Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia coli and Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Strains
2.3. Preparation of SAMs
2.4. Characterization of Morphology
2.5. Assessment of Loading Capacity
2.6. Determination of Minimum Inhibitory and Bactericidal Concentrations
2.7. Growth Inhibition Tests
2.8. Time-Kill Assays
2.9. Antibacterial Efficacy of SAMs in Hand Sanitizers
2.10. Analysis of Cell Wall Permeability
2.11. Measurement of Cell Membrane Permeability
2.12. Data Analysis
3. Results and Discussion
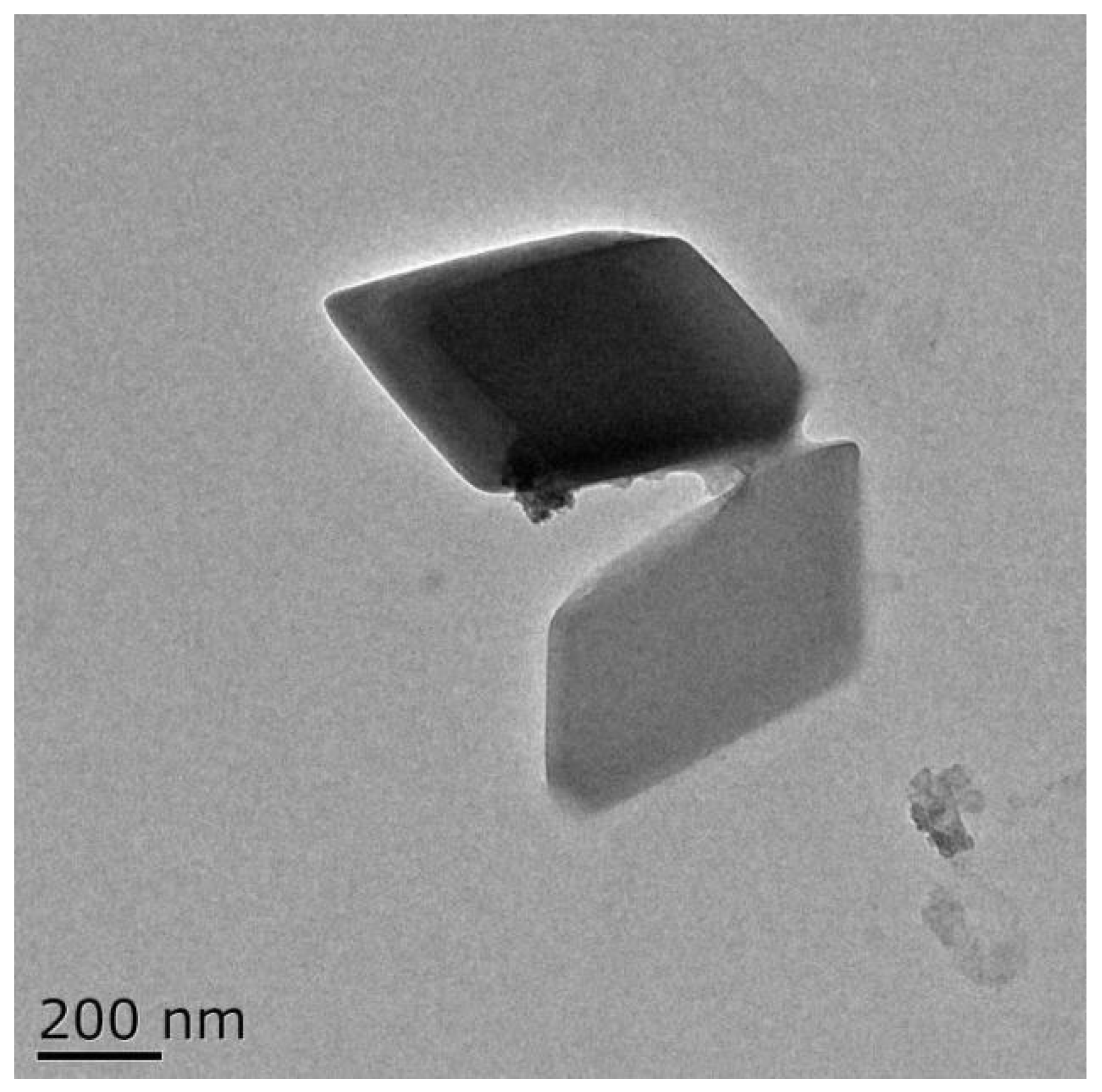
3.1. TEM Morphology of SAMs
3.2. Loading Capacity of SAMs
3.3. MIC and MBC Values of SAMs
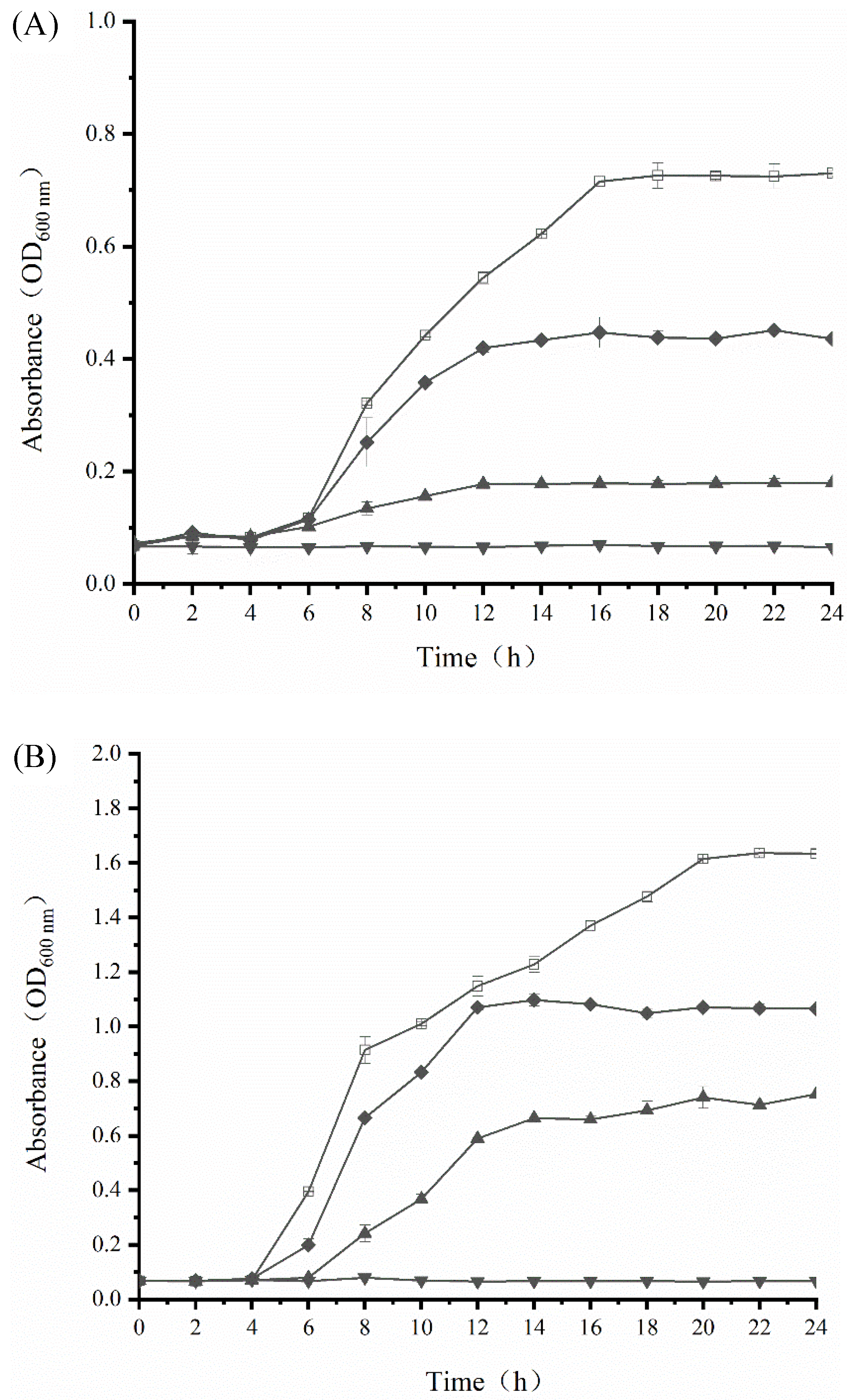
3.4. Growth Inhibition Activity of SAMs
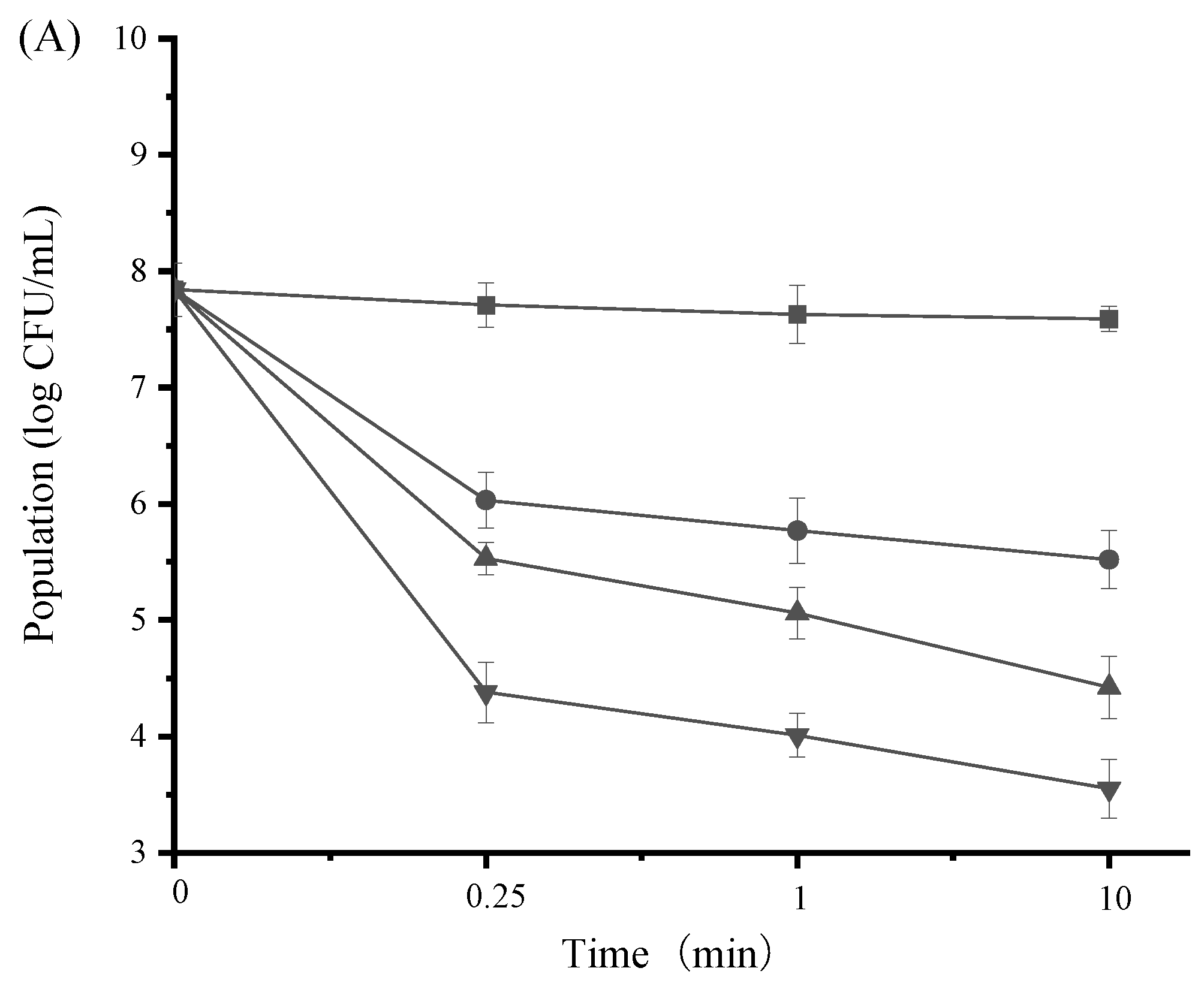
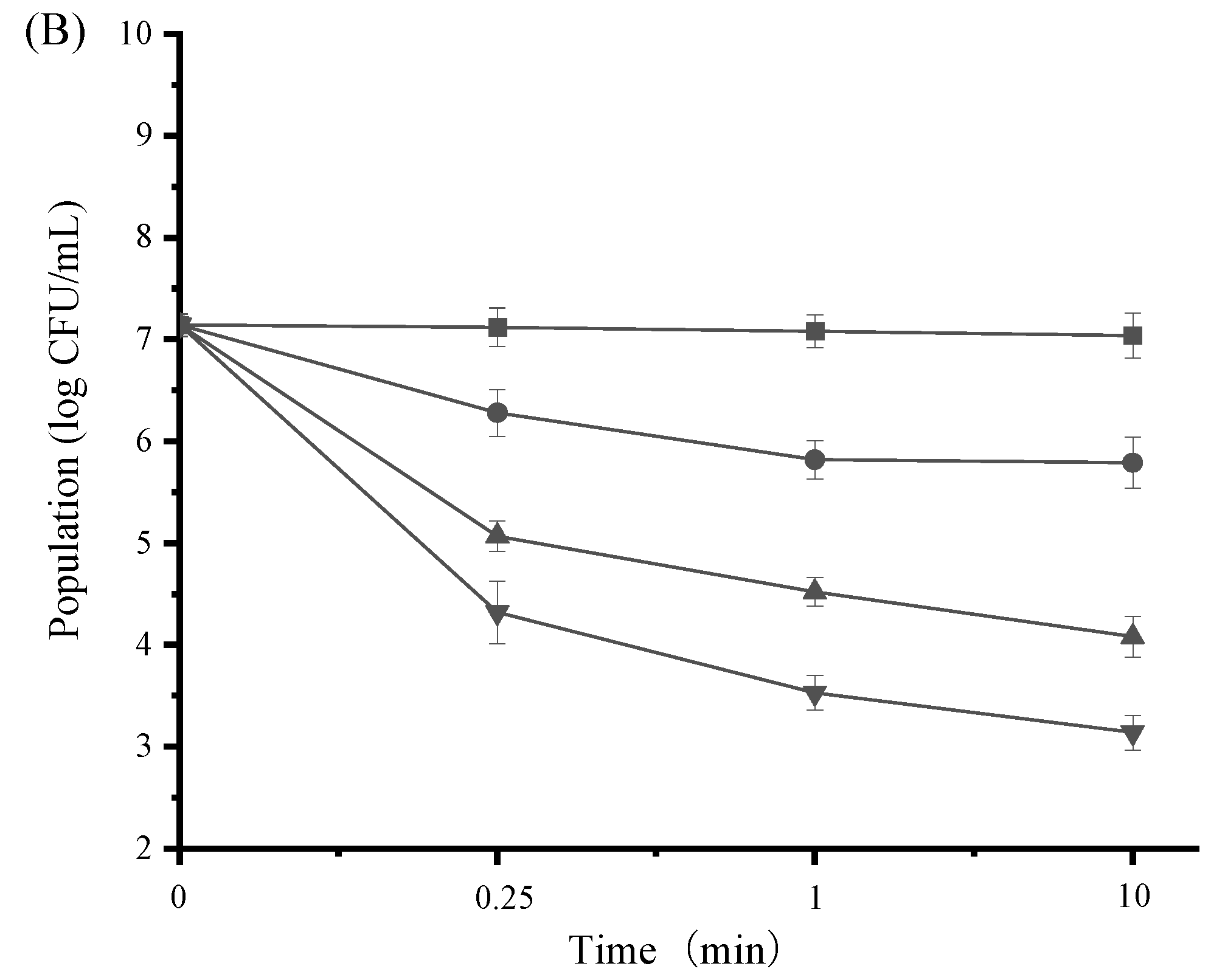
3.5. Time-Kill Activity of SAMs
3.6. Antibacterial Efficacy of SAMs in Hand Sanitizers
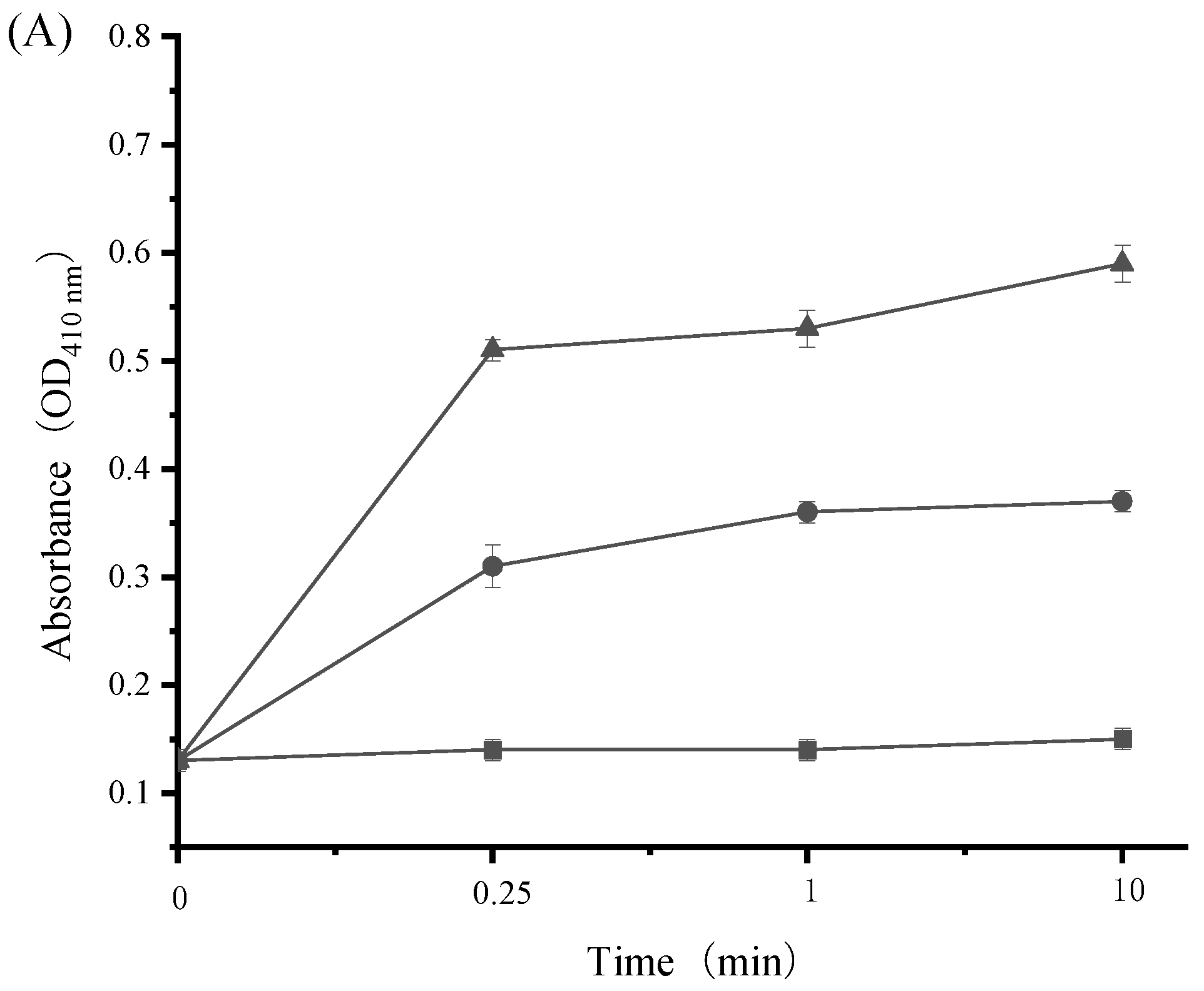
3.7. Impact of SAMs on Bacterial Cell Wall Integrity
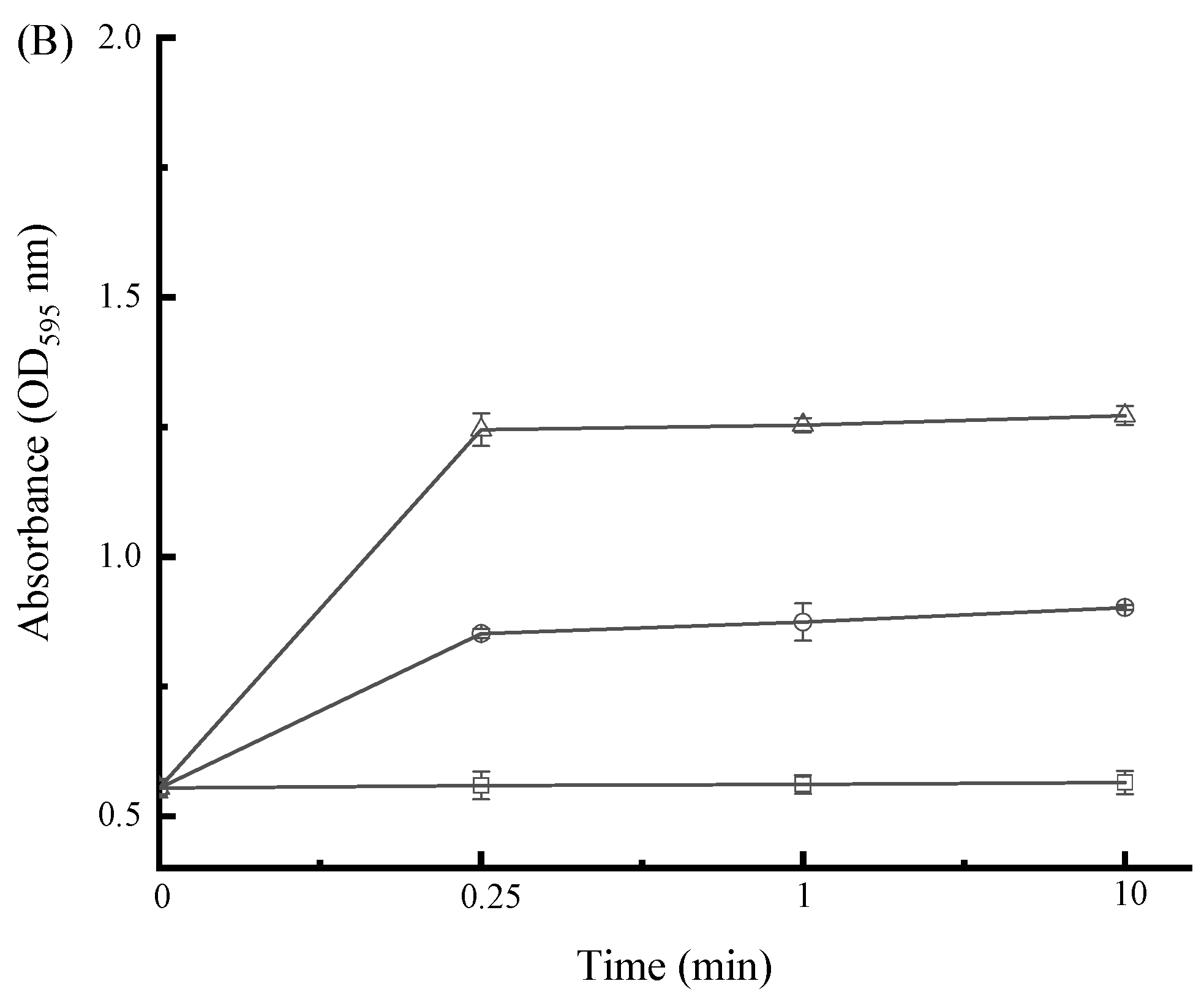
3.8. Impact of SAMs on Bacterial Cell Membrane Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Y.; Fu, J.; Tao, C.; Liu, P.; Cui, B. Mechanical properties and antibacterial activities of novel starch-based composite films incorporated with salicylic acid. Int. J. Biol. Macromol. 