Combined Effects of Diet and Physical Activity on Inflammatory Joint Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Searching and Selection Processes
2.2. Study Inclusion and Exclusion Criteria
- Randomized controlled trials (RCT), quasi RCT, controlled trials (CT), and single arm design studies that integrated: (a) combined interventions of any diet/nutrition and any physical activity/exercise, AND (b) were performed on RA patients and/or SpA patients AND (c) participants of any ethnicity, age, and gender, AND (d) participants under any pharmacological treatment. As a control situation, we accepted RCT, quasi RCT, and CT that used a control group (i.e., usual care) as well as single arm design studies that used baseline measurements as a control situation that were compared with post intervention measurements.
- Epidemiological studies that integrated: (a) combined measurements of diet/nutrition and physical activity/exercise, AND (b) were performed on RA patients and/or SpA patients, as well as in the general population (adults) to explore associations with the above-mentioned diseases, AND (c) participants of any ethnicity, age, and gender, AND (d) participants under any pharmacological treatment.
- Animal studies, reviews, study protocols, editorials, conference proceedings, magazines, and grey literature publications were excluded.
2.3. Study Quality Assessment
2.4. Data Extraction Strategy
2.5. Data Synthesis and Presentation
2.6. Evidence of Effectiveness
3. Results
3.1. Results of Searching and Selection Processes
3.2. Characteristics of Included Studies
3.3. Study Quality Assessment Outcomes
3.4. Narrative Data Synthesis Results
3.4.1. Narrative Data Synthesis Results for Controlled Trials
3.4.2. Narrative Data Synthesis Results for Epidemiological Studies
3.5. Meta-Analyses Outcomes
3.6. GRADE Analysis Outcomes
4. Discussion
4.1. Summary of Main Findings
4.2. Completeness and Applicability of Evidence
4.3. Strengths and Potential Biases in the Review Process
4.4. Statement on Significant Deviations in Methods from the Published Protocol
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Full Name | ||
| 1 | Adriana Machaira | [email protected] |
| 2 | Aggelos Mpaltos | [email protected] |
| 3 | Anastasia Grigoriadou | [email protected] |
| 4 | Anastasia Prokopiou | [email protected] |
| 5 | Anastasia-Foteini Mpanti | [email protected] |
| 6 | Anna Daskalaki | [email protected] |
| 7 | Anna-Maria Stamathioudaki | [email protected] |
| 8 | Anthi Karambelia | [email protected] |
| 9 | Anthi Kousi | [email protected] |
| 10 | Argyri Nerantzoglou | [email protected] |
| 11 | Antonia Daskalaki | [email protected] |
| 12 | Antonia-Kiriaki Kampioti | [email protected] |
