Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review
Abstract
:1. Introduction
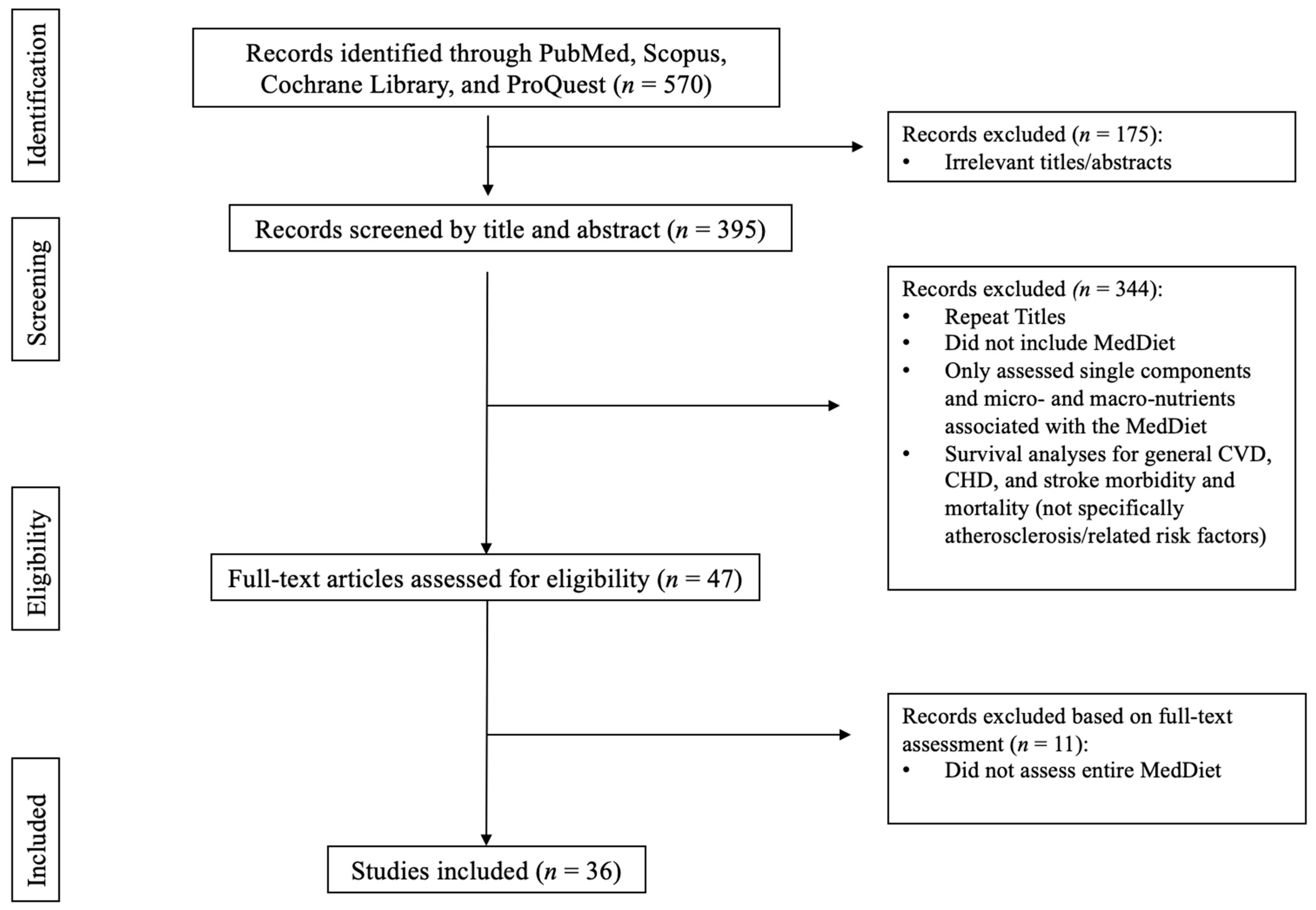
2. Materials and Methods
3. Results
3.1. Clinical Trials
3.2. Genetics
3.3. Biomarkers for Stress and Inflammation
3.4. Blood Pressure, Lipids, and Anthropometric Measurements
3.5. Carotid Intima-Media Thickness, Flow-Mediated Dilation, and Plaque Height
3.6. MedDiet Adherence
3.7. Observational Studies
3.8. Biomarkers for Stress and Inflammation
3.9. Insulin Measures, Lipids, and Lipoproteins
3.10. Plaque and Arterial Health
3.11. Subclinical Atherosclerosis Measurements
3.12. Scoring Measures of Heart and Vascular Health
3.13. MedDiet Adherence
4. Discussion
4.1. Clinical Studies
4.2. Observational Studies
4.3. Strengths and Limitations
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Heart Disease Facts; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 14 September 2021).
