Self-Administered Six-Minute Walk Test Using a Free Smartphone App in Asymptomatic Adults: Reliability and Reproducibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample, Recruitment, and Design
2.2. Application Choice
2.3. Phase 1 Protocol
2.4. Health Screening
2.5. Supervised 6MWT
2.6. Phase 2 Protocol
2.7. Self-Administered 6MWT
2.8. Statistical Analysis
2.8.1. Phase 1
2.8.2. Phase 2
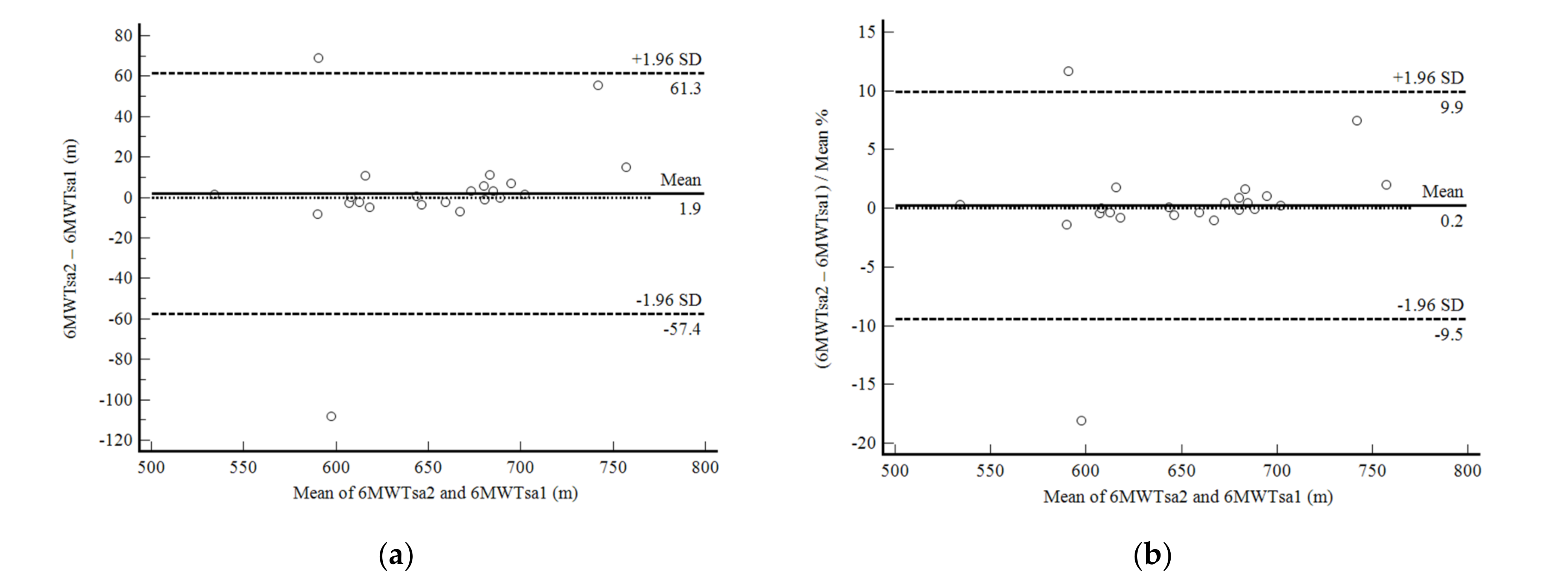
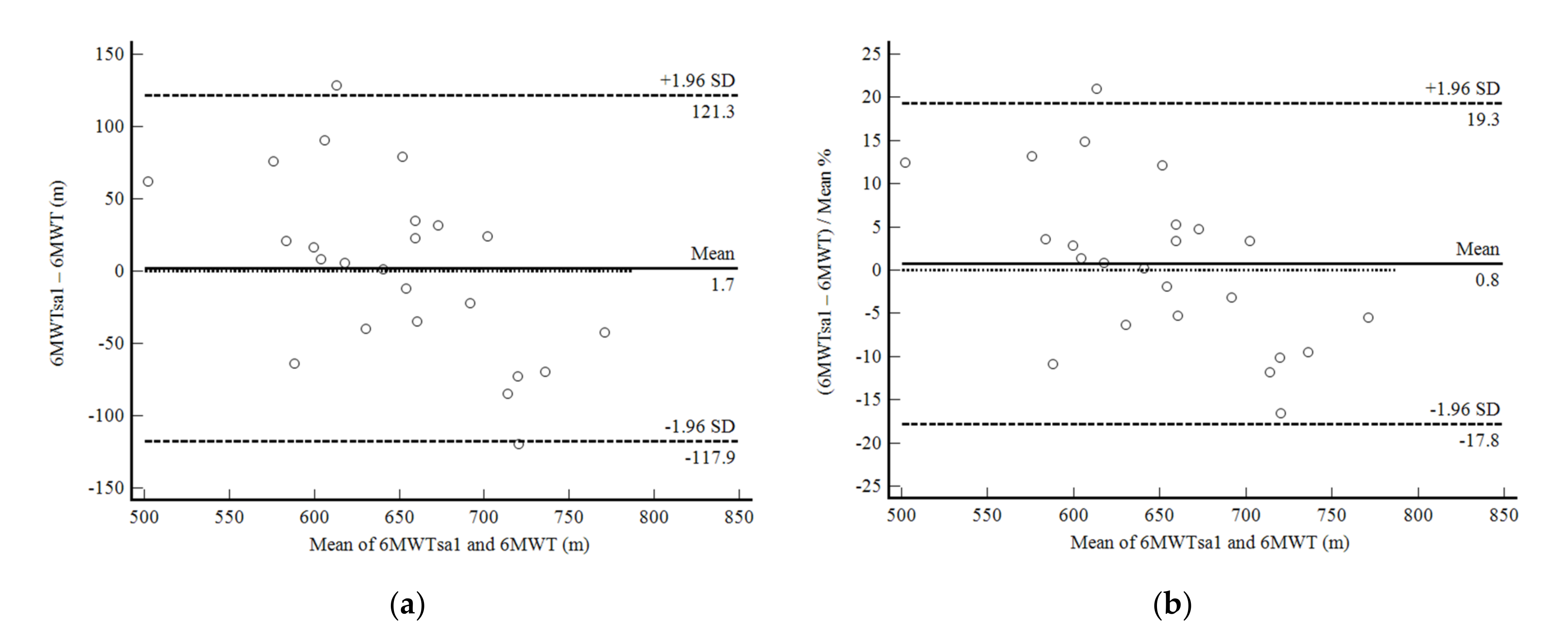
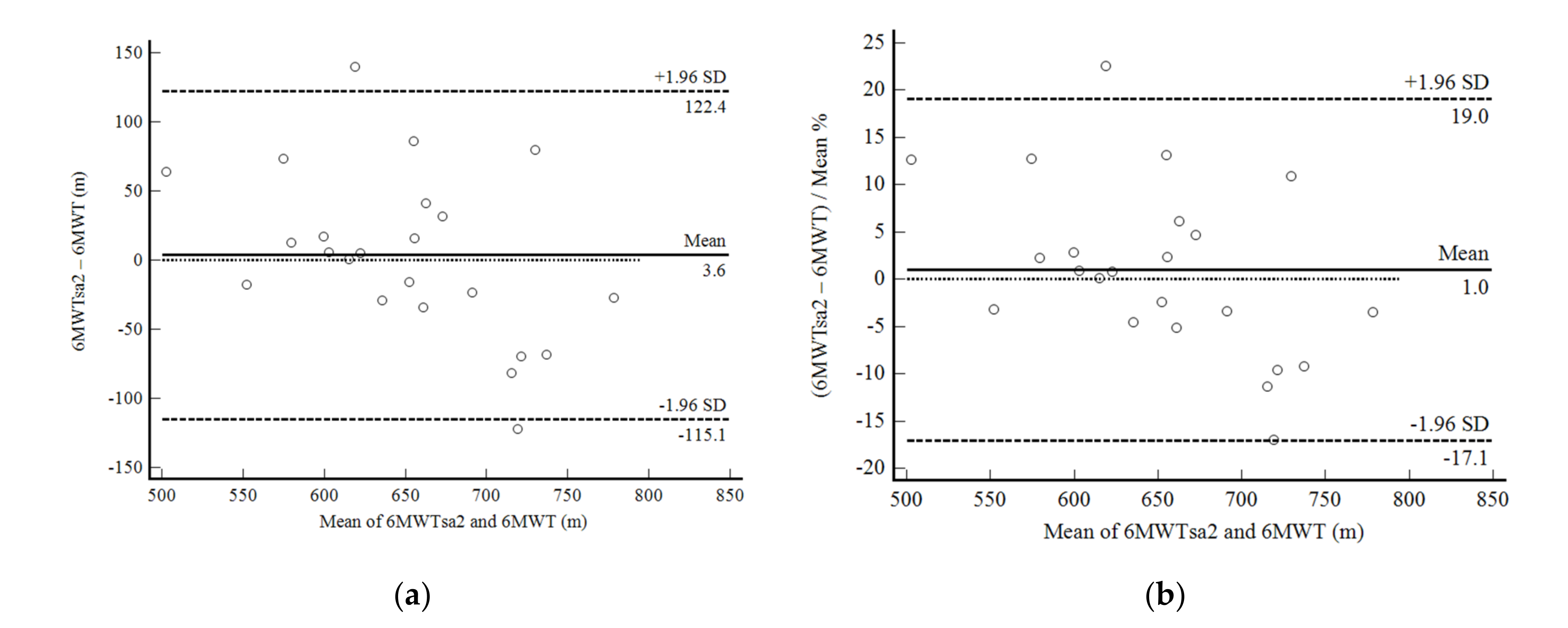
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herdy, A.H.; Uhlendorf, D. Reference values for cardiopulmonary exercise testing for sedentary and active men and women. Arq. Bras. Cardiol. 2011, 96, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Liao, P.A.; Chang, H.H.; Wang, J.H.; Wu, M.C. Examining the relationship between cardiorespiratory fitness and body weight status: Empirical evidence from a population-based survey of adults in Taiwan. Sci. World J. 2014, 2014, 463736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.; Kaminsky, L.A.; Lima, R.; Christle, J.W.; Ashley, E.; Arena, R. A reference equation for normal standards for VO2 max: Analysis from the fitness registry and the importance of exercise national database (FRIEND Registry). Prog. Cardiovasc. Dis. 2017, 60, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Belli, K.C.; Callegaro, C.C.; Richter, C.M.; Klafke, J.Z.; Stein, R.; Viecili, P.R. Cardiorespiratory fitness of a Brazilian regional sample distributed in different tables. Arq. Bras. Cardiol. 2012, 99, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Dourado, V.Z. Reference equations for the 6-minute walk test in healthy individuals. Arq. Bras. Cardiol. 2011, 96, e128–e138. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, E.F.; Arantes, R.L.; Matheus, A.C.; Silva, R.P.; Lauria, V.T.; Romiti, M.; Gagliardi, A.R.T.; Dourado, V.Z. Intensity and physiological responses to the 6-minute walk test in middle-aged and older adults: A comparison with cardiopulmonary exercise testing. Braz. J. Med. Biol. Res. 2015, 48, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Bittner, V.; Weiner, D.H.; Yusuf, S.; Rogers, W.J.; McIntyre, K.M.; Bangdiwala, S.I.; Kronenberg, M.V.; Kostis, J.B.; Kohn, R.M.; Woods, P.A.; et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993, 270, 1702–1707. [Google Scholar] [CrossRef]