| 13 | Athanasia-Vakchi Miliou | [email protected] |
| 14 | Athina-Efraimia Papoutsaki | [email protected] |
| 15 | Christina Mikelopoulou | [email protected] |
| 16 | Christina Strotou | [email protected] |
| 17 | Chrysoula Psifogianni | [email protected] |
| 18 | Despoina Pantazelou | [email protected] |
| 19 | Dimitra Falkou | [email protected] |
| 20 | Dimitra Theodorou | [email protected] |
| 21 | Dimitra Zapounidou | [email protected] |
| 22 | Dimitra-Evdokia Kelepouri | [email protected] |
| 23 | Dimitrios Marinakis | [email protected] |
| 24 | Dimitrios-Ioannis Kasartzian | [email protected] |
| 25 | Dimitrios-Paisios Koungoulas | [email protected] |
| 26 | Eleni-Anna Tsima | [email protected] |
| 27 | Eleftheria-Paraskevi Sinani | [email protected] |
| 28 | Eleftheria Thomaidi | [email protected] |
| 29 | Elem Agko | [email protected] |
| 30 | Eleni Mpalasopoulou | [email protected] |
| 31 | Eleni Tsironi | [email protected] |
| 32 | Eleni-Anna Tsavou | [email protected] |
| 33 | Evaggelia-Tsampika Athanasa | [email protected] |
| 34 | Evaggelia-Christina Papadopoulou | [email protected] |
| 35 | Georgia Georgiou | [email protected] |
| 36 | Georgia Lavasa | [email protected] |
| 37 | Georgia Loupsati | [email protected] |
| 38 | Georgia Tsakalou | [email protected] |
| 39 | Georgios Charmantzis | [email protected] |
| 40 | Galatiani-Maria Fragkou | [email protected] |
| 41 | Ilia-Anna Verdou | [email protected] |
| 42 | Ioanna Chatzigianni | [email protected] |
| 43 | Ioanna Gialou | [email protected] |
| 44 | Ioanna Goula | [email protected] |
| 45 | Katherina Karampet | [email protected] |
| 46 | Kyriaki Gyltidou | [email protected] |
| 47 | Klaountio Ntemai | [email protected] |
| 48 | Konstantina Kaziani | [email protected] |
| 49 | Maria Damala | [email protected] |
| 50 | Maria Kosmatou | [email protected] |
| 51 | Maria Mavreli | [email protected] |
| 52 | Maria Moschopoulou | [email protected] |
| 53 | Maria Ntalouka | [email protected] |
| 54 | Maria-Niki Daniil | [email protected] |
| 55 | Marianna Grigoriadi | [email protected] |
| 56 | Marianna Nikoletopoulou | [email protected] |
| 57 | Marios-Ioannis Kyrilis | [email protected] |
| 58 | Melek Kalentzi | [email protected] |
| 59 | Nikoleta Adamidi | [email protected] |
| 60 | Nina Michailidou | [email protected] |
| 61 | Ntorina Chotza | [email protected] |
| 62 | Olympios Stergiou | [email protected] |
| 63 | Panagiota Chatzoudi | [email protected] |
| 64 | Panagiota Karamvasi | [email protected] |
| 65 | Panagiota Nasopoulou | [email protected] |
| 66 | Panagiotis Efthimiadis | [email protected] |
| 67 | Paraskevi Apostolou | [email protected] |
| 68 | Philipos Papathemistokleous | [email protected] |