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, A.; Januszewski, A.; O’Neal, D. The early detection of atherosclerosis in type 1 DIABETES: Why, how and what to do about it. Cardiovasc. Endocrinol. Metab. 2019, 8, 14–27. [Google Scholar] [CrossRef]
- Watson, K.E.; Harmel, A.L.P.; Matson, G. Atherosclerosis in type 2 diabetes mellitus: The role of insulin resistance. J. Cardiovasc. Pharmacol. Ther. 2003, 8, 253–260. [Google Scholar] [CrossRef]
- Soeki, T.; Sata, M. Inflammatory biomarkers and atherosclerosis. Int. Heart J. 2016, 57, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Halcox, J.P.J. Endothelial dysfunction. In Primer on the Autonomic Nervous System; Academic Press: Cambridge, MA, USA, 2012; pp. 319–324. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothe-lium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, T.; Gräfe, K.; Senn, F.; Sur, P.; Stangl, G.I.; Dawczynski, C.; März, W.; Kleber, M.E.; Lorkowski, S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: A systematic analysis of the Global Burden of Disease Study. Eur. J. Epidemiol. 2019, 34, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Rumawas, M.E.; Meigs, J.B.; Dwyer, J.T.; McKeown, N.M.; Jacques, P.F. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2009, 90, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.E.; Cross, A.J.; Subar, A.F.; Krebs-Smith, S.M.; Park, Y.; Powell-Wiley, T.; Hollenbeck, A.; Reedy, J. Comparison of 4 established DASH diet indexes: Examining associations of index scores and colorectal cancer. Am. J. Clin. Nutr. 2013, 98, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Up-date of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, L.; Arroyo-Olivares, R.; Bittencourt, M.S.; Tada, M.T.; Lima, I.; Jannes, C.E.; Krieger, J.E.; Pereira, A.C.; Quintana-Navarro, G.; Muñiz-Grijalvo, O.; et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Nicolucci, A.; Mattina, A.; Rosafio, G.; Massenti, F.M.; Lucisano, G.; Galvano, F.; Amodio, E.; Pellegrini, F.; Barile, A.M.; et al. Association of dietary patterns with insulin resistance and clinically silent carotid atherosclerosis in apparently healthy people. Eur. J. Clin. Nutr. 2013, 67, 1284–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet Decreases Inflammatory bi-omarkers related to atherosclerosis: A Substudy of the Predimed trial. Br. J. Clin. Pharmacol. 2016, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Torres-Peña, J.D.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Alcala-Diaz, J.F.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Leon-Acuña, A.; Lopez-Moreno, J.; Camargo, A.; et al. Mediterranean diet IMPROVES Endothelial function in patients with diabetes And prediabetes: A report from the CORDIOPREV study. Atherosclerosis 2018, 269, 50–56. [Google Scholar] [CrossRef]
- Aoun, C.; Papazian, T.; Helou, K.; El Osta, N.; Khabbaz, L.R. Comparison of five international indices of adherence to the Mediterranean diet among healthy adults: Similarities and differences. Nutr. Res. Pract. 2019, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-Martí, A.; Cabañero-Martínez, M.J.; Hurtado-Sánchez, J.A.; Laguna-Pérez, A.; Ferrer-Cascales, R. Evaluation of Mediterranean diet adherence scores: A systematic review. BMJ Open 2018, 8, e019033. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Zito, F.P.; Polese, B.; Vozzella, L.; Gala, A.; Genovese, D.; Verlezza, V.; Medugno, F.; Santini, A.; Barrea, L.; Cargiolli, M.; et al. Good adherence to Mediterranean diet can prevent gastrointestinal symptoms: A survey from southern Italy. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 564. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; di Minno, G.; Ritieni, A. Red wine consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.-C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 59, 2772–2795. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive Oil Intake and Risk of Cardiovascular Disease and Mortality in the Predimed Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Rifler, J.-P. Is a meal without wine good for health? Diseases 2018, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.; Masulli, M.; Calabrese, I.; Rivellese, A.; Bonora, E.; Signorini, S.; Perriello, G.; Squatrito, S.; Buzzetti, R.; Sartore, G.; et al. Impact of a Mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: A real-life study. Nutrients 2018, 10, 1067. [Google Scholar] [CrossRef] [Green Version]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, C.M.; Valentini, A.; Calabrese, G. Gut microbiota and type 1 Diabetes MELLITUS: The effect of Mediterranean diet. Front. Nutr. 2021, 7, 612773. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A Journey into a Mediterranean diet and type 2 diabetes: A systematic review with Meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [Green Version]
- Leroux, C.; Brazeau, A.-S.; Gingras, V.; Desjardins, K.; Strychar, I.; Rabasa-Lhoret, R. Lifestyle and Cardiometabolic risk in adults with type 1 Diabetes: A review. Can. J. Diabetes 2014, 38, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association (ADA). Diabetes and Cardiovascular Disease. Available online: https://www.diabetes.org/diabetes/complications/cardiovascular-disease (accessed on 13 September 2021).
- Dokken, B.B. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spectr. 2008, 21, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.T.; Neylon, O.M.; O’Brien, T. Dyslipidaemia in type 1 Diabetes: Molecular mechanisms and therapeutic Op-portunities. Biomedicines 2021, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and control of type 2 diabetes by Mediterranean Diet: A systematic review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2019, 177, 1241–1257. [Google Scholar] [CrossRef] [Green Version]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2016, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 60, 1207–1227. [Google Scholar] [CrossRef]
