Novel Soft Dosage Forms for Paediatric Applications: Can We 3D-Print Them or Not?
Abstract
:1. Introduction
2. Methods of 3D Printing
2.1. Fused Deposition Modelling (FDM)
2.2. Stereolithography (SLA)
2.3. Embedded 3D Printing (e-3DP)
2.4. Inkjet 3DP (IJP)
2.5. Semisolid Extrusion (EXT or SSE)
2.6. Selective Laser Sintering (SLS)
2.7. Digital Light Processing (DLP)
3. 3D-Printed Hydrogels
4. 3D-Printed Buccal & Mucoadhesive Films
4.1. Buccal Films
4.2. Orodispersible Film (ODF)
4.3. Oral Films and Fast Dissolving Oral Films (FDFs)
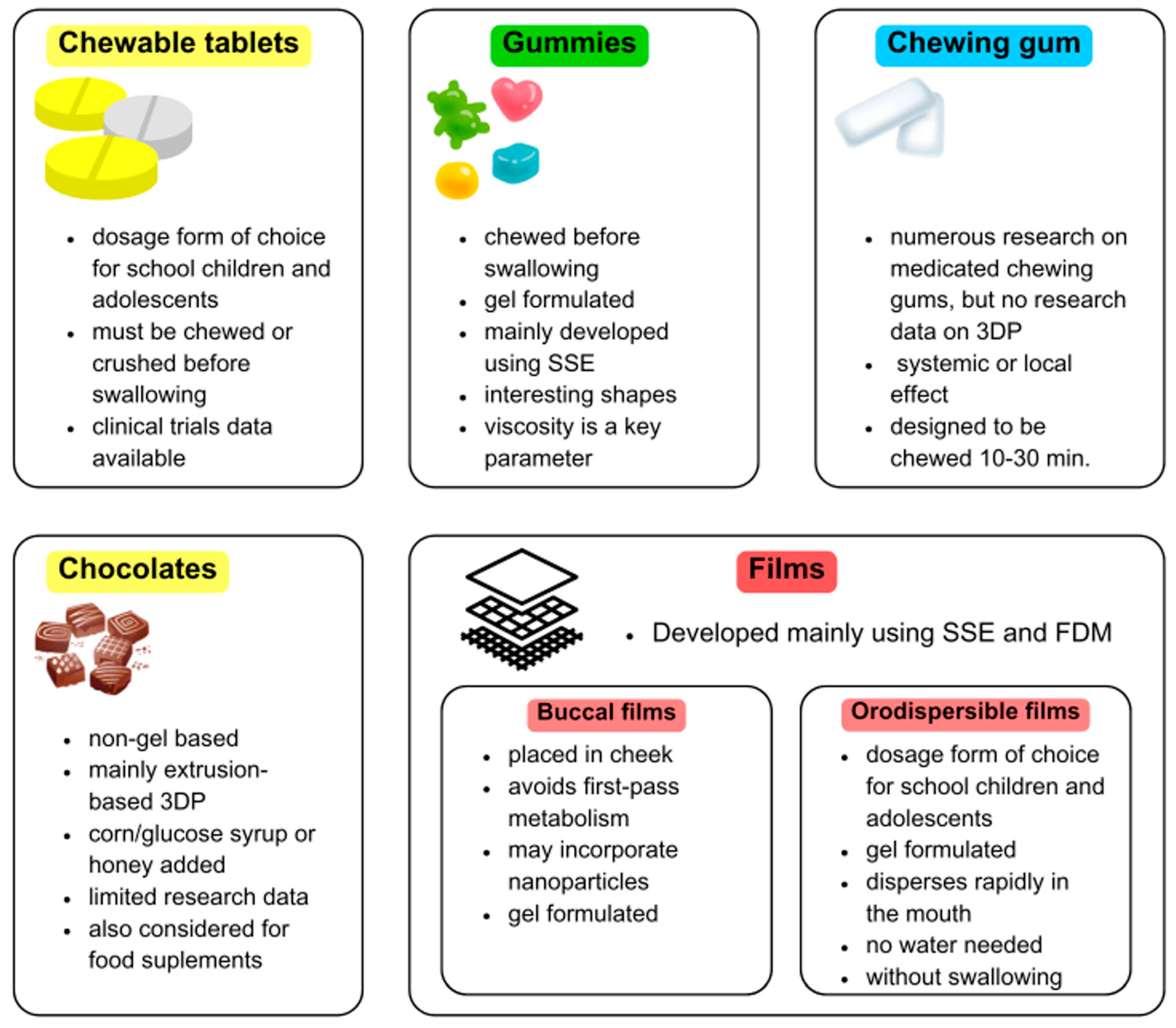
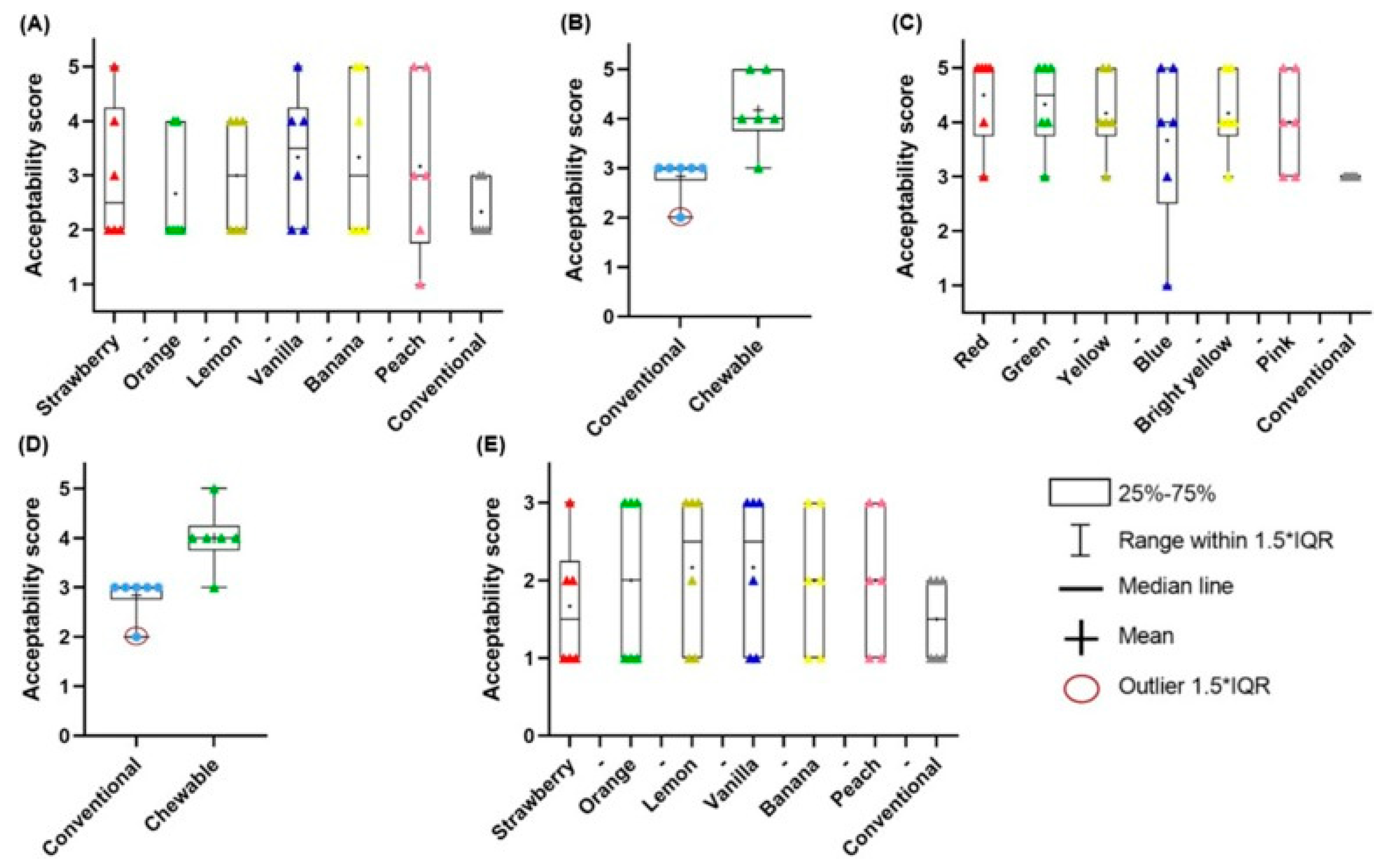
5. 3D-Printed Gummies & Chewable Tablets
5.1. Gummies
5.2. Chewable Tablets
6. 3D-Printed Chocolates & Novel Delivery Forms
6.1. Chewing Gums
6.2. Chocolate
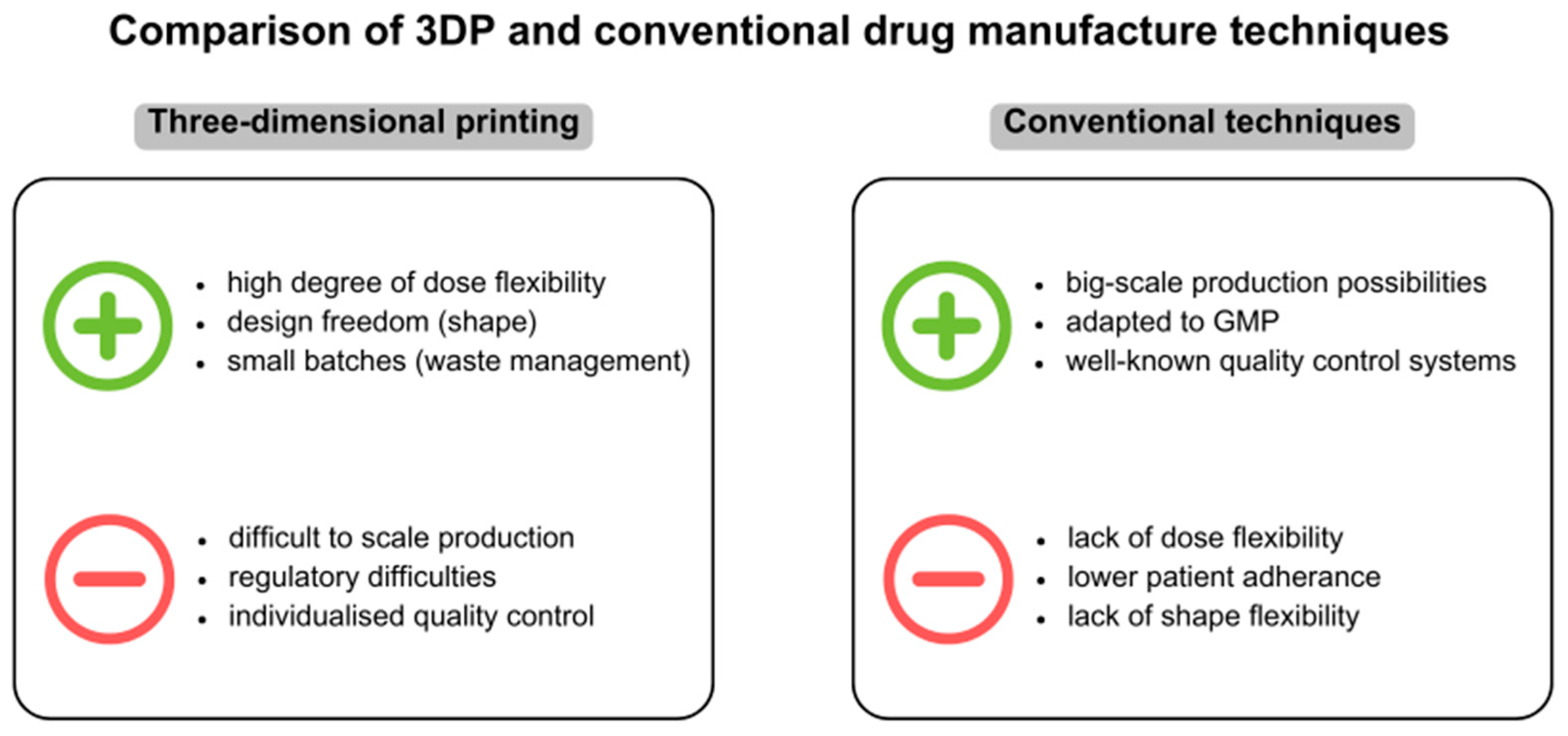
7. Challenges and Benefits of 3DP in Paediatric Drug Development
7.1. The Role of 3D Printing in Addressing Challenges of Paediatric Drug Formulations
7.2. Cost Effectiveness and Scalability
7.3. Spatial Arrangement
7.4. Toxicity Considerations of Excipients—Processing Issue
7.5. Waste Management
7.6. Standardization
7.7. Ensuring Safety and Quality
8. Future Perspectives and Conclusions
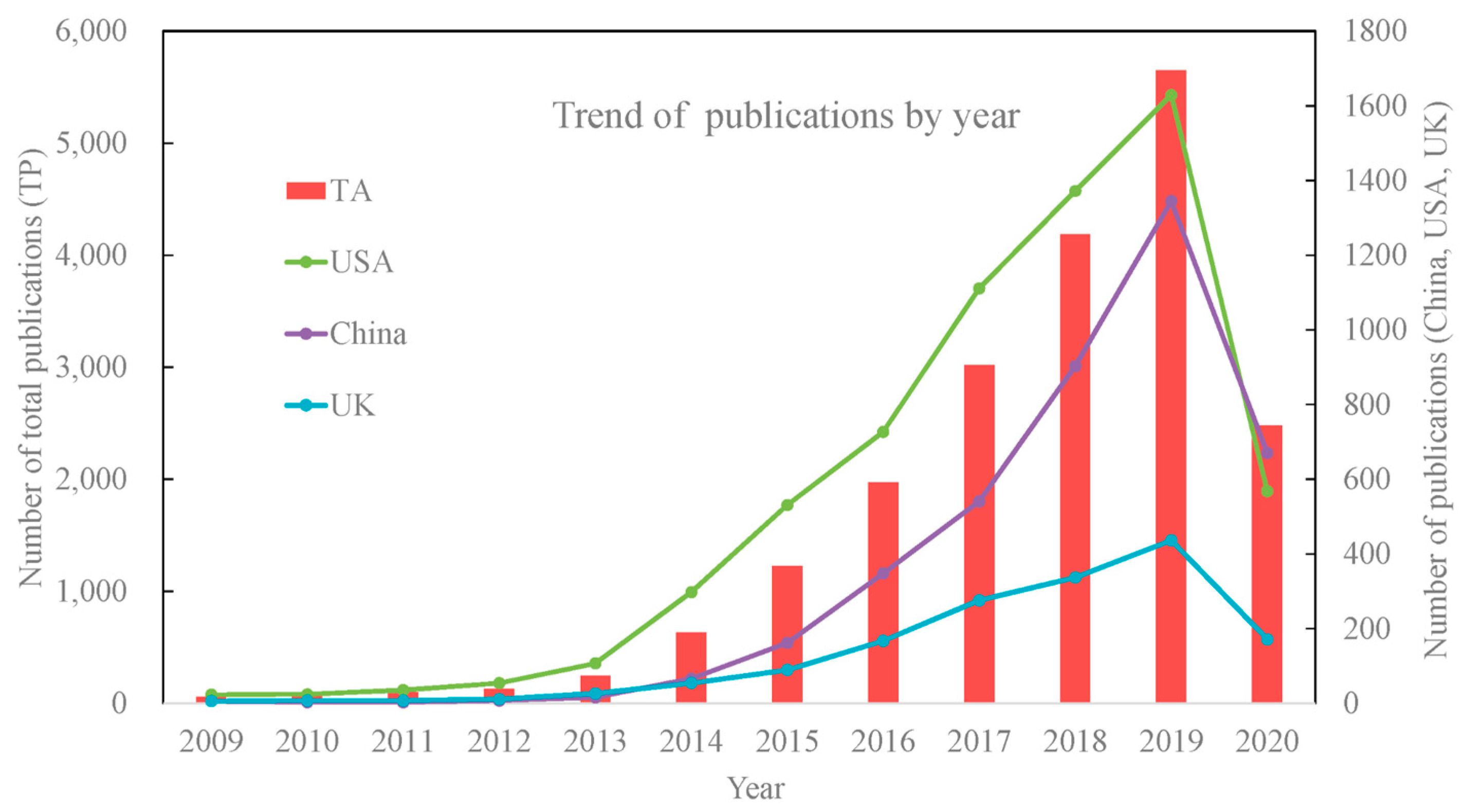
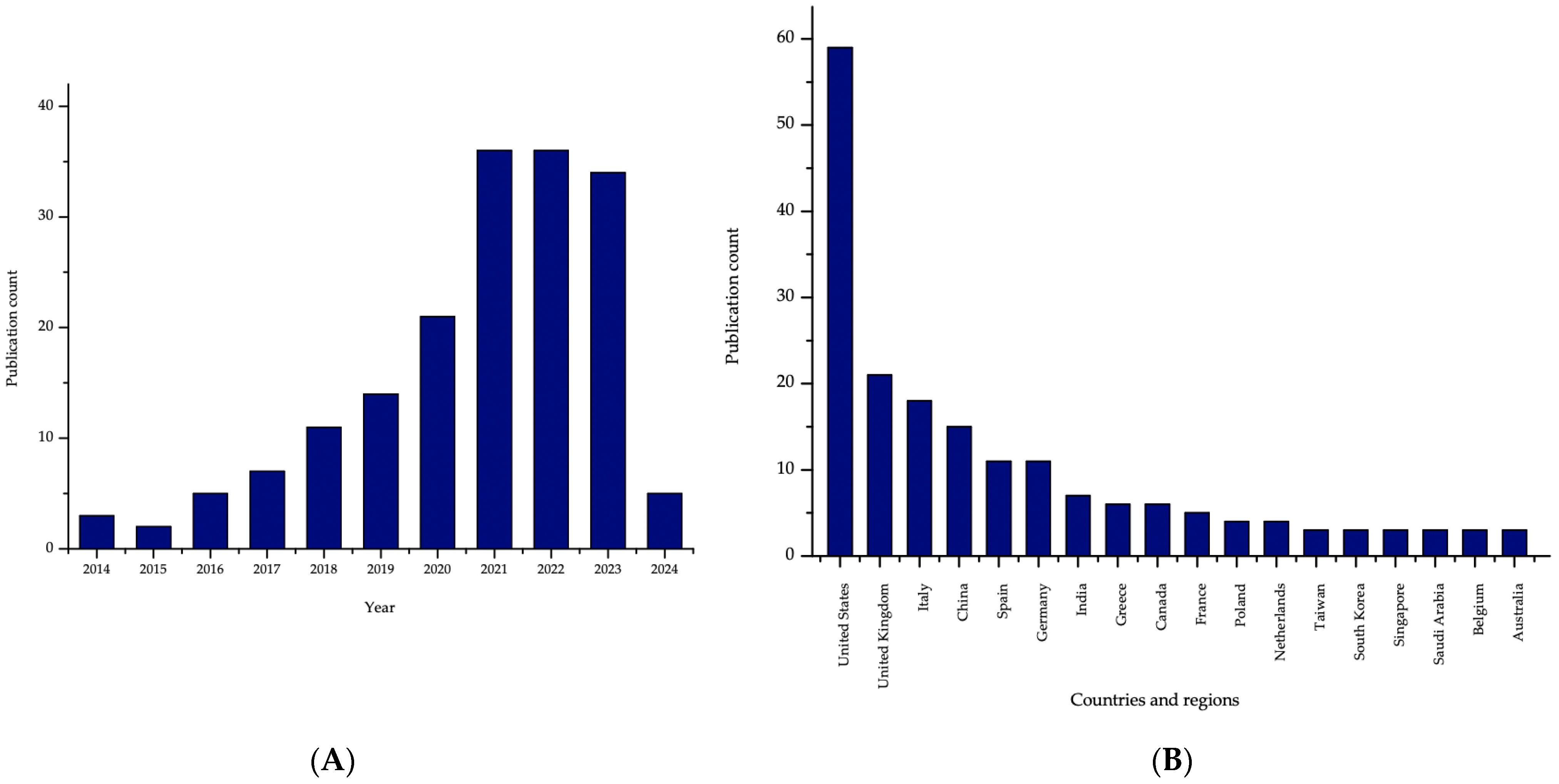
8.1. Research Trends
8.2. 3DP & Patents
8.3. Clinical Trials and Approved Drugs
8.4. 4D Bioprinting
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3DP | Three-dimensional printing |
| AA | Ascorbic acid |
| AM | Additive manufacturing |
| API | Active pharmaceutical ingredient |
| CAD | Computer-aided design |
| CAF | Caffeine |
| CBS | Clobetasol propionate |
| CS | Chitosan |
| CMC-Na | Carboxymethyl cellulose sodium |
| DDS | Drug delivery system |
| DLP | Digital light processing |
| DNa | Diclofenac sodium |
| DOD | Droplets-on-demand |
| DOP | Drop-on-powder |
| e-3DP | Embedded 3D printing |
| EC | Ethyl cellulose |
| EE | Eucalypt extract |
| FDA | U.S. Food and Drug Administration |
| FDM | Fused deposition modeling |
| HP-β-CD | Hydroxypropyl-β-cyclodextrin |
| HPMC | Hydroxypropyl methylcellulose |
| ICH | International Congress on Harmonization |
| IJP | Inkjet 3DP |
| JH | Jerusalem sage honey |
| LH | Lavandula angustifolia honey |
| MSUD | Maple syrup urine disease |
| ODF | Orodispersible films |
| OLP | Oral lichen planus |
| PEG | Polyethylene glycol |
| PEO | Polyethylene oxide |
| PIB | Polyisobutylene |
| PLA | Polylactic acid |
| PVAc | Polyvinyl acetate |