| 69 | Rentona Nourtsia | [email protected] |
| 70 | Sofia Chatzivasileiou | [email protected] |
| 71 | Sofia Karava | [email protected] |
| 72 | Sofia Panidou | [email protected] |
| 73 | Spyridon Stathopoulos | [email protected] |
| 74 | Stamatia Tzima | [email protected] |
| 75 | Stamatina Matiatou | [email protected] |
| 76 | Stamatis Vragkas | [email protected] |
| 77 | Styliani Ferentinou | [email protected] |
| 78 | Theodora Fragista | [email protected] |
| 79 | Theodora-Iouliana Cojocariu | [email protected] |
| 80 | Theodora Parpa | [email protected] |
| 81 | Thiresia Chondrou | [email protected] |
| 82 | Thomais Karanika | [email protected] |
| 83 | Vaia Ntinti | [email protected] |
| 84 | Vasiliki Doulaki | [email protected] |
| 85 | Vasiliki Vythoulka | [email protected] |
| 86 | Violeta Dionisidou-Konstantinidou | [email protected] |
| 87 | Vivjana Moulliri | [email protected] |
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Dalal, D.S.; Zhang, T.; Shireman, T.I. Medicare expenditures for conventional and biologic disease modifying agents commonly used for treatment of rheumatoid arthritis. Semin. Arthritis Rheum. 2020, 50, 822–826. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Metsios, G.S.; Pace, A.V.; John, H.; Treharne, G.J.; Banks, M.J.; Kitas, G.D. Hypertension in rheumatoid arthritis. Rheumatology 2008, 47, 1286–1298. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Douglas, K.M.J.; Smith, J.P.; Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Nightingale, P.; Kitas, G.D. Transforming growth factor-beta1 869T/C, but not interleukin-6 -174G/C, polymorphism associates with hypertension in rheumatoid arthritis. Rheumatology 2008, 48, 113–118. [Google Scholar] [CrossRef]
- Toms, T.E.; Panoulas, V.F.; Smith, J.P.; Douglas, K.M.; Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Kitas, G.D. Rheumatoid arthritis susceptibility genes associate with lipid levels in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1025–1032. [Google Scholar] [CrossRef]
- Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Koutedakis, Y.; Kitas, G.D. Obesity in rheumatoid arthritis. Rheumatology 2011, 50, 450–462. [Google Scholar] [CrossRef]
- Sandoo, A.; Carroll, D.; Metsios, G.S.; Kitas, G.D.; Veldhuijzen van Zanten, J.J. The association between microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 2011, 13, R99. [Google Scholar] [CrossRef]
- Sandoo, A.; Veldhuijzen van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. Vascular function and morphology in rheumatoid arthritis: A systematic review. Rheumatology 2011, 50, 2125–2139. [Google Scholar] [CrossRef]