- Martínez-González, M.; Hershey, M.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet TO Non-Mediterranean Countries. what is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Mattei, J. Mediterranean Diet and Cardiometabolic Diseases in Racial/Ethnic Minority Populations in the United States. Nutrients 2018, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Drouin-Chartier, J.-P.; Côté, J.A.; Labonté, M.-È.; Brassard, D.; Tessier-Grenier, M.; Desroches, S.; Couture, P.; Lamarche, B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv. Nutr. Int. Rev. J. 2016, 7, 1041–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, L.E.; Paddon-Jones, D.; Wright, A.J.; Campbell, W.W. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am. J. Clin. Nutr. 2018, 108, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.-H.; Estruch, R.; Clish, C.B.; Ros, E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J. Nutr. 2015, 146, 920S–927S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy Fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Majid, S. Bioactive Components in Milk and Dairy Products. Ph.D. Thesis, SLU/Department of Food Science, Uppsala, Sweden, 2016. [Google Scholar]
- Koutelidakis, A.; Dimou, C. Grape pomace: A challenging renewable resource of bioactive phenolic compounds with diversified health benefits. MOJ Food Process. Technol. 2016, 2, 016. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of selected antioxidative and bioactive compounds in meat and animal origin products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Camargo, A.; Delgado-Lista, J.; Garcia-Rios, A.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Lora-Aguilar, P.; Rodriguez-Cantalejo, F.; Fuentes-Jimenez, F.; et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br. J. Nutr. 2011, 108, 500–508. [Google Scholar] [CrossRef]
- Di Renzo, L.; Cioccoloni, G.; Salimei, P.S.; Ceravolo, I.; De Lorenzo, A.; Gratteri, S. Alcoholic beverage and meal choices for the Prevention of Noncommunicable Diseases: A randomized nutrigenomic trial. Oxid. Med. Cell. Longev. 2018, 2018, 5461436. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2015, 56, 89–97. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Sacanella, E.; Corella, D.; Andrés-Lacueva, C.; Llorach, R.; Garrabou, G.; Cardellach, F.; Sala-Vila, A.; Ros, E.; et al. The 3-year effect of the Mediterranean diet intervention on inflammatory biomarkers related to cardiovascular disease. Biomedicines 2021, 9, 862. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.-M.; Salas-Salvadó, J.; Martínez-González, M.-A.; Ros, E.; Estruch, R. Long-term immunomodulatory effects of a Mediterranean diet in adults at high risk of cardiovascular disease in the prevención con Dieta Mediterránea (predimed) randomized controlled trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañer, O.; Corella, D.; Covas, M.-I.; Sorlí, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bulló, M.; de la Torre, R.; Portolés, O.; et al. In vivo transcriptomic profile after a Mediterranean diet in high–cardiovascular risk patients: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duś-Żuchowska, M.; Bajerska, J.; Krzyżanowska, P.; Chmurzyńska, A.; Miśkiewicz-Chotnicka, A.; Muzsik, A.; Walkowiak, J. The Central European Diet as an alternative to the Mediterranean diet in atherosclerosis prevention in postmenopausal obese women with a high risk of metabolic syndrome—A randomized nutrition-al trial. Acta Sci. Pol. Technol. Aliment. 2018, 17, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Gutierrez-Mariscal, F.M.; Gomez, P.; et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am. J. Clin. Nutr. 2010, 93, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuñiga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Marin, C.; Lopez-Moreno, J.; Castaño, J.P.; Delgado-Lista, J.; et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Gicchino, M.; Caputo, M.; Giugliano, D.; Esposito, K. Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: Follow-up of a randomized trial. Eur. J. Prev. Cardiol. 2016, 24, 399–408. [Google Scholar] [CrossRef]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Gudban, N.; Yehuda, I.; Nasir, W.; Soboh, S.; Tamir, S.; Blum, A. Effect of Telemedicine Dietary Intervention on Endothelial Function in Patients with Type 2 Diabetes Mellitus on Mediterranean Diet. Isr. Med. Assoc. J. 2021, 23, 89–93. [Google Scholar] [PubMed]
- Murie-Fernandez, M.; Irimia, P.; Toledo, E.; Martínez-Vila, E.; Buil-Cosiales, P.; Serrano-Martínez, M.; Ruiz-Gutiérrez, V.; Ros, E.; Estruch, R.; Martínez-González, M.Á. Carotid intima-media thickness changes with Mediterranean diet: A randomized trial (predimed-navarra). Atherosclerosis 2011, 219, 158–162. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Romero-Mamani, E.-S.; Gilabert, R.; Núñez, I.; de la Torre, R.; Corella, D.; Ruiz-Gutiérrez, V.; López-Sabater, M.-C.; Pintó, X.; Rekondo, J.; et al. Changes in ultrasound-assessed carotid intima-media thickness and plaque with a Mediterranean diet. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Cesari, F.; Sofi, F.; Molino Lova, R.; Vannetti, F.; Pasquini, G.; Cecchi, F.; Marcucci, R.; Gori, A.M.; Macchi, C.; Boni, R.; et al. Aging process, adherence to Mediterranean diet and nutritional status in a large cohort of nonagenarians: Effects on endothelial progenitor cells. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 84–90. [Google Scholar] [CrossRef]