| PVAl | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| R&D | Research and development |
| SA | Sodium alginate |
| SC | Sodium croscarmellose |
| SLA | Stereolithography |
| SLS | Selective laser sintering |
| SSE | Semisolid extrusion |
| TEC | Triethyl citrate |
| VitD3 | Vitamin D3 |
| VH | Vital honey |
| Xyl | Xylitol |
| SSG | Sodium starch glycolate |
| SBR | Styrene-butadiene |
References
- Integrated Management of Childhood Illness (WHO). Available online: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/child-health/integrated-management-of-childhood-illness (accessed on 10 January 2025).
- Tan, D.C.T.; Khong, Y.M.; Mount, S.; Galella, E.; Mitra, B.; Charlton, S.; Kuhli, M.; Ternik, R.; Walsh, J.; Rajapakshe, A.; et al. Pediatric Formulation Development–Challenges of Today and Strategies for Tomorrow: Summary Report from M−CERSI Workshop 2019. Eur. J. Pharm. Biopharm. 2021, 164, 54–65. [Google Scholar] [CrossRef]
- Litalien, C.; Bérubé, S.; Tuleu, C.; Gilpin, A.; Landry, É.K.; Valentin, M.; Strickley, R.; Turner, M.A. From Paediatric Formulations Development to Access: Advances Made and Remaining Challenges. Brit J. Clin. Pharma 2022, 88, 4349–4383. [Google Scholar] [CrossRef]
- Rocchi, F.; Tomasi, P. The Development of Medicines for Children. Pharmacol. Res. 2011, 64, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Webb, E.A.; Kerr, S.; Davies, J.H.; Stirling, H.; Batchelor, H. How Close Is the Dose? Manipulation of 10 Mg Hydrocortisone Tablets to Provide Appropriate Doses to Children. Int. J. Pharm. 2018, 545, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ernest, T.B.; Craig, J.; Nunn, A.; Salunke, S.; Tuleu, C.; Breitkreutz, J.; Alex, R.; Hempenstall, J. Preparation of Medicines for Children–A Hierarchy of Classification. Int. J. Pharm. 2012, 435, 124–130. [Google Scholar] [CrossRef]
- Simšič, T.; Planinšek, O.; Baumgartner, A. Taste-Masking Methods in Multiparticulate Dosage Forms with a Focus on Poorly Soluble Drugs. Acta Pharm. 2024, 74, 177–199. [Google Scholar] [CrossRef] [PubMed]
- on behalf of European Paediatric Formulation Initiative (EuPFI); Salunke, S.; Liu, F.; Batchelor, H.; Walsh, J.; Turner, R.; Ju, T.R.; Tuleu, C. European Paediatric Formulation Initiative (EuPFI)—Formulating Ideas for Better Medicines for Children. AAPS PharmSciTech 2017, 18, 257–262. [Google Scholar] [CrossRef]
- How Many Children Are There in the World? (UNICEF). Available online: https://data.unicef.org/how-many/how-many-children-under-18-are-in-the-world/ (accessed on 10 October 2024).