- Dessein, P.H.; Joffe, B.I.; Stanwix, A.E. Inflammation, insulin resistance, and aberrant lipid metabolism as cardiovascular risk factors in rheumatoid arthritis. J. Rheumatol. 2003, 30, 1403–1405. [Google Scholar]
- Drosos, G.C.; Vedder, D.; Houben, E.; Boekel, L.; Atzeni, F.; Badreh, S.; Boumpas, D.T.; Brodin, N.; Bruce, I.N.; González-Gay, M.; et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2022, 81, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and inflammation. Best. Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welgh, V. (Eds.) Cochrane Handbook for Systematic Review of Interventions Version 6.2 (Updated February 2021); John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=370993 (accessed on 17 December 2022).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Viswanathan, M.; Berkman, N.D. Development of the RTI item bank on risk of bias and precision of observational studies. J. Clin. Epidemiol. 2012, 65, 163–178. [Google Scholar] [CrossRef]
- The Nordic Cochrane Centre. Review Manager (RevMan) [Computer Program], version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014.
- Bruce, B.; Fries, J.F. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23, S14–S18. [Google Scholar] [PubMed]
- Engelhart, M.; Kondrup, J.; Høie, L.H.; Andersen, V.; Kristensen, J.H.; Heitmann, B.L. Weight reduction in obese patients with rheumatoid arthritis, with preservation of body cell mass and improvement of physical fitness. Clin. Exp. Rheumatol. 1996, 14, 289–293. [Google Scholar]
- Gordon, M.M.; Thomson, E.A.; Madhok, R.; Capell, H.A. Can intervention modify adverse lifestyle variables in a rheumatoid population? Results of a pilot study. Ann. Rheum. Dis. 2002, 61, 66–69. [Google Scholar] [CrossRef]
- Pineda-Juárez, J.A.; Lozada-Mellado, M.; Hinojosa-Azaola, A.; García-Morales, J.M.; Ogata-Medel, M.; Llorente, L.; Alcocer-Varela, J.; Orea-Tejeda, A.; Martín-Nares, E.; Castillo-Martínez, L. Changes in hand grip strength and body weight after a dynamic exercise program and Mediterranean diet in women with rheumatoid arthritis: A randomized clinical trial. Physiother. Theory Pract. 2022, 38, 504–512. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Garner, S.; Fenton, T.; Martin, L.; Creaser, C.; Johns, C.; Barnabe, C. Personalized diet and exercise recommendations in early rheumatoid arthritis: A feasibility trial. Musculoskelet. Care 2018, 16, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A.E. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach; GRADE Working Group: Rochester, MN, USA, 2013. [Google Scholar]