- Diniz, L.S.; Neves, V.R.; Starke, A.C.; Barbosa, M.P.T.; Britto, R.R.; Ribeiro, A.L.P. Safety of early performance of the six-minute walk test following acute myocardial infarction: A cross-sectional study. Braz. J. Phys. Ther. 2017, 21, 167–174. [Google Scholar] [CrossRef]
- Sperandio, E.F.; Arantes, R.L.; Silva, R.P.; Matheus, A.C.; Lauria, V.T.; Bianchim, M.S.; Romiti, M.; Gagliardi, A.R.T.; Dourado, V.Z. Screening for physical inactivity among adults: The value of distance walked in the six-minute walk test. A cross-sectional diagnostic study. Sao Paulo Med. J. 2016, 134, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Dourado, V.Z.; Kan, R.; Simões, M.S.M.P.; Lauria, V.T.; Tanni, S.E.; Godoy, I.; Gagliardi, A.R.T.; Romiti, M.; Arante, R.L. Classification of cardiorespiratory fitness using the six-minute walk test in adults: Comparison with cardiopulmonary exercise testing. Pulmonology 2021, 27, 500–508. [Google Scholar] [CrossRef]
- Direito, A.; Dale, L.P.; Shields, E.; Dobson, R.; Whittaker, R.; Maddison, R. Do physical activity and dietary smartphone applications incorporate evidence-based behaviour change techniques? BMC Public Health 2014, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.C.; Vittinghoff, E.; Iyer, S.; Tandon, D.; Kuhar, P.; Madsen, K.A.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Accuracy and usability of a self-administered 6-minute walk test smartphone application. Circ. Heart Fail. 2015, 8, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Douma, J.A.J.; Verheul, H.M.W.; Buffart, L.M. Feasibility, validity and reliability of objective smartphone measurements of physical activity and fitness in patients with cancer. BMC Cancer 2018, 18, 3. [Google Scholar] [CrossRef]