- The State of the World’s Children 2024 Statistical Compendium (UNICEF). Available online: https://data.unicef.org/resources/sowc-2024/ (accessed on 10 January 2025).
- ICH Harmonised Guideline. Addendum to Ich E11: Clinical Investigation of Medicinal Products in the Pediatric Population, E11 (R1). Available online: https://database.ich.org/sites/default/files/E11_R1_Addendum.pdf (accessed on 7 March 2025).
- Food and Drug Administration Safety and Innovation Act (FDASIA). Available online: https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/food-and-drug-administration-safety-and-innovation-act-fdasia (accessed on 10 January 2025).
- Paediatric Regulation (EMA). Available online: https://www.ema.europa.eu/en/human-regulatory-overview/paediatric-medicines-overview/paediatric-regulation (accessed on 10 January 2025).
- UN News. Available online: https://news.un.org/en/story/2007/12/242332 (accessed on 10 January 2025).
- Barbieri, E.; Minotti, C.; Cavagnis, S.; Giaquinto, C.; Cappello, B.; Penazzato, M.; Lallemant, M. Paediatric Medicine Issues and Gaps from Healthcare Workers Point of View: Survey Results and a Narrative Review from the Global Accelerator for Paediatric Formulations Project. Front. Pharmacol. 2023, 14, 1200848. [Google Scholar] [CrossRef]
- Stegemann, S.; Klingmann, V.; Reidemeister, S.; Breitkreutz, J. Patient-Centric Drug Product Development: Acceptability across Patient Populations–Science and Evidence. Eur. J. Pharm. Biopharm. 2023, 188, 1–5. [Google Scholar] [CrossRef]
- Aa, D.; Azbs, B. Advantages of Jelly over Liquid Formulations for Pediatrics. J. Formul. Sci. Bioavailab. 2018, 1, 1000102. [Google Scholar] [CrossRef]
- Moreira, M.; Sarraguça, M. How Can Oral Paediatric Formulations Be Improved? A Challenge for the XXI Century. Int. J. Pharm. 2020, 590, 119905. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Fuh, J.; Lu, W. 3D Printing and 3D Bioprinting in Pediatrics. Bioengineering 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. A Graphical Review on the Escalation of Fused Deposition Modeling (FDM) 3D Printing in the Pharmaceutical Field. J. Pharm. Sci. 2020, 109, 2943–2957. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D Printing: An Appealing Route for Customized Drug Delivery Systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Milliken, R.L.; Quinten, T.; Andersen, S.K.; Lamprou, D.A. Application of 3D Printing in Early Phase Development of Pharmaceutical Solid Dosage Forms. Int. J. Pharm. 2024, 653, 123902. [Google Scholar] [CrossRef]
- Cunha-Filho, M.; Araújo, M.R.; Gelfuso, G.M.; Gratieri, T. FDM 3D Printing of Modified Drug-Delivery Systems Using Hot Melt Extrusion: A New Approach for Individualized Therapy. Ther. Deliv. 2017, 8, 957–966. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of Controlled-Release Budesonide Tablets via Desktop (FDM) 3D Printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef]
- Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Investigation of the Degradation and In-Situ Amorphization of the Enantiomeric Drug Escitalopram Oxalate during Fused Deposition Modeling (FDM) 3D Printing. Eur. J. Pharm. Sci. 2023, 185, 106423. [Google Scholar] [CrossRef]
- Chung, S.; Zhang, P.; Repka, M.A. Fabrication of Timed-Release Indomethacin Core–Shell Tablets for Chronotherapeutic Drug Delivery Using Dual Nozzle Fused Deposition Modeling (FDM) 3D Printing. Eur. J. Pharm. Biopharm. 2023, 188, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef]
- Shim, K.W. Medical Applications of 3D Printing and Standardization Issues. Brain Tumor Res. Treat. 2023, 11, 159. [Google Scholar] [CrossRef]
- Weber, S.; Block, A.; Bärenfänger, F. Assessment of Attenuation Properties for SLA and SLS 3D-Printing Materials in X-Ray Imaging and Nuclear Medicine. Z. Med. Phys. 2024, in press. [Google Scholar] [CrossRef]
- Rahman, Z.; Barakh Ali, S.F.; Ozkan, T.; Charoo, N.A.; Reddy, I.K.; Khan, M.A. Additive Manufacturing with 3D Printing: Progress from Bench to Bedside. AAPS J. 2018, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D Printing of a Bladder Device for Intravesical Drug Delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D Printing of Ascorbic Acid Loaded Hydrogels: A Controlled Release Study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D Printing of Oral Modified-Release Dosage Forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D Printing of Strain Sensors within Highly Stretchable Elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Mitchell, K.; Raymond, L.; Godina, B.; Zhao, D.; Zhou, W.; Jin, Y. Fluid Bath-Assisted 3D Printing for Biomedical Applications: From Pre- to Postprinting Stages. ACS Biomater. Sci. Eng. 2021, 7, 4736–4756. [Google Scholar] [CrossRef]
- Zhao, J.; He, N. A Mini-Review of Embedded 3D Printing: Supporting Media and Strategies. J. Mater. Chem. B 2020, 8, 10474–10486. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, C.R.M.; Okafor-Muo, O.L.; Hassanin, H.; ElShaer, A. 3DP Printing of Oral Solid Formulations: A Systematic Review. Pharmaceutics 2021, 13, 358. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Akkineni, A.R.; Förster, Y.; Köhler, T.; Knaack, S.; Gelinsky, M.; Lode, A. Design and Fabrication of Complex Scaffolds for Bone Defect Healing: Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann. Biomed. Eng. 2017, 45, 224–236. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef]
- Castrejon-Pita, J.R.; Baxter, W.R.S.; Morgan, J.; Temple, S.; Martin, G.D.; Hutchings, I.M. Future, Opportunities and Challenges of Inkjet Technologies. At. Spr. 2013, 23, 541–565. [Google Scholar] [CrossRef]
- Shipp, L.; Liu, F.; Kerai-Varsani, L.; Okwuosa, T.C. Buccal Films: A Review of Therapeutic Opportunities, Formulations & Relevant Evaluation Approaches. J. Control. Release 2022, 352, 1071–1092. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Rodríguez-Pombo, L.; Ong, J.J.; Basit, A.W.; Santoveña-Estévez, A.; Fariña, J.B.; Alvarez-Lorenzo, C.; Goyanes, A. Integrating Pressure Sensor Control into Semi-Solid Extrusion 3D Printing to Optimize Medicine Manufacturing. Int. J. Pharm. X 2022, 4, 100133. [Google Scholar] [CrossRef]
- Roche, A.; Sanchez-Ballester, N.M.; Aubert, A.; Rossi, J.-C.; Begu, S.; Soulairol, I. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS PharmSciTech 2023, 24, 122. [Google Scholar] [CrossRef]
- Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. [Google Scholar] [CrossRef]
- Firth, J.; Basit, A.W.; Gaisford, S. The Role of Semi-Solid Extrusion Printing in Clinical Practice. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 133–151. ISBN 978-3-319-90754-3. [Google Scholar]
- Fina, F.; Gaisford, S.; Basit, A.W. Powder Bed Fusion: The Working Process, Current Applications and Opportunities. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 81–105. ISBN 978-3-319-90754-3. [Google Scholar]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing: Principles and Pharmaceutical Applications of Selective Laser Sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef] [PubMed]
- Balasankar, A.; Anbazhakan, K.; Arul, V.; Mutharaian, V.N.; Sriram, G.; Aruchamy, K.; Oh, T.H.; Ramasundaram, S. Recent Advances in the Production of Pharmaceuticals Using Selective Laser Sintering. Biomimetics 2023, 8, 330. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-Dimensional Printing for Medical Applications. Int. J. Bioprinting 2019, 6, 242. [Google Scholar] [CrossRef]
- Liu, X.; Tao, J.; Liu, J.; Xu, X.; Zhang, J.; Huang, Y.; Chen, Y.; Zhang, J.; Deng, D.Y.B.; Gou, M.; et al. 3D Printing Enabled Customization of Functional Microgels. ACS Appl. Mater. Interfaces 2019, 11, 12209–12215. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography Apparatus and Digital Light Processing-Based 3D Bioprinting for Tissue Fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital Light Processing (DLP) 3D-Printing Technology and Photoreactive Polymers in Fabrication of Modified-Release Tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Cao, L.; Lu, Y.; Chen, H.; Su, Y.; Cheng, Y.; Xu, J.; Sun, H.; Song, K. A 3D Bioprinted Antibacterial Hydrogel Dressing of Gelatin/Sodium Alginate Loaded with Ciprofloxacin Hydrochloride. Biotechnol. J. 2024, 19, 2400209. [Google Scholar] [CrossRef]
- Liu, H.; Deng, X.; Zhao, X.; Cao, P.; Li, Y. Preparation and Performance Study of Sodium Alginate/Bamboo Fiber/Gelatin Ionic Conductive Self-Healing Hydrogel. Int. J. Biol. Macromol. 2024, 278, 134549. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Al-Mahmood, S.M.A.; Chatterjee, B.; Hadi, H.A.; Doolaanea, A.A. Carbamazepine Gel Formulation as a Sustained Release Epilepsy Medication for Pediatric Use. Pharmaceutics 2019, 11, 488. [Google Scholar] [CrossRef]