2020, 155, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Curá, J.A. Role of beneficial microorganisms and salicylic acid in improving rainfed agriculture and future food safety. Microorganisms 2020, 8, 1018. [Google Scholar] [CrossRef]
- Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef]
- Panahirad, S.; Zaare-Nahandi, F.; Safaralizadeh, R.; Alizadeh-Salteh, S. Postharvest control of Rhizopus stolonifer in peach (Prunus persica L. Batsch) fruits using salicylic acid. J. Food Saf. 2012, 32, 502–507. [Google Scholar] [CrossRef]
- Hussain, M.; Hamid, M.I.; Ghazanfar, M.U. Salicylic acid induced resistance in fruits to combat against postharvest pathogens: A review. Arch. Phytopathol. Plant Prot. 2015, 48, 34–42. [Google Scholar] [CrossRef]
- Hu, F.; Sun, T.; Xie, J.; Shao, Z. Functional properties of chitosan films with conjugated or incorporated salicylic acid. J. Mol. Struct. 2021, 1223, 129237. [Google Scholar] [CrossRef]
- Malaquias, L.F.B.; Sá-Barreto, L.C.L.; Freire, D.O.; Silva, I.C.R.; Karan, K.; Durig, T.; Lima, E.M.; Marreto, R.N.; Gelfuso, G.M.; Gratieri, T. Taste masking and rheology improvement of drug complexed with beta-cyclodextrin and hydroxypropyl-β-cyclodextrin by hot-melt extrusion. Carbohyd. Polym. 2018, 185, 19–26. