriction on spirometry: Findings from the First National Health and Nutrition Examination Survey and follow-up. Int. J. Tuberc. Lung Dis. 2005, 9, 613–621. [Google Scholar]
- Kurth, L.; Hnizdo, E. Change in prevalence of restrictive lung impairment in the U.S. population and associated risk factors: The National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 2007–2010. Multidiscip. Respir. Med. 2015, 10, 7. [Google Scholar] [CrossRef]
- Guerra, S.; Sherrill, D.L.; Venker, C.; Ceccato, C.M.; Halonen, M.; Martinez, F.D. Morbidity and mortality associated with the restrictive spirometric pattern: A longitudinal study. Thorax 2010, 65, 499–504. [Google Scholar] [CrossRef]
- Soriano, J.B.; Miravitlles, M.; García-Río, F.; Muñoz, L.; Sánchez, G.; Sobradillo, V.; Durán, E.; Guerrero, D.; Ancochea, J. Spirometrically-defined restrictive ventilatory defect: Population variability and individual determinants. Prim. Care Respir. J. 2012, 21, 187–193. [Google Scholar] [CrossRef]
- Mannino, D.M.; Ford, E.S.; Redd, S.C. Obstructive and restrictive lung disease and functional limitation: Data from the Third National Health and Nutrition Examination. J. Intern. Med. 2003, 254, 540–547. [Google Scholar] [CrossRef]
- Nakajima, K.; Kubouchi, Y.; Muneyuki, T.; Ebata, M.; Eguchi, S.; Munakata, H. A possible association between suspected restrictive pattern as assessed by ordinary pulmonary function test and the metabolic syndrome. Chest 2008, 134, 712–718. [Google Scholar] [CrossRef]
- van den Borst, B.; Gosker, H.R.; Zeegers, M.P.; Schols, A.M. Pulmonary function in diabetes: A metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef]
- Sattari, M.; Serwint, J.R.; Levine, D.M. Maternal Implications of Breastfeeding: A Review for the Internist. Am. J. Med. 2019, 132, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Koh, K. Maternal breastfeeding and children’s cognitive development. Soc. Sci. Med. 2017, 187, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Perrine, C.G.; Nelson, J.M.; Corbelli, J.; Scanlon, K.S. Lactation and Maternal Cardio-Metabolic Health. Annu. Rev. Nutr. 2016, 36, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.N.; Wu, Q.J.; Gong, T.T.; Vogtmann, E.; Wang, Y.L.; Lin, B. Breastfeeding and ovarian cancer risk: A meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2013, 98, 1020–1031. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Aggarwal, A.N.; Agarwal, R. The new ATS/ERS guidelines for assessing the spirometric severity of restrictive lung disease differ from previous standards. Respirology 2007, 12, 759–762. [Google Scholar] [CrossRef]
- Mannino, D.M.; Davis, K.J.; Disantostefano, R.L. Chronic respiratory disease, comorbid cardiovascular disease and mortality in a representative adult US cohort. Respirology 2013, 18, 1083–1088. [Google Scholar] [CrossRef]
- Fox, M.; Berzuini, C.; Knapp, L.A. Maternal breastfeeding history and Alzheimer’s disease risk. J. Alzheimer’s Dis. 2013, 37, 809–821. [Google Scholar] [CrossRef]