- Kammona, O.; Tsanaktsidou, E.; Kiparissides, C. Recent Developments in 3D-(Bio)Printed Hydrogels as Wound Dressings. Gels 2024, 10, 147. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The Formation Mechanism of Hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2022, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, G.; Xiang, T.; Situ, W. Effect of Crosslinking Processing on the Chemical Structure and Biocompatibility of a Chitosan-Based Hydrogel. Food Chem. 2021, 354, 129476. [Google Scholar] [CrossRef]
- Ou, Y.-H.; Ou, Y.-H.; Gu, J.; Kang, L. Personalized Anesthetic Patches for Dental Applications. Int. J. Bioprint 2019, 5, 203. [Google Scholar] [CrossRef]
- Moreira, M.F.; Kanaan, A.F.; Piedade, A.P. Indirect Additive Manufacturing: A Valid Approach to Modulate Sorption/Release Profile of Molecules from Chitosan Hydrogels. Polymers 2022, 14, 2530. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Pearce, H.A.; Hogan, K.J.; Smoak, M.M.; Guo, J.L.; Melchiorri, A.J.; Scott, D.W.; Mikos, A.G. Swelling Behaviors of 3D Printed Hydrogel and Hydrogel-Microcarrier Composite Scaffolds. Tissue Eng. Part. A 2021, 27, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chu, C.-C. Visible Light Induced Dextran-Methacrylate Hydrogel Formation Using (−)-Riboflavin Vitamin B2 as a Photoinitiator and L-Arginine as a Co-Initiator. Fibers Polym. 2009, 10, 14–20. [Google Scholar] [CrossRef]
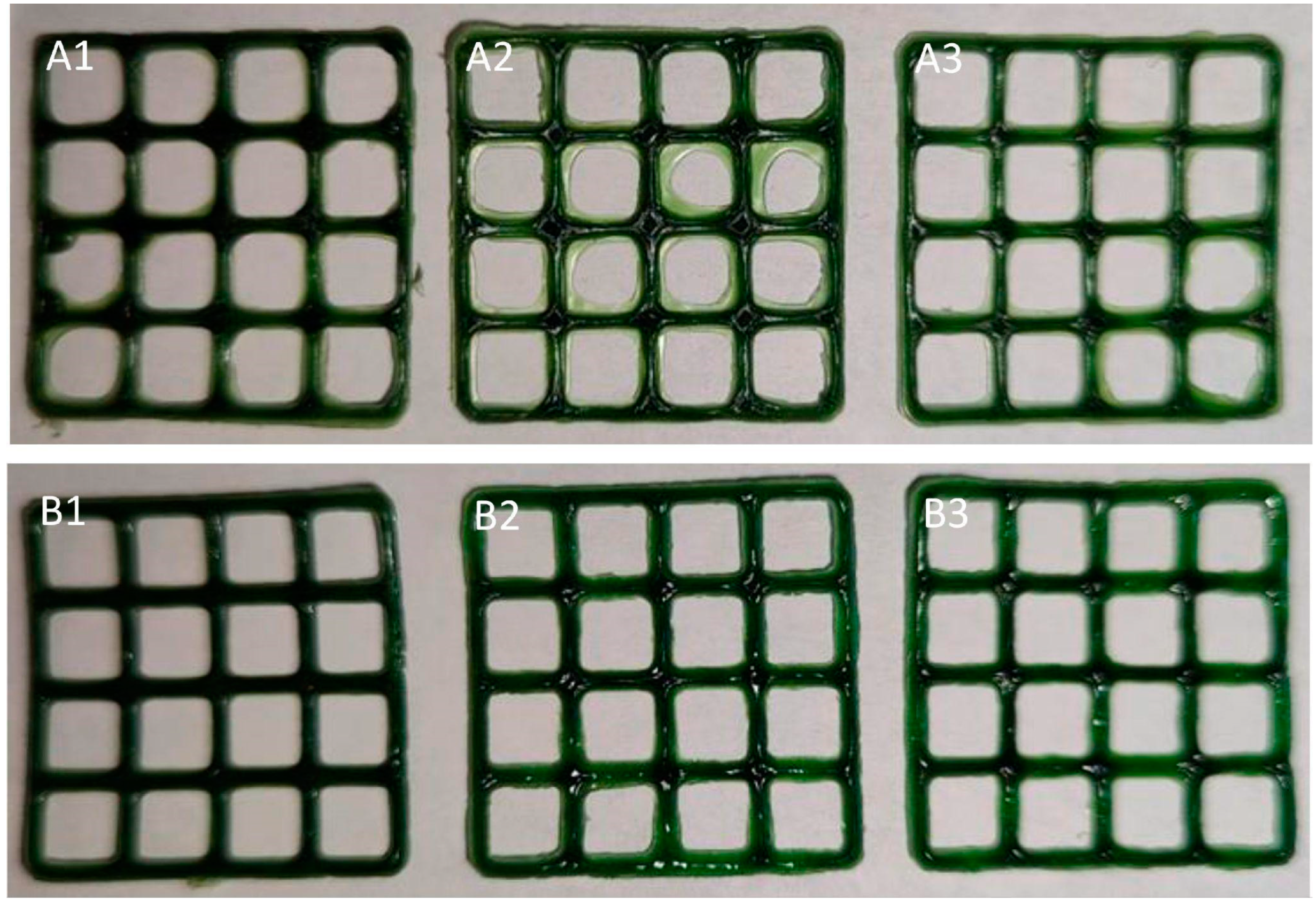
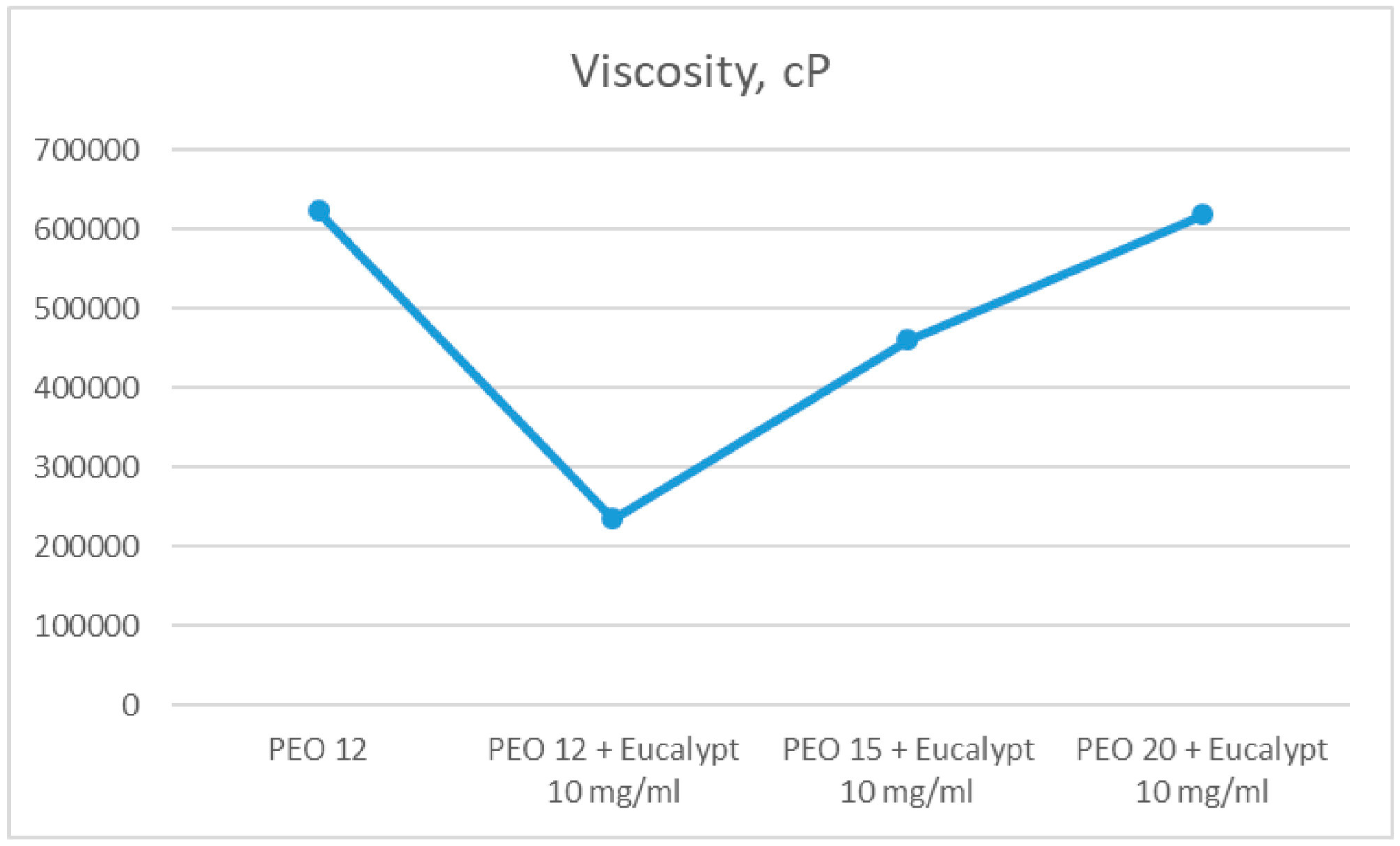
- Koshovyi, O.; Heinämäki, J.; Laidmäe, I.; Topelius, N.S.; Grytsyk, A.; Raal, A. Semi-Solid Extrusion 3D-Printing of Eucalypt Extract-Loaded Polyethylene Oxide Gels Intended for Pharmaceutical Applications. Ann. 3D Print. Med. 2023, 12, 100123. [Google Scholar] [CrossRef]
- Kovalenko, V.N. Compendium 2020—Medicines; Morion: Kyiv, Ukraine, 2020. [Google Scholar]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-Printing of Nanoemulsified Eucalypt Extracts and Their Antimicrobial Activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef]
- Vetchý, D.; Landová, H.; Gajdziok, J.; Doležel, P.; Daněk, Z.; Štembírek, J. Determination of Dependencies among in Vitro and in Vivo Properties of Prepared Mucoadhesive Buccal Films Using Multivariate Data Analysis. Eur. J. Pharm. Biopharm. 2014, 86, 498–506. [Google Scholar] [CrossRef]
- Auel, T.; Mentrup, A.F.C.; Oldfield, L.R.; Seidlitz, A. 3D Printing of Pharmaceutical Dosage Forms: Recent Advances and Applications. Adv. Drug Deliv. Rev. 2025, 217, 115504. [Google Scholar] [CrossRef] [PubMed]
- Do Couto, R.O.; Cubayachi, C.; Calefi, P.L.; Lopez, R.F.V.; Pedrazzi, V.; De Gaitani, C.M.; De Freitas, O. Combining Amino Amide Salts in Mucoadhesive Films Enhances Needle-Free Buccal Anesthesia in Adults. J. Control. Release 2017, 266, 205–215. [Google Scholar] [CrossRef]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of Glycerol on the Physical and Mechanical Properties of Thin Gellan Gum Films for Oral Drug Delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and Evaluation of Buccal Films as Paediatric Dosage Form for Transmucosal Delivery of Ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Iordanopoulou, A.; Katsamenis, O.L.; Tsitsos, A.; Koltsakidis, S.; Anastasiadou, P.; Andreadis, D.; Economou, V.; Ritzoulis, C.; Tzetzis, D.; et al. Tailored Sticky Solutions: 3D-Printed Miconazole Buccal Films for Pediatric Oral Candidiasis. AAPS PharmSciTech 2024, 25, 190. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.D.; Durai, R.D.; Veluri, S.; Narayanan, V.H.B. Semisolid Extrusion 3D Printing of Dolutegravir-Chitosan Nanoparticles Laden Polymeric Buccal Films: Personalized Solution for Pediatric Treatment. Biomed. Mater. 2024, 19, 025046. [Google Scholar] [CrossRef]
- Abruzzo, A.; Crispini, A.; Prata, C.; Adduci, R.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Freeze-Dried Matrices for Buccal Administration of Propranolol in Children: Physico-Chemical and Functional Characterization. J. Pharm. Sci. 2021, 110, 1676–1686. [Google Scholar] [CrossRef]
- Sliefert, M.; Maloba, M.; Wexler, C.; Were, F.; Mbithi, Y.; Mugendi, G.; Maliski, E.; Nicolay, Z.; Thomas, G.; Kale, S.; et al. Challenges with Pediatric Antiretroviral Therapy Administration: Qualitative Perspectives from Caregivers and HIV Providers in Kenya. PLoS ONE 2024, 19, e0296713. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional Drug Release from 3D Printed Mucoadhesive Buccal Films Using FDM Technology: In Vitro and Ex Vivo Evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of Orodispersible Films for Delivery of Medicines to Infants and Preschool Children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Hanff, L.M.; Frijlink, H.W. Personalized Medicine in Pediatrics: The Clinical Potential of Orodispersible Films. AAPS PharmSciTech 2017, 18, 267–272. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized Orodispersible Films by Hot Melt Ram Extrusion 3D Printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, G.F.; Pistone, M.; Meazzini, C.; Lopedota, A.; Arduino, I.; Rizzi, R.; Lopalco, A.; Musazzi, U.M.; Cilurzo, F.; Denora, N. 3D Printed Mucoadhesive Orodispersible Films Manufactured by Direct Powder Extrusion for Personalized Clobetasol Propionate Based Paediatric Therapies. Int. J. Pharm. 2023, 643, 123214. [Google Scholar] [CrossRef] [PubMed]
- Oussedik, E.; Saleem, M.D.; Feldman, S.R. A Randomized, Double-Blind, Placebo-Controlled Study of the Vasoconstrictor Potency of Topical 0.25% Desoximetasone Spray: A High to Super High Range of Potency (Class I to Class II) Corticosteroid Formulation. J. Drugs Dermatol. 2017, 16, 972–975. [Google Scholar] [PubMed]
- Canto, A.M.D.; Müller, H.; Freitas, R.R.D.; Santos, P.S.D.S. Líquen Plano Oral (LPO): Diagnóstico Clínico e Complementar. An. Bras. Dermatol. 2010, 85, 669–675. [Google Scholar] [CrossRef]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A.H. Orodispersible Films–Recent Developments and New Applications in Drug Delivery and Therapy. Biochem. Pharmacol. 2022, 200, 115036. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Laquintana, V.; Summonte, S.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. Spray-Dried Mucoadhesive Microparticles Based on S-Protected Thiolated Hydroxypropyl-β-Cyclodextrin for Budesonide Nasal Delivery. Int. J. Pharm. 2021, 603, 120728. [Google Scholar] [CrossRef]
- Taketomo, C.K.; Hodding, J.H. Pediatric & Neonatal Dosage Handbook, 29th ed.; ACCP: Lenexa, KS, USA, 2022. [Google Scholar]
- Cendejas-Hernandez, J.; Sarafian, J.T.; Lawton, V.G.; Palkar, A.; Anderson, L.G.; Larivière, V.; Parker, W. Paracetamol (Acetaminophen) Use in Infants and Children Was Never Shown to Be Safe for Neurodevelopment: A Systematic Review with Citation Tracking. Eur. J. Pediatr. 2022, 181, 1835–1857. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Jacobs, I.C. Semi-Solid Formulations. In Pediatric Formulations; Bar-Shalom, D., Rose, K., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2014; Volume 11, pp. 171–179. ISBN 978-1-4899-8010-6. [Google Scholar]
- Ganatra, P.; Jyothish, L.; Mahankal, V.; Sawant, T.; Dandekar, P.; Jain, R. Drug-Loaded Vegan Gummies for Personalized Dosing of Simethicone: A Feasibility Study of Semi-Solid Extrusion-Based 3D Printing of Pectin-Based Low-Calorie Drug Gummies. Int. J. Pharm. 2024, 651, 123777. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D Printed Gummies: Personalized Drug Dosage in a Safe and Appealing Way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Rouaz-El Hajoui, K.; Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Suñé-Pou, M.; Nardi-Ricart, A.; Pérez-Lozano, P.; García-Montoya, E. Pellets and Gummies: Seeking a 3D Printed Gastro-Resistant Omeprazole Dosage for Paediatric Administration. Int. J. Pharm. 2023, 643, 123289. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D Printing of Gummy Drug Formulations Composed of Gelatin and an HPMC-Based Hydrogel for Pediatric Use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef]
- Santamaría, K.J.; Anaya, B.J.; Lalatsa, A.; González-Barranco, P.; Cantú-Cárdenas, L.; Serrano, D.R. Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use. Gels 2024, 10, 620. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [Google Scholar] [CrossRef]
- Pharmacopeial Forum Volume 35(5). Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/pharmaceuticalDosageForms.pdf (accessed on 19 January 2025).
