Effects of Exercise and Physical Activity Levels on Childhood Cancer: An Umbrella Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Types of Interventions
2.1.4. Types of Outcome Measures
2.2. Search Methods for Identification of Reviews
Electronic Searches and Other Resources
2.3. Data Collection and Analysis
2.3.1. Selection of Reviews
2.3.2. Data Extraction and Management
2.3.3. Quality of Evidence
2.3.4. Data Synthesis
3. Results
3.1. Description of Included Reviews
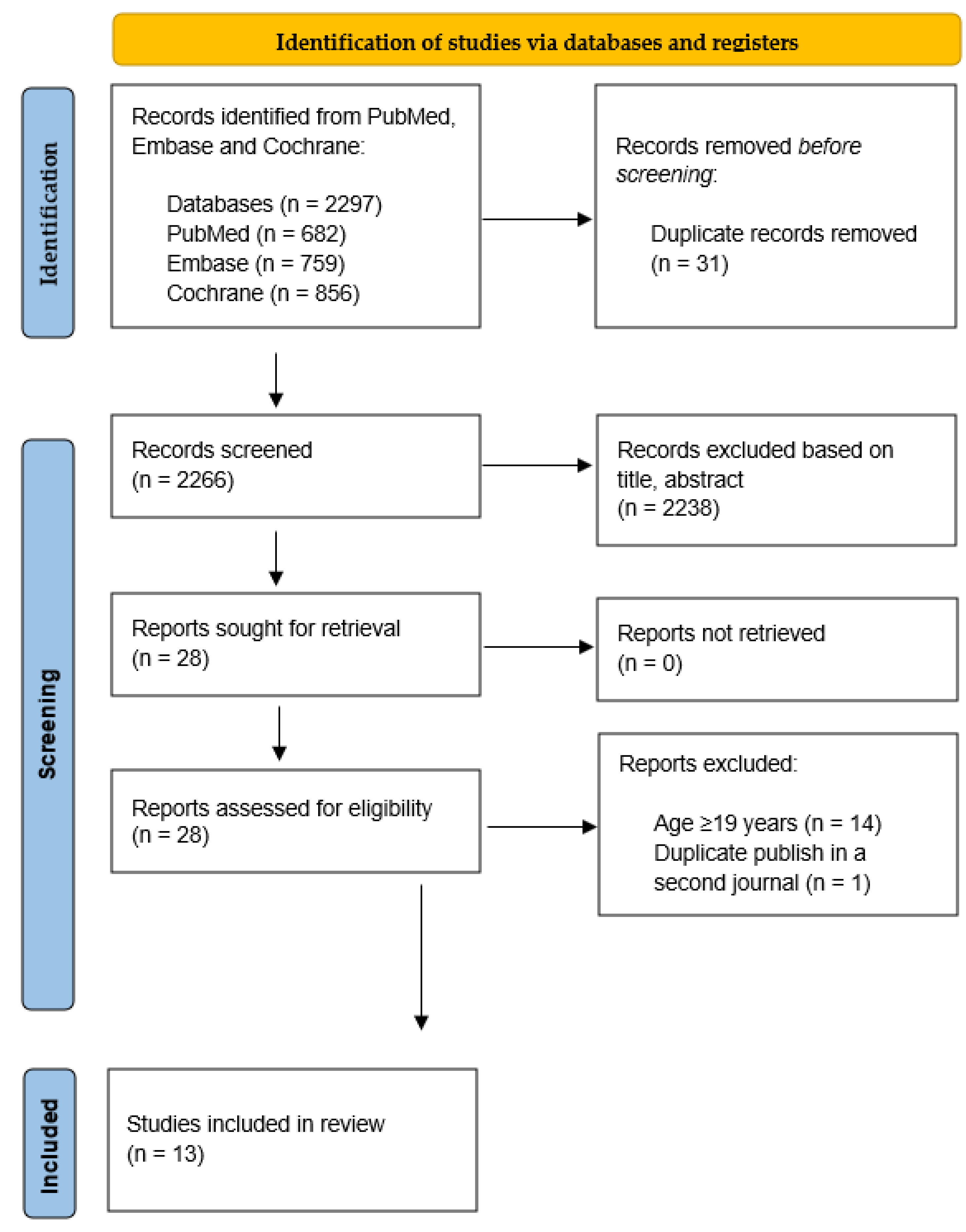
3.1.1. Search Outcomes
3.1.2. Characteristics of Eligible Systematic Reviews
3.1.3. Methodological Quality of Included Reviews
3.2. Effects of Interventions
3.2.1. Cancer-Related Fatigue
3.2.2. Muscle Strength
3.2.3. Aerobic Capacity
3.2.4. Cancer-Related Pain
3.2.5. Body Composition
3.2.6. Activity and Participation Levels
3.2.7. Psychosocial Health Indices
3.2.8. Health-Related Quality of Life
3.2.9. Cardiorespiratory Fitness
3.2.10. Cardiovascular Fitness
3.2.11. Cardiovascular Function and Structure
3.2.12. Flexibility/Range of Motion
3.2.13. Coordination
3.2.14. Physical Fitness
3.2.15. Physical Function
3.2.16. Functional Capacity
3.2.17. Physical Capacity
3.2.18. Biochemical Indicators
3.2.19. Bone Mineral Density
3.2.20. Brain Volume and Structure
3.2.21. Cognitive Function
3.2.22. Energy Consumption
3.2.23. General Health Domain
3.2.24. Adverse Effects
4. Discussion
4.1. Summary of Main Results and Overall Completeness and Applicability of Evidence
4.2. Quality of the Evidence
4.3. Study Limitations
4.4. Potential Biases in the Review Process
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antwi, G.O.; Jayawardene, W.; Lohrmann, D.K.; Mueller, E.L. Physical activity and fitness among pediatric cancer survivors: A meta-analysis of observational studies. Support. Care Cancer 2019, 27, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Banks, L.; Schneiderman, J.E.; Caterini, J.E.; Stephens, S.; White, G.; Dogra, S.; Wells, G.D. Physical activity for children with chronic disease; a narrative review and practical applications. BMC Pediatr. 2019, 19, 12. [Google Scholar] [CrossRef]
- WHO. Childhood Cancer; WHO: Geneva, Switzerland, 2021.
- NCI. Childhood Cancers; NCI: Rockville Pike, MD, USA, 2022.