- Pignanelli, M.; Just, C.; Bogiatzi, C.; Dinculescu, V.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.; Ruetz, K.; Velenosi, T.; et al. Mediterranean diet score: Associations with metabolic products of the intestinal microbiome, carotid plaque burden, and renal function. Nutrients 2018, 10, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.; Makarem, N.; Emin, M.; Liao, M.; Jelic, S.; Aggarwal, B. Mediterranean diet components are linked to greater endothelial function and lower inflammation in a pilot study of ethnically diverse women. Nutr. Res. 2020, 75, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Zujko, M.E. Markers of endothelial dysfunction in young non-overweight women—Effect of serum lipids, body measures and nutrition. Prog. Health Sci. 2014, 4, 24–30. [Google Scholar]
- Millar, S.R.; Navarro, P.; Harrington, J.M.; Shivappa, N.; Hébert, J.R.; Perry, I.J.; Phillips, C.M. Comparing dietary score associations with lipoprotein particle subclass profiles: A cross-sectional analysis of a middle-to older-aged population. Clin. Nutr. 2021, 40, 4720–4729. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Coppi, F.; Migaldi, M.; Scicchitano, P.; Ciccone, M.M.; Farinetti, A. Relationship between Mediterra-nean diet and asymptomatic peripheral arterial disease in a population of pre-menopausal women. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 985–990. [Google Scholar] [CrossRef]
- Woo, J.; Yu, B.W.; Chan, R.S.; Leung, J. Influence of dietary patterns and inflammatory markers on atherosclerosis using ankle brachial index as a surrogate. J. Nutr. Health Aging 2018, 22, 619–626. [Google Scholar] [CrossRef]
- Gardener, H.; Wright, C.B.; Cabral, D.; Scarmeas, N.; Gu, Y.; Cheung, K.; Elkind, M.S.V.; Sacco, R.L.; Rundek, T. Mediterranean diet and carotid atherosclerosis in the northern Manhattan study. Atherosclerosis 2014, 234, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Angelis, A.; Chrysohoou, C.; Tzorovili, E.; Laina, A.; Xydis, P.; Terzis, I.; Ioakeimidis, N.; Aznaouridis, K.; Vlachopoulos, C.; Tsioufis, K. The Mediterranean diet benefit on cardiovascular hemodynamics and erectile function in chronic heart failure male patients by Decoding Central and peripheral vessel rheology. Nutrients 2020, 13, 108. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Fernández-Friera, L.; López-Melgar, B.; Uzhova, I.; Oliva, B.; Fernández-Alvira, J.M.; Laclaustra, M.; Pocock, S.; Mocoroa, A.; Mendiguren, J.M.; et al. Association between a social-business eating pattern and early asymptomatic atherosclerosis. J. Am. Coll. Cardiol. 2016, 68, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Uzhova, I.; Mateo-Gallego, R.; Moreno-Franco, B.; Molina-Montes, E.; Leon-Latre, M.; Lenguas, J.A.C.; Civeira, F.; Peñalvo, J.L. The additive effect of adherence to multiple healthy lifestyles on subclinical atherosclerosis: Insights from the AWHS. J. Clin. Lipidol. 2018, 12, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Frölich, S.; Lehmann, N.; Weyers, S.; Wahl, S.; Dragano, N.; Budde, T.; Kälsch, H.; Mahabadi, A.A.; Erbel, R.; Moebus, S.; et al. Association of Dietary Patterns with five-year degree and progression of coronary artery calcification in the Heinz Nixdorf Recall Study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 999–1007. [Google Scholar] [CrossRef]
- Whelton, S.P.; Silverman, M.G.; McEvoy, J.W.; Budoff, M.J.; Blankstein, R.; Eng, J.; Blumenthal, R.S.; Szklo, M.; Nasir, K.; Blaha, M.J. Predictors of long-term healthy arterial aging. JACC Cardiovasc. Imaging 2015, 8, 1393–1400. [Google Scholar] [CrossRef]
- Višković, K.; Rutherford, G.W.; Sudario, G.; Stemberger, L.; Brnić, Z.; Begovac, J. Ultrasound measurements of carotid intima-media thickness and plaque in HIV-infected patients on the Mediterranean diet. Croat. Med. J. 2013, 54, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgullu, C. The relation between compliance to the Mediterranean diet and the extensiveness of coronary artery disease. Turk Kardiyol. Dern. Ars. Arch. Turk. Soc. Cardiol. 2015, 43, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, M.G.; Sánchez, L.G.; Patino-Alonso, M.C.; Alonso-Domínguez, R.; Sánchez-Aguadero, N.; Lugones-Sánchez, C.; Sánchez, E.R.; Ortiz, L.G.; Gómez-Marcos, M.A. Adherence to the Mediterranean diet in Spanish population and its relationship with early vascular aging according to sex and age: Eva Study. Nutrients 2020, 12, 1025. [Google Scholar] [CrossRef] [Green Version]
- Cowell, O.R.; Mistry, N.; Deighton, K.; Matu, J.; Griffiths, A.; Minihane, A.M.; Mathers, J.C.; Shannon, O.M.; Siervo, M. Effects of a Mediterranean diet on blood pressure: A systematic review and meta-analysis of randomized controlled trials and observational studies. J. Hypertens. 2021, 39, 729–739. [Google Scholar] [CrossRef]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vacca-rino, V. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean diet: From dietary sources to microrna modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: A narrative review of the evidence. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the predimed. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Mendes Furtado, M.; Lima Rocha, J.É.; da Silva Mendes, A.V.; Mello Neto, R.S.; Brito, A.K.; Sena de Almeida, J.O.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Cunha Sales, A.L.; Gomes Vasconcelos, A.; et al. Effects of ω-3 PUFA-rich oil supplementation on cardiovascular morphology and aortic vascular reactivity of adult male rats submitted to an hypercholesterolemic diet. Biology 2022, 11, 202. [Google Scholar] [CrossRef]