- Pharmacopoeial Standards (WHO) Ensuring the Efficacy of a Deworming Medicine: Albendazole Chewable Tablets. Available online: https://iris.who.int/bitstream/handle/10665/331049/DI294-440-445-eng.pdf?sequence=1&isAllowed=y (accessed on 7 March 2025).
- Quality Attribute Considerations for Chewable Tablets Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Quality-Attribute-Considerations-for-Chewable-Tablets-Guidance-for-Industry.pdf (accessed on 19 January 2025).
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated Therapy Preparation of Isoleucine Formulations Using 3D Printing for the Treatment of MSUD: First Single-Centre, Prospective, Crossover Study in Patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; De Castro-López, M.J.; Sánchez-Pintos, P.; Giraldez-Montero, J.M.; Januskaite, P.; Duran-Piñeiro, G.; Dolores Bóveda, M.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; et al. Paediatric Clinical Study of 3D Printed Personalised Medicines for Rare Metabolic Disorders. Int. J. Pharm. 2024, 657, 124140. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Gallego-Fernández, C.; Jørgensen, A.K.; Parramon-Teixidó, C.J.; Cañete-Ramirez, C.; Cabañas-Poy, M.J.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. 3D Printed Personalized Therapies for Pediatric Patients Affected by Adrenal Insufficiency. Expert. Opin. Drug Deliv. 2024, 21, 1665–1681. [Google Scholar] [CrossRef]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of Developing Hospital Preparation by Semisolid Extrusion 3D Printing: Personalized Amlodipine Besylate Chewable Tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Jójárt, I.; Kása, P.; Kelemen, A.; Pintye-Hódi, K. Study of the Compressibility of Chewing Gum and Its Applicability as an Oral Drug Delivery System. Pharm. Dev. Technol. 2016, 21, 321–327. [Google Scholar] [CrossRef]
- Viljoen, J.M.; Van Der Walt, S.; Hamman, J.H. Formulation of Medicated Chewing Gum Containing Sceletium Tortuosum and Process Optimization Utilizing the SeDeM Diagram Expert System. AAPS PharmSciTech 2021, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Chewing Gums, Medicated. In European Pharmacopoeia, 9th ed.; European Directorate for the Quality of Medicines, Council of Europe: Strasbourg, Fance, 2016; p. 855.
- General Rules for Preparations; Preparations for Oro-Mucosal Application. In The Japanese Pharmacopoeia, English Version, 17th ed.; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016; p. 12.
- <1151> Pharmaceutical Dosage Forms. In United States Pharmacopeia and National Formulary USP 41–NF 36; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2018; pp. 1543–1568.
- Nilsson, H.; Ekberg, O.; Olsson, R.; Hindfelt, B. Quantitative Aspects of Swallowing in an Elderly Nondysphagic Population. Dysphagia 1996, 11, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.A.; Shahiwala, A.F. Medicated Chewing Gum–a Potential Drug Delivery System. Expert. Opin. Drug Deliv. 2010, 7, 871–885. [Google Scholar] [CrossRef]
- Mostafavi, S.; Varshosaz, J.; Arabian, S. Formulation Development and Evaluation of Metformin Chewing Gum with Bitter Taste Masking. Adv. Biomed. Res. 2014, 3, 92. [Google Scholar] [CrossRef]
- Wadgave, U.; Nagesh, L. Nicotine Replacement Therapy: An Overview. Int. J. Health Sci. 2016, 10, 425–435. [Google Scholar] [CrossRef]
- Paradkar, M.; Gajra, B.; Patel, B. Formulation Development and Evaluation of Medicated Chewing Gum of Anti-Emetic Drug. Saudi Pharm. J. 2016, 24, 153–164. [Google Scholar] [CrossRef]
- Stojanov, M.; Larsen, K.L. Cetirizine Release from Cyclodextrin Formulated Compressed Chewing Gum. Drug Dev. Ind. Pharm. 2012, 38, 1061–1067. [Google Scholar] [CrossRef]
- Pedersen, M.; Rassing, M.R. Miconazole Chewing Gum as a Drug Delivery System Application of Solid Dispersion Technique and Lecithin. Drug Dev. Ind. Pharm. 1990, 16, 2015–2030. [Google Scholar] [CrossRef]
- Andersen, T.; Gram-Hansen, M.; Pedersen, M.; Rassing, M.R. Chewing Gum as a Drug Delivery System for Nystatin Influence of Solubilising Agents Upon the Release of Water Insoluble Drugs. Drug Dev. Ind. Pharm. 1990, 16, 1985–1994. [Google Scholar] [CrossRef]
- Moradi, A.; Ghahremaninejad, F.; Hoseini, E.; Talebi, M.N.; Lohrasbi, S.; Farahimanesh, S.; Nami, M.; Khazaei, H.; Kazemi, K.; Mohammadi, M. The Effectiveness of Caffeinated Chewing Gum in Ameliorating Cognitive Functions Affected by Sleep Deprivation. Sleep. Sci. 2022, 15, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.A.; Darwish, M.K.; Abd El-Fattah, M.A. Development of Medicated Chewing Gum of Taste Masked Levocetirizine Dihydrochloride Using Different Gum Bases: In Vitro and in Vivo Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 395–402. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Altomare, C.; Kamal, M.M.; Nazzal, S. Mechanical Characterization and Dissolution of Chewing Gum Tablets (CGTs) Containing Co-Compressed Health in Gum® and Curcumin/Cyclodextrin Inclusion Complex. AAPS PharmSciTech 2018, 19, 3742–3750. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Scholz, K.J.; Schenke, I.M.; Pfab, F.; Cieplik, F.; Hiller, K.-A.; Buchalla, W.; Sahm, C.; Kirschneck, C.; Paddenberg-Schubert, E. Randomized Controlled Clinical Trial on the Efficacy of a Novel Antimicrobial Chewing Gum in Reducing Plaque and Gingivitis in Adolescent Orthodontic Patients. Clin. Oral. Invest. 2024, 28, 272. [Google Scholar] [CrossRef]
- Esmaeelian, M.; Esmaeelian, E. Effect of Neroli-Flavored Chewing Gum on Anxiety. Explore 2024, 20, 103028. [Google Scholar] [CrossRef]
- Potineni, R. Mechanisms of Flavor Release and Perception in Sugar-Free Chewing Gum. Ph.D. Thesis, The Pennsylvania State University, University Park, PA, USA, 2007. [Google Scholar]
- Konno, M.; Takeda, T.; Kawakami, Y.; Suzuki, Y.; Kawano, Y.; Nakajima, K.; Ozawa, T.; Ishigami, K.; Takemura, N.; Sakatani, K. Relationships Between Gum-Chewing and Stress. In Oxygen Transport to Tissue XXXVII; Elwell, C.E., Leung, T.S., Harrison, D.K., Eds.; Springer: New York, NY, USA, 2016; Volume 876, pp. 343–349. ISBN 978-1-4939-3022-7. [Google Scholar]
- Yaman-Sözbir, Ş.; Ayaz-Alkaya, S.; Bayrak-Kahraman, B. Effect of Chewing Gum on Stress, Anxiety, Depression, Self-focused Attention, and Academic Success: A Randomized Controlled Study. Stress. Health 2019, 35, 441–446. [Google Scholar] [CrossRef]
- Hetherington, M.M.; Regan, M.F. Effects of Chewing Gum on Short-Term Appetite Regulation in Moderately Restrained Eaters. Appetite 2011, 57, 475–482. [Google Scholar] [CrossRef]
- Newton, J.T.; Awojobi, O.; Nasseripour, M.; Warburton, F.; Di Giorgio, S.; Gallagher, J.E.; Banerjee, A. A Systematic Review and Meta-Analysis of the Role of Sugar-Free Chewing Gum in Dental Caries. JDR Clin. Transl. Res. 2020, 5, 214–223. [Google Scholar] [CrossRef]
- Mantihal, S.; Kobun, R.; Lee, B.-B. 3D Food Printing of as the New Way of Preparing Food: A Review. Int. J. Gastron. Food Sci. 2020, 22, 100260. [Google Scholar] [CrossRef]
- Dankar, I.; Haddarah, A.; Omar, F.E.L.; Sepulcre, F.; Pujolà, M. 3D Printing Technology: The New Era for Food Customization and Elaboration. Trends Food Sci. Technol. 2018, 75, 231–242. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. (Ed.) Polymers for Food Applications; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-94624-5. [Google Scholar]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Soon, A. You’ll Be Able to 3D Print Chewing Gum with GumJet 3D Printer. Available online: https://www.3ders.org/articles/20150218-two-london-students-develop-3d-printed-chewing-gum.html (accessed on 22 January 2025).
- Krassenstein, B. GumJet—3D Print Your Own Chewing Gum. Available online: https://3dprint.com/44851/gumjet-3d-printer/ (accessed on 20 January 2025).
- Chewing Gum Composition and Method of Shaping Chewing Gum in a 3d Printer (US20190313663A1). Available online: https://patents.google.com/patent/US20190313663A1/en (accessed on 20 January 2025).