- Liu, B.; Beral, V.; Balkwill, A. Childbearing, breastfeeding, other reproductive factors and the subsequent risk of hospitalization for gallbladder disease. Int. J. Epidemiol. 2009, 38, 312–318. [Google Scholar] [CrossRef]
- Karlson, E.W.; Mandl, L.A.; Hankinson, S.E.; Grodstein, F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004, 50, 3458–3467. [Google Scholar] [CrossRef] [PubMed]
- Bjørnerem, A.; Ahmed, L.A.; Jørgensen, L.; Størmer, J.; Joakimsen, R.M. Breastfeeding protects against hip fracture in postmenopausal women: The Tromsø study. J. Bone Miner Res. 2011, 26, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, M.S.; Jankowich, M.D. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest 2016, 149, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Gold, L.; Järvholm, B.; Alavanja, M.C.; Ward, M.H.; Vermeulen, R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2007, 62, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Roterman, I.; Yigla, M.; Kerner, A.; Avizohar, O.; Sella, R.; Bartha, P.; Levy, Y.; Markiewicz, W. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am. J. Respir. Crit. Care Med. 2006, 174, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, B.; Jacobs, D.R.; Apostol, G.G.; Smith, L.J.; Lewis, C.E.; Williams, O.D. Plasma fibrinogen and lung function: The CARDIA Study. Int. J. Epidemiol. 2006, 35, 1001–1008. [Google Scholar] [CrossRef]
- Engström, G.; Lind, P.; Hedblad, B.; Wollmer, P.; Stavenow, L.; Janzon, L.; Lindgärde, F. Lung function and cardiovascular risk: Relationship with inflammation-sensitive plasma proteins. Circulation 2002, 106, 2555–2560. [Google Scholar] [CrossRef]
- Ahn, S.; Corwin, E.J. The association between breastfeeding, the stress response, inflammation, and postpartum depression during the postpartum period: Prospective cohort study. Int. J. Nurs. Stud. 2015, 52, 1582–1590. [Google Scholar] [CrossRef]
- Groer, M.W.; Davis, M.W. Cytokines, infections, stress, and dysphoric moods in breastfeeders and formula feeders. J. Obstet Gynecol. Neonatal. Nurs. 2006, 35, 599–607. [Google Scholar] [CrossRef]
- Tafuro, F.; Corradi, M. An approach to interpreting restrictive spirometric pattern results in occupational settings. Med. Lav. 2016, 107, 419–436. [Google Scholar]
- Enright, P.; Brusasco, V. Counterpoint: Should we abandon FEV₁/FVC < 0.70 to detect airway obstruction? Yes. Chest 2010, 138, 1040–1042, discussion 1042–1044. [Google Scholar] [CrossRef]
- Natland, S.T.; Andersen, L.F.; Nilsen, T.I.; Forsmo, S.; Jacobsen, G.W. Maternal recall of breastfeeding duration twenty years after delivery. BMC Med. Res. Methodol. 2012, 12, 179. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n) | RSP | Non-RSP | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 9261 | 913 | 9.9 | 8348 | 90.1 | |||
| Breastfeeding duration (months) | 0.0006 | ||||||
| None | 1328 | 14.3 | 128 | 9.6 | 1200 | 90.4 | |
| Yes | 7933 | 85.7 | 785 | 9.9 | 7148 | 90.1 | |