- Braam, K.I.; van der Torre, P.; Takken, T.; Veening, M.A.; van Dulmen-den Broeder, E.; Kaspers, G.J. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016, 3, CD008796. [Google Scholar] [CrossRef]
- Grimshaw, S.L.; Taylor, N.F.; Shields, N. The Feasibility of Physical Activity Interventions During the Intense Treatment Phase for Children and Adolescents with Cancer: A Systematic Review. Pediatr. Blood Cancer 2016, 63, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Betz, G.; Marchese, V. Exploring pulmonary function and physical function in childhood cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 160, 103279. [Google Scholar] [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Herrera-Olivares, A.M.; Bano-Rodrigo, A.; Castillo-Garcia, A.; Rincon-Castanedo, C.; Martin-Ruiz, A.; San-Juan, A.F.; Fiuza-Luces, C.; Lucia, A. Exercise Interventions and Cardiovascular Health in Childhood Cancer: A Meta-analysis. Int. J. Sports Med. 2020, 41, 141–153. [Google Scholar] [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Velazquez-Diaz, D.; Castillo-Garcia, A.; Jimenez-Pavon, D.; Lucia, A.; Fiuza-Luces, C. Exercise and Childhood Cancer-A Historical Review. Cancers 2021, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Gawade, P.L.; Ness, K.K. Impairments that influence physical function among survivors of childhood cancer. Children 2015, 2, 1–36. [Google Scholar] [CrossRef]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005-2017). PM&R 2017, 9, S347–S384. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Bhardwaj, T.; Koffman, J. Non-pharmacological interventions for management of fatigue among children with cancer: Systematic review of existing practices and their effectiveness. BMJ Support. Palliat. Care 2017, 7, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Mu, P.F.; Jou, S.T.; Wong, T.T.; Chen, Y.C. Systematic review and meta-analysis of nonpharmacological interventions for fatigue in children and adolescents with cancer. Worldviews Evid. Based Nurs. 2013, 10, 208–217. [Google Scholar] [CrossRef]
- Coombs, A.; Schilperoort, H.; Sargent, B. The effect of exercise and motor interventions on physical activity and motor outcomes during and after medical intervention for children and adolescents with acute lymphoblastic leukemia: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 152, 103004. [Google Scholar] [CrossRef]
- Khaleqi-Sohi, M.; Sadria, G.; Ghalibafian, M.; Khademi-Kalantari, K.; Irannejad, S. The Effects of Physical Activity and Exercise Therapy on Pediatric Brain Tumor Survivors: A systematic review. J. Bodyw. Mov. Ther. 2022, 30, 1–9. [Google Scholar] [CrossRef]
- Martha, B.A.; Vacchi, C.O.; Fattori, R.A.; Macagnan, F.E. Effect of physical exercise on the functional capacity of children and adolescents submitted to transplantation of hematopoietic stem cells-A systematic review with meta-analysis. J. Child Health Care 2021, 25, 18–30. [Google Scholar] [CrossRef]
- Mizrahi, D.; Wakefield, C.E.; Fardell, J.E.; Quinn, V.F.; Lim, Q.; Clifford, B.K.; Simar, D.; Ness, K.K.; Cohn, R.J. Distance-delivered physical activity interventions for childhood cancer survivors: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 118, 27–41. [Google Scholar] [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Herrera-Olivares, A.M.; Rincón-Castanedo, C.; Martín-Ruiz, A.; Castillo-García, A.; Fiuza-Luces, C.; Lucia, A. What are the effects of exercise training in childhood cancer survivors? A systematic review. Cancer Metastasis Rev. 2020, 39, 115–125. [Google Scholar] [CrossRef]
- Santos, S.D.S.; Moussalle, L.D.; Heinzmann-Filho, J.P. Effects of Physical Exercise during Hospitalization in Children and Adolescents with Cancer: A Systematic Review. Rev. Paul. Pediatr. 2020, 39, e2019313. [Google Scholar] [CrossRef]
- Shi, Q.; Zheng, J.; Liu, K. Supervised Exercise Interventions in Childhood Cancer Survivors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Children 2022, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Ruiz, J.R.; Tuchman, H.; Lucia, A. Exercise in adult and pediatric hematological cancer survivors: An intervention review. Leukemia 2010, 24, 1113–1120. [Google Scholar] [CrossRef]
- Winter, C.; Muller, C.; Brandes, M.; Brinkmann, A.; Hoffmann, C.; Hardes, J.; Gosheger, G.; Boos, J.; Rosenbaum, D. Level of activity in children undergoing cancer treatment. Pediatr. Blood Cancer 2009, 53, 438–443. [Google Scholar] [CrossRef]
- Thong, M.S.Y.; van Noorden, C.J.F.; Steindorf, K.; Arndt, V. Cancer-Related Fatigue: Causes and Current Treatment Options. Curr. Treat. Options Oncol. 2020, 21, 17. [Google Scholar] [CrossRef]
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom. Manag. 2016, 51, 1070–1090.e1079. [Google Scholar] [CrossRef]
- Eilertsen, M.E.; Rannestad, T.; Indredavik, M.S.; Vik, T. Psychosocial health in children and adolescents surviving cancer. Scand. J. Caring Sci. 2011, 25, 725–734. [Google Scholar] [CrossRef]
- Klassen, A.F.; Anthony, S.J.; Khan, A.; Sung, L.; Klaassen, R. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: A systematic review. Support. Care Cancer 2011, 19, 1275–1287. [Google Scholar] [CrossRef]
- Goodenough, C.G.; Wogksch, M.D.; Kundu, M.; Lear, M.; Thomas, P.G.; Srivastava, D.K.; Wang, Z.; Armstrong, G.T.; Hudson, M.M.; Robison, L.L.; et al. Associations between exercise capacity, p16INK4a expression and inflammation among adult survivors of childhood cancer. Front. Oncol. 2022, 12, 1014661. [Google Scholar] [CrossRef]
- Invernizzi, M.; Lippi, L.; Folli, A.; Turco, A.; Zattoni, L.; Maconi, A.; de Sire, A.; Fusco, N. Integrating molecular biomarkers in breast cancer rehabilitation. What is the current evidence? A systematic review of randomized controlled trials. Front. Mol. Biosci. 2022, 9, 930361. [Google Scholar] [CrossRef]
- LaPak, K.M.; Burd, C.E. The Molecular Balancing Act of p16INK4a in Cancer and Agingp16INK4a in Cancer and Aging. Mol. Cancer Res. 2014, 12, 167–183. [Google Scholar] [CrossRef]
- Takken, T.; van der Torre, P.; Zwerink, M.; Hulzebos, E.H.; Bierings, M.; Helders, P.J.M.; van der Net, J. Development, feasibility and efficacy of a community-based exercise training program in pediatric cancer survivors. Psycho-Oncology 2009, 18, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Post-White, J.; Fitzgerald, M.; Savik, K.; Hooke, M.C.; Hannahan, A.B.; Sencer, S.F. Massage Therapy for Children With Cancer. J. Pediatr. Oncol. Nurs. 2008, 26, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Hinds, P.S.; Hockenberry, M.; Rai, S.N.; Zhang, L.; Razzouk, B.; Cremer, L.; McCarthy, K.; Rodriguez-Galindo, C. Clinical Field Testing of an Enhanced-Activity Intervention in Hospitalized Children with Cancer. J. Pain Symptom Manag. 2007, 33, 686–697. [Google Scholar] [CrossRef]