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-Friendly Chocolate-Based Dosage Forms for the Oral Administration of Both Hydrophilic and Lipophilic Drugs Fabricated with Extrusion-Based 3D Printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef]
- Lesutan, V.-N.; Milliken, R.L.; Browne, A.; Ferguson, T.; Lamprou, D.A. Enhancing 3D-Printed Chocolate Drug Delivery: The Role of Soy Lecithin in Improving Formulation Printability and Consistency. RPS Pharm. Pharmacol. Rep. 2025, 4, rqae030. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Karavasili, C.; Mavrokefalou, E.-E.; Gioumouxouzis, C.I.; Ritzoulis, C.; Fatouros, D.G. Quality Control Evaluation of Paediatric Chocolate-Based Dosage Forms: 3D Printing vs Mold-Casting Method. Int. J. Pharm. 2022, 624, 121991. [Google Scholar] [CrossRef] [PubMed]
- Milliken, R.L.; Dedeloudi, A.; Vong, E.; Irwin, R.; Andersen, S.K.; Wylie, M.P.; Lamprou, D.A. 3D Printed Cacao-Based Formulations as Nutrient Carriers for Immune System Enhancement. Curr. Res. Food Sci. 2025, 10, 100949. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Paediatric Pharmacokinetics: Key Considerations. Brit J. Clin. Pharma 2015, 79, 395–404. [Google Scholar] [CrossRef]
- O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. Int. J. Mol. Sci. 2019, 20, 2688. [Google Scholar] [CrossRef]
- Neal-Kluever, A.; Fisher, J.; Grylack, L.; Kakiuchi-Kiyota, S.; Halpern, W. Physiology of the Neonatal Gastrointestinal System Relevant to the Disposition of Orally Administered Medications. Drug Metab. Dispos. 2019, 47, 296–313. [Google Scholar] [CrossRef]
- Monteil, M.; Sanchez-Ballester, N.M.; Devoisselle, J.M.; Begu, S.; Soulairol, I. Regulations on Excipients Used in 3D Printing of Pediatric Oral Forms. Int. J. Pharm. 2024, 662, 124402. [Google Scholar] [CrossRef]
- ISO/ASTM 52900; Additive Manufacturing-General Principles-Fundamentals and Vocabulary. ISO/ASTM International: Geneva, Switzerland, 2021.
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Kumari, J.; Pandey, S.; Jangde, K.K.; Kumar, P.V.; Mishra, D.K. Evolution, Integration, and Challenges of 3D Printing in Pharmaceutical Applications: A Comprehensive Review. Bioprinting 2024, 44, e00367. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D Printing for Oral Drug Delivery: A New Tool to Customize Drug Delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Basit, A.W.; Gaisford, S. Energy Consumption and Carbon Footprint of 3D Printing in Pharmaceutical Manufacture. Int. J. Pharm. 2023, 639, 122926. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. 3D Printing in Healthcare: A Review on Drug Printing, Challenges and Future Perspectives. Mater. Today Commun. 2024, 40, 110199. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Li, L.; Fang, D.; Sun, H.; Qiao, S.; Li, X.; Pan, W. Exploration and Preparation of Patient-Specific Ciprofloxacin Implants Drug Delivery System Via 3D Printing Technologies. J. Pharm. Sci. 2021, 110, 3678–3689. [Google Scholar] [CrossRef]
- Youssef, S.H.; Abdella, S.; Garg, S. Development and Validation of a Novel Tool for Assessing the Environmental Impact of 3D Printing Technologies: A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. A Review of Three-Dimensional Printing for Pharmaceutical Applications: Quality Control, Risk Assessment and Future Perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102571. [Google Scholar] [CrossRef]
- Saydam, M.; Takka, S. Improving the Dissolution of a Water-Insoluble Orphan Drug through a Fused Deposition Modelling 3-Dimensional Printing Technology Approach. Eur. J. Pharm. Sci. 2020, 152, 105426. [Google Scholar] [CrossRef]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; Von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality Considerations on the Pharmaceutical Applications of Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2021, 592, 119901. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, S.; Ben Ali, M.S.; Mert, M. Sources of Economic Growth in MENA Countries: Technological Progress, Physical or Human Capital Accumulations. In Economic Development in the Middle East and North Africa; Ben Ali, M.S., Ed.; Palgrave Macmillan US: New York, NY, USA, 2016; pp. 27–69. ISBN 978-1-349-55918-3. [Google Scholar]
- Columbus, L. The State Of 3D Printing. 2017. Available online: https://www.forbes.com/sites/louiscolumbus/2017/05/23/the-state-of-3d-printing-2017/ (accessed on 27 February 2025).
- Bai, X.; Liu, Y. Technology Resources Distribution Characteristics of 3D Printing: Based on Patent Bibliometric Analysis. Int. J. Technol. Transf. Commer. 2016, 14, 171. [Google Scholar] [CrossRef]
- Bai, W.; Fang, H.; Wang, Y.; Zeng, Q.; Hu, G.; Bao, G.; Wan, Y. Academic Insights and Perspectives in 3D Printing: A Bibliometric Review. Appl. Sci. 2021, 11, 8298. [Google Scholar] [CrossRef]
- Jewell, C.; Stones, J. 3D Printing Techniques in the Pharmaceutical Sciences–Intellectual Property Issues. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 183–213. ISBN 978-3-319-90754-3. [Google Scholar]
- Stones, J.A.; Jewell, C.M. 3D Printing of Pharmaceuticals: Patent and Regulatory Challenges. Pharm. Pat. Anal. 2017, 6, 147–151. [Google Scholar] [CrossRef]
- Karavasili, C.; Gkaragkounis, A.; Fatouros, D.G. Patent Landscape of Pediatric-Friendly Oral Dosage Forms and Administration Devices. Expert. Opin. Ther. Pat. 2021, 31, 663–685. [Google Scholar] [CrossRef]
- Jacob, J.; Coyle, N.; West, T.G.; Monkhouse, D.C.; Surprenant, H.L.; Jain, N.B. Rapid Disperse Dosage Form Containing Levetiracetam. U.S. Patent 20140271862A1, 17 May 2016. [Google Scholar]
- Alhnan, M.A. Solid Dosage form Production. WO 2016/038356A1, 8 September 2015. [Google Scholar]
- Deng, F.; Li, X.; Cheng, S. Dosage Forms with Desired Release Profiles and Methods of Designing and Making Thereof. U.S. Patent 10350822B1, 16 July 2019. [Google Scholar]
- Li, X. 3D Printing Methods for Compartmented Pharmaceutical Dosage Forms. U.S. Patent 12042562B2, 23 July 2024. [Google Scholar]
- Zheng, Y. A Kind of Amlodipine Besylate Tablet Oral Disintegrating Tablet Prepared Using 3D Printing and Preparation Method Thereof. CN107823153A, 9 February 2021. [Google Scholar]
- Zheng, Y. A Kind of Preparation Method Based on Ink-Jet 3D Printing Olanzapine Orally-Disintegrating Tablet. CN110269844A, 24 September 2019. [Google Scholar]
- Siyawamwaya, M.; Du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.P.D.; Pillay, V. 3D Printed, Controlled Release, Tritherapeutic Tablet Matrix for Advanced Anti-HIV-1 Drug Delivery. Eur. J. Pharm. Biopharm. 2019, 138, 99–110. [Google Scholar] [CrossRef]
- Berg, S.; Krause, J.; Björkbom, A.; Walter, K.; Harun, S.; Granfeldt, A.; Janzén, D.; Nunes, S.F.; Antonsson, M.; Van Zuydam, N.; et al. In Vitro and In Vivo Evaluation of 3D Printed Capsules with Pressure Triggered Release Mechanism for Oral Peptide Delivery. J. Pharm. Sci. 2021, 110, 228–238. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Gómez-Lado, N.; Lázare-Iglesias, H.; García-Otero, X.; Antúnez-López, J.R.; Ruibal, Á.; Varela-Correa, J.J.; Aguiar, P.; Basit, A.W.; Otero-Espinar, F.J.; et al. 3D Printed Tacrolimus Rectal Formulations Ameliorate Colitis in an Experimental Animal Model of Inflammatory Bowel Disease. Biomedicines 2020, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Sitkowski, M.; Zuzak, T.; Coles-Black, J.; Chuen, J.; Major, P.; Pdziwiatr, M. From Ideas to Long-Term Studies: 3D Printing Clinical Trials Review. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1473–1478. [Google Scholar] [CrossRef]
- Lai, J.; Liu, Y.; Lu, G.; Yung, P.; Wang, X.; Tuan, R.S.; Li, Z.A. 4D Bioprinting of Programmed Dynamic Tissues. Bioact. Mater. 2024, 37, 348–377. [Google Scholar] [CrossRef]
- Lukin, I.; Musquiz, S.; Erezuma, I.; Al-Tel, T.H.; Golafshan, N.; Dolatshahi-Pirouz, A.; Orive, G. Can 4D Bioprinting Revolutionize Drug Development? Expert. Opin. Drug Discov. 2019, 14, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, A.; Foppoli, A.; Cerea, M.; Palugan, L.; Cirilli, M.; Moutaharrik, S.; Melocchi, A.; Maroni, A. Towards 4D Printing in Pharmaceutics. Int. J. Pharm. X 2023, 5, 100171. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, L.A.; Oyewumi, M.O. Integration of 3D Printing Technology in Pharmaceutical Compounding: Progress, Prospects, and Challenges. Ann. 3D Print. Med. 2021, 4, 100035. [Google Scholar] [CrossRef]
- Yang, G.H.; Yeo, M.; Koo, Y.W.; Kim, G.H. 4D Bioprinting: Technological Advances in Biofabrication. Macromol. Biosci. 2019, 19, 1800441. [Google Scholar] [CrossRef]
- Rodríguez-Maciñeiras, X.; Bendicho-Lavilla, C.; Rial, C.; Garba-Mohammed, K.; Worsley, A.; Díaz-Torres, E.; Orive-Martínez, C.; Orive-Mayor, Á.; Basit, A.W.; Alvarez-Lorenzo, C.; et al. Advancing Medication Compounding: Use of a Pharmaceutical 3D Printer to Auto-Fill Minoxidil Capsules for Dispensing to Patients in a Community Pharmacy. Int. J. Pharm. 2025, 671, 125251. [Google Scholar] [CrossRef]
- Aprecia.com. Available online: https://aprecia.com/capabilities/ (accessed on 10 October 2024).