| 1–6 | 1247 | 13.5 | 96 | 7.7 | 1151 | 92.3 | |
| 7–12 | 1051 | 11.3 | 87 | 8.3 | 964 | 91.7 | |
| 13–18 | 780 | 8.4 | 65 | 8.3 | 715 | 91.7 | |
| 19–24 | 1487 | 16.1 | 150 | 10.1 | 1337 | 89.9 | |
| 25- | 3368 | 36.4 | 387 | 11.5 | 2981 | 88.5 | |
| Age (years) | 57.5 (10.7) † | 61.7 (10.4) † | 57.0 (10.6) † | <0.0001 | |||
| 40–49 | 2519 | 27.2 | 126 | 5.0 | 2393 | 95.0 | |
| 50–59 | 2925 | 31.6 | 263 | 9.0 | 2662 | 91.0 | |
| 60–69 | 2306 | 24.9 | 269 | 11.7 | 2037 | 88.3 | |
| 70–79 | 1369 | 14.8 | 222 | 16.2 | 1147 | 83.8 | |
| 80+ | 142 | 1.5 | 33 | 23.2 | 109 | 76.8 | |
| BMI (kg/m2) | 24.0 (3.2) † | 25.4 (3.8) † | 23.9 (3.2) † | <0.0001 | |||
| Underweight | 189 | 2.0 | 20 | 10.6 | 169 | 89.4 | |
| Obese | 3127 | 33.8 | 466 | 14.9 | 2661 | 85.1 | |
| Normal | 5945 | 64.2 | 427 | 7.2 | 5518 | 92.8 | |
| FEV1 (L) | 2.30 (0.45) † | 1.81 (0.30) † | 2.36 (0.43) † | ||||
| FVC (L) | 2.91 (0.51) † | 2.27 (0.32) † | 2.98 (0.48) † | ||||
| FVC Percentage (%) | 92.76 (11.58) † | 74.22 (5.30) † | 94.79 (10.20) † | ||||
| FEV1/FVC | 0.79 (0.06) † | 0.80 (0.05) † | 0.79 (0.06) † | ||||
| Smoking status | 0.6915 | ||||||
| Ever (less than 100) | 76 | 0.8 | 8 | 10.5 | 68 | 89.5 | |
| Ever (more than 100) | 619 | 6.7 | 55 | 8.9 | 564 | 91.1 | |
| Never | 8566 | 92.5 | 850 | 9.9 | 7716 | 90.1 | |
| Asthma | 0.0580 | ||||||
| Diagnosed | 307 | 3.3 | 40 | 13.0 | 267 | 87.0 | |
| Never diagnosed | 8954 | 96.7 | 873 | 9.7 | 8081 | 80.3 | |
| Pulmonary tuberculosis | 0.0937 | ||||||
| Diagnosed | 326 | 3.5 | 41 | 12.6 | 285 | 87.4 | |
| Never diagnosed | 8935 | 96.5 | 872 | 09.8 | 8063 | 90.2 | |
| Hypertension | <0.0001 | ||||||
| Diagnosed | 2515 | 27.2 | 368 | 14.6 | 2147 | 85.4 | |
| Never diagnosed | 6746 | 72.8 | 545 | 08.1 | 6201 | 91.9 | |
| Diabetes Mellitus | <0.0001 | ||||||
| Diagnosed | 860 | 9.3 | 153 | 17.8 | 707 | 82.2 | |
| Never diagnosed | 8401 | 90.7 | 760 | 9.0 | 7641 | 91.0 | |
| Region | 0.1346 | ||||||
| Capital | 4366 | 47.1 | 409 | 9.4 | 3957 | 90.6 | |
| Non-Capital | 4895 | 52.9 | 504 | 10.3 | 4391 | 89.7 | |
| Employment status | <0.0001 | ||||||
| Blue-collar worker | 1863 | 20.1 | 194 | 10.4 | 1669 | 89.6 | |
| White-collar worker | 2900 | 31.3 | 220 | 7.6 | 2680 | 92.4 | |
| Unemployed | 4498 | 48.6 | 499 | 11.1 | 3999 | 88.9 | |
| Education level | <0.0001 | ||||||
| Elementary or lower | 2863 | 30.9 | 366 | 12.8 | 2497 | 87.2 | |
| Middle school | 1334 | 14.4 | 149 | 11.2 | 1185 | 88.8 | |
| High school | 3054 | 33.0 | 263 | 8.6 | 2791 | 91.4 | |
| College or higher | 2010 | 21.7 | 135 | 6.7 | 1875 | 93.3 | |
| House income level | <0.0001 | ||||||
| Very low | 1940 | 20.9 | 253 | 13.0 | 1687 | 87.0 | |
| Low | 2340 | 25.3 | 233 | 10.0 | 2107 | 90.0 | |
| High | 2329 | 25.1 | 226 | 9.7 | 2103 | 90.3 | |
| Very high | 2652 | 28.6 | 201 | 7.6 | 2451 | 92.4 | |
| Parity | 0.0052 | ||||||
| Primipara | 347 | 3.7 | 19 | 5.5 | 328 | 94.5 | |
| Multipara | 8914 | 96.3 | 894 | 10.0 | 8020 | 90.0 | |