- As’ari, H. Decrease of LDL Cholesterol through the Increase of HDL Cholesterol by Administering Garcinia mangostana L. Peel Extract in White Mice. Folia Med. Indones. 2021, 57, 298–302. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Hassan, M.; Al-Qaba, S.F. Protective Effect of Fermented Camel Milk Containing Bifidobacterium longum BB536 on Blood Lipid Profile in Hypercholesterolemic Rats. J. Nutr. Metab. 2021, 2021, 1557945. [Google Scholar] [CrossRef]
- Hébert, J.R.; Frongillo, E.A.; Adams, S.A.; Turner-McGrievy, G.M.; Hurley, T.G.; Miller, D.R.; Ockene, I.S. Perspective: Randomized controlled trials are not a panacea for diet-related research. Adv. Nutr. 2016, 7, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Childers, A. A Prospective Study of Mediterranean Diet and Cognitive Decline. Ph.D. Thesis, Appalachian State University, Boone, NC, USA, 2018. [Google Scholar]
- Obeid, C.A.; Gubbels, J.S.; Jaalouk, D.; Kremers, S.P.; Oenema, A. Adherence to the Mediterranean diet among adults in Mediterranean countries: A Systematic Literature Review. Eur. J. Nutr. 2022, 61, 3327–3344. [Google Scholar] [CrossRef]
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean diet and Cardiovascular Risk Factors: A systematic review. Crit. Rev. Food Sci. Nutr. 2013, 54, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Polychronopoulos, E.; Chrysohoou, C.; Zampelas, A.; Trichopoulou, A. Can a Mediterra-nean diet moderate the development and clinical progression of coronary heart disease? A systematic review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004, 10, RA193–RA198. [Google Scholar]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 58, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Type 2 Diabetes; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/diabetes/basics/type2.html#:~:text=Type%202%20diabetes%20most%20often,adults%20are%20also%20developing%20it (accessed on 15 June 2022).
- Centers for Disease Control and Prevention. Just Diagnosed with Type 1 Diabetes; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/diabetes/basics/diabetes-type-1-diagnosis.html#:~:text=Did%20You%20Know%3F,older%20(even%20over%2040) (accessed on 23 June 2022).
- Overcash, F.; Crusan, A. Substitution modeling shows simple dietary changes increase Mediterranean-style diet pattern scores for U.S. adults. Curr. Dev. Nutr. 2022, 6 (Suppl. 1), 936. [Google Scholar] [CrossRef]
| Food Group | Bioactive Compounds | Association with CVD Risk Factors |
|---|---|---|
| Fruits | Polyphenols 3 Fiber 2 Ascorbic acid 6 Vitamin C 6 Myricetin 6 Quercetin 6 Anthocyanins 6 Cyanidins 6 Flavonols 6 Ascorbic Acid 6 | Decrease CVD 1 Decrease cholesterol 6 Inhibit LDL-C oxidation 6 Decrease Blood Pressure 6 |
| Vegetables | Polyphenols 3 Fiber 2 Carotenoids 6 Vitamin C 6 Lycopene 6 | Decrease CVD 1 Decrease Blood Pressure 3 Decrease cholesterol 6 Inhibit LDL-C oxidation 6 |
| Whole Grains | Phytochemicals 6 Fiber 3 K+, Mg2+, and Ca2+ 3 | Decrease Cholesterol 3 Increase Glycemic Control 3 Reduce T2DM Risk 3 Decrease Blood Pressure 3 Decrease Adiposity 3 |
| Nuts | Fiber 3 K+, Mg2+, and Ca2+ 3 Phytosterols 6 Linolenic acids 2 Tocopherols 6 Omega-3s 6 | Decrease Cholesterol 3 Decrease Triglycerides 3 Decrease Blood Pressure 3 Reduce T2DM Risk 3 |
| Legumes | Polyphenols 6 Fiber 3 K+, Mg2+, and Ca2+ 3 | Decrease CVD 1 Decrease Blood Pressure 3 Decrease cholesterol 6 |
| Olive Oil | Polyphenols 3 Phytosterols 3 MUFA 3 | Decrease CVD 1 Decrease Adiposity 3 Decrease Blood Pressure 3 Inhibit LDL-C oxidation 6 Decrease Inflammation 3 Reduce T2DM Risk 3 |
| Fish | Omega-3s 6 | Decrease Triglycerides 3 Increase HDL 3 Decrease cholesterol 6 Inhibit LDL-C oxidation 6 Decrease Blood Pressure 6 |
| Red Wine (Moderate) | Polyphenols 3 Resveratrol 2 Myricetin 6 Quercetin 6 Anthocyanins 6 Cyanidins 6 Flavonols 6 | Decrease Adiposity 3 Decrease Blood Pressure 3 Decrease Inflammation 3 Reduce T2DM Risk 3 |
| Meat and meat products § | L-carnitine 7 L-carnosine 7 Choline 7 Lipoic acid 7 Conjugated dienes of linoleic acid (CLA) 7 Glutathione 7 Taurine 7 Coenzyme Q10 7 Creatine 7 | Decrease Blood Pressure 7 Decrease Inflammation 7 Reduction of Fat Mass 7 |
| Dairy | Calcium 5 Conjugated linoleic acid 5 Glycomacropeptide 5 Lactoferrin 5 β–Lactoglobulin 5 | Decrease Blood Pressure 4 Decrease Inflammation 4 Reduce T2DM Risk 4 |
| Author/Year [Location] | Study Design/Length | Intervention | Participants/Major Cohort | Comparison Group | Diabetes: Y/N | Clinical Measurements | Outcome Associated w/Higher MedDiet Adherence |
|---|---|---|---|---|---|---|---|
| Medina-Remon et al. (2017) [Spain] [17] | RCT, 1y | MD-EVOO, MD-Nuts, LFD | High CVD Risk, 55–80 yo men & 60–80 yo women (n = 1139)/PREDIMED | Baseline Comparison | Y | Urine Total Polyphenols (UTP), SBP, DBP, Fasting Glucose, TC, LDL-C, HDL-C, TG, VCAM-1, ICAM-1, IL-6, TNF-α, MCP-1 | UTP T1: ↓UTP, ⦸SBP, ⦸DBP, ⦸Fasting Glucose, ⦸TC, ↓LDL-C, ↑HDL-C, ⦸TG, ⦸VCAM-1, ⦸ICAM-1, ⦸IL-6, ⦸TNF-α, ⦸MCP-1 UTP T2: ↑UTP, ↓SBP, ↓DBP, ⦸Fasting Glucose, ⦸TC, ↓LDL-C, ↑HDL-C, ⦸TG, ⦸VCAM-1, ⦸ICAM-1, ↓IL-6, ↓TNF-α, ⦸MCP-1 UTP T3: ↑UTP, ↓SBP, ↓DBP, ⦸Fasting Glucose, ⦸TC, ↓LDL-C, ↑HDL-C, ⦸TG, ↓VCAM-1, ↓ICAM-1, ↓IL-6, ↓TNF-α, ↓MCP-1 |
| Torres-Pena et al. (2018) [Cordoba, Spain] [18] | RCT, 1.5y | MedDiet v. LFD | CHD Patients w/no events in the last 6 months, 20–75 yo, life expectancy of at least 5 years, prediabetes (n = 289), T2D (n = 438), and no T2D (n = 78) (n = 805)/CORDIOPREV | LFD (n = 387) | Y | FMD | ↑FMD in MedDiet + Prediabetes and MedDiet + Diabetes (Baseline to 1.5y) MedDiet v. LFD in patients with Diabetes: ↑FMD MedDiet & LFD in patients w/o Diabetes: ⦸ FMD |