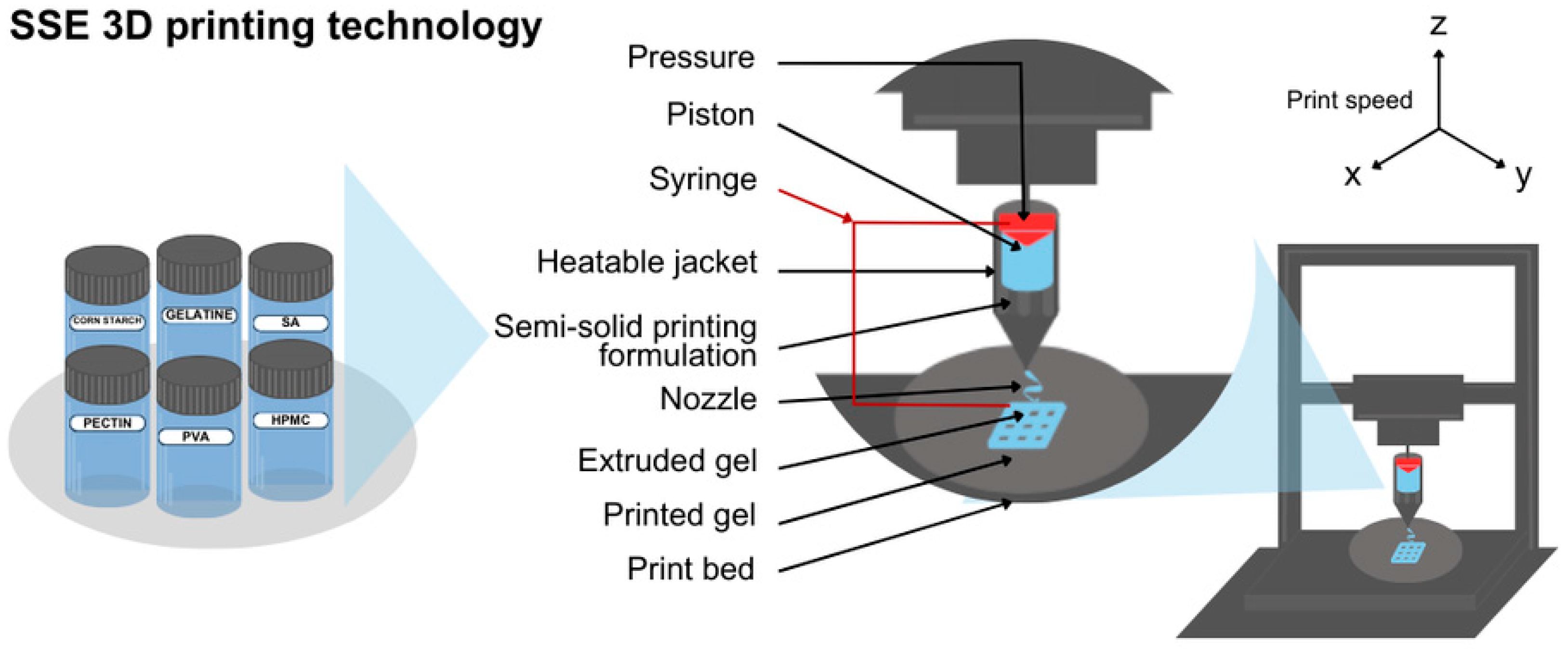
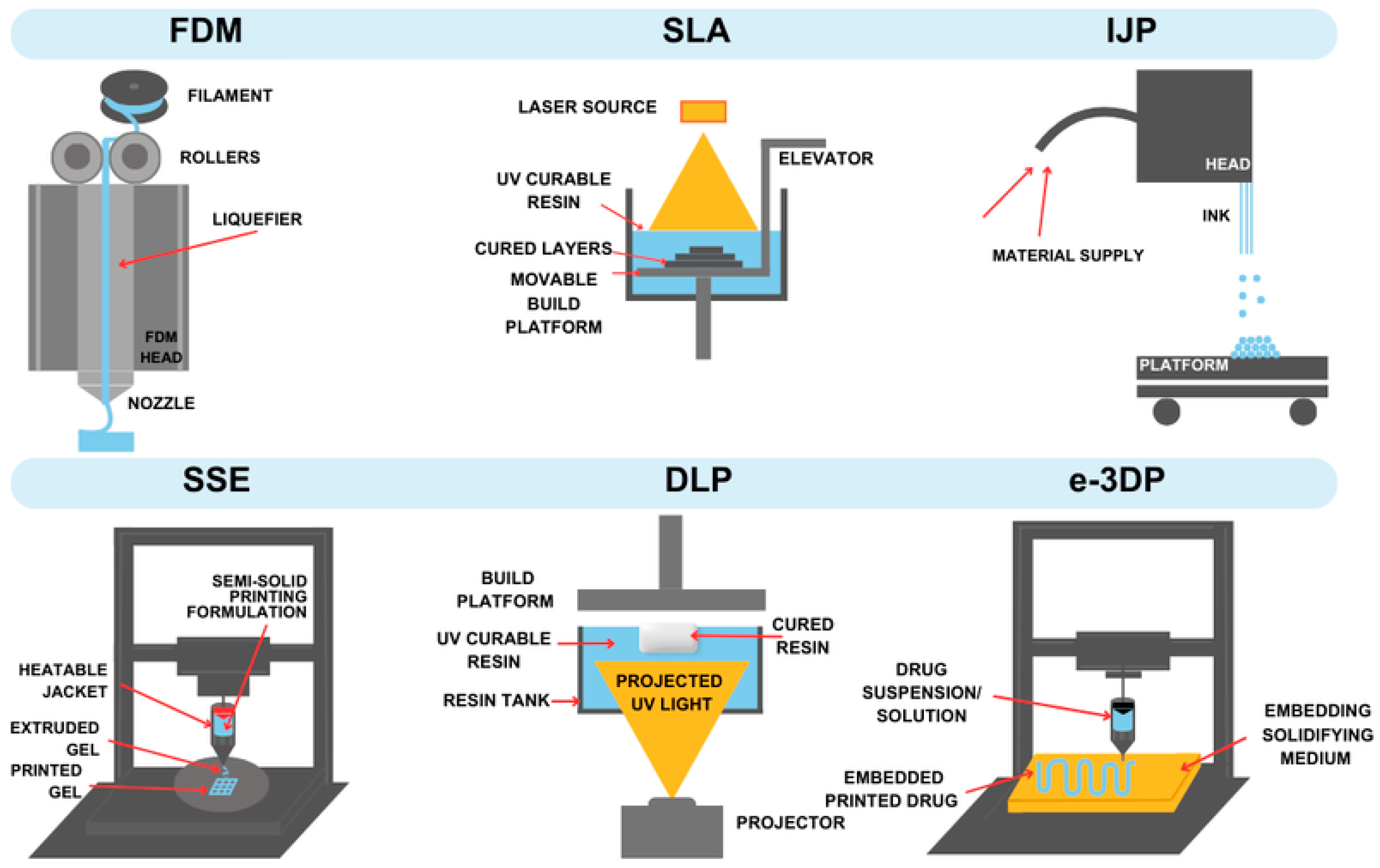
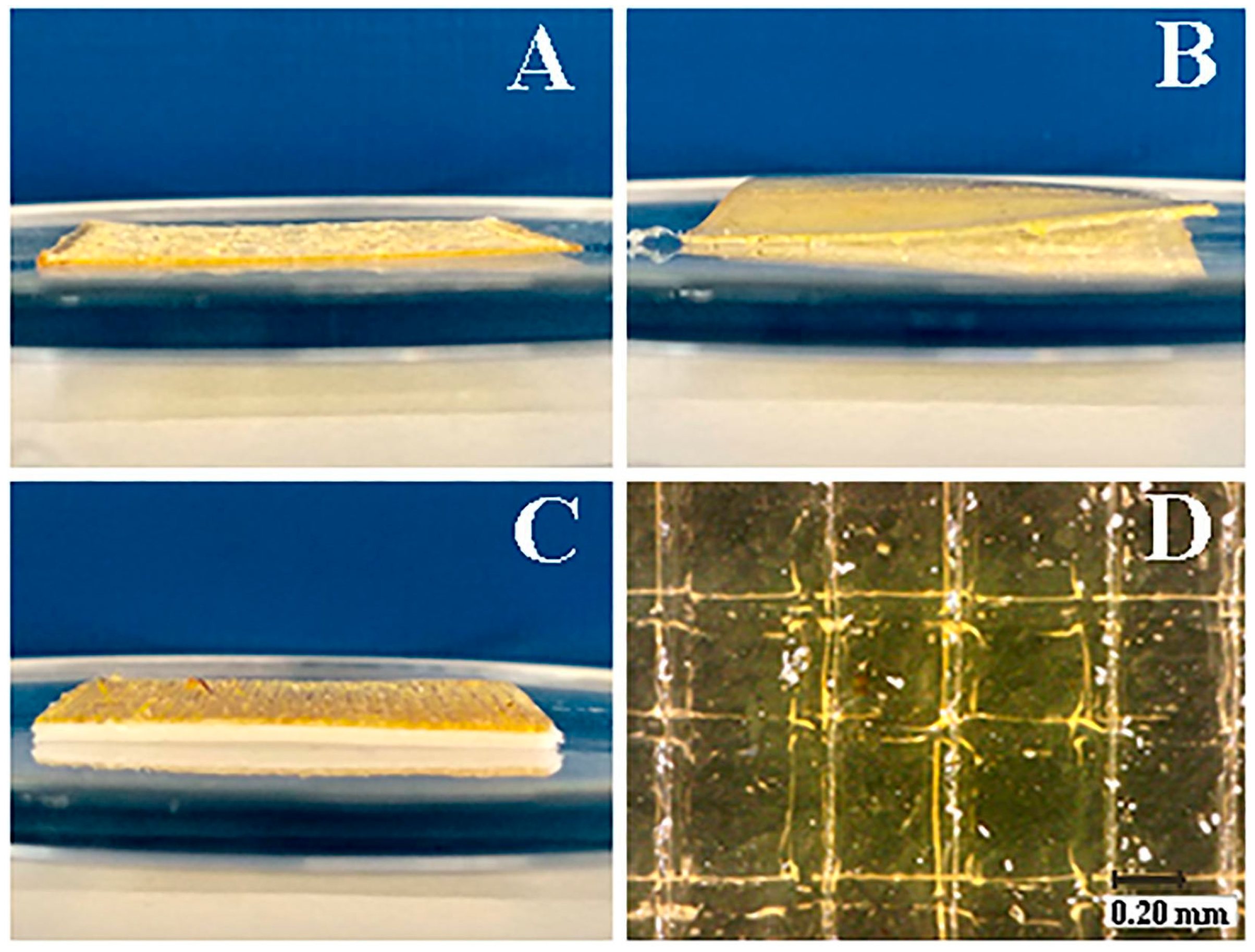
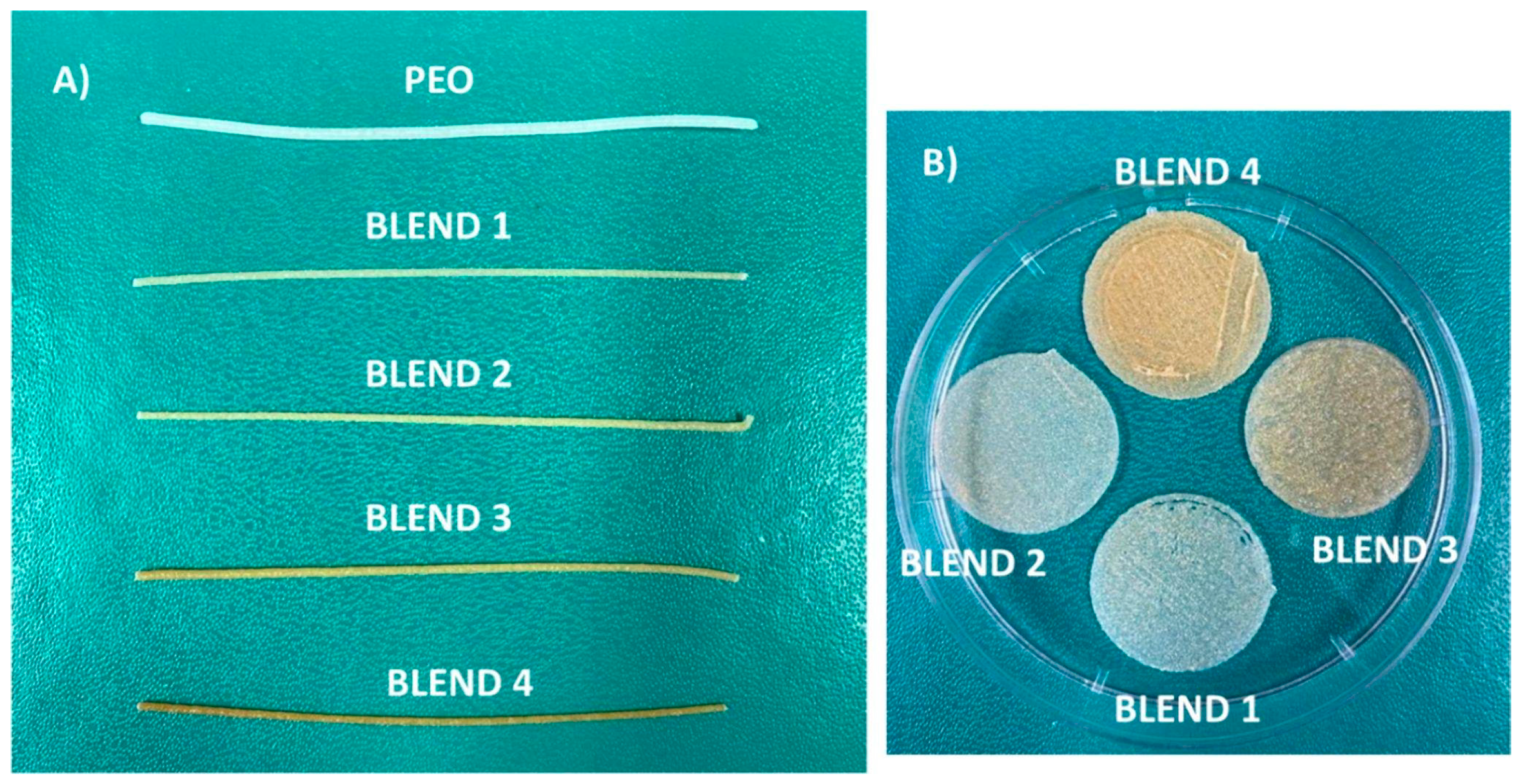
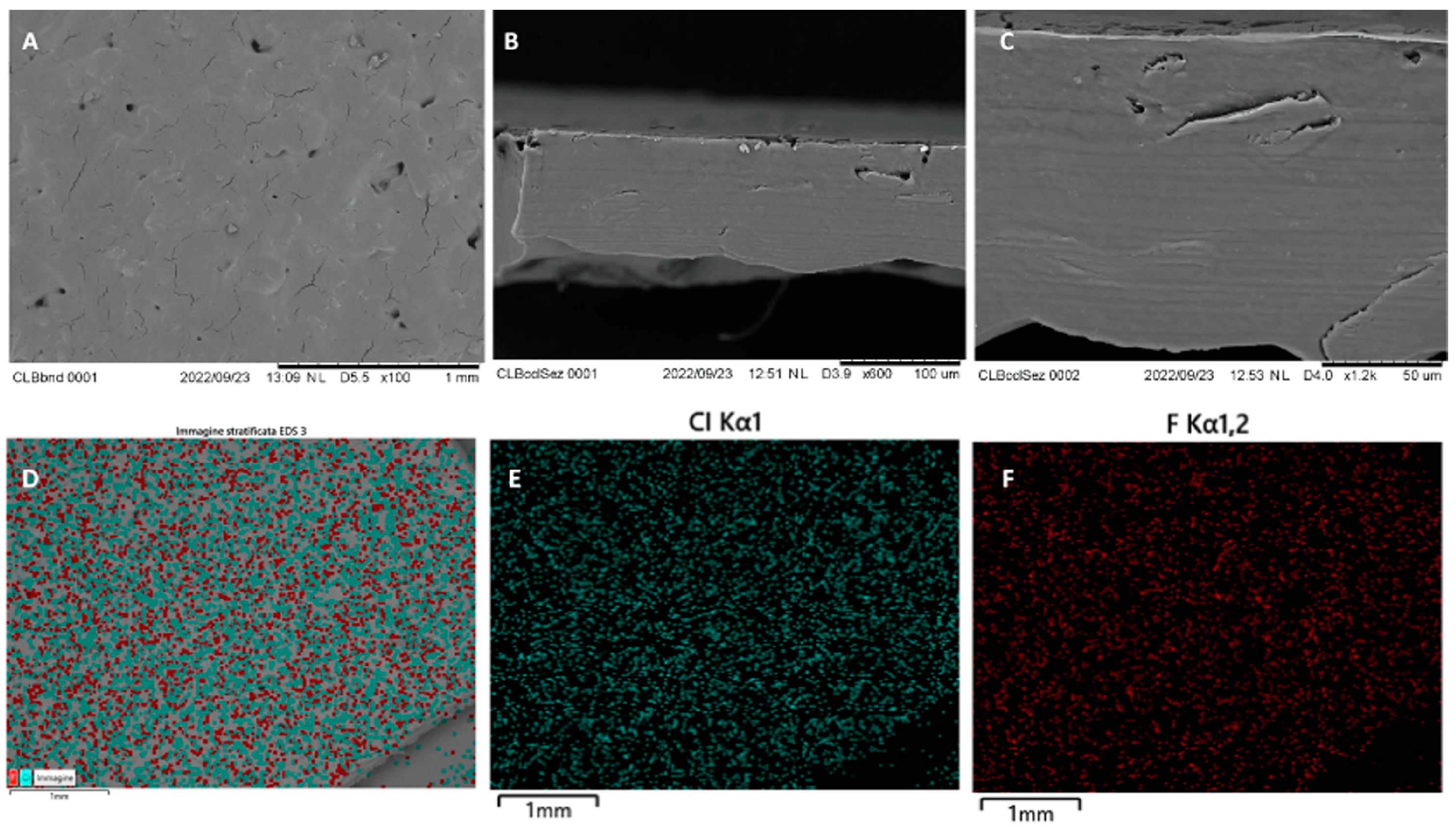


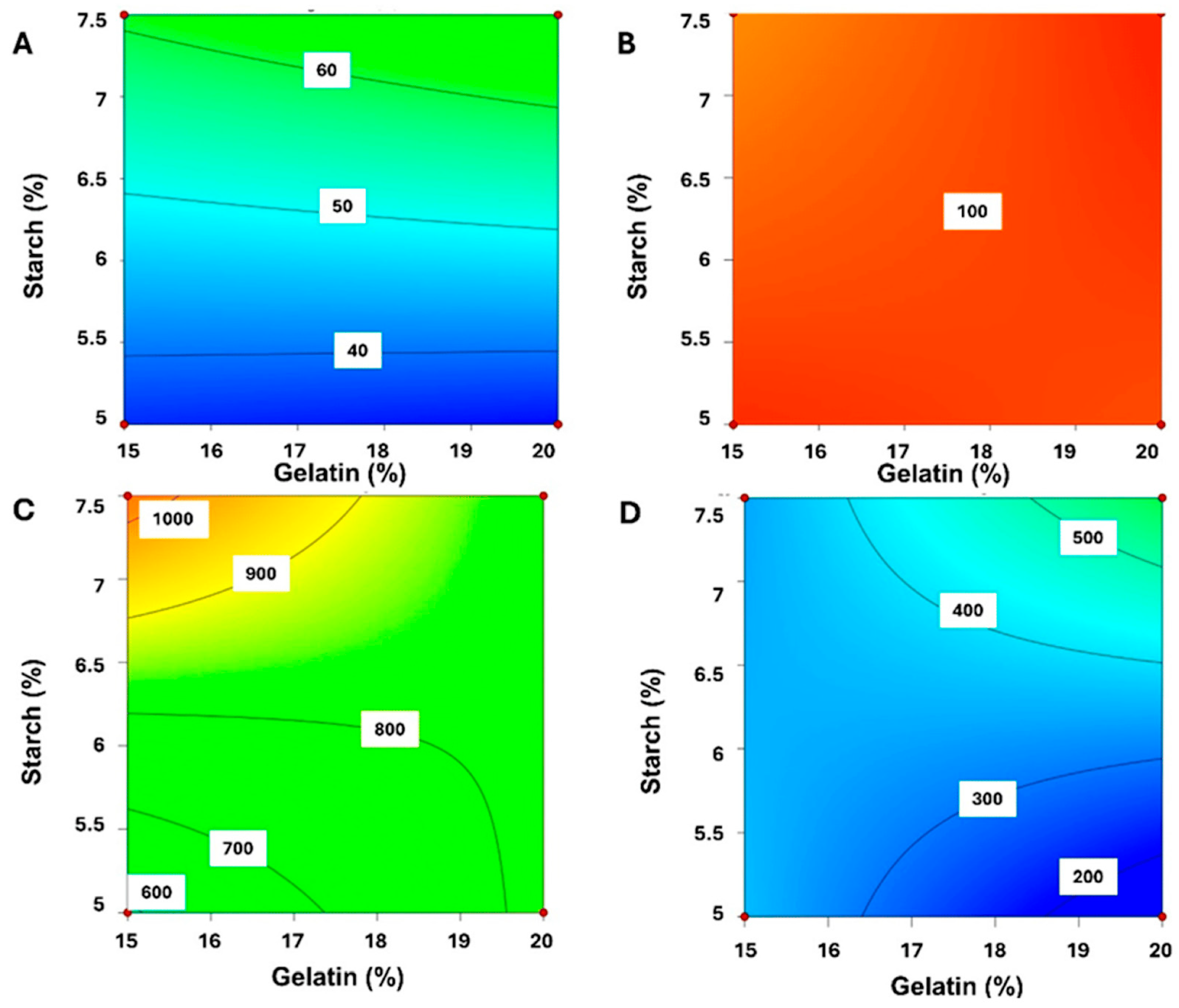


| Technique | Material Applied | Conditions | Potential Drawbacks | Advantages |
|---|---|---|---|---|
| FDM | thermoplastic polymer | high temperature | thermal decomposition of the drug | good equipment availability |
| SLA | photocurable resin | irradiation | resin toxicity | good equipment availability, good resolution |
| IJP | liquid ink | mild conditions depending on the material | nozzle clogging | good dose accuracy |
| SSE | gel/paste material | mild conditions depending on the material | low resolution, post-processing requirements | mild conditions, possibility to use biocompatible materials |
| DLP | photocurable resin | irradiation | resin toxicity | fast, cost-efficient, good resolution |
| e-3DP | viscoelastic ink and gel embedding phase | depending on the material | phases incompatibility, low resolution | interesting design, taste masking |
| Dosage Form | Active Ingredient | Printing Technique | Additives | Reference |
|---|---|---|---|---|
| hydrogel | L-ascorbic acid | SLA | PEDMA, triethanolamine, Riboflavin | [35] |
| gels | eucalypt extract | SSE | PEO, ascorbic acid, Eumulgin | [68] |
| buccal films | Miconazole | SSE | zein-PVP | [76] |
| oral films | Caffeine | SSE | SA, HPMC, SC | [47] |
| orodispersible mucoadhesive films | clobetasol propionate | DPE | HPMC, CS, HP-β-CD, PEO | [84] |
| buccal films with incorporated nanoparticles | Dolutegravir | SSE | PVAl, SA | [77] |
| ODF | paracetamol | hot melt ram extrusion | MDX, GLN, Span® 80, GLY, TiO2 | [83] |
| mucoadhesive buccal films | diclofenac sodium | FDM | PVAl, CS, EC, TEC, Xyl | [80] |
| FDF | paracetamol, ibuprofen | FDM | PVAl, PEO, ethylene, PEG, starch, sodium starch glycolate, croscarmellose, sodium lauryl sulfate | [91] |
| Formulation | Active Compound | Printing Method | Printing Material | Reference |
|---|---|---|---|---|
| gummies | paracetamol and ibuprofen | e-3DP and SSE | water, glycerol, gelatine | [38] |
| gummies | ranitidine hydrochloride | SSE | corn starch | [94] |