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in β-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Soliman, E.A.; El-Moghazy, A.Y.; El-Din, M.; Massoud, M.A. Microencapsulation of essential oils within alginate: Formulation and in vitro evaluation of antifungal activity. J. Enca. Adsor. Sci. 2013, 3, 48–55. [Google Scholar]
- Piletti, R.; Zanetti, M.; Jung, G.; De Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Mat. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT—Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial activity and membrane-disruptive mechanism of 3-p-trans-coumaroyl-2-hydroxyquinic acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Song, X.; Ye, L.; Zhang, J.; Wang, L.; Sun, Y.; He, J. Composite wall material microcapsules loaded with salicylic acid and preparation method thereof. CN Patent 108210346 B, 23 June 2020. [Google Scholar]
- Belyakova, L.A.; Varvarin, A.M.; Lyashenko, D.Y.; Khora, O.V.; Oranskaya, E.I. Complexation in a β-cyclodextrin-salicylic acid system. Colloid J. 2007, 69, 546–551. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, H.; Lin, L. The specific antibacterial effect of the Salvia oil nanoliposomes against Staphylococcus aureus biofilms on milk container. Food Control 2016, 61, 92–98. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT—Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Li, K.; Guan, G.; Zhu, J.; Wu, H.; Sun, Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control 2019, 96, 234–243. [Google Scholar] [CrossRef]
