1. Introduction
Appropriate and regular exercise training is considered to be effective in preventing injuries, improving muscle mass and strength performance, improving the physiological metabolism, and so on [
1]. In addition to exercise training, appropriate nutritional supplementation also plays a considerable role in exercise performance and promoting muscle growth and synthesis [
2]. In the past, common sports nutrition supplements were nothing more than antioxidant or protein supplements; however, in recent years, more and more studies have shown that probiotics are supplements with the potential to increase muscle mass and exercise performance. In a past meta-trial combining the results of 24 studies, it was found that probiotic supplementation significantly improved skeletal muscle mass and strength performance, although it did not help total lean mass [
3]. In addition, another study has also shown that supplementation with
Lactiplantibacillus plantarum TWK10 for six consecutive weeks without exercise training significantly improved the muscle mass as well as reducing the body fat rate and exercise fatigue indicators of non-athletes [
4].
Probiotics are live micro-organisms that, when taken orally for several weeks and ingested in sufficient amounts, increase the number of beneficial bacteria in the gut, thereby providing health benefits to the host [
5,
6]. More and more evidence has demonstrated that the gut microbiota can stimulate the IGF-1/p70s6k/mTOR pathway in muscle cells through the synthesis of amino acids, thereby promoting muscle synthesis [
7]. The exact mechanism by which probiotics stimulate increases in muscle mass or strength is currently unknown, but the most commonly accepted factor may be through improved protein digestion and amino acid absorption into the bloodstream [
8], thereby promoting skeletal muscle anabolism and improving muscle mass and functional performance [
9]. Some past studies have confirmed that probiotic supplementation can modulate the gut microbiota to enhance protein assimilation, upregulate mTOR activation and mitochondrial function, and reduce inflammatory cytokine activity, thereby improving muscle anabolism and reducing muscle loss [
10,
11]. A meta-analysis of the overall effects reported in seven studies has also shown that probiotic supplementation improved muscle mass and strength [
12].
Lactobacillus brevis is a heterofermentative Gram-positive organism which is widely present in fermented foods of plant and animal origin, highly adhesive to low-pH bile salts and pancreatic juice, competitive against
Salmonella and
Escherichia coli, and promotes primary bile acid biosynthesis, amino acids, and other metabolic pathways [
13,
14]. A previous study has pointed out that daily supplementation with
L. brevis FZU0713 for 8 weeks significantly regulated liver genes (including Acat2, Acox1, Hmgcr, Cd36, Srebp-1c, and Cyp7a1), thereby promoting the lipid metabolism and maintaining bile acid stability. In addition, they also found that the levels of short-chain fatty acids (SCFAs) in the feces of HFD-fed rats were significantly increased, which are considered to be able to effectively change the composition and metabolic function of gut micro-organisms [
15]. Another previous study has shown that SCFAs play an important role in skeletal muscle function and exercise capacity [
16], possibly through activating multiple regulatory pathways (e.g., UCP2-AMPK-ACC and PGC1-α) to increase ATP production and muscle fiber metabolic efficiency to affect skeletal muscle performance [
17]. In addition, they act by inhibiting histone deacetylase, thereby preventing apoptosis and preventing muscle protein catabolism [
18].
While there are no studies directly confirming the relationship between
L.
brevis and muscle and exercise performance, past studies have shown that
L.
brevis can increase the production of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the mammalian central nervous system [
19]. It can increase plasma growth hormone concentrations, promoting amino acid transport, cellular amino acid uptake, and skeletal muscle growth [
20]. A previous study demonstrated that when whey protein was combined with GABA supplementation and resistance exercise training for 12 weeks, it significantly increased growth hormone levels and total body lean tissue weight [
21]. Therefore, this study aims to investigate whether supplementation with
L. brevis combined with resistance exercise training can provide benefits in increasing muscle mass and strength performance, and further explore its adaptability effects on the human body. We hypothesize that the synergistic effect of
L. brevis combined with resistance exercise training can promote muscle mass and strength performance. This will be the first study to explore the effects of
L.
brevis on muscle and exercise performance, serving not only as a foundation for understanding its mechanisms and applications but also as a potential choice for sports nutrition supplementation.