- Keats, M.R.; Culos-Reed, S.N. A community-based physical activity program for adolescents with cancer (project TREK): Program feasibility and preliminary findings. J. Pediatr. Hematol. Oncol. 2008, 30, 272–280. [Google Scholar] [CrossRef]
- Genc, R.E.; Conk, Z. Impact of Effective Nursing Interventions to the Fatigue Syndrome in Children Who Receive Chemotherapy. Cancer Nurs. 2008, 31, 312–317. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Yeh, C.H. The effects of a home-based aerobic exercise intervention on fatigue in children with acute lymphoblastic leukemia during the maintenance stage of chemotherapy. Ann. Behav. Med. 2008, 35, 3–12. [Google Scholar]
- Vina, C.C.; Ruiz, J.; Santana-Sosa, E.; Vicent, M.G.; Madero, L.; Pérez, M.; Fleck, S.J.; Pérez, A.; Ramírez, M.; Lucia, A. Exercise during Hematopoietic Stem Cell Transplant Hospitalization in Children. Med. Sci. Sports Exerc. 2010, 42, 1045–1053. [Google Scholar] [CrossRef]
- Cortés-Reyes, É.; Escobar-Zabala, P.; González-García, L. The effect of game-based exercise on infant acute lymphocytic leukaemia patients. Rev. Fac. Med. Univ. Nac. Colomb. 2013, 61, 349–355. [Google Scholar]
- Diorio, C.; Schechter, T.; Lee, M.; O’Sullivan, C.; Hesser, T.; Tomlinson, D.; Piscione, J.; Armstrong, C.; Tomlinson, G.; Sung, L. A pilot study to evaluate the feasibility of individualized yoga for inpatient children receiving intensive chemotherapy. BMC Complement. Altern. Med. 2015, 15, 2. [Google Scholar] [CrossRef]
- Geyer, R.; Lyons, A.; Amazeen, L.; Alishio, L.; Cooks, L. Feasibility study: The effect of therapeutic yoga on quality of life in children hospitalized with cancer. Pediatr. Phys. Ther. 2011, 23, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Gohar, S.F.; Comito, M.; Price, J.; Marchese, V. Feasibility and parent satisfaction of a physical therapy intervention program for children with acute lymphoblastic leukemia in the first 6 months of medical treatment. Pediatr. Blood Cancer 2011, 56, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Götte, M.; Kesting, S.; Winter, C.; Rosenbaum, D.; Boos, J. Experience of barriers and motivations for physical activities and exercise during treatment of pediatric patients with cancer. Pediatr. Blood Cancer 2014, 61, 1632–1637. [Google Scholar] [CrossRef]
- Hartman, A.; Winkel, M.T.; van Beek, R.; Keizer-Schrama, S.D.M.; Kemper, H.; Hop, W.; Heuvel-Eibrink, M.V.D.; Pieters, R. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 53, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Winter, C.; Boos, J.; Gosheger, G.; Hardes, J.; Vieth, V.; Rosenbaum, D. Effects of an Exercise Intervention on Bone Mass in Pediatric Bone Tumor Patients. Int. J. Sports Med. 2014, 35, 696–703. [Google Scholar] [CrossRef]
- Rosenhagen, A.; Bernhörster, M.; Vogt, L.; Weiss, B.; Senn, A.; Arndt, S.; Siegler, K.; Jung, M.; Bader, P.; Banzer, W. Implementation of Structured Physical Activity in the Pediatric Stem Cell Transplantation. Klin. Pädiatr. 2011, 223, 147–151. [Google Scholar] [CrossRef]
- Speyer, E.; Herbinet, A.; Vuillemin, A.; Briançon, S.; Chastagner, P. Effect of adapted physical activity sessions in the hospital on health-related quality of life for children with cancer: A cross-over randomized trial. Pediatr. Blood Cancer 2010, 55, 1160–1166. [Google Scholar] [CrossRef]
- Winter, C.C.; Müller, C.; Hardes, J.; Gosheger, G.; Boos, J.; Rosenbaum, D. The effect of individualized exercise interventions during treatment in pediatric patients with a malignant bone tumor. Support. Care Cancer 2013, 21, 1629–1636. [Google Scholar] [CrossRef]
- Inspiratory Muscle Training in Patients with Acute Leukemia: Preliminary Results. Available online: https://www.scielo.br/j/rpp/a/bwpjS4NsQ8Q6jyd6PcPLjrJ/abstract/?lang=en (accessed on 7 July 2022).
- Marchese, V.G.; Chiarello, L.A.; Lange, B.J. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2003, 42, 127–133. [Google Scholar] [CrossRef]
- Moyer-Mileur, L.J.; Ransdell, L.; Bruggers, C.S. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: Response to a home-based exercise and nutrition program. J. Pediatr. Hematol. Oncol. 2009, 31, 259–266. [Google Scholar] [CrossRef]
- Tanir, M.K.; Kuguoglu, S. Impact of Exercise on Lower Activity Levels in Children with Acute Lymphoblastic Leukemia: A Randomized Controlled Trial from Turkey. Rehabilitation Nurs. 2013, 38, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Wai, J.P.M.; Lin, U.-S.; Chiang, Y.-C. A Pilot Study to Examine the Feasibility and Effects of a Home-Based Aerobic Program on Reducing Fatigue in Children With Acute Lymphoblastic Leukemia. Cancer Nurs. 2011, 34, 3–12. [Google Scholar] [CrossRef]
- Wong, J.; Ghiasuddin, A.; Kimata, C.; Patelesio, B.; Siu, A. The Impact of Healing Touch on Pediatric Oncology Patients. Integr. Cancer Ther. 2012, 12, 25–30. [Google Scholar] [CrossRef]
- Blaauwbroek, R.; Bouma, M.J.; Tuinier, W.; Groenier, K.H.; Greef, M.H.G.D.; Jong, B.M.; Kamps, W.A.; Postma, A. The effect of exercise counselling withfeedback from a pedometer on fatigue in adult survivors of childhood cancer: A pilot study. Support. Care Cancer 2009, 17, 1041–1048. [Google Scholar] [CrossRef]
- Gilliam, M.B.; Ross, K.; Futch, L.; Walsh, A.; Klapow, J.; Davis, D.; Whelan, K.; Madan-Swain, A. A Pilot Study Evaluation of a Web-Based Token Economy to Increase Adherence with a Community-Based Exercise Intervention in Child and Adolescent Cancer Survivors. Rehabilit. Oncol. 2011, 29, 16–22. [Google Scholar] [CrossRef]
- Järvelä, L.S.; Kemppainen, J.; Niinikoski, H.; Hannukainen, J.C.; Lähteenmäki, P.M.; Kapanen, J.; Arola, M.; Heinonen, O.J. Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2011, 59, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Stratton, E.; Giblin, J.; Esiashvili, N.; Mertens, A. Pilot results of an online intervention targeting health promoting behaviors among young adult cancer survivors. Psycho-Oncology 2014, 23, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Esbenshade, A.J.; Friedman, D.L.; Smith, W.A.; Jeha, S.; Pui, C.-H.; Robison, L.L.; Ness, K.K. Feasibility and Initial Effectiveness of Home Exercise During Maintenance Therapy for Childhood Acute Lymphoblastic Leukemia. Pediatr. Phys. Ther. 2014, 26, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Dillon, L.; Terrones, L.; Schubert, L.; Roberts, W.; Finklestein, J.; Swartz, M.C.; Norman, G.J.; Patrick, K. Fit4Life: A weight loss intervention for children who have survived childhood leukemia. Pediatr. Blood Cancer 2014, 61, 894–900. [Google Scholar] [CrossRef]
- Smith, W.A.; Ness, K.K.; Joshi, V.; Hudson, M.M.; Robison, L.L.; Green, D.M. Exercise training in childhood cancer survivors with subclinical cardiomyopathy who were treated with anthracyclines. Pediatr. Blood Cancer 2013, 61, 942–945. [Google Scholar] [CrossRef]