- García-Morales, J.M.; Lozada-Mellado, M.; Hinojosa-Azaola, A.; Llorente, L.; Ogata-Medel, M.; Pineda-Juárez, J.A.; Alcocer-Varela, J.; Cervantes-Gaytán, R.; Castillo-Martínez, L. Effect of a Dynamic Exercise Program in Combination With Mediterranean Diet on Quality of Life in Women With Rheumatoid Arthritis. J. Clin. Rheumatol. 2020, 26, S116–S122. [Google Scholar] [CrossRef] [PubMed]
- Bilberg, A.; Larsson, I.; Björkman, S.; Eliasson, B.; Klingberg, E. The impact of a structured weight-loss treatment on physical fitness in patients with psoriatic arthritis and obesity compared to matched controls: A prospective interventional study. Clin. Rheumatol. 2022, 41, 2745–2754. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, U.R.; Dideriksen, K.; Andersen, M.B.; Boesen, A.; Malmgaard-Clausen, N.M.; Sorensen, I.J.; Schjerling, P.; Kjaer, M.; Holm, L. Preserved skeletal muscle protein anabolic response to acute exercise and protein intake in well-treated rheumatoid arthritis patients. Arthritis Res. Ther. 2015, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Viggiani, M.T.; Anelli, M.G.; Fanizzi, R.; Lorusso, O.; Lopalco, G.; Cantarini, L.; Di Leo, A.; Lapadula, G.; Iannone, F. Sarcopenia in Patients with Rheumatic Diseases: Prevalence and Associated Risk Factors. J. Clin. Med. 2018, 7, 504. [Google Scholar] [CrossRef]
- Elkan, A.C.; Hakansson, N.; Frostegard, J.; Hafstrom, I. Low level of physical activity in women with rheumatoid arthritis is associated with cardiovascular risk factors but not with body fat mass—A cross sectional study. BMC Musculoskelet. Disord. 2011, 12, 13. [Google Scholar] [CrossRef]
- Matsunaga, M.; Lim, E.; Davis, J.; Chen, J.J. Dietary Quality Associated with Self-Reported Diabetes, Osteoarthritis, and Rheumatoid Arthritis among Younger and Older US Adults: A Cross-Sectional Study Using NHANES 2011–2016. Nutrients 2021, 13, 545. [Google Scholar] [CrossRef]
- Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Smith, J.P.; Panoulas, V.F.; Douglas, K.M.J.; Jamurtas, A.Z.; Koutedakis, Y.; Kitas, G.D. What predicts obesity in patients with rheumatoid arthritis An investigation of the interactions between lifestyle and inflammation. Int. J. Obes. 2010, 34, 295–301. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Wieczorek, M.; Balanescu, A.; Bischoff-Ferrari, H.A.; Boonen, A.; Cavalli, G.; de Souza, S.; de Thurah, A.; Dorner, T.E.; Moe, R.H.; et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2023, 82, 48. [Google Scholar] [CrossRef]
- Organisation, W.H. Diet, Nutrition and the Prevention of Chronic Diseases. Report of the Joint WHO/FAO Expert Consultation. Who Technical Report Series. Available online: https://www.who.int/publications/i/item/924120916X (accessed on 17 December 2022).
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Huang, R.Y.; Huang, C.C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef]