2. Materials and Methods
2.1. Sample Preparation
L. brevis GKEX was isolated from douchi (fermented black soybeans) and preserved at the National Institute of Technology and Evaluation, Biological Resource Center (NBRC, Chiba, Japan), with collection number NITE BP-03696. The bacterial strain was cultured in MRS broth (BD, Franklin Lakes, NJ, USA) for 16 h at 32 °C with agitation at 100 rpm. Subsequently, the seed culture was 0.01% inoculated in a 15T bioreactor containing 80% culture medium as the working volume. The scaled-up culture was maintained at 32 °C for 16 h with pH control set to 6.0. The bioreactor medium comprised 5% glucose, 2% yeast extract, 0.05% MgSO
4, 0.1% K
2HPO
4, and 0.1% Tween 80. Following harvest,
L. brevis GKEX was centrifuged (MBPX810, Alfa Laval AB, Lund, Sweden) at 7000 rpm and the bacterial pellet was mixed with 10% skim milk for lyophilization (FD24, Kingmech Scientific, Taoyuan, Taiwan) under 30 °C for 5 days. The resulting freeze-dried GKEX powder was then filled into No. 0 vegetable capsules, with each capsule containing 1.0 × 10
10 CFU of live bacteria. Since the bacterial powder was mixed with microcrystalline α-cellulose and then filled into capsules, we used microcrystalline α-cellulose as a placebo substitute and filled it into the capsules accordingly. All the manufacturing processes were conducted at Grape King Bio Ltd. (Taoyuan, Taiwan) in compliance with the ISO22000 system [
22].
2.2. Participants
The Harvard calculator (
http://hedwig.mgh.harvard.edu/sample_size/size.html, accessed on 20 June 2022) was used to determine the sample size, assuming a parallel design with a significance level of 0.05, a power of 0.8, and a standard deviation of 0.8 for the difference. The trial included a total of 52 subjects (including 20 males and 36 females) from the National Sports University, aged 20–39 years old, who were not athletes. None of them met our exclusion criteria, including (1) BMI ≥ 27; (2) a history of metabolic disease, asthma, cancer, cardiovascular disease, or hypertension; (3) limb and neuromuscular movement disorders occurring within the last 6 months, indicating that the subject is unable to exercise; (4) had taken anti-inflammatory, analgesic, acute, or chronic disease or other drugs in the past month; (5) previous hepatobiliary and gastrointestinal surgery (except hernia and polypectomy); (6) those who smoke or drink; (7) having taken probiotic powder, capsules, or lozenges (including yogurt and other related foods) within two weeks; (8) allergy to food or lactic acid bacteria products; (9) students or stakeholders of the principal investigator. All eligible participants gave written informed consent before starting the experiment. The experiment was performed after receiving approval from the Institutional Review Board of Landseed International Hospital (Taoyuan, Taiwan; LSHIRB No. 22-022-A2) and was registered at
clinicaltrials.gov under registration number NCT05909475. This study was conducted according to the guidelines of the Declaration of Helsinki.
2.3. Experimental Design
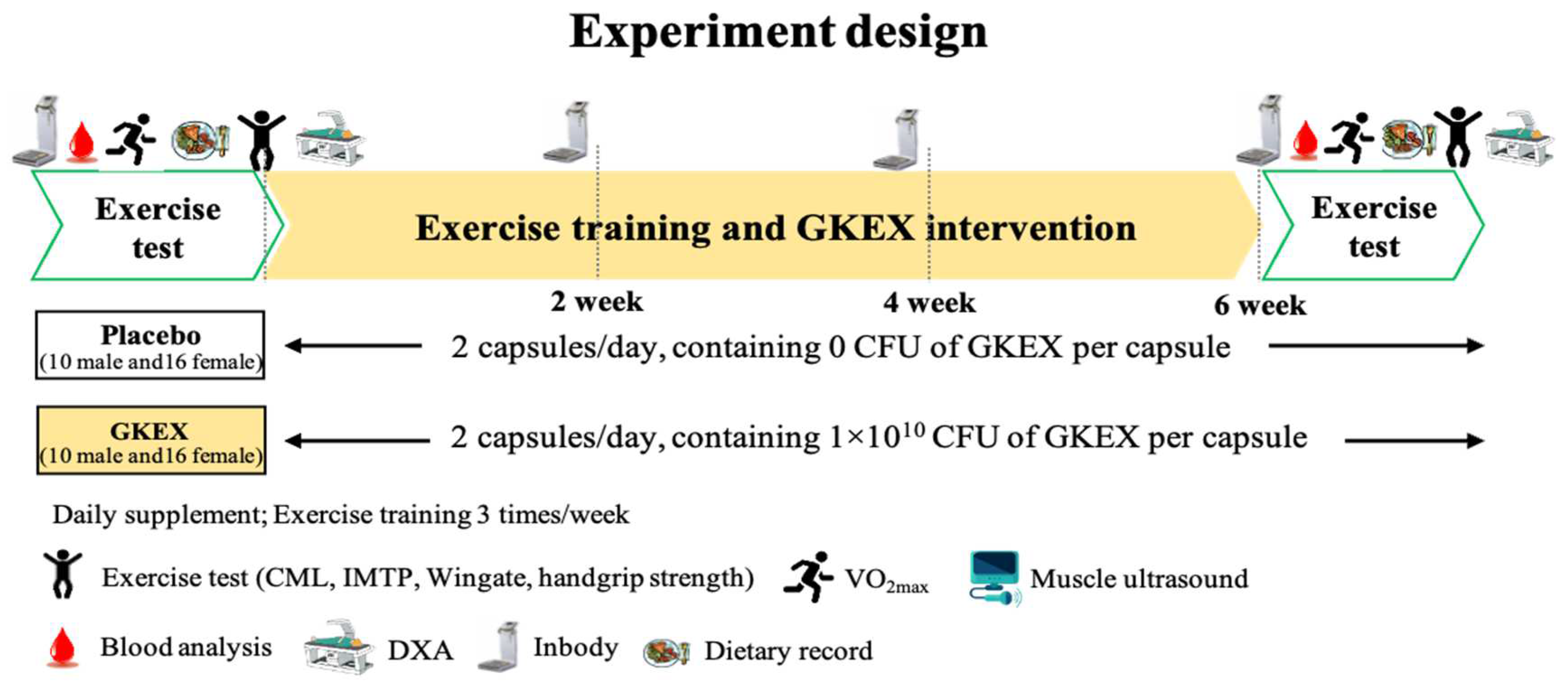
After all subjects were recruited, numbers were assigned according to the order of registration. Then, when the gender ratio of the two groups was the same, we randomly divided them into two groups through numbered sampling and conducted a double-blind experiment (with 10 male and 16 female participants in each group): a placebo group (2 capsules/day, containing 0 CFU of GKEX per capsule) and a GKEX group (2 capsules/day, containing 1 × 10