- Salvi, D.; Poffley, E.; Orchard, E.; Tarassenko, L. The mobile-based 6-minute walk test: Usability study and algorithm development and validation. JMIR mHealth uHealth 2020, 8, e13756. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines on Physical Activity and Sedentary Behavior. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 18 August 2021).
- Poh, M.Z.; Poh, Y.C. Validation of a standalone smartphone application for measuring heart rate using imaging photoplethysmography. Telemed. e-Health 2017, 23, 678–683. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Green, A.; Coopoo, Y.; Borman, A. Physical activity tracking using mobile devices: Can a heterogeneous sample of smart phones accurately quantify steps? Afr. J. Phys. Act. Health Sci. 2018, 24, 514–524. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage Publications: London, UK, 2013. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção de Saúde. Vigitel Brasil 2019: Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco.pdf (accessed on 13 December 2021).
- Du, H.; Newton, P.J.; Salamonson, Y.; Carrieri-Kohlman, V.L.; Davidson, P.M. A review of the six-minute walk test: Its implication as a self-administered assessment tool. Eur. J. Cardiovasc. Nurs. 2009, 8, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Takacs, J.; Pollock, C.L.; Guenther, J.R.; Bahar, M.; Napier, C.; Hunt, M.A. Validation of the Fitbit One activity monitor device during treadmill walking. J. Sci. Med. Sport 2014, 17, 496–500. [Google Scholar] [CrossRef]
- Arena, R.; McNeil, A. Let’s talk about moving: The impact of cardiorespiratory fitness, exercise, steps and sitting on cardiovascular risk. Braz. J. Cardiovasc. Surg. 2017, 32, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Maranhao Neto, G.A.A.; Lourenco, P.M.C.; Farinatti, P.d.T.V. Prediction of aerobic fitness without stress testing and applicability to epidemiological studies: A systematic review. Cad. Saude Publica 2004, 20, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Jurca, R.; Jackson, A.S.; LaMonte, M.J.; Morrow, J.R., Jr.; Blair, S.N.; Wareham, N.J.; Haskell, W.L.; Van Mechelen, W.; Churc, T.S.; Jakicic, J.M.; et al. Assessing cardiorespiratory fitness without performing exercise testing. Am. J. Prev. Med. 2005, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Nes, B.M.; Janszky, I.; Wisloff, U.; Stoylen, A.; Karlsen, T. Age-predicted maximal heart rate in healthy subjects: The HUNT fitness study. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Manolis, A.; Pittaras, A.; Doumas, M.; Giannelou, A.; Panagiotakos, D.B.; Faselis, C.; Narayan, P.; Singh, S.; Myers, J. Exercise capacity and mortality in hypertensive men with and without additional risk factors. Hypertension 2009, 53, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, E.; Hamer, M.; O’Donovan, G.; Batty, G.D.; Kivimaki, M. A non-exercise testing method for estimating cardiorespiratory fitness: Associations with all-cause and cardiovascular mortality in a pooled analysis of eight population-based cohorts. Eur. Heart J. 2013, 34, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- Morakami, F.K.; Morita, A.A.; Bisca, G.W.; Felcar, J.M.; Ribeiro, M.; Furlanetto, K.C.; Hernandes, N.A.; Pitta, F. Can the six-minute walk distance predict the occurrence of acute exacerbations of COPD in patients in Brazil? J. Bras. Pneumol. 2017, 43, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Gary, R.A.; Sueta, C.A.; Rosenberg, B.; Cheek, D. Use of the 6-minute walk test for women with diastolic heart failure. J. Cardiopulm. Rehabil. 2004, 24, 264–268. [Google Scholar] [CrossRef]


| Variables | Males (n = 39) | Females (n = 54) | Total Sample (n = 93) |
|---|---|---|---|
| Age (years) | 42 ± 6 | 46 ± 11 | 44 ± 10 |
| Weight (kg) | 81.0 ± 17.7 | 70.4 ± 11.8 | 74.8 ± 15.4 |
| Height (cm) | 173 ± 6 | 59 ± 6 | 165 ± 9 |
| BMI (kg/m2) | 26.8 ± 4.4 | 27.6 ± 5.2 | 27.3 ± 4.9 |
| Cardiovascular risk factors, n (%) | |||
| Arterial hypertension a | 6 (15.4) | 11 (20.4) | 17 (18.3) |
| Diabetes a | 2 (5.1) | 8 (14.8) | 10 (10.8) |
| Dyslipidemia a | 6 (15.4) | 11 (20.4) | 17 (18.3) |
| Obesity | 8 (20.5) | 16 (29.6) | 24 (25.8) |
| Current smoking a | 3 (7.7) | 7 (13) | 10 (10.8) |
| Physical inactivity | 4 (10.3) | 3 (5.6) | 7 (7.5) |
| Six-minute walk distance (m) | 670 ± 65 | 602 ± 81 | 633 ± 81 |