- Metsios, G.S.; Kitas, G.D. Physical activity, exercise and rheumatoid arthritis: Effectiveness, mechanisms and implementation. Best. Pract. Res. Clin. Rheumatol. 2018, 32, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Dzięcioł-Anikiej, Z.; Kuryliszyn-Moskal, A.; Hryniewicz, A.; Kaniewska, K.; Chilińska-Kopko, E.; Dzięcioł, J. Gait disturbances in patients with rheumatoid arthritis. Arch. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Rome, K.; Dixon, J.; Gray, M.; Woodley, R. Evaluation of static and dynamic postural stability in established rheumatoid arthritis: Exploratory study. Clin. Biomech. 2009, 24, 524–526. [Google Scholar] [CrossRef]
- Hayashibara, M.; Hagino, H.; Katagiri, H.; Okano, T.; Okada, J.; Teshima, R. Incidence and risk factors of falling in ambulatory patients with rheumatoid arthritis: A prospective 1-year study. Osteoporos. Int. 2010, 21, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Moschou, D.; Krikelis, M.; Georgakopoulos, C.; Mole, E.; Chronopoulos, E.; Tournis, S.; Mavragani, C.; Makris, K.; Dontas, I.; Gazi, S. Sarcopenia in Rheumatoid arthritis. A narrative review. J. Frailty Sarcopenia Falls 2023, 8, 44–52. [Google Scholar] [CrossRef]
- Sveaas, S.H.; Smedslund, G.; Hagen, K.B.; Dagfinrud, H. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: A systematic review and meta-analysis. Br. J. Sport. Med. 2017, 51, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Lanspa, M.; Kothe, B.; Pereira, M.R.; Kesselman, M.M.; Petrosky, S.N. A Systematic Review of Nutritional Interventions on Key Cytokine Pathways in Rheumatoid Arthritis and Its Implications for Comorbid Depression: Is a More Comprehensive Approach Required? Cureus 2022, 14, e28031. [Google Scholar] [CrossRef]
- Veldhuijzen van Zanten, J.J.; Rouse, P.C.; Hale, E.D.; Ntoumanis, N.; Metsios, G.S.; Duda, J.L.; Kitas, G.D. Perceived Barriers, Facilitators and Benefits for Regular Physical Activity and Exercise in Patients with Rheumatoid Arthritis: A Review of the Literature. Sport. Med. 2015, 45, 1401–1412. [Google Scholar] [CrossRef]
- Benito-Garcia, E.; Feskanich, D.; Hu, F.B.; Mandl, L.A.; Karlson, E.W. Protein, iron, and meat consumption and risk for rheumatoid arthritis: A prospective cohort study. Arthritis Res. Ther. 2007, 9, R16. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Feng, M.; Zhang, X.; Jin, Y.B.; Li, X.; Su, L.C.; Liu, S.; Wang, A.X.; Chen, X.M.; et al. Dietary intake and risk of rheumatoid arthritis-a cross section multicenter study. Clin. Rheumatol. 2016, 35, 2901–2908. [Google Scholar] [CrossRef]