10 CFU of GKEX per capsule). There were no significant differences between the placebo and GKEX groups in terms of age (placebo: 22.2 ± 1.9; GKEX: 22.6 ± 2.4 years old) and height (placebo: 166.2 ± 8.9; GKEX: 168.6 ± 8.7 cm). The trial lasted for 6 weeks, during which all subjects were required to supplement with two capsules daily and perform resistance exercise training three times a week. Before and after the intervention, all subjects underwent tests for dietary record, an exercise performance test, and blood samples were collected to analyze and measure body composition indicators. Among these, only body composition was required to be measured every two weeks. Furthermore, all subjects were required to maintain their original lifestyle and habits. The nutrient intake was analyzed by a nutritionist.
Table 1 presents the daily nutritional intake of participants, including carbohydrates, proteins, fats, and total calories, both before the experiment and six weeks after the intervention. No notable distinctions were observed between the groups, and there was no statistically significant alteration when comparing the pre- and post-intervention values within each group. The experimental design is shown in
Figure 1.
2.4. Resistance Exercise Training
All subjects were instructed to engage in resistance training sessions three times per week, utilizing pneumatic resistance training equipment. The equipment comprised abdominal/back 5310, abduction/adduction 3520-HI5, push-up/pull-down 3120, leg extension/curl 3530, twist rehab 5340, and leg press 5540 (AB Hur Oy, Kokkola, Finland). This equipment selection was designed to target the enhancement of strength in specific muscle groups, including the upper limbs, back, abdomen, and legs. The incorporation of pneumatic resistance training equipment provided a standardized platform for participants to engage in targeted exercises, fostering comprehensive strength development in the upper limbs, back, abdomen, and legs. Before initiation of the training regimen, each participant underwent an assessment of their 3RM (repetition maximum) and, subsequently, their 1RM (repetition maximum) was calculated. This calculation involved the application of a coefficient formula derived from the prior literature, ensuring the determination of an appropriate resistance training intensity [
23]. The training protocol spanned four weeks, with distinct progressions in intensity. In the initial week, participants executed a set of 15 repetitions at an intensity equivalent to 60% of their 1RM. Progressing to the second week, the intensity was elevated to 70% of 1RM, with a set comprising 12 repetitions. In the subsequent week, there was a further increase in intensity to 75% of 1RM, maintaining a set of 12 repetitions. Until the last week, subjects consistently maintained an intensity of 75% of 1RM, completing 2 sets of 10 repetitions. Researchers adjusted the resistance equipment weight and number of sets based on each subject’s individual 1RM in accordance with the exercise protocol. Subjects were required to complete the designated movements and sets during each training session, with multiple professionals present to monitor the integrity and safety of their movements.