| Age at menarche | 14.6 (1.9) † | 14.8 (2.0) † | 14.5 (1.9) † | 0.0010 | |||
| <15 years | 4850 | 52.4 | 431 | 8.9 | 4419 | 91.1 | |
| ≥15 years | 4411 | 47.6 | 482 | 10.9 | 3929 | 89.1 | |
| Age at the first delivery | 25.0 (3.9) † | 24.4 (3.8) † | 25.1 (3.9) † | <0.0001 | |||
| <25 years | 4541 | 49.0 | 514 | 11.3 | 4027 | 88.7 | |
| ≥25 years | 4720 | 51.0 | 399 | 8.5 | 4321 | 91.5 | |
| Age at the last delivery | 29.5 (4.3) † | 29.2 (4.2) † | 29.5 (4.3) † | 0.2097 | |||
| <30 years | 5093 | 55.0 | 520 | 10.2 | 4573 | 89.8 | |
| ≥30 years | 4168 | 45.0 | 393 | 9.4 | 3775 | 90.6 | |
| Examined year | <0.0001 | ||||||
| 2013 | 1478 | 16.0 | 127 | 8.6 | 1351 | 91.4 | |
| 2014 | 1413 | 15.3 | 96 | 6.8 | 1317 | 93.2 | |
| 2015 | 1455 | 15.7 | 119 | 8.2 | 1336 | 91.8 | |
| 2016 | 1687 | 18.2 | 218 | 12.9 | 1469 | 87.1 | |
| 2017 | 1551 | 16.7 | 176 | 11.3 | 1375 | 88.7 | |
| 2018 | 1677 | 18.1 | 177 | 10.6 | 1500 | 89.4 | |
| Variables | OR | 95% CI | p-Value | p-Value for Trend |
|---|---|---|---|---|
| Breastfeeding duration | ||||
| Yes | 0.75 | (0.60–0.92) | 0.0067 | 0.0004 |
| 1–6 | 0.86 | (0.65–1.14) | 0.2918 | |
| 7–12 | 0.79 | (0.59–1.06) | 0.1195 | |
| 13–18 | 0.82 | (0.60–1.14) | 0.2358 | |
| 19–24 | 0.74 | (0.57–0.96) | 0.0257 | |
| 25– | 0.63 | (0.49–0.81) | 0.0003 | |
| None | 1.00 | |||
| Age | <0.0001 | |||
| 40–49 | 0.22 | (0.13–0.36) | <0.0001 | |
| 50–59 | 0.39 | (0.25–0.63) | <0.0001 | |
| 60–69 | 0.51 | (0.33–0.79) | 0.0028 | |
| 70–79 | 0.74 | (0.48–1.14) | 0.1671 | |
| 80+ | 1.00 | |||
| BMI | <0.0001 | |||
| Underweight | 1.76 | (1.08–2.85) | 0.0224 | |
| Obese | 2.00 | (1.73–2.31) | <0.0001 | |
| Normal | 1.00 | |||
| Smoking status | 0.8768 | |||
| Ever (less than 100) | 1.40 | (0.66–2.98) | 0.3816 | |
| Ever (more than 100) | 0.96 | (0.71–1.29) | 0.7746 | |
| Never | 1.00 | |||
| Asthma | 0.5878 | |||
| Diagnosed | 1.10 | (0.78–1.57) | 0.5878 | |
| Never diagnosed | 1.00 | |||
| Pulmonary tuberculosis | 0.1737 | |||
| Diagnosed | 1.27 | (0.90–1.79) | 0.1737 | |
| Never diagnosed | 1.00 | |||
| Hypertension | 0.0437 | |||
| Diagnosed | 1.19 | (1.01–1.40) | 0.0437 | |
| Never diagnosed | 1.00 | |||
| Diabetes Mellitus | 0.0003 | |||
| Diagnosed | 1.46 | (1.19–1.79) | 0.0003 | |
| Never diagnosed | 1.00 | |||
| Region | 0.4792 | |||
| Capital | 0.95 | (0.82–1.10) | 0.4792 | |
| Non-Capital | 1.00 | |||
| Employment status | 0.7790 | |||
| Blue-collar worker | 1.01 | (0.84–1.21) | 0.9578 | |
| White-collar worker | 0.97 | (0.81–1.17) | 0.7492 | |
| Unemployed | 1.00 | |||
| Education level | 0.2958 | |||
| Elementary or lower | 0.91 | (0.68–1.21) | 0.5028 | |
| Middle school | 1.03 | (0.77–1.37) | 0.8614 | |
| High school | 1.08 | (0.86–1.36) | 0.5124 | |
| College or higher | 1.00 | |||
| House income level | 0.7543 | |||
| Very high | 1.00 | (0.79–1.26) | 0.9784 | |
| High | 1.16 | (0.93–1.44) | 0.1852 | |
| Low | 1.02 | (0.83–1.25) | 0.8766 | |
| Very low | 1.00 | |||
| Parity | 0.0912 | |||