| Casas et al. (2018) [Barcelona, Spain] [30] | RCT, 5y | MD-EVOO, MD-Nuts, LFD | High CVD Risk, 55–80 yo men & 60–80 yo women (n = 160)/PREDIMED | LFD (n = 52) | Y | CVD Risk factors: SBP, DBP, TGs, Total-C, HDL-C, LDL-C, Total:HDL-C, Glucose, Glycated hemoglobin, Wt, BMI, WC T-lymphocytes: CD11a, CD49d, & CD40 Monocytes: CD11a, CD11b, CD49d, & CD40 Circulating Markers: MCP-1, IL-6, TNF-α, & hs-CRP | CVD Risk Factors: Baseline v. 5y: ↓SBP, DBP, LDL-C, Total-C, Total:HDL-C, TG, Wt, BMI, & WC; ↑HDL-C; ⦸Glucose, Glycated Hemoglobin, 5y: MD v. LFD: ↓SBP, DBP, LDL-C, Wt, & BMI ⦸ TG, Total-C, HDL-C, Total-HDL-C, Glucose, Glycated Hemoglobin, & WC T-Lymphocytes: Baseline v. 5y: ↓CD11a, CD49d, & CD40 5y MD v. LFD: ↓CD11a, CD49d, & CD40 Monocytes: Baseline v. 5y: ↓CD11a, CD11b, CD49d, & CD40 5y MD v. LFD: ↓CD11a, CD11b, CD49d, & CD40 Circulating Markers: Baseline v. 5y: ↓MCP-1, IL-6, TNF-α, & hs-CRP 5y MD v. LFD: ↓MCP-1, IL-6, TNF-α, & hs-CRP |
| Camargo et al. (2012) [Spain] [51] | RCT-Crossover, 9w | MD-VOO, SFA-Rich, CHO-PUFA | Healthy, elderly (n = 20) | SFA-Rich and CHO-PUFA | N | Inflammation Genes: NF-kB (p65 & IkBα), MCP-1, TNF-α, IL-6 Plaque Stability Gene: MMP-9 & MIF-1 | Compared to SFA-Rich: Fasting: ↓p65, ⦸NF-kB (IkBα), MCP-1, TNF-α, IL-6, MIF-1, MMP-9 Postprandial: ↓p65, MCP-1, MMP-9 ↑IkBα, ⦸TNF-α, IL-6, Compared to CHO-PUFA-Rich: Fasting: ⦸ All measures Postprandial: ↓p65 & TNF-α, ↑IkBα, ⦸MCP-1, IL-6, MMP-9, & MIF-1 |
| Di Renzo et al. (2018) [Rome, Italy] [52] | RCT, 2h | Only: (a) Red Wine (RW), (b) White Wine (WW), (c) Vodka (V); Only: (d) MedDiet or (e) a High-Fat Meal (HFM); Combination: (f)MedDiet + RW (g) MedDiet + WW (h) MedDiet + V (i) HFM + RW (j) HFM + WW (k) HFM + V | 18–65 yo, BMI between 18.5–35 kg/m2, otherwise healthy adults (n = 55) | Baseline Comparison | N | oxLDL-C Gene expression: Catalase (CAT), superoxide dismutase 2 (SOD2), and glutathione peroxidase 1 (GPX1) | Baseline to post-intervention: ↑ oxLDL-C (HFM-only), ⦸ All other beverages, ⦸ All diet, and ⦸All combinations; Among treatments: MedDiet v. HFM: ↓oxLDL-C (MedDiet), ↑oxLDL-C (HFM), MedDiet + RW v. HFM: ↓oxLDL-C (MedDiet + RW), ↑oxLDL-C (HFM) CAT Regulation: ↑RW, ↓WW, V, HFM + WW, & HFM+V SOD2 Regulation: ↑ WW, MedDiet + V, and RW ↓HFM+V GPX1 Regulation: ↑RW, MedDiet+RW, and HFM+RW |
| Storniolo et al. (2017) [Reus and Barcelona, Spain] [53] | RCT, 1y | MD-EVOO, MD-Nuts, LFD | Non-smoker, hypertensive women, 60–80 yo, not consuming non-steroidal anti-inflammatory drugs w/o CVD, but at high risk of CVD (n = 90)/PREDIMED | LFD (n = 30) | Y | BP, serum nitric oxide (NO) and endothelin-1 (ET-1) Antioxidant capacities: total antioxidant capacity (TAC), malondialdehyde (MDA) Gene expression: endothelial NO synthase (eNOS), caveolin 2 (CAV2) and endothelin-1 receptors (ETAR and ETBR) | MD-Nuts: ↓DBP, serum ET-1 MD-EVOO: ↑NO; ⦸ antioxidant capacity and MDA Change in Gene Expression: MD-Nuts: ↓eNOS, CAV2, ET-1 receptors (A and B); MD-EVOO: ↓CAV2 and ET-Receptor B |
| Urpi-Sarda et al. (2021) [Barcelona and Valencia, Spain] [54] | RCT, 3y | MD-EVOO, MD-Nuts, LFD | High CVD Risk, 55–80 yo men & 60–80 yo women (n = 285)/PREDIMED | LFD (n = 100) | Y | Plasma Markers: CRP, IL-1β, IL-6, IL-8, IL-12p70, IL-18, TNF-α, MCP-1, RANTES/CCL5, MIP-1β/CCL4, IP-10/CXCL10, ENA78/CXCL5, I-TAC/CXCL11 & IFN-γ Genes: TLR2, TLR4, TLR6, NLRP3, CASP-1, IL1R1, CCR2, CCR5, CXCR2, CXCR3 | ↓ CRP, IL-1β, IL-6, IL-8, IL-12p70, TNF-α, MCP-1, RANTES/CCL5, MIP-1β/CCL4, ENA78/CXCL5, & IFN-γ ⦸ IP-10, I-TAC, & IL-18 ⦸ All Genes |
| Casas et al. (2016) [Barcelona, Spain] [55] | RCT, 1y | MD-EVOO, MD-Nuts, LFD | High CVD Risk, 55–80 yo men & 60–80 yo women (n = 164)/PREDIMED | LFD (n = 54) | Y | CVD Risk factors: SBP, DBP, TGs, Total-C, HDL-C, LDL-C, Total:HDL-C, Glucose, Glycated hemoglobin, Wt, BMI, WCT-lymphocytes: CD11a, CD49d, CD40 Monocytes: CD11a, CD11b, CD49d, & CD40 Circulating Markers: sVCAM, sICAM, sE-SEL, sP-SEL, IL-6, CRP, IL-18, IL-10, IL-18/IL-10 Ratio, MMP-9, TIMP-1, MMP-9/TIMP-1 ratio, TGF-β1 | CVD Risk Factors: Baseline v. 1y: ↓WC, SBP, DBP, Total-C, LDL-C, & Total:HDL-C; ⦸WT, BMI, Glucose, Glycated Hemoglobin, TG, HDL-C MD v. LFD: ↓SBP, DBP, Total-C, LDL-C, Total:HDL-C; ⦸Wt, BMI, WC, Glucose, Glycated Hemoglobin, TG, & HDL-C T-Lymphocytes: Baseline v. 1y:↓ CD11a, CD49d, CD40 MD v. LFD: ⦸CD11a, CD49d, CD40 Monocytes: Baseline v. 1y: ↓CD40, CD11a, CD11b, & CD49d MD v. LFD: ↓CD40 ⦸CD11a, CD11b, & CD49d Circulating Markers: Baseline v. 1y:↓sVCAM, sICAM, sE-SEL, sP-SEL, IL-6, CRP, IL-18, IL-18/IL-10 ratio; ↑ TGF-β1; ⦸ IL-10, MMP-9, TIMP-1, MMP-9/TIMP-1 ratio MD v. LFD: ↓sP-SEL, IL-6, CRP, IL-18/IL-10 Ratio |
| Castaner et al., 2013 [Spain] [56] | RCT, 3m | MD-EVOO, MD-Nuts, LFD | men aged 55–80 y, women aged 60–80 y with at least one of the following criteria: (1) T2D or (2) 3 or more CVD risk factors [current smoking, hypertension (BP > 140/90 mm Hg or treatment with antihypertensive drugs), LDL-C concentration > 160 mg/dL (or treatment with hypolipidemic drugs), HDL-Cconcentration < 40 mg/dL, BMI (in kg/m2) > 25, or a family history of premature CAD (n = 34)/PREDIMED | LFD (n = 12) | Y | BMI, WC, SBP, DBP, Glucose, TC, LDL-C, HDL-C, TG, ApoA-I, Apo B-100, OxLDL, CRP, Hydroxytyrosol, IL1β, IL1RN, TNF-α, ICAM1, VEGF Signaling Pathways: Role of NFAT in Cardiac Hypertrophy, P2 gamma Purigenic Receptor Signaling Pathway, Hypoxia Signaling in the CVD, Cardiac Hypertrophy Signaling, Renin-Angiotensin Signaling, Inhibition of Angiogenesis by TSP1, Angiopoietin Signaling, Nitric Oxide Signaling in the CVS, Atherosclerosis Signaling, eNOS Signaling, Factors Promoting Cardiogenesis in Vertebrates, Aldostrone Signaling in Epithelial Cells, Cardiac Beta-adrenergic Signaling, HIF1alpha Signaling, Cardiomyocyte Differentiation via BMP Receptors, Thrombin Signaling, Endothelin-1 signaling, and Cellular Effects of Sildenafil | ↓BMI (MD-Nuts & MD-EVOO), ↓WC (MD-Nuts), ↓SBP (MD-VOO), ⦸DBP, ⦸Glucose, ⦸TC, ⦸LDL-C, ⦸HDL-C, TG, ⦸ApoA-I, ⦸Apo B-100, ⦸OxLDL, ⦸CRP, ⦸Hydroxytyrosol Signaling Pathways: (MD-EVOO + MD-Nuts):↓Hypoxia Signaling in the CVS, ↓eNOS Signaling, ↓Nitric Oxide Signaling in the CVS, ↓Renin-Angiotensin Signaling, ↓Aldosterone Signaling in Epithelial Cells, ↓P2 gamma Purigenic Receptor Signaling Pathway, ↓Cardiac Hypertrophy Signaling MD-EVOO (only): ↓Atherosclerosis, ↓Nitric Oxide Signaling in the CVS, ↓Angiopoietin Signaling, ↓Renin-Angiotensin Signaling, ↓Role of NFAT in Cardiac Hypertrophy, and ↓Cardiac Hypertrophy Signaling |