2.5. Maximal Oxygen Uptake (VO2max)
Assessment of the maximum oxygen consumption and exercise performance in the subjects involved the utilization of a treadmill (Pulsar, h/p/cosmos, Traunstein, Bavaria, Germany) and an automated breathing analyzer (Vmax 29c, Sensor Medics, Yorba Linda, CA, USA). Following the manufacturer’s instructions, the flow sensor, as well as the O
2 and CO
2 sensors, underwent calibration before each test to guarantee accurate measurements. An automated calibration function was employed for the O
2 and CO
2 sensors. Heart rate (HR) monitoring was performed using a Polar heart rate device. Following the Bruce protocol, the treadmill was initiated at a speed of 7.2 km/h, progressively increasing by 1.8 km/h every 2 min until subjects reached a point of fatigue, as outlined in the Bruce protocol [
24]. The determination of maximum O
2 consumption occurred when the respiratory exchange rate (VCO
2/VO
2, the volume ratio of CO
2 produced to O
2) exceeded 1.10 and coincided with reaching the maximum heart rate (maximum HR = 220 − age). The average of the three highest peaks in VO
2max was computed to derive individual VO
2max values.
2.6. Handgrip Strength Test
Handgrip strength was quantified in kilograms using a Takei digital grip strength meter (T. K. K. 5401, Takei Scientific Instruments Co., Ltd., Kamo, Niigata, Japan). Prior to the formal testing, subjects were instructed to apply minimal force to the gripper as a preliminary measure, in order to ensure their comprehension of the operating procedure and gripping distance. The researchers randomly assigned either the dominant or non-dominant hand as the starting point for the test. During the examination, participants were directed to exert maximal effort while squeezing the gripper with one hand, sustaining the squeeze for a minimum of 5 s. To mitigate the effects of fatigue, subjects alternated hands at 60 s intervals and repeated the test. This exchange method was repeated three times, and the maximum grip strengths of both the dominant and non-dominant hands were individually recorded [
25].
2.7. Countermovement Jump Assessment (CMJ)
The CMJ test serves as a means to assess the maximal speed, strength, and explosiveness of the lower body. During this evaluation, participants were directed to place their hands on their hips and position their feet on a Kistler force-measuring platform (9260AA, Kistler GmbH, Winterthur, Switzerland). Subsequently, they were instructed to execute a squat until their knees reached a 90-degree bend, followed with an immediate jump with maximal force to attain the highest possible vertical height. Each participant performed three replicates of the CMJ test, and data were acquired at specified points. The instrument was calibrated for each individual’s weight to ensure accuracy [
26]. The parameters measured included the rate of force development (RFD), relative peak force, and jump height. These metrics collectively provided insights into the dynamic capabilities of the lower body, reflecting the participant’s ability to generate force and power during vertical jumping movements.
2.8. Isometric Mid-Thigh Pull (IMTP)
The isometric mid-thigh pull (IMTP) serves as a force–time diagnostic tool [
27]. For this purpose, customized IMTP test equipment and two force plates (type 9287BA, Kistler Instruments AG, Winterthur, Switzerland) were employed. All participants were positioned with their feet at a consistent width and a rod was placed between the thighs, ensuring an upright torso, neutral spine, and knee and hip angles set at 140° to acquaint them with the IMTP test method. A careful evaluation was conducted to confirm the symmetry of the force trace in the practice test and the stability of the weighing cycle. To minimize the risk of measurement errors associated with changes in posture, each test phase involved repeating the specific measurement at 2 min intervals. The recorded parameters encompassed the average relative peak force (N/kg), rate of force development (RFD), and peak rate of force development (pRFD). The collection of these parameters aimed to provide a nuanced understanding of the isometric force production capabilities of the participants and their potential alterations in response to the strenuous exercise regimen.
2.9. Wingate Anaerobic Test (WAnT)
Following a standardized warm-up routine, all participants underwent assessment using the classical Wingate Anaerobic Test (WAnT) on a cycloergometer (Monark 894E, Varberg, Sweden) in a 30 s “go all out” ultramax test. The seat height was adjusted to each participant’s satisfaction, and toe clips were utilized to prevent their feet from slipping off the pedals. Prior to the initial test, a 5 min warm-up at approximately 50 W was conducted. Following the warm-up, two 3 s preparation exercises, with a load of 3% of their own body weight, were administered to familiarize the participants with the resistance [
28]. The test commenced with the resistance set on the friction belt of the dynamometer. External loading was individually estimated at 5% of body weight. The recorded results included the relative mean power (W/kg), relative peak power (W/kg), and fatigue index (%), which provide insights into the anaerobic power of the participants, showcasing their mean and peak power outputs, as well as their resistance to fatigue during intense, short-duration efforts.