- Asoudeh, F.; Jayedi, A.; Kavian, Z.; Ebrahimi-Mousavi, S.; Nielsen, S.M.; Mohammadi, H. A systematic review and meta-analysis of observational studies on the association between animal protein sources and risk of rheumatoid arthritis. Clin. Nutr. 2021, 40, 4644–4652. [Google Scholar] [CrossRef]
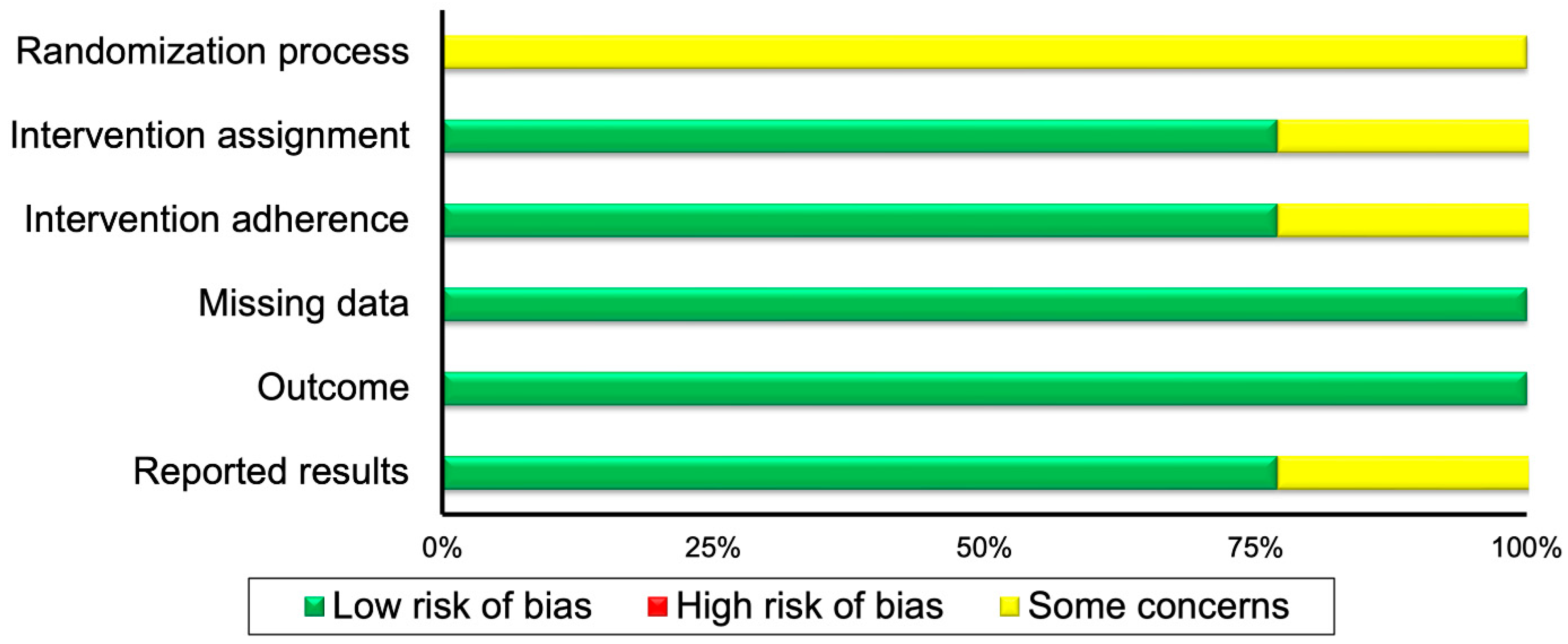
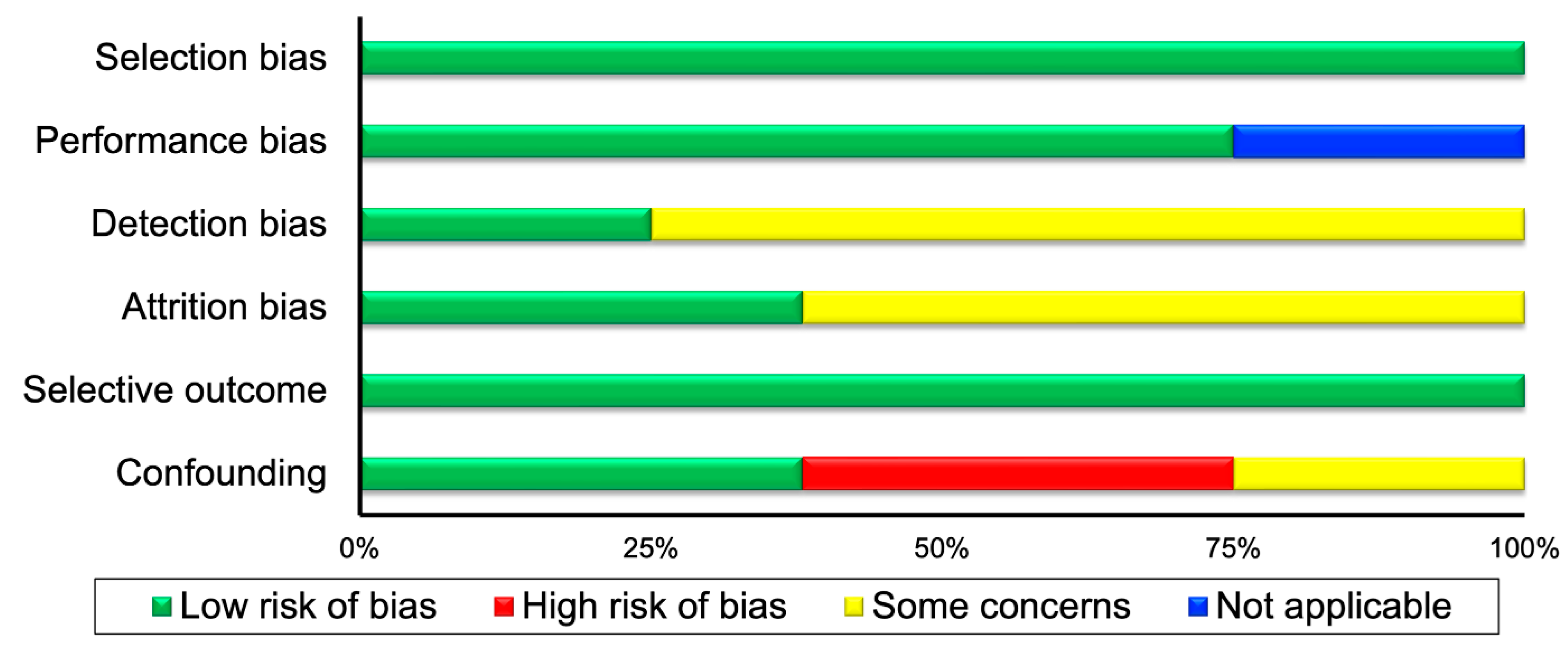

| Randomized Controlled Trials | ||||||
|---|---|---|---|---|---|---|
| First author | Randomization process | Intervention assignment | Intervention adherence | Missing data | Outcome | Reported results |
| Garcia-Morales 2020 [28] | ? | ? | + | + | + | + |
| Garner 2018 [25] | ? | + | ? | + | + | ? |
| Pineda-Juarez 2022 [23] | ? | + | + | + | + | + |
| Controlled trials and epidemiological studies | ||||||
| First author | Selection | Performance | Detection | Attrition | Selective Outcome | Confounding |
| Barone 2018 [31] | + | N | ? | ? | + | − |
| Bilberg 2022 [29] | + | + | ? | + | + | − |
| Elkan 2011 [32] | + | + | ? | ? | + | + |
| Engelhart 1996 [21] | + | + | ? | + | + | + |
| Gordon 2002 [22] | + | + | ? | ? | + | − |
| Matsunaga 2021 [33] | + | N | + | ? | + | ? |
| Mikkelsen 2015 [30] | + | + | + | + | + | + |
| Stavropoulos-Kalinoglou 2010 [34] | + | + | ? | ? | + | ? |
| Study | Type of Patients | Type of Diet/Nutrition and Exercise/Physical Activity | Outcome |
|---|---|---|---|
| Controlled Trials | |||
| Engelhart, 1996 [21] | RA | 12-week resistance exercise and walking. Reduction of 30% of energy intake, 62 gof high-quality protein intake per day, vitamins, and mineral supplements | Reduction of body fat mass and fat free mass |
| Mikkelsen, 2015 [30] | RA | A protein drink consisting of 0.5 g intact whey protein isolate (Lacprodan-9224, Arla Foods Ingredients, Viby, Denmark)/kg lean body mass (12.5% enriched with ring-13C6-phenylalanine) dissolved in 190 mL water. Acute exercise of unilateral leg extension, 8 × 10 repetitions at 70% of 1 repetition maximum | Muscle protein synthesis and transcriptional regulation can be stimulated with both protein intake and physical exercise in patients with RA to a similar degree as in healthy individuals |
| Bilberg, 2022 [29] | PsA | Recommendation for physical activity, ≥150 min/week and weight loss treatment | Patients improved quality of life (SF-36 score), total fat mass (kg), and reduced total lean mass from baseline to 6-month (p < 0.01) and 12-month (p < 0.01). |
| Epidemiological studies | |||
| Elkan, 2011 [32] | RA | Questionnaires assessed dietary habits and physical activity levels | RA patients who consumed saturated fatty acids and had low level of total physical activity displayed significantly lower levels of HDL, apolipoprotein A1, the atheroprotective anti-phosphorylcholine antibodies, and significantly higher levels of insulin |