| Duś-Zuchowska et al. (2018) [57] [Poland] | RCT, 16w | MedDiet v. Central European Diet (CED) | Obese, postmenopausal women, nonsmokers with a risk of Metabolic Syndrome (MS) (n = 144) | Baseline Comparison | N | hs-CRP and asymmetrical dimethylarginine (ADMA) | Within group comparisons: ↓hs-CRP Between Group Comparison: ⦸hs-CRP Within group comparisons: ↓ADMA (CED-only) Between Group Comparison: ⦸ADMA |
| Marin et al. (2011) [58] [Spain] | RCT, Crossover, 4w | saturated fatty acid (SFA) diet; a LFHC diet; and a MedDiet | Free living, elderly (>65 yo), free of chronic illness (hepatic, renal, thyroid, or cardiac dysfunction) (n = 20) | Cross comparison | Y | TC, TG, HDL-C, LDL-C, ApoA-I, ApoB, ischemic reactive hyperemia (IRH), Superoxide dismutase activity, β-Carotene, Catalase activity, Isoprostane, Lipoperoxides, ⍺-Tocopherol, OxLDL, Nitric Oxide, Protein Carbonyl activity, Nitrotyrosine, Glutathione peroxidase activity Total MPs, Apoptotic EMPs, Activated EMPs, %EPCs | ↓TC, LDL-C, ApoB, Superoxide dismutase activity, Isoprostane, Lipoperoxides, and Nitrotyrosine ↑IRH, β-Carotene ⦸ TG, HDL-C, ApoA-I, Catalase Activity, ⍺-Tocopherol, OxLDL, Nitric Oxide, Protein Carbonyl activity ↓ Total MPs, Apoptotic EMPs, Activated EMPs, ↑ %EPCs |
| Yubero-Serrano et al. (2020) [Cordoba, Spain] [59] | RCT, 1y | MedDiet v. LFD | CHD Patients w. no events in the last 6 months, 20–75 yo, life expectancy of at least 5 years (n = 805)/CORDIOPREV | LFD (n = 387) | Y | Flow-Mediated Dilation (FMD), endothelial microparticles (EMPs), and endothelial progenitor cells (EPCs) | When FMD <2% and FMD>2%: MedDiet v. LFD: ↑FMD, ↓EMP (Activated & Apoptotic), and ↑EPC MedDiet v. LFD: ⦸BMI, ⦸LDL-C, ↑HDL-C, ⦸TC, ⦸TG, ↓Fasting Glucose, ⦸Fasting Insulin, ⦸HbA1c, ↓hsCRP ↓EMPs, ROS, cellular apoptosis, senescence ↑ cellular proliferation and angiogenesis |
| Maiorino et al. (2016) [Naples, Italy] [60] | RCT, 8.1y | MedDiet v. LFD | Men and women with newly diagnosed T2D, overweight, never treated with antihyperglycemic drugs, HbA1c levels <11% (n = 215)/MEDITA | LFD (n = 107) | Y | endothelial progenitor cells (EPCs) and cIMTWeight, WC, HbA1c, Plasma Glucose, HOMA of insulin sensitivity, TC, HDL-C, Non-HDL-C, SBP, DBP, cIMT, CRP | MedDiet (Baseline, Year 2, Year 4, EOT) and compared to LFD(starting at Year 2): ↑CD34+KDR+, ↑CD34+KDR+CD133+ MedDiet: ↓cIMT; LFD: ⦸cIMT MedDiet (v. LFD): ↓Weight, WC, HbA1c, HOMA-IS, TC, SBP, ↑HDL-C |
| Carnevale et al. (2014) [Italy] [61] | RCT, Crossover, 4w | Study 1: MedDiet w/EVOO and MedDiet w/o EVOO Study 2: MedDiet w/EVOO and MedDiet w/Corn Oil | Healthy adults working in research institute (n = 25) | Cross comparison | N | Platelet reactive oxidant species (ROS) and 8-iso-PGF2a-III, activity of NOX2, the catalytic sub-unit of NADPH oxidase, as assessed in platelets and serum, serum vitamin E and endothelial dysfunction | Study 1: MedDiet w/o EVOO: ↑platelet ROS, 8-iso-PGF2a-III, NOX2 activity, sE-selectin, sVCAM1 and ↓serum vitamin E; ⦸ in all measures for MedDiet + EVOO Study 2: Corn Oil: ↑platelet ROS, 8-iso-PGF2a-III, NOX2 activity, sE-selectin, sVCAM1 and ↓serum vitamin E; ⦸ in all measures for MedDiet + EVOO |
| Gudban et al. (2021) [Israel] [62] | RCT, 3m | MedDiet Intervention v. No intervention | T2D patients, 18yo+, adults, otherwise healthy (no other chronic diseases or conditions) (n = 22) | No intervention (n = 10) | Y | BMI, FMD%, CRP, ICAM-1, TC, TG, and HbA1c | ⦸ BMI, HbA1c, TC, TG ↓ CRP, ICAM-1 ↑FMD% |
| Murie-Fernandez et al. (2011) [Navarra, Spain] [63] | RCT, 1y | MD-EVOO, MD-Nuts, LFD | High CVD Risk, 55–80 yo men & 60–80 yo women (n = 187)/PREDIMED | LFD (n = 62) | Y | Carotid intima-media thickness (cIMT) | 1-year change: ↓cIMT in MD+Nuts, ⦸ MD+EVOO, ⦸LFDAdjusted 1 year change: ↓cIMT in MD+Nuts and ↓MD+EVOO, ⦸LFD Univariate & Multivariate analysis: When cIMT >= 0.9 mm, MD-EVOO + MD-Nuts: ↓cIMT, ⦸LFD; For Multivariate Analysis: When cIMT >= 0.9 mm, MD-EVOO ↓cIMT and MD-Nuts ↓cIMT, ⦸LFD When cIMT < 0.9 mm, ⦸All diets |
| Sala-Vila et al. (2014) [Spain] [64] | RCT, 2.4y | MD-EVOO, MD-Nuts, LFD | High CVD Risk, 55–80 yo men & 60–80 yo women (n = 164)/PREDIMED | LFD (n = 61) | Y | plaque height and cIMT of three prespecified segments (ICA, bifurcation (BIF), and common (CCA)) | LFD: ↑ICA-IMT(Mean), ↑ plaque (max); MD-Nuts: ↓ICA-IMT (mean) ⦸ ICA-IMT (Max) and Plaque (Max); MD-EVOO: ⦸ICA-IMT (Mean), ICA-IMT (Max), and Plaque (MAX) ⦸ Between Group Differences: CCA-IMT (Max and Mean), BIF-IMT (Max and Mean) |
| Authors, Year [Location] | Study Design/Length | Participants | Diabetes: Y/N | Clinical Measurements | Outcome Associated w/Higher MedDiet Adherence |
|---|---|---|---|---|---|
| Antoniazzi et al. (2021) [Brazil and Spain] [15] | Cross section | Confirmed Familial Hypercholesterolemia or LDL receptor variants, ≥20 yo (n = 190) | N | plasma LDL-C, apolipoprotein-B (ApoB), high sensitivity C-reactive protein (hs-CRP) | ⦸ LDL-C, ↓ApoB, ↓hs-CRP |