- Sabel, M.; Sjölund, A.; Broeren, J.; Arvidsson, D.; Saury, J.-M.; Blomgren, K.; Lannering, B.; Emanuelson, I. Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil. Rehabilit. 2016, 38, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Hooke, M.C.; Gilchrist, L.S.; Tanner, L.; Hart, N.; Withycombe, J.S. Use of a Fitness Tracker to Promote Physical Activity in Children With Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2016, 63, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Dubnov-Raz, G.; Azar, M.; Reuveny, R.; Katz, U.; Weintraub, M.; Constantini, N.W. Changes in fitness are associated with changes in body composition and bone health in children after cancer. Acta Paediatr. 2015, 104, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Järvelä, L.S.; Niinikoski, H.; Heinonen, O.J.; Lähteenmäki, P.M.; Arola, M.; Kemppainen, J. Endothelial function in long-term survivors of childhood acute lymphoblastic leukemia: Effects of a home-based exercise program. Pediatr. Blood Cancer 2013, 60, 1546–1551. [Google Scholar] [CrossRef]
- Järvelä, L.S.; Saraste, M.; Niinikoski, H.; Hannukainen, J.C.; Heinonen, O.J.; Lähteenmäki, P.M.; Arola, M.; Kemppainen, J. Home-based exercise training improves left ventricle diastolic function in survivors of childhoodALL: A tissue doppler and velocity vector imaging study. Pediatr. Blood Cancer 2016, 63, 1629–1635. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S. Feasibility and benefits of a combined programme of exercise and play for paediatric cancer survivors: A pilot study. Eur. J. Cancer Care 2019, 28, e13111. [Google Scholar] [CrossRef]
- Long, T.M.; Rath, S.R.; Wallman, K.E.; Howie, E.K.; Straker, L.; Bullock, A.; Walwyn, T.S.; Gottardo, N.; Cole, C.H.; Choong, C.S.; et al. Exercise training improves vascular function and secondary health measures in survivors of pediatric oncology related cerebral insult. PLoS ONE 2018, 13, e0201449. [Google Scholar] [CrossRef]
- Piscione, P.; Bouffet, E.; Timmons, B.; Courneya, K.; Tetzlaff, D.; Schneiderman, J.; de Medeiros, C.; Bartels, U.; Mabbott, D. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur. J. Cancer 2017, 80, 63–72. [Google Scholar] [CrossRef]
- Rath, S.R.; Long, T.M.; Bear, N.L.; Miles, G.C.; Bullock, A.M.; Gottardo, N.G.; Cole, C.H.; Naylor, L.H.; Choong, C.S. Metabolic and Psychological Impact of a Pragmatic Exercise Intervention Program in Adolescent and Young Adult Survivors of Pediatric Cancer-Related Cerebral Insult. J. Adolesc. Young Adult Oncol. 2018, 7, 349–357. [Google Scholar] [CrossRef]
- Riggs, L.; Piscione, J.; Laughlin, S.; Cunningham, T.; Timmons, B.W.; Courneya, K.S.; Bartels, U.; Skocic, J.; de Medeiros, C.; Liu, F.; et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: A controlled clinical trial with crossover of training versus no training. Neuro-Oncology 2016, 19, 440–450. [Google Scholar] [CrossRef]
- Szulc-Lerch, K.U.; Timmons, B.W.; Bouffet, E.; Laughlin, S.; de Medeiros, C.B.; Skocic, J.; Lerch, J.P.; Mabbott, D.J. Repairing the brain with physical exercise: Cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. NeuroImage Clin. 2018, 18, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.S.; Valenzuela, P.L.; Rincón-Castanedo, C.; Takken, T.; Fiuza-Luces, C.; Santos-Lozano, A.; Lucia, A. Exercise training in childhood cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2018, 70, 154–167. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; .Padilla, J.R.; Valentín, J.; Santana-Sosa, E.; Santos-Lozano, A.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Morales, J.S.; Fleck, S.J.; Pérez, M.; et al. Effects of Exercise on the Immune Function of Pediatric Patients With Solid Tumors: Insights From the PAPEC Randomized Trial. Am. J. Phys. Med. Rehabil. 2017, 96, 831–837. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; .Padilla, J.R.; Soares-Miranda, L.; Santana-Sosa, E.; Quiroga, J.V.; Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Lorenzo-González, R.; Verde, Z.; et al. Exercise Intervention in Pediatric Patients with Solid Tumors: The Physical Activity in Pediatric Cancer Trial. Med. Sci. Sports Exerc. 2017, 49, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bogg, T.F.T.; Broderick, C.; Shaw, P.; Cohn, R.; Naumann, F.L. Feasibility of an inpatient exercise intervention for children undergoing hematopoietic stem cell transplant. Pediatr. Transplant. 2015, 19, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Perondi, M.B.; Gualano, B.; Artioli, G.G.; Painelli, V.D.S.; Filho, V.O.; Netto, G.; Muratt, M.; Roschel, H.; Pinto, A.L.D.S. Effects of a combined aerobic and strength training program in youth patients with acute lymphoblastic leukemia. J. Sports Sci. Med. 2012, 11, 387–392. [Google Scholar]
- Ruiz, J.R.; Fleck, S.J.; Vingren, J.L.; Ramírez, M.; Madero, L.; Fragala, M.S.; Kraemer, W.J.; Lucia, A. Preliminary Findings of a 4-Month Intrahospital Exercise Training Intervention on IGFs and IGFBPs in Children with Leukemia. J. Strength Cond. Res. 2010, 24, 1292–1297. [Google Scholar] [CrossRef]
- Juan, A.F.S.; Fleck, S.J.; Chamorro-Viña, C.; Maté-Muñoz, J.L.; Moral, S.; Pérez, M.; Cardona, C.; DEL Valle, M.F.; Hernández, M.; Ramírez, M.; et al. Effects of an Intrahospital Exercise Program Intervention for Children with Leukemia. Med. Sci. Sports Exerc. 2007, 39, 13–21. [Google Scholar] [CrossRef]
- Kaushal, B.D.; Narendra, D.B.; Smitha, D. A Comparative Study between Relaxation Technique and Aerobic Exercise in Fatigue During Chemotherapy in Acute Lymphoblastic Leukemia in Children. Indian J. Physiother. Occup. Ther. 2013, 7, 140. [Google Scholar]
- Waked, I.; Albenasy, K. Bone mineral density, lean body mass and bone biomarkers following physical exercise in children with acute lymphoblastic leukemia undergoing chemotherapy. Iran. J. Blood Cancer 2018, 10, 69–75. [Google Scholar]
- Wright, M.J.P.; Collins, L.B.; Christie, A.M.; Birken, K.M.; Dettmer, E.P.; Nathan, P.C.M. A Comprehensive Healthy Lifestyle Program for Children Receiving Treatment for Acute Lymphoblastic Leukemia: Feasibility and Preliminary Efficacy Data. Rehabilit. Oncol. 2013, 31, 6–13. [Google Scholar] [CrossRef]
- Baky, A.; Elhakk, S. Impact of Aerobic Exercise on Physical Fitness and Fatigue in Children with Acute Lymphoblastic Leukemia. Int. J. Ther. Rehabilit. Res. 2017, 6, 137. [Google Scholar] [CrossRef]
- Manchola-González, J.D.; Bagur-Calafat, C.; Girabent-Farrés, M.; Serra-Grima, J.R.; Pérez, R.; Garnacho-Castaño, M.V.; Badell, I.; Ramírez-Vélez, R. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: Results of a randomised clinical trial. Support. Care Cancer 2019, 28, 3171–3178. [Google Scholar] [CrossRef]
- Cox, C.L.; Zhu, L.; Kaste, S.C.; Srivastava, K.; Barnes, L.; Nathan, P.C.; Wells, R.J.; Ness, K.K. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2017, 65, e26929. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, E.; Badiee, Z.; Sohrabi, M.; Ghassemi, A.; Hosseinzade, V. The effect of an aerobic exercise program on the quality of life in children with cancer. Turk. J. Pediatr. 2017, 59, 678. [Google Scholar] [CrossRef] [PubMed]