2.10. Body Composition
To assess body composition, a multi-frequency approach utilizing a bioelectrical impedance analyzer (BIA) was employed; specifically, an InBody 570 (In-body, Seoul, Republic of Korea) was utilized. This advanced device conducts measurements at frequencies of 1, 5, 50, 260, 500, and 1000 kHz within a 60 s timeframe. During the measurement process, participants stood on the foothold electrodes following clearance of the palms and soles. Holding the sensing handle with both hands, participants maintained an open-arm position at a 30° angle away from their body, refraining from speaking or making movements throughout the measurement period. Additionally, participants observed an 8 h fasting period prior to the tests, ensuring optimal conditions for the assessment of body composition. We conducted measurements every two weeks during the experimental period.
Because BIA is measured in skeletal muscle, it may be affected by the amount of water in the body. Therefore, we additionally used comprehensive and reliable dual-energy X-ray absorptiometry (DXA) measurements before and after the intervention and facilitated the acquisition of additional bone quality data. The Lunar iDXA system from GE Healthcare (Chicago, IL, USA) was used. Subjects were instructed to lie flat on the test bed, aligning their bodies along the centerline with limbs positioned within the detection range to measure the distribution of lean tissue, fat mass, and bone density. The DXA technology involved the use of two X-ray beams with different energies to scan the designated body regions. The scintillation detector received the X-rays that penetrated the inspected parts, facilitating the calculation and analysis of parameters such as muscle mass and body fat.
2.11. Ultrasonic Measurement of Muscle and Fascia Thickness
Muscle and fascia thickness measurements of the biceps brachii and quadriceps femoris muscles were conducted using an ultrasonic scanner (H1300, BENQ, Taipei, Taiwan). Participants were positioned lying flat on their backs with palms facing upward, assuming an anatomical position. For the biceps brachii, the measurement position involved determining the straight-line distance from the acromion to the transverse crease of the elbow. The ultrasonic probe was then placed at one-third of the transverse crease of the elbow. Regarding the quadriceps femoris, the muscle probe was positioned from the anterior superior iliac spine (ASIS) to the patella at a one-half straight-line distance from the edge [
29]. Muscle thickness analysis was comprised of calculating the average thickness by dividing the sum of the thickness at both ends of the muscle by two. The method for calculating muscle fascia thickness was based on the muscle fascia calculation method [
30], defined as muscle thickness × sin(θ), where θ represents the angle between the muscle fascia and the horizontal line. Image analysis was performed using ImageJ version 2.3.0/1.53f (National Institutes of Health, Bethesda, MD, USA).
2.12. Clinical Biochemistry and Hematology Analysis
Blood samples were obtained from arm venous catheters both before and after the 6-week intervention as part of the study. The collected samples underwent a comprehensive analysis encompassing liver function, renal function, blood lipids, and glucose levels to assess the metabolic and health status of the subjects. To ensure standardized conditions for blood analysis, all participants were instructed to observe a fasting period of 8 h at minimum prior to blood collection. The blood serum obtained from the samples was then assessed using an autoanalyzer (Hitachi 717, Tokyo, Japan) for various biochemical markers, including aspartate transaminase (AST), aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen (BUN), creatinine (CREA), uric acid (UA), and glucose levels. Complete blood count (CBC) profiles (Sysmex XE-2100, Sysmex Corporation, Kobe, Japan) were also analyzed at the 90 min time point in the recovery phase.
2.13. Statistics
All data are expressed as the mean ± SD. Statistical analyses were performed using SPSS Statistics 25 (IBM Co., Armonk, NY, USA). To assess differences within the groups before and after the intervention, a paired Student’s t-test was applied for parametric analysis. Post hoc comparisons were analyzed using the Bonferroni test. For non-parametric data, the Wilcoxon signed-rank test was employed. Between-group comparisons were conducted using an unpaired Student’s t-test for parametric analyses, while non-parametric data were analyzed using the Mann–Whitney U-test. A significance level of p < 0.05 was considered indicative of a statistically significant difference in all analyses.
4. Discussion
In recent years, a growing body of research has explored the concept of the gut–muscle axis [
31], and further evidence has emerged to support the beneficial effects of probiotics in promoting muscle growth and improving exercise performance [
32]. However, this study is the first to examine the benefits of supplementation with
L. brevis to increase muscle mass and exercise performance. In the current study, we found that 6 weeks of supplementation with GKEX and combined with resistance training three times/week could significantly improve muscle strength performance, explosive force, muscle mass, and muscle thickness compared with before the intervention. Supplementation with GKEX combined with resistance exercise training not only effectively increased muscle mass and strength, but it was also found to effectively reduce the body fat.