| Barone, 2018 [31] | RA | Measurements of physical activity and daily calorie and protein intake | No association of physical activity and daily calorie and protein intake with sarcopenia in patients |
| Matsunaga, 2021 [33] | RA | Questionnaires assessed dietary habits and physical activity levels | No association of healthy eating index total scores with self-reported RA, adjusted for physical activity |
| Stavropoulos-Kalinoglou, 2010 [34] | RA | Questionnaires assessed dietary habits and physical activity levels | Physical activity and energy intake were not associated with HAQ, DAS28, body fat, BMI, interleukin-1β, interleukin-6, tumor necrosis factor alpha, ESR, and CRP |
| Barone, 2018 [31] | PsA | Measurements of physical activity and daily calorie and protein intake | No association of physical activity and daily calorie and protein intake with sarcopenia in patients |
| Barone, 2018 [31] | AS | Measurements of physical activity and daily calorie and protein intake | No association of physical activity and daily calorie and protein intake with sarcopenia in patients |
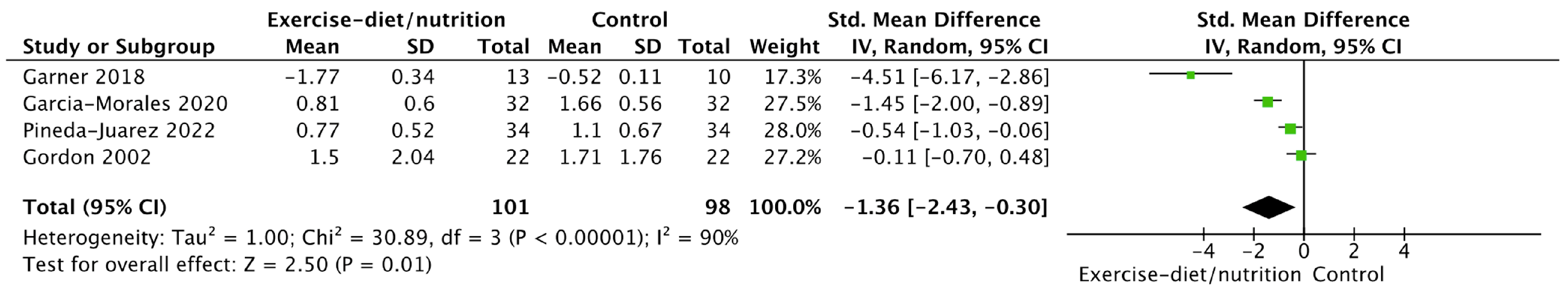
| Outcomes | No of Participants (Studies/Entries) | Quality of the Evidence (GRADE) | Relative Effect (95% CI) |
|---|---|---|---|
| Diet/nutrition and physical activity/exercise HAQ score vs. control | 199 (4 studies/entries) | Low ⊕⊕◯◯ due to inconsistency of results | Standardized mean difference = −1.36 (−2.43)–(−0.30) |
| Diet/nutrition and physical activity/exercise ESR vs. control | 67 (2 studies/entries) | Low ⊕⊕◯◯ due to imprecision | Mean difference = 0.2 (0.09–0.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinas, P.C.; on behalf of the students of module 5104 (Introduction to Systematic Reviews); Moe, R.H.; Boström, C.; Kosti, R.I.; Kitas, G.D.; Metsios, G.S. Combined Effects of Diet and Physical Activity on Inflammatory Joint Disease: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 1427. https://doi.org/10.3390/healthcare11101427
Dinas PC, on behalf of the students of module 5104 (Introduction to Systematic Reviews), Moe RH, Boström C, Kosti RI, Kitas GD, Metsios GS. Combined Effects of Diet and Physical Activity on Inflammatory Joint Disease: A Systematic Review and Meta-Analysis. Healthcare. 2023; 11(10):1427. https://doi.org/10.3390/healthcare11101427
Chicago/Turabian StyleDinas, Petros C., on behalf of the students of module 5104 (Introduction to Systematic Reviews), Rikke Helene Moe, Carina Boström, Rena I. Kosti, George D. Kitas, and George S. Metsios. 2023. "Combined Effects of Diet and Physical Activity on Inflammatory Joint Disease: A Systematic Review and Meta-Analysis" Healthcare 11, no. 10: 1427. https://doi.org/10.3390/healthcare11101427