- Tanner, L.R.; Hooke, M.C. Improving body function and minimizing activity limitations in pediatric leukemia survivors: The lasting impact of the Stoplight Program. Pediatr. Blood Cancer 2019, 66, e27596. [Google Scholar] [CrossRef] [PubMed]
- Wallek, S.; Senn-Malashonak, A.; Vogt, L.; Schmidt, K.; Bader, P.; Banzer, W. Impact of the initial fitness level on the effects of a structured exercise therapy during pediatric stem cell transplantation. Pediatr. Blood Cancer 2017, 65, e26851. [Google Scholar] [CrossRef]
- Msc, V.Y.K.; Duger, T.; Cetinkaya, D.U. Investigation of the Effects of an Exercise Program on Physical Functions and Activities of Daily Life in Pediatric Hematopoietic Stem Cell Transplantation. Pediatr. Blood Cancer 2016, 63, 1643–1648. [Google Scholar] [CrossRef]
- Juan, A.S.; Chamorro-Viña, C.; Moral, S.; del Valle, M.F.; Madero, L.; Ramírez, M.; Pérez, M.; Lucia, A. Benefits of Intrahospital Exercise Training after Pediatric Bone Marrow Transplantation. Int. J. Sports Med. 2007, 29, 439–446. [Google Scholar] [CrossRef]
- Ladha, A.B.; Courneya, K.S.; Bell, G.J.; Field, C.; Grundy, P. Effects of Acute Exercise on Neutrophils in Pediatric Acute Lymphoblastic Leukemia Survivors: A Pilot Study. J. Pediatr. Hematol. 2006, 28, 671–677. [Google Scholar] [CrossRef]
- Oldervoll, L.; Kaasa, S.; Knobel, H.; Loge, J. Exercise reduces fatigue in chronic fatigued Hodgkins disease survivors—results from a pilot study. Eur. J. Cancer 2003, 39, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.F.S.; Fleck, S.J.; Chamorro-Viña, C.; Maté-Muñoz, J.L.; Moral, S.; García-Castro, J.; Ramírez, M.; Madero, L.; Lucia, A. Early-phase adaptations to intrahospital training in strength and functional mobility of children with leukemia. J. Strength Cond. Res. 2007, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, A.M.; Carey, A.B.; Heise, C.T.; Barber, G. Cardiac rehabilitation after cancer therapy in children and young adults. Am. J. Cardiol. 1993, 71, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.; Shepard, R.J. Immune responses to exercise in children treated for cancer. J. Sports Med. Phys. Fit. 1999, 39, 240. [Google Scholar] [CrossRef]
- Cox, E.; Bells, S.; Timmons, B.W.; Laughlin, S.; Bouffet, E.; de Medeiros, C.; Beera, K.; Harasym, D.; Mabbott, D.J. A controlled clinical crossover trial of exercise training to improve cognition and neural communication in pediatric brain tumor survivors. Clin. Neurophysiol. 2020, 131, 1533–1547. [Google Scholar] [CrossRef]
- Sabel, M.; Sjölund, A.; Broeren, J.; Arvidsson, D.; Saury, J.-M.; Gillenstrand, J.; Emanuelson, I.; Blomgren, K.; Lannering, B. Effects of physically active video gaming on cognition and activities of daily living in childhood brain tumor survivors: A randomized pilot study. Neuro-Oncol. Pr. 2016, 4, 98–110. [Google Scholar] [CrossRef]
- Müller, C.; Krauth, K.A.; Gerß, J.; Rosenbaum, D. Physical activity and health-related quality of life in pediatric cancer patients following a 4-week inpatient rehabilitation program. Support. Care Cancer 2016, 24, 3793–3802. [Google Scholar] [CrossRef]
- Wurz, A.; Chamorro-Vina, C.; Guilcher, G.M.; Schulte, F.; Culos-Reed, S.N. The feasibility and benefits of a 12-week yoga intervention for pediatric cancer out-patients. Pediatr. Blood Cancer 2014, 61, 1828–1834. [Google Scholar] [CrossRef]
- Braam, K.; van Dijk-Lokkart, E.; van Dongen, J.; van Litsenburg, R.; Takken, T.; Huisman, J.; Merks, J.; Bosmans, J.; Hakkenbrak, N.; Bierings, M.; et al. Cost-effectiveness of a combined physical exercise and psychosocial training intervention for children with cancer: Results from the quality of life in motion study. Eur. J. Cancer Care 2016, 26, e12586. [Google Scholar] [CrossRef]
- Lam, K.K.; Li, W.H.; Chung, J.; Ho, K.; Chiu, S.; Lam, H.; Chan, G.C. An integrated experiential training programme with coaching to promote physical activity, and reduce fatigue among children with cancer: A randomised controlled trial. Patient Educ. Couns. 2018, 101, 1947–1956. [Google Scholar] [CrossRef]
- Li, H.C.W.; Chung, O.K.J.; Ho, K.Y.; Chiu, S.Y.; Lopez, V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psycho-Oncology 2013, 22, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Ho, K.; Lam, K.; Lam, H.; Chui, S.; Chan, G.C.; Cheung, A.; Ho, L.; Chung, O. Adventure-based training to promote physical activity and reduce fatigue among childhood cancer survivors: A randomized controlled trial. Int. J. Nurs. Stud. 2018, 83, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Saultier, P.; Vallet, C.; Sotteau, F.; Hamidou, Z.; Gentet, J.-C.; Barlogis, V.; Curtillet, C.; Verschuur, A.; Revon-Riviere, G.; Galambrun, C.; et al. A Randomized Trial of Physical Activity in Children and Adolescents with Cancer. Cancers 2021, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Stössel, S.; Neu, M.A.; Wingerter, A.; Bloch, W.; Zimmer, P.; Paret, C.; El Malki, K.; Baumann, F.T.; Russo, A.; Henninger, N.; et al. Benefits of Exercise Training for Children and Adolescents Undergoing Cancer Treatment: Results From the Randomized Controlled MUCKI Trial. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef]
- Beulertz, J.; Prokop, A.; Rustler, V.; Bloch, W.; Felsch, M.; Baumann, F.T. Effects of a 6-Month, Group-Based, Therapeutic Exercise Program for Childhood Cancer Outpatients on Motor Performance, Level of Activity, and Quality of Life. Pediatr. Blood Cancer 2015, 63, 127–132. [Google Scholar] [CrossRef]
- Braam, K.I.; van Dijk-Lokkart, E.M.; Kaspers, G.J.L.; Takken, T.; Huisman, J.; Buffart, L.M.; Bierings, M.B.; Merks, J.H.M.; Heuvel-Eibrink, M.M.V.D.; Veening, M.A.; et al. Effects of a combined physical and psychosocial training for children with cancer: A randomized controlled trial. BMC Cancer 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Morales, J.S.; Santana-Sosa, E.; Santos-Lozano, A.; Baño-Rodrigo, A.; Valenzuela, P.L.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Vicent, M.G.; Pérez-Somarriba, M.; Madero, L.; et al. Inhospital exercise benefits in childhood cancer: A prospective cohort study. Scand. J. Med. Sci. Sports 2019, 30, 126–134. [Google Scholar] [CrossRef]
- Su, H.-L.; Wu, L.-M.; Chiou, S.-S.; Lin, P.-C.; Liao, Y.-M. Assessment of the effects of walking as an exercise intervention for children and adolescents with cancer: A feasibility study. Eur. J. Oncol. Nurs. 2018, 37, 29–34. [Google Scholar] [CrossRef]
| Study | Assessment of Bias | Sample Demographics | Type of Cancer | Cancer Treatment-Stage of Cancer | Intervention Characteristics | Main Results |
|---|---|---|---|---|---|---|