Resistance training has also been shown to improve the diversity and composition of the gut microbiota. It can increase the richness of SCFA-producing gut microbiota and reduce the relative abundance of pro-inflammatory-inducing species [
33]. Previous research has shown that elite rugby players have a higher gut microbial diversity compared to sedentary individuals. Additionally, athletes have a greater proportion of bacteria that are involved in the carbohydrate and amino acid metabolism, resulting in an increased production of SCFAs in the gut, such as acetate, butyrate, and propionate [
34]. Among these, butyrate has been shown to prevent cell apoptosis and muscle protein degradation by inhibiting histone deacetylase, and can also increase ATP and muscle fiber metabolic efficiency by activating the UCP2-AMPK-ACC and PGC1-α pathways [
18]. In addition, some studies have shown that butyric acid can activate AMPK signaling pathways, stimulate mitochondrial fatty acid oxidation, enhance the glucose metabolism in muscle cells, reduce lipid deposition in muscles, and thereby improve muscle quality and function [
18]. It may also promote the proliferation of C2C12 myoblasts by upregulating ERK phosphorylation, in which the expression of myogenic regulatory factors including Myf5 and MyoD are significantly increased, thereby regulating the activation of muscle satellite cells [
35]. In one of our previous animal studies, we found that supplementation with
L. plantarum PL-02 for 4 weeks combined with resistance exercise training significantly increased the muscle mass and strength performance in mice [
36]. Moreover, a study in humans without regular exercise habits has also found that supplementation with
L. plantarum TWK10 for 6 weeks significantly increased muscle mass and endurance performance [
4]. Although the
L. brevis intervention in our study was combined with resistance exercise training, similar results were found in terms of the promotion of muscle mass gain (
Table 2 and
Table 3). However, we need further research to verify the mechanism by which GKEX promotes muscle growth.
Improvements in muscle strength are thought to be positively correlated with increases in muscle mass [
37]. Furthermore, there is also a strong positive correlation between the ability of muscles to produce force and their cross-sectional area (CSA) [
38]. Previous research has confirmed that muscle strength is typically determined by the product of muscle mass and the relative strength to muscle volume. Therefore, increasing muscle thickness serves as the basis for achieving strength gains [
39]. Despite the use of a muscle ultrasound in our study for measurements, we observed a significant increase in muscle thickness in the GKEX supplementation group compared to before the intervention, which was accompanied by an increase in fascial thickness (
Table 4). As greater muscle strength and body mass require thicker connective tissue to withstand and transmit greater forces, increases in muscle thickness may also be accompanied by increases in fascia thickness [
40]. This seems to explain our results. In a previous study, continuous supplementation of pea peptides combined with resistance exercise training for 8 weeks significantly enhanced the expression of the insulin-like growth factor 1 receptor and AMP-activated protein kinase. Simultaneously, there was a significant reduction in the expression of myostatin—a muscle growth inhibitor—in the skeletal muscles. This led to an increase in the cross-sectional area of muscles and muscle strength in rats [
41]. Although one possible way that probiotics can enhance muscle mass or strength is by improving protein digestion and the absorption of amino acids into the bloodstream [
3], the
L. brevis used in the intervention of this study has not been proven to have this effect. Increased muscle mass is thought to have a positive impact on the rate of strength development, thereby improving movements such as sprinting, jumping, and the ability to change direction [
42]. In this study, grip strength, CMJ, IMTP, and anaerobic power tests were performed to assess changes in muscle strength and explosive performance [
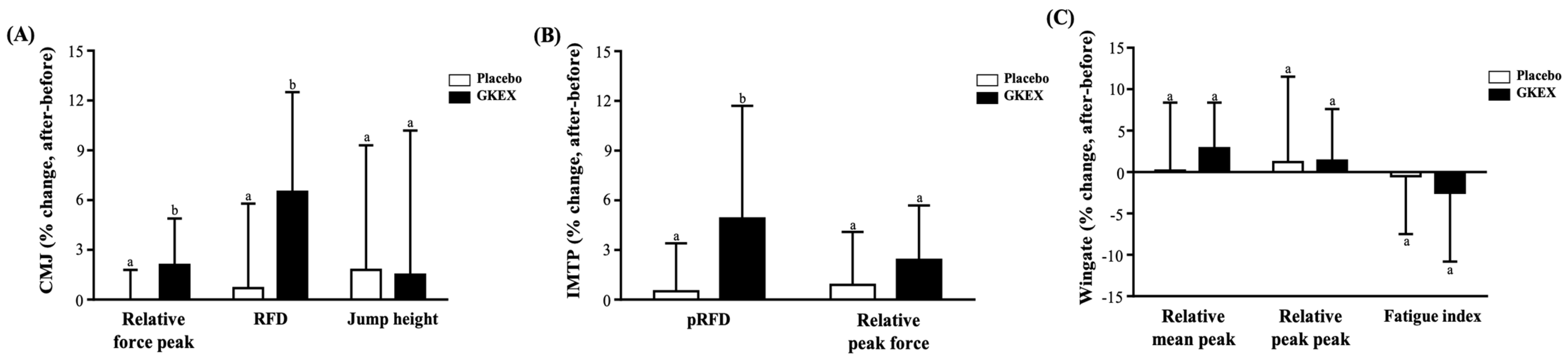
43]. Although there were no significant differences in various measurements between the two groups, we observed a notable increase in performance only in the group supplemented with GKEX when compared to that before the intervention (
Figure 1 and
Table 5). Moreover, the GKEX supplementation group exhibited more significant improvements in muscle strength and explosive performance compared to the placebo group, as demonstrated by the magnitude of changes (
Figure 4). Previous research in patients with sarcopenia has reported that daily supplementation for 16 weeks with a mixture of various strains totaling 1.12 trillion led to significant improvements in grip strength and gait speed [
44]. Another study, considering supplementation with inactivated and coagulated spore-forming bacteria and conducted in soldiers participating in a self-defense course, observed an increase in vertical jump power [
45]. These findings support the potential of probiotics in promoting the development of muscle strength. However, further exploration is needed to understand the mechanisms through which