| Step counts (accelerometer) | 767 ± 84 | 758 ± 76 | 762 ± 79 |
| Step counts (application) | 721 ± 112 | 686 ± 111 | 704 ± 112 |
| Variables | Males (n = 16) | Females (n = 9) | Total Sample (n = 25) |
|---|---|---|---|
| Age (years) | 38 ± 8 | 41 ± 10 | 36 ± 9 |
| Weight (kg) | 88.6 ± 18.4 | 70.2 ± 13.5 | 82.0 ± 18.8 |
| Height (cm) | 176 ± 6 | 162 ± 5 | 171 ± 9 |
| BMI (kg/m2) | 28.4 ± 5.0 | 26.6 ± 5.0 | 27.8 ± 5.0 |
| Cardiovascular risk factors, n (%) | |||
| Arterial hypertension a | 2 (12) | 0 (0) | 2 (8) |
| Diabetes a | 0 (0) | 0 (0) | 0 (0) |
| Dyslipidemia a | 3 (18) | 3 (33) | 6 (24) |
| Obesity | 3 (18) | 2 (22) | 5 (20) |
| Current smoking a | 3 (18) | 1 (11) | 4 (16) |
| Physical inactivity | 4 (25) | 5 (55) | 9 (36) |
| Six-minute walk distance (m) | 663 ± 79 | 640 ± 69 | 648 ± 84 |
| Step counts (accelerometer) | 754 ± 105 | 761 ± 93 | 754 ± 98 |
| Step counts (application) | 707 ± 112 | 644 ± 138 | 680 ± 123 |
| Variables | 6MWD (m) | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | Accelerometer-Based Step Counts | App-Based Step Counts | |
|---|---|---|---|---|---|---|---|---|
| 6MWD (m) | r | 1 | −0.499 ** | 0.459 ** | −0.166 | −0.512 ** | 0.350 ** | 0.351 ** |
| p | 0.000 | 0.000 | 0.073 | 0.000 | 0.000 | 0.003 | ||
| Age (years) | r | −0.499 ** | 1 | −0.427 ** | −0.124 | 0.155 | −0.215 * | −0.021 |
| p | 0.000 | 0.000 | 0,180 | 0.094 | 0.020 | 0.864 | ||
| Height (cm) | r | 0.459 ** | −0.427 ** | 1 | 0.505 ** | −0.091 | −0.130 | 0.061 |
| p | 0.000 | 0.000 | 0.000 | 0.327 | 0.162 | 0.611 | ||
| Weight (kg) | r | −0.166 | −0.124 | 0.505 ** | 1 | 0.805 ** | −0.378 ** | 0.016 |
| p | 0.073 | 0.180 | 0.000 | 0.000 | 0.000 | 0.894 | ||
| BMI (kg/m2) | r | −0.512 ** | 0.155 | −0.091 | 0.805 ** | 1 | −0.347 ** | −0.032 |
| p | 0.000 | 0.094 | 0.327 | 0.000 | 0.000 | 0.792 | ||
| Accelerometer-based step counts | r | 0.350 ** | −0.215 * | −0.130 | −0.378 ** | −0.347 ** | 1 | 0.025 |
| p | 0.000 | 0.020 | 0.162 | 0.000 | 0.000 | 0.834 | ||
| App-based step counts | r | 0.351 ** | −0.021 | 0.061 | 0.016 | −0.032 | 0.025 | 1 |