| [24] | Critically low | − 4–18 y (and adults) − n = 209 | Any type of hematological cancer | − Pediatric survivors not receiving hematopoietic stem cell transplantation − Pediatric survivors undergoing HSCT | Type: Aerobic or a combination of aerobic and resistance exercise Frequency: NR Intervention duration: Not receiving HSCT: 12–20 weeks; Receiving HSCT: 8 weeks Exercise duration: ranged from 30–120 min Intensity: NR Supervised: home-based, intrahospital | − Strong evidence for a benefit on muscle strength (particularly if training was conducted in the hospital setting) − Body composition: weak evidence − ↑ Cardiorespiratory fitness − Ankle dorsiflexion: weak evidence − Physical functioning: weak evidence − No adverse effects |
| [16] | Low | − 6–18 y − n = 155 | ALL (the most common), solid tumors, AML, and lymphoma | − Maintenance stage of chemotherapy − Just received their first round of chemotherapy − Had completed two courses of treatment (4–8 weeks each) − Survivor stage | Type: home-based aerobic exercise using a video compact disc; use of a bicycle-style exerciser; aerobics and various types of physical activities and strength-building exercises Frequency: ranged from 2 to 3 days/week to twice/day Intervention duration: ranged from 2 days–16 weeks Exercise duration: ranged from 10–45 min Intensity: heart rate of >90% of the maximum heart rate (HR max), or the increase in the percentage of heart rate reserve (% HRR) of 40–60% Supervised: intrahospital, home-based, community | − ↓ Fatigue (11% mean reduction) after 12 weeks of training in 6–14 years for ALL survivors − ↓ General fatigue levels after 8 weeks of training and at a 3-month follow-up in 14–18-year-old patients with cancer − No adverse effects |
| [6] | Low | − 0–18 y − n = 278 | Mixed cancer diagnoses, ALL, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological cancer, solid tumor, hematological disorders | − Intense phase − Intense cancer treatment included hematopoietic stem cell transplantation and all treatment phases except the ‘maintenance phase’ of leukemia therapy | Type: aerobic, strengthening and stretching exercises, games, and yoga Frequency: ranged from 1 day/week to twice/day Intervention duration: ranged from 3 weeks–3 months Exercise duration: ranged from 15–60 min Intensity: NR Supervised: intrahospital supervised, home-based | − ↑ Muscle strength − ↑ Aerobic capacity − ↓ Pain − ↑ Role/social–physical, self-esteem, and mental health − HRQOL: ↑ when assessed by the Oncology Module KINDL scale, but ↓ when assessed by the generic version of the KINDL − ↑ Physical function − General health domain: parent’s responses-significant findings, child data-non-significant findings − No adverse effects |
| [5] | High | − <19 y at diagnosis − n = 171 (males: n = 98, females: n = 70) − Participants in the training program needed to be no more than 5 years from diagnosis | ALL | − During chemotherapy − During the maintenance treatment period − Shortly after diagnosis | Type: strength and inspiratory training Frequency: NR Intervention duration: ranged from 10 weeks–2 years Exercise duration: ranged from 15–60 min Intensity: NR Supervised: at least a home-based exercise program with guidance from a therapist of the treating hospital | − Fatigue, general fatigue: non-significant differences between the control and intervention groups (limited data) − Sleep/rest fatigue, cognitive fatigue: no intervention effect − ↑ back and leg strength combination score − BMI: no statistically significant differences − ↑ BMD for the intervention group compared to the control group − Activity levels: no statistically significant differences (limited data) − HRQOL: some positive effects in favor of the intervention group (assessed by the PedsQL Cancer Module) − ↑ Cardiorespiratory fitness (defined as: VO2 peak, Wmax, or endurance time) was significantly improved by the 9-min run-walk test, timed up and down stairs test, the timed up and go time test, and the 20-m shuttle run test, but not the timed up and down stairs test − Flexibility: ↑ Passive ankle dorsiflexion but no active ankle dorsiflexion and body flexibility − No adverse effects (limited data) |
| [15] | Critically low | − 0–18 y − n = 116 (55 of all participants in exercise interventions) | Cancer | − Active treatment − Post-chemotherapy and following treatment | Type: aerobics, pedaling a stationary bicycle, and physiotherapy practice sessions Frequency: NR Intervention duration: NR Exercise duration: NR Intensity: NR Supervised: intrahospital, home-based | − ↓ Fatigue scores among intervention groups (with no statistically significant differences) |
| [20] | Critically low | − < 18 y at diagnoses − n = 270 (54% females) | ALL, solid tumor survivors, brain tumor survivors, childhood cancer survivors of mixed diagnoses | − Maintenance chemotherapy > 20 years following intensive treatment −Within 5 years of treatment completion − More than 5 years following intensive treatment completion | Type: aerobic based only, combinations of aerobic, resistance, interval, functional, and flexibility training Frequency: ranged from daily to twice/week and from 60–420 min/week Intervention duration: ranged from 2 weeks–1 year Exercise duration: NR Intensity: between 40 and 70% of heart rate reserve and 66–90% of maximum heart rate, at a ‘moderate to vigorous’ intensity Supervised: ≥50% of the intervention was unsupervised, home-based, supervised in the treating clinic, or community-based | − Muscle strength: collectively improved − Activity levels: non-significantly increased self-reported physical activity levels − Psychosocial health indices: collectively improved negative mood, interpersonal problems, self-esteem, ineffectiveness, anhedonia, and fatigue − ↑ Cardiovascular fitness − ↑ Flexibility − Biochemical indicators: did not improve hemoglobin and HbA1c levels − No adverse effects (limited data) |
| [21] | Critically low | − 6–41 y (the age at diagnosis, the time since diagnosis, and the time since the end of treatment ranged from 0–15 y, from 1–22 y, and from 1–21 y) − n = 296 (intervention: n = 189, females: n = 96) (control: n = 107, females: n = 48) | Different types of childhood cancer (the most common being hematological malignancies (leukemia) and brain tumors in childhood cancer), and survivors who had finished anticancer therapy ≥ 1 year before the study | N/R | Type: aerobic or a combination of aerobic and resistance exercise Frequency: ranged from two to five sessions/week Intervention duration: from 8 weeks–6 months Exercise duration: N/R Intensity: 50–60% of one-repetition maximum for resistance exercise and between 40% of heart rate (HR) reserve and > 90% of maximum HR for aerobic exercise Supervised: NR | − Body composition: ↓ central adiposity (waist circumference and waist-to-hip ratio) − ↑ in total body bone mineral content and femoral neck bone mineral density − ↑ Activity levels − Cardiovascular function and structure: improved endothelial function − Brain volume and structure: benefits on white matter fractional anisotropy and hippocampal volume and on cortical thickness and white matter volume (preliminary evidence) − Some adverse effects or problems regarding tolerance or safety (limited data) |