| gummies | omeprazole | SSE | xanthan gum, corn starch | [95] |
| gummies | lamotrigine | SSE | gelatine, HPMC | [96] |
| gummies | simethicone | SSE | pectin | [93] |
| gummies | propranolol Hydrochloride | SSE | gelatin and γ-Aminobutyric acid | [97] |
| gummies | metformin | SSE and convective casting method | gelatine | [98] |
| chewable tablets | isoleucine | SSE | pectin, sucrose and maltodextrin | [103] |
| chewable tablets | citrulline, isoleucine, valine | SSE | pectin, sucrose, maltodextrin, water, maltitol | [104] |
| chewable tablets | hydrocortisone | SSE | pectin, sucrose, maltodextrin, water, maltitol | [105] |
| chewable tablets | amlodipine | SSE | CMC-Na, SSG, glycerine | [106] |
| Formulation | Active Compound | Printing Method | Printing Material | Reference |
|---|---|---|---|---|
| chocolate | paracetamol, ibuprofen | extrusion-based 3DP | chocolate, corn syrup | [137] |
| chocolate | paracetamol, ibuprofen | extrusion-based 3DP | cacao, soy lecithin, glucose syrup | [138] |
| chocolate | paracetamol | SSE | chocolate | [139] |
| chocolate | VitD3 | chocolate 3D printer | cacao, honey | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, A.; Krysztofiak, J.; Hozakowska, A.; Wojszel, Z.; Osmałek, T.; Wojtyłko, M.; Froelich, A. Novel Soft Dosage Forms for Paediatric Applications: Can We 3D-Print Them or Not? Gels 2025, 11, 187. https://doi.org/10.3390/gels11030187
Białek A, Krysztofiak J, Hozakowska A, Wojszel Z, Osmałek T, Wojtyłko M, Froelich A. Novel Soft Dosage Forms for Paediatric Applications: Can We 3D-Print Them or Not? Gels. 2025; 11(3):187. https://doi.org/10.3390/gels11030187
Chicago/Turabian StyleBiałek, Antoni, Julia Krysztofiak, Aleksandra Hozakowska, Zuzanna Wojszel, Tomasz Osmałek, Monika Wojtyłko, and Anna Froelich. 2025. "Novel Soft Dosage Forms for Paediatric Applications: Can We 3D-Print Them or Not?" Gels 11, no. 3: 187. https://doi.org/10.3390/gels11030187
APA StyleBiałek, A., Krysztofiak, J., Hozakowska, A., Wojszel, Z., Osmałek, T., Wojtyłko, M., & Froelich, A. (2025). Novel Soft Dosage Forms for Paediatric Applications: Can We 3D-Print Them or Not? Gels, 11(3), 187. https://doi.org/10.3390/gels11030187