| Buscemi et al. (2013) [Italy] [16] | Cross section | No DM, CHD, or Renal Failure, ≥18 yo (n = 929) | N | cIMT, HOMA-IR, Triglycerides (Tri)/HDL-C, asymptomatic carotid atherosclerosis (PC) | ⦸ cIMT, ↓HOMA-IR, ↓Tri/HDL-C, ⦸ asymptomatic carotid atherosclerosis (plaques and/or cIMT ≥ 0.9 mm) |
| Barrea et al. (2019) [Italy] [65] | Cross section | Healthy, normal weight, 18–50 yo (n = 302) | N | TMAO | ↓TMAO |
| Cesari et al. (2018) [Italy] [66] | Cross section | >90 yo, men and women in Mugello area, Tuscany, Italy (n = 421) | N | endothelial progenitor (EPCs) and circulating progenitor (CPCs) cells | 4th MedDiet Quartile v. Other Quartiles: ↑ EPCs (CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+) ⦸ CPCs (CD34+, CD133+, CD34+/CD133+) |
| Pignanelli et al. (2018) [Ontario, Canada] [67] | Prospective, 1y | Stroke, transient ischemic attack, and/or atherosclerotic patients (n = 276) | N | Total plaque area (TPA), trimethylamine N-oxide (TMAO), p-cresyl sulfate (PCS), hippuric acid (HA), indoxyl sulfate (IS), p-cresyl glucuronide (PCG), phenyl acetyl glutamine (PAG), & phenyl sulfate (PS) | ⦸ TPA, TMAO, PCS, HA, IS, PCG, PAG, & PS |
| Shah et al. (2020) [USA] [68] | Cross section | Women enrolled in American Heart Association Go Red for Women Strategically Focused Research Network at Columbia University Irving Medical Center, BMI 25–33 kg/m2 or BMI 20–25 kg/m2 w/o immediate fam history of obesity, hypertension, or DM; absence of chronic diseases and chornic disease medication (n = 25) | N | NF- κB and eNOS | ⦸NF- κB and eNOS |
| Witkowska & Zujko (2014) [Poland] [69] | Cross section | 19–22 yo, women, BMI ≤ 25 kg/m2, without inflammatory, autoimmune, or metabolic diseases (n = 25) | N | sICAM-1, sVCAM-1, and E-selectins | ⦸ VCAM-1 and ICAM-1 ↓sE-selectin |
| Millar et al. (2021) [Ireland] [70] | Cross section | Clinical random sample, 46–73 yo, White European (n = 1862) | Y | Plasma Lipids: TotChol, TG, LDL-C, HDL-C Lipoprotein particle concentrations: TotTRL, S-, M-,&L-VLDL; S-, L- & T-LDL; S-, M-, L-, T-HDL; IDL Lipoprotein particle size: VLDL, LDL, HDL, LP-IR | ⦸ All Plasma Lipids ⦸ Lipoprotein Particle Concentrations ⦸Lipoprotein particle size |
| Mattioli et al. (2017) [Italy] [71] | Retrospective, 7d | Asymptomatic for CVD, premenopausal women, 45–54 yo (n = 425) | N | ABI | ↑ABI (normal range) |
| Woo et al. (2018) [China] [72] | Cross section | Healthy, ≥65 yo (n = 4000) | N | ABI (ankle brachial index) | ⦸ ABI |
| Gardener et al. (2014) [USA] [73] | Cross section | No stroke history, resided in Northern Manhattan, >40 yo (n = 1374) | N | cIMT, Plaque Presence, Plaque Thickness, and Total Plaque Area (TPA) | ⦸ cIMT, ⦸ Plaque Presence, ↓Plaque Thickness, ↓TPA |
| Angelis et al. (2020) [Athens, Greece] [74] | Cross section | Clinically stable congestive heart failure (CHF) Males, 18 yo+, left ventricular ejection fraction less than or equal to 40%, symptoms according to New York Heart Association class II or higher, and taking medication (n = 150) | Y | PWV: Pulse Wave Velocity; AIx: Augmentation index; cIMT: Carotid Intima Media Thickness; EF: Ejection Fraction; SRV: Systolic Wave of Tricuspid Annulus; LA: Left atrium; GLPS: global longitudinal strain of the left ventricle, VO2 Max, VE/VCO2, Pulse Pressure | ↓cIMT, AI x ↑SRV ⦸all other variables |
| Peñalvo et al. (2016) [Spain] [75] | Cross section | Free of clinical CVD, 40–54 yo (n = 4082) [Progression of Early Subclinical Atherosclerosis] | N | Agatston Score (AG), CAC, Any Plaque Presence, Plaque in Aorta (PA), Carotids (PC), Femorals (PF), & Iliac (PI) | (When compared to Western and Social-Business DP):↓AG, ↓CAC Presence, ↓Any Plaque, ↓PA, PC, PF, & PI |
| Uzhova et al. (2018) [Madrid, Spain] [76] | Cross section | Men, 40–55 yo (n = 1798) | N | CACS, Plaque in Femorals (PF) and/or Carotids (PC), and Atherosclerosis | ↓PF, ⦸CACS, ⦸PC, ↓Atherosclerosis |
| Frölich et al. (2017) [Germany] [77] | Prospective, 5y | Free of Clinical CHD, 45–75 yo (n = 3718) | Y | CAC | ↓CAC progression & CAC degree |
| Whelton et al. (2015) [USA] [78] | Prospective, 9.6y | Free of clinical CVD, Multi-ethnic, 45–84 yo (n = 1850) | N | CAC | ⦸ CAC |
| Viskovic et al. (2013) [Zagreb, Croatia] [79] | Cross section | HIV-infected on ART for 12-months or more, and non-HIV infected, adults 18+ yo, no other serious health conditions or medications (n = 241) | N | Subclinical atherosclerosis (defined as CIMT ≥ 0.9 mm and/or the presence of ≥1 carotid plaque) | HIV-Infected: ↓subclinical atherosclerosis (cIMT ≥ 0.9 mm or carotid plaques) |
| Akgüllü et al. (2015) [Turkey] [80] | Cross section | CAD Diagnosis, 35–80 yo (n = 200) | N | GS (Gensini Score) | ↓GS |
| Gomez Sanchez et al. (2020) [Spain] [81] | Cross section | 35–75 yo, without CVD or any other chronic conditions (n = 500) | Y | early vascular aging (vascular damage in carotid arteries or peripheral artery disease were classified as EVA and subjects at the percentile of the combined Vascular Aging Index (VAI) were classified; ≥p90 was considered EVA and <p90 was considered normal vascular aging (NVA)), carotid-femoral pulse wave velocity (cfPWV), and cIMT | ↑MedDiet: ↓EVA ↑NVA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, L.A.; Izuora, K.; Basu, A. Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 12762. https://doi.org/10.3390/ijerph191912762
Richardson LA, Izuora K, Basu A. Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(19):12762. https://doi.org/10.3390/ijerph191912762
Chicago/Turabian StyleRichardson, Leigh Ann, Kenneth Izuora, and Arpita Basu. 2022. "Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 19: 12762. https://doi.org/10.3390/ijerph191912762