| [22] | Critically low | − 4–18 y − n = 172 | Solid or hematologic (most of them ALL) cancer with no previous organ transplantation, post radiotherapy/chemotherapy sessions, or medical contraindication for exercise (solid tumors, extracranial solid tumors, ALL, AA, ALCL, AML, MPD, hematological malignancy, rhabdomyosarcoma, neuroblastoma, undetermined) | N/R | Type: combination of strength and aerobic training, balance activities, stretching, and games Frequency: ranged from two to five sessions/week Intervention duration: from 3 to 22 weeks Exercise duration: ranged from 10–120 min Intensity: N/R Supervised: during hospitalization | − ↑ Muscle strength and maintained for 20 weeks after the end of the study − HRQOL: non-significant differences − ↑ Physical fitness and maintained for 20 weeks after the end of the study − Functional capacity: ↑ when measured by the TUDS test, but non-significant differences when measured by 6MWT − No adverse effects |
| [17] | Critically low | − 0–18 y − n = 508 (intervention: n = 282, control: n = 226) | ALL | Acute chemotherapy, maintenance chemotherapy, post-treatment survivorship, multiple phases | Type: Aerobic training, general strengthening and/or ankle dorsiflexor strengthening, gastrocsoleus stretching and/or general stretching, bone strengthening, balance training, and motor skill training Frequency: − Maintenance chemotherapy: 2 days/month–7 days/week − Post-treatment survivorship: 2 days/month–7 days/week − Across multiple phases of treatment: 1–2 days/month–7 days/week Intervention duration: − Acute chemotherapy: 3 weeks − Maintenance chemotherapy: 2 weeks–12 months − Post-treatment survivorship: 2 weeks–4 months − Across multiple phases of treatment: 12–135 weeks Exercise duration: − Maintenance chemotherapy: 15–120 min - Post-treatment survivorship: 15–120 min − Across multiple phases of treatment: 45–60 min Intensity: NR Supervised: NR | − Fatigue: ↓ after aerobic training intervention during acute chemotherapy and maintenance chemotherapy − Muscle strength: ↑ during maintenance chemotherapy, post-treatment survivorship and multiple-phase interventions − Activity levels: ↑ during maintenance chemotherapy and multiple-phase interventions − Participation: ↑ during multiple-phase interventions − Flexibility: ↑ range of motion during maintenance chemotherapy and post-treatment survivorship − Functional mobility: ↑ during post-treatment survivorship − Coordination: ↑ during multiple-phase interventions − Bone mineral density: ↑ during maintenance chemotherapy − Some specific adverse events (none of them reported harm, injury, or adverse effects associated with motor interventions) |
| [19] | Critically low | − 3–18 y − n = 91 (intervention: n = 45, control: n = 46) | Cancer, HSCT | HSCT | Type: mixed exercise program with aerobic and strength training Frequency: ranged from 3 to 7 days/week Intervention duration: from 6–8 weeks Exercise duration: ranged from 20–120 min Intensity: mild to moderate Supervised: predominantly or partially supervised | − Muscle strength: positive responses regarding peripheral muscle strength (not clearly demonstrated) − HRQOL: significant ↑ in children’s comfort and resilience − Functional capacity: significant ↑ in TUDS test but no difference in 6MWT − No adverse effects |
| [9] | Critically low | − 5–38 y (the age at diagnosis ranged from 0–15 y, the time since diagnosis from 1–22 y, and the time since the end of treatment of those with CCS who had already finished treatment from 0–21 y) − n = 697 (CCS: n = 669, healthy: n = 28) | Childhood cancer survivors, different types of childhood cancer (the most common being ALL) | During or after treatment | Type: aerobic or a combination of aerobic and resistance exercise Frequency: ranged from 1 to 6 days/week Intervention duration: from 3 weeks–2.5 years Exercise duration: NR Intensity: ranged from 50–60% of 1 repetition maximum for resistance exercise and between 50 and >90% heart | − Cardiorespiratory fitness: non-significant trend towards an improvement in peak oxygen uptake (VO2peak) − Cardiovascular function and structure: preserved left ventricular ejection fraction from decline − ↑ Physical capacity or attenuating the decline − Some adverse effects: a patella dislocation, a fall during an exercise session, headache, muscle soreness, fatigue, and hyperventilation during the exercise interventions |
| [18] | Critically low | − 1–23 (1–10 years from the conclusion Of the treatment, or 1–5 years from the conclusion of the treatment, or during treatment in two studies that include ages between 4–18) − n = 306 | Pediatric brain tumors (hemispheric or posterior fossa brain tumors in most studies) | Patients or survivors: children who had undergone either cranial or craniospinal radiation therapy (a number of them had also undergone surgical operations with or without chemotherapy) | Type: aerobic exercise, combinations of aerobic and strengthening exercises, yoga Frequency: ranged from 2 to 5 days/week Intervention duration: from 12–24 weeks Exercise duration: NR Intensity: NR Supervised: − intrahospital: under the supervision of a physiotherapist or a kinesiologist − home-based: under the supervision of their parents | − Aerobic capacity: submaximal aerobic capacity enhancement and endurance improvement − ↑ Physical activity levels − Psychosocial health indices: ↓ depression level showed a positive correlation with the increased thickness of the cortex (assessed by the CDI-2 questionnaire) − ↑ HRQOL: after 4 weeks of inpatient rehabilitation and after 12 weeks of yoga intervention (but the result did not last for a year) (assessed by the KINDL health related quality of life and the Peds 4.0 General Module) − Cardiovascular fitness: the distance covered in 6 min increased after a 12-week training period (assessed by 6 min walk test) − ↑ Hamstring flexibility after a 12-week yoga intervention in pediatric cancer out-patients − Physical fitness: after a 12-week yoga program, the participants performed the TUG-3m test significantly faster than the pre-test − Coordination: after a 12-week exercise training, the bilateral coordination increased while balance remained unchanged (improved performance maintained even 12 weeks after the training had ended) (measured by the Bruininks-Oseretsky Test2) − Brain volume and structure: ▪ Increased right somatosensory cortical thickness ▪ Increased fractional anisotropy (FA) in the corpus callosum, in the right corticospinal pathway, and in the cingulum − Cognitive function: improvement of reaction time after 12 weeks of training that was maintained for 12 weeks after training had ended (measured by the Cambridge Neuropsychological Test Automated Battery) − Energy consumption: no noticeable changes with active video gaming [assessed by the Metabolic Equivalent Task (MET)] |