L. brevis can enhance muscle mass and strength performance.
In addition to influencing muscle mass, probiotics are thought to have the potential to improve endurance performance, although several potential mechanisms remain uncertain. Previous research has suggested that supplementation with a mixture of different probiotics can attenuate the exercise-induced decrease in circulating tryptophan concentrations. This is thought to maintain normal serotonin metabolism, thereby slowing fatigue and preserving exercise performance [
46]. Studies have suggested that an increase in training volume and compliance might lead to a reduction in the frequency of upper respiratory tract infections (URTIs). In a trial involving female adolescent swimmers, it has been observed that continuous daily supplementation with 400 mL of probiotic yogurt containing 4 × 10
10 CFU/mL (including
Lactobacillus acidophilus SPP,
Lactobacillus delbrueckii Bulgaricus,
Bifidobacterium bifidum, and
Streptococcus salivarius subsp. thermophilus) for 8 weeks led to a significant reduction in the frequency and duration of URTIs, as well as an improvement in maximal oxygen consumption [
47]. In our previous study, we found that continuous supplementation with
L. plantarum TWK10 for six weeks led to a significant improvement in exercise endurance performance in mice. Additionally, there were notable increases in muscle mass and glycogen storage [
48]. Glycogen is a polymer of glucose that is related to muscle mass. It is catabolized by glycogen to produce adenosine triphosphate (ATP), which provides the primary fuel source for muscles during exercise [
49]. Bacteria in the gut may produce SCFAs, of which butyrate can maintain blood sugar stability and promote liver glucose metabolism through the GPR43-AKT-GSK3 signaling pathway [
50]. This can improve exercise endurance performance, delay fatigue, and accelerate recovery [
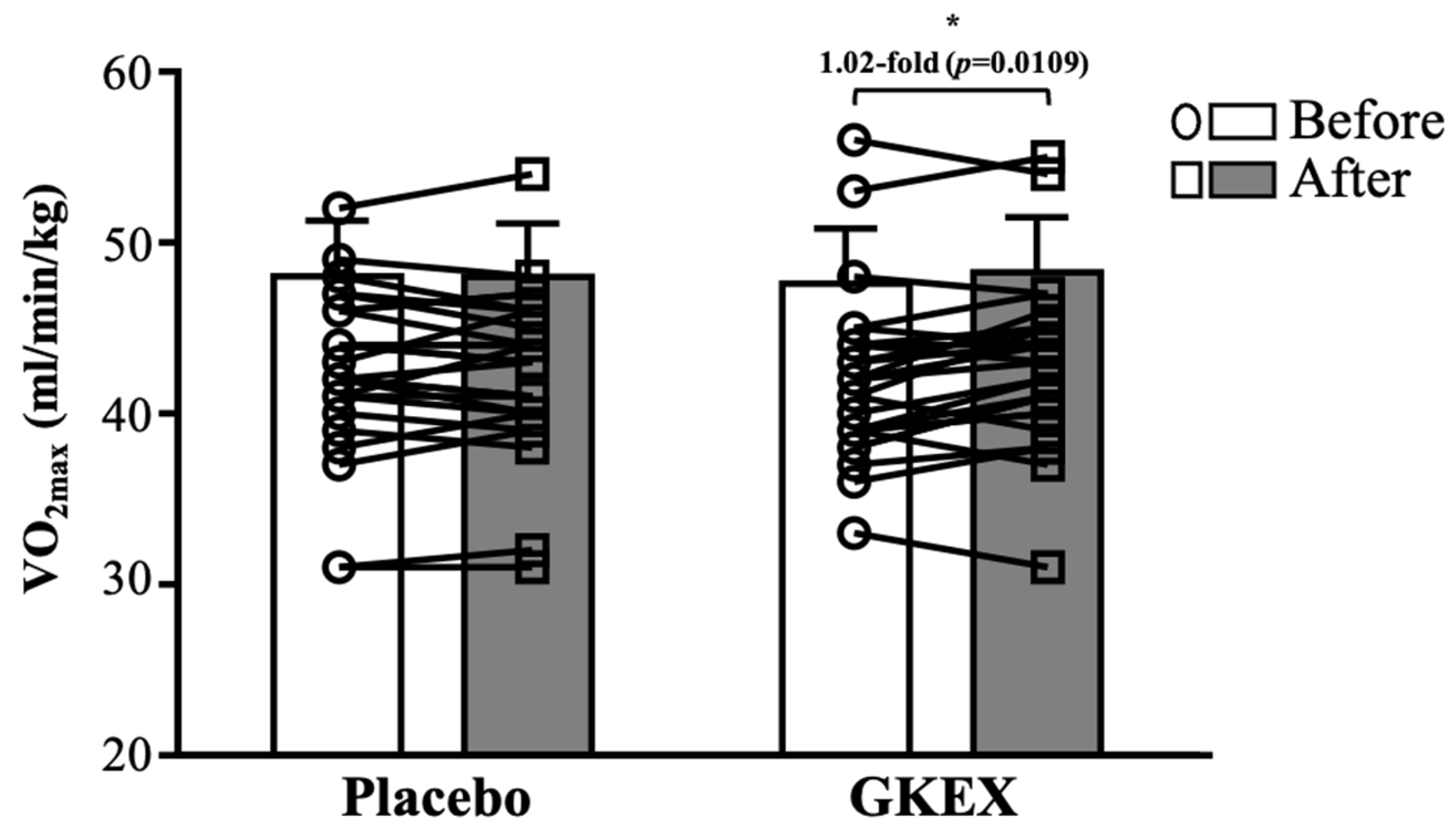
51]. Although it was found that 6 weeks of resistance exercise training combined with GKEX supplementation significantly increased VO
2max (
Figure 3) in this study, further research is required to gain a deeper understanding of the mechanisms involved.
Although this study confirmed the benefits of
L. brevis GKEX in terms of improving muscle mass and strength performance, there are limitations and possible mechanisms that require further exploration. To ensure gender equality, no special restrictions were imposed on recruitment, resulting in a slightly different gender ratio between the two groups of subjects. However, the implications of gender differences in the sample and the composition of the sample being comprised of physical education non-majors, specifically, physiological differences between males and females, such as in skeletal muscles, energy metabolism, and hormonal profiles, can lead to larger standard deviations within the group. These differences can potentially affect the statistical results and interpretations of the study [