| p | 0.003 | 0.864 | 0.611 | 0.894 | 0.792 | 0.834 |
| Variables | Unstandardized Coefficients | Standardized Coefficients | 95% Confidence Interval of B | |||||
|---|---|---|---|---|---|---|---|---|
| B | Standard Error | Beta | Partial R2 | ΔR2 | p | Lower Limit | Upper LIMIT | |
| Constant | 795.456 | 593.820 | 0.000 | 676.021 | 914.891 | |||
| Heightm × app-based step counts | 0.815 | 0.121 | 2.114 | 0.230 | 0.230 | 0.000 | 0.574 | 1.056 |
| Age (years) | −1.620 | 0.754 | −0.185 | 0.348 | 0.119 | 0.035 | −3.125 | −0.115 |
| Weight (kg) | −3.005 | 0.482 | −0.597 | 0.437 | 0.088 | 0.000 | −3.967 | −2.042 |
| App-based step counts | −1.155 | 0.214 | −1.634 | 0.609 | 0.172 | 0.000 | −1.583 | −0.727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, M.O.d.; Ostolin, T.L.V.D.P.; Proença, N.L.; Silva, R.P.d.; Dourado, V.Z. Self-Administered Six-Minute Walk Test Using a Free Smartphone App in Asymptomatic Adults: Reliability and Reproducibility. Int. J. Environ. Res. Public Health 2022, 19, 1118. https://doi.org/10.3390/ijerph19031118
Jesus MOd, Ostolin TLVDP, Proença NL, Silva RPd, Dourado VZ. Self-Administered Six-Minute Walk Test Using a Free Smartphone App in Asymptomatic Adults: Reliability and Reproducibility. International Journal of Environmental Research and Public Health. 2022; 19(3):1118. https://doi.org/10.3390/ijerph19031118
Chicago/Turabian StyleJesus, Matheus Oliveira de, Thatiane Lopes Valentim Di Paschoale Ostolin, Neli Leite Proença, Rodrigo Pereira da Silva, and Victor Zuniga Dourado. 2022. "Self-Administered Six-Minute Walk Test Using a Free Smartphone App in Asymptomatic Adults: Reliability and Reproducibility" International Journal of Environmental Research and Public Health 19, no. 3: 1118. https://doi.org/10.3390/ijerph19031118
APA StyleJesus, M. O. d., Ostolin, T. L. V. D. P., Proença, N. L., Silva, R. P. d., & Dourado, V. Z. (2022). Self-Administered Six-Minute Walk Test Using a Free Smartphone App in Asymptomatic Adults: Reliability and Reproducibility. International Journal of Environmental Research and Public Health, 19(3), 1118. https://doi.org/10.3390/ijerph19031118