| [23] | High | − 4–18 y − n = 642 (intervention: n = 322, control: n = 320) | Childhood cancer survivors with mixed types of cancer | During or after treatment | Type: aerobic, anaerobic, resistance, or combined physical exercise training Frequency: mean 2.25 sessions/week Intervention duration: mean 16.6 weeks Exercise duration: mean 152.36 min Intensity: low, Medium, high Supervised: supervised by health professionals, medical staff, or coaches | − ↓ Fatigue − ↑ Muscle strength − ↑ BMI, but non-significant effects on body composition − ↑ Level of daily physical activity − ↑ self-efficacy − HRQOL: no significant effect − ↑ cardiopulmonary fitness − ↑ Flexibility and balance (limited data) − Physical function: no intervention effect in pediatric patients with solid tumors (limited data) |
| Outcome (Number of Studies) | Effect | Study | Type of Cancer |
|---|---|---|---|
| Fatigue (5) | ↓ | [15,16,17,23] | ALL (the most common), solid tumors, AML, lymphoma, CCS with mixed types of cancer |
| ↔ | [5] | ALL | |
| Physical function: | |||
| Muscle strength (8) | ↑ | [5,6,17,19,20,22,23,24] | Hematological cancer (ALL, AA, ALCL, AML, MPD), osteosarcoma or Ewing’s sarcoma of the lower limb, solid tumor, extracranial solid tumor, rhabdomyosarcoma, neuroblastoma, undetermined hematological disorders, hematopoietic stem cell transplantation, CCS with mixed types of cancer |
| Flexibility (5) | ↑ | [18,20,23] | ALL, brain tumors, CCS with mixed types of cancer |
| ↔ | [5] | ALL | |
| Weak evidence | [24] | Hematological cancer | |
| Range of motion (1) | ↑ | [16] | ALL (undergoing maintenance chemotherapy and during post-treatment survivorship) |
| Coordination (2) | ↑ | [17,18] | ALL, pediatric brain tumors |
| Physical fitness (2) | ↑ | [18,22] | Solid tumors, extracranial solid tumor, hematological cancer (ALL, AA, ALCL, AML, MPD), rhabdomyosarcoma, neuroblastoma, brain tumors |
| Motor performance (4) | ↑ | [6,20] | ALL, solid tumors, solid tumor survivors, brain tumor survivors, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological cancer, hematological disorders, CCS with mixed types of cancer |
| ↔ | [23] | CCS with mixed types of cancer | |
| Weak evidence | [24] | Hematological cancer | |
| Functional capacity (3) | ↑ | [17,19,22] | ALL, solid tumors, extracranial solid tumor, hematological cancer (AA, ALCL, AML, MPD), rhabdomyosarcoma, neuroblastoma, hematopoietic stem cell transplantation |
| Physical capacity (1) | ↑ | [8] | Different types of childhood cancer, CCS with mixed types of cancer |
| CRF: | |||
| Aerobic capacity (2) | ↑ | [6,18] | ALL, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological cancer, solid tumor, hematological disorders, brain tumors |
| Cardiorespiratory fitness (4) | ↑ | [5,23,24] | Hematological cancer (ALL and others), CCS with mixed types of cancer |
| ↔ | [8] | Different types of childhood cancer, CCS with mixed types of cancer | |
| Cardiovascular fitness (2) | ↑ | [18,20] | ALL, brain tumors, CCS with mixed types of cancer (solid tumor survivors, brain tumor survivors, and others) |
| Cardiovascular function and structure (2) | ↑ | [8,22] | Different types of childhood cancer, hematological malignancies, brain tumors, CCS with mixed types of cancer |
| Pain (1) | ↓ | [6] | ALL, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological cancer, solid tumor, hematological disorders |
| Body Weight/composition: | |||
| Body Composition (3) | ↔ | [22,23] | Hematological cancer, brain tumors, CCS with mixed types of cancer |
| Weak evidence | [24] | Hematological cancer | |
| BMI (2) | ↔ | [5] | ALL |
| ↑ | [23] | CCS with mixed types of cancer | |
| Activity/participation levels (6) | ↑ | [16,18,22,23] | ALL, hematological cancer, brain tumors, CCS with mixed types of cancer |
| ↔ | [5,20] | ALL, solid tumor survivors, brain tumor survivors, CCS with mixed types of cancer | |
| Energy consumption (1) | ↔ | [18] | Brain tumors |
| Psychosocial health indices (4) | ↑ | [6,18,20,23] | ALL, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological cancer, solid tumor, brain tumors, hematological disorders, CCS with mixed types of cancer (solid tumor survivors, brain tumor survivors and others) |
| HRQL (6) | ↑ | [5,6,18,19] | Hematological cancer (ALL and others), osteosarcoma or Ewing’s sarcoma of the lower limb, solid tumor, brain tumors, hematological disorders, hematopoietic stem cell transplantation |
| ↔ | [22,23] | Solid tumors, extracranial solid tumor, hematological cancer (ALL, AA, ALCL, AML, MPD), rhabdomyosarcoma, neuroblastoma, CCS with mixed types of cancer | |
| Biochemical indicators (1) | ↔ | [20] | ALL, CCS with mixed types of cancer (solid tumor survivors, brain tumor survivors) |
| Bone mineral density (3) | ↑ | [5,19,22] | ALL, hematological cancer, brain tumors |
| Brain Volume/structure (2) | ↑ | [18,22] | Hematological cancer, brain tumors |
| General health domain (1) | ↔ | [6] | ALL, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological cancer, solid tumor, hematological disorders |
| Cognitive function (1) | ↑ | [18] | Brain tumors |
| Adverse effects (11) | No adverse effects | [5,6,16,19,20,22,23,24] | Hematological cancer (ALL, AML, AA, ALCL, MPD, lymphoma), solid tumors, osteosarcoma or Ewing’s sarcoma of the lower limb, hematological disorders, extracranial solid tumor, rhabdomyosarcoma, neuroblastoma, hematopoietic stem cell transplantation, CCS with mixed types of cancer (solid tumor survivors, brain tumor survivors) |
| Some adverse effects | [8,17,22] | Hematological cancer (ALL and others), brain tumors, CCS with mixed types of cancer | |
| Not reported | [15,18] | Brain tumors, cancer in general |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapti, C.; Dinas, P.C.; Chryssanthopoulos, C.; Mila, A.; Philippou, A. Effects of Exercise and Physical Activity Levels on Childhood Cancer: An Umbrella Review. Healthcare 2023, 11, 820. https://doi.org/10.3390/healthcare11060820
Rapti C, Dinas PC, Chryssanthopoulos C, Mila A, Philippou A. Effects of Exercise and Physical Activity Levels on Childhood Cancer: An Umbrella Review. Healthcare. 2023; 11(6):820. https://doi.org/10.3390/healthcare11060820
Chicago/Turabian StyleRapti, Christina, Petros C. Dinas, Costas Chryssanthopoulos, Alexandra Mila, and Anastassios Philippou. 2023. "Effects of Exercise and Physical Activity Levels on Childhood Cancer: An Umbrella Review" Healthcare 11, no. 6: 820. https://doi.org/10.3390/healthcare11060820
APA StyleRapti, C., Dinas, P. C., Chryssanthopoulos, C., Mila, A., & Philippou, A. (2023). Effects of Exercise and Physical Activity Levels on Childhood Cancer: An Umbrella Review. Healthcare, 11(6), 820. https://doi.org/10.3390/healthcare11060820