- Lee, D.S.; Je, J.Y. Gallic acid-grafted-chitosan inhibits foodborne pathogens by a membrane damage mechanism. J. Agric. Food Chem. 2013, 61, 6574–6579. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Tang, C.; Xiao, J.; Xie, B.; Sun, Z. Synergistic effect of B-type oligomeric procyanidins from lotus seedpod in combination with water-soluble Poria cocos polysaccharides against E. coli and mechanism. J. Funct. Foods. 2018, 48, 134–143. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Liu, G.; Ren, G.; Zhao, L.; Cheng, L.; Wang, C.; Sun, B. Antibacterial activity and mechanism of bifidocin A against Listeria monocytogenes. Food Control 2017, 73, 854–861. [Google Scholar] [CrossRef]
- Anaya-Castro, M.A.; Ayala-Zavala, J.F.; Muñoz-Castellanos, L.; Hernández-Ochoa, L.; Peydecastaing, J.; Durrieu, V. β-Cyclodextrin inclusion complexes containing clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils: Preparation, physicochemical and antimicrobial characterization. Food Packag. Shelf Life 2017, 14, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Santana, A.A.; Cano-Higuita, D.M.; De Oliveira, R.A.; Telis, V.R. Influence of different combinations of wall materials on the microencapsulation of jussara pulp (Euterpe edulis) by spray drying. Food Chem. 2016, 212, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, Y.; Zhang, L.; Adhikari, B.; Xu, B.; Li, J.; Zheng, T. Synthesis and characterization of lotus seed protein-based curcumin microcapsules with enhanced solubility, stability, and sustained release. J. Sci. Food Agric. 2022, 102, 2220–2231. [Google Scholar] [CrossRef]
- Javad, S.; Gopirajah, R.; Rizvi, S.S. High internal phase oil-in-water emulsions stabilized by supercritical carbon dioxide extruded whey protein concentrate. Food Chem. 2022, 372, 131362. [Google Scholar] [CrossRef]
- Ghasemi, H.; Darjani, S.; Mazloomi, H.; Mozaffari, S. Preparation of stable multiple emulsions using food-grade emulsifiers: Evaluating the effects of emulsifier concentration, W/O phase ratio, and emulsification process. SN Appl. Sci. 2020, 2, 2002. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, Y.; Ji, H. Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan. Chin. J. Chem. Eng. 2016, 24, 421–426. [Google Scholar] [CrossRef]
- Song, X.; Xu, Y.; Wang, Y.; Chen, J.; Xiao, Y. Effects of cinnamon oil microcapsules on antioxidant activities and gut microbiota in mice. Food Sci. 2021, 42, 143–152. [Google Scholar]
- Oulkheir, S.; Aghrouch, M.; Mourabit, F.E.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial activity of essential oils extracts from cinnamon, thyme, clove and geranium against a Gram negative and Gram positive pathogenic bacteria. J. Med. Plants Res. 2017, 3, 1–5. [Google Scholar]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef] [PubMed]