52]. This study primarily investigated the impact of GKEX on enhancing muscle mass and exercise performance, necessitating the inclusion of both male and female subjects in the same group for comparison. Therefore, it may be necessary to include more subjects in future trials to further compare the effects and mechanisms of this sample in both genders. Second, all participants were non-physical education majors from the same university. Although their living conditions were similar and variability was low, this does not necessarily mean that the results apply to the wider ethnic group or population. Third, the original purpose of this study was to explore efficacy and, so, the group design did not compare whether sedentary control or supplementation alone had substantial benefits. Furthermore, apart from this study, no research has been conducted on the correlation between
L. brevis GKEX and muscle and exercise performance. In summary, the current study demonstrates that
L. brevis GKEX supplementation combined with resistance exercise training improves muscle mass and strength performance. Although there have been many studies in the past showing the benefits of combining probiotics with exercise training on athletic performance and even competitive athletes [
53], this study aimed to investigate its efficacy. To minimize the burden on participants, we collected a limited number of specimens. Once the efficacy test results are confirmed, we plan to delve deeper into the mechanisms using more rigorous experimental designs and analysis projects. Finally, to gain a deeper understanding of the cellular mechanisms underlying our findings, it may be necessary to conduct muscle biopsies or in vitro animal studies. Based on the above, a broader perspective is needed to confirm our findings. We aim to delve deeper into the mechanisms of muscle growth and the role of the gut–muscle axis by analyzing the gut microbiota. We anticipate that, through continued research into these mechanisms and the efficacy results obtained from this study, our findings can be applied to athletes, offering a potential avenue for sports nutrition supplements. Further research in the future needs to develop more stringent experimental design and condition restrictions, and make appropriate adjustments based on the characteristics of the probiotics and the intervention time [
54].