| Primipara | 0.66 | (0.41–1.07) | 0.0912 | |
| Multipara | 1.00 | |||
| Age at menarche | 0.3458 | |||
| <15 years | 1.08 | (0.92–1.26) | 0.3458 | |
| ≥15 years | 1.00 | |||
| Age at the first delivery | 0.9210 | |||
| <25 years | 0.99 | (0.84–1.17) | 0.921 | |
| ≥25 years | 1.00 | |||
| Age at the last delivery | 0.1757 | |||
| <30 years | 1.11 | (0.95–1.30) | 0.1757 | |
| ≥30 years | 1.00 | |||
| Examined year | 0.0002 | |||
| 2013 | 0.83 | (0.65–1.06) | 0.1403 | |
| 2014 | 0.63 | (0.49–0.83) | 0.0007 | |
| 2015 | 0.75 | (0.58–0.96) | 0.0232 | |
| 2016 | 1.20 | (0.96–1.49) | 0.1056 | |
| 2017 | 1.06 | (0.85–1.33) | 0.6027 | |
| 2018 | 1.00 | |||
| Values are presented as adjusted odds ratio (95% confidence interval). | ||||
| FT Results | Variables | Coefficient | p-value | p-Value for Trend |
|---|---|---|---|---|
| FEV1 | Breastfeeding | |||
| Ever | 0.0390 | 0.0001 | 0.0004 | |
| 1–6 | 0.0303 | 0.0213 | ||
| 7–12 | 0.0421 | 0.0023 | ||
| 13–18 | 0.0296 | 0.0498 | ||
| 19–24 | 0.0385 | 0.0033 | ||
| 25– | 0.0506 | <0.0001 | ||
| Never | (reference) | |||
| FVC | Breastfeeding | |||
| Ever | 0.0521 | <0.0001 | <0.0001 | |
| 1–6 | 0.0407 | 0.0112 | ||
| 7c12 | 0.0461 | 0.0062 | ||
| 13–18 | 0.0422 | 0.0219 | ||
| 19–24 | 0.0521 | 0.0011 | ||
| 25– | 0.0732 | <0.0001 | ||
| Never | (reference) | |||
| FVC percentage (%) | Breastfeeding | |||
| Ever | 0.9540 | 0.0051 | 0.0002 | |
| 1–6 | 0.7174 | 0.1007 | ||
| 7–12 | 0.6514 | 0.1552 | ||
| 13–18 | 0.3390 | 0.4989 | ||
| 19–24 | 1.0267 | 0.0181 | ||
| 25– | 1.6906 | <0.0001 | ||
| Never | (reference) | |||
| FEV1/FVC | Breastfeeding | |||
| Ever | −0.0006 | 0.7038 | 0.1956 | |
| 1–6 | −0.0002 | 0.9264 | ||
| 7–12 | 0.0015 | 0.4928 | ||
| 13–18 | −0.0012 | 0.6094 | ||
| 19–24 | −0.0007 | 0.7334 | ||
| 25– | −0.0023 | 0.2588 | ||
| Never | (reference) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Kwon, S.; Lee, B.; Kim, G.; Chae, W.; Jang, S.-I. Association between Breastfeeding and Restrictive Spirometric Pattern in Women Aged over 40 Years: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 16291. https://doi.org/10.3390/ijerph192316291
Jang H, Kwon S, Lee B, Kim G, Chae W, Jang S-I. Association between Breastfeeding and Restrictive Spirometric Pattern in Women Aged over 40 Years: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(23):16291. https://doi.org/10.3390/ijerph192316291
Chicago/Turabian StyleJang, Hyeokjoo, Sebin Kwon, Bumyeol Lee, Gahyeon Kim, Wonjeong Chae, and Sung-In Jang. 2022. "Association between Breastfeeding and Restrictive Spirometric Pattern in Women Aged over 40 Years: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 23: 16291. https://doi.org/10.3390/ijerph192316291
APA StyleJang, H., Kwon, S., Lee, B., Kim, G., Chae, W., & Jang, S.-I. (2022). Association between Breastfeeding and Restrictive Spirometric Pattern in Women Aged over 40 Years: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(23), 16291. https://doi.org/10.3390/ijerph192316291