- Bauza, B.; Carranza, H.; Pérez, C.; Sosa, Á.; López, R.; Velasco, O. Antimicrobial activity of ginger (Zingiber Officinale) and its application in food products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Trigui, M.; Mansour, R.B.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Momeni, S.S.; Tomlin, N.; Ruby, J.D. Isolation of Raoultella planticola from refillable antimicrobial liquid soap dispensers in a dental setting. J. Am. Dent. Assoc. 2015, 146, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Fong, K.; Wang, S.; Shi, X. Meat juice contributes to the stability of ethanol adaptation in Salmonella enterica serovar Enteritidis. Food Qual. Saf. 2021, 5, fyab017. [Google Scholar] [CrossRef]
- He, S.; Fong, K.; Wang, S.; Shi, X. Ethanol adaptation in foodborne bacterial pathogens. Crit. Rev. Food Sci. 2021, 61, 777–787. [Google Scholar] [CrossRef]
- He, S.; Zhan, Z.; Shi, C.; Wang, S.; Shi, X. Ethanol at subinhibitory concentrations enhances biofilm formation in Salmonella Enteritidis. Foods 2022, 11, 2237. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Li, C.; Vittayapadung, S.; Cui, H. Antibacterial mechanism of ε-Poly-lysine against Listeria monocytogenes and its application on cheese. Food Control 2018, 91, 76–84. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, X.; Xu, L.; Wang, S. Antibacterial properties and possible action mechanism of chelating peptides-zinc nanocomposite against Escherichia coli. Food Control 2019, 106, 106675. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, T.; Liang, S.; Ma, M.; Deng, S. Antibacterial activity and action mechanism of microencapsulated dodecyl gallate with methyl-β-cyclodextrin. Food Control 2020, 109, 106953. [Google Scholar] [CrossRef]
- He, S.; Yang, Q.; Ren, X.; Zi, J.; Lu, S.; Wang, S. Antimicrobial efficiency of chitosan solutions and coatings incorporated with clove oil and/or ethylenediaminetetraacetate. J. Food Saf. 2014, 34, 345–352. [Google Scholar] [CrossRef]
- He, S.; Zhou, X.; Shi, C.; Shi, X. Ethanol adaptation induces direct protection and cross-protection against freezing stress in Salmonella enterica serovar Enteritidis. J. Appl. Microbiol. 2016, 120, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.D.; Qian, H.L.; Feng, C.M.; Wang, T.; Guo, Z.Y.; Wu, X.K.; Zhang, S.H. Study on the mechanism of epigallocatechin gallate (EGCG) to the cell membrane of Escherichia coli. Sci. Adv. Mater. 2019, 11, 262–268. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghosh, D.; Bhattacharya, S.; Sarkar, S.; Karmakar, P.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of Kombucha against Vibrio cholerae: Targeting cell membrane. Lett. Appl. Microbiol. 2018, 66, 145–152. [Google Scholar] [CrossRef]
- Magdalena, E.S.; Agnieszka, N.; Agata, C. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. 2021, 61, 149–178. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Li, R.; Zhang, Q.; He, S.; Wang, Y. Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia coli and Staphylococcus aureus. Int. J. Environ. Res. Public Health 2022, 19, 12761. https://doi.org/10.3390/ijerph191912761
Song X, Li R, Zhang Q, He S, Wang Y. Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia coli and Staphylococcus aureus. International Journal of Environmental Research and Public Health. 2022; 19(19):12761. https://doi.org/10.3390/ijerph191912761
Chicago/Turabian StyleSong, Xiaoqiu, Rui Li, Qian Zhang, Shoukui He, and Yifei Wang. 2022. "Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia coli and Staphylococcus aureus" International Journal of Environmental Research and Public Health 19, no. 19: 12761. https://doi.org/10.3390/ijerph191912761
APA StyleSong, X., Li, R., Zhang, Q., He, S., & Wang, Y. (2022). Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia coli and Staphylococcus aureus. International Journal of Environmental Research and Public Health, 19(19), 12761. https://doi.org/10.3390/ijerph191912761






