Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Methodological Assessment
2.4. Data Synthesis and Analysis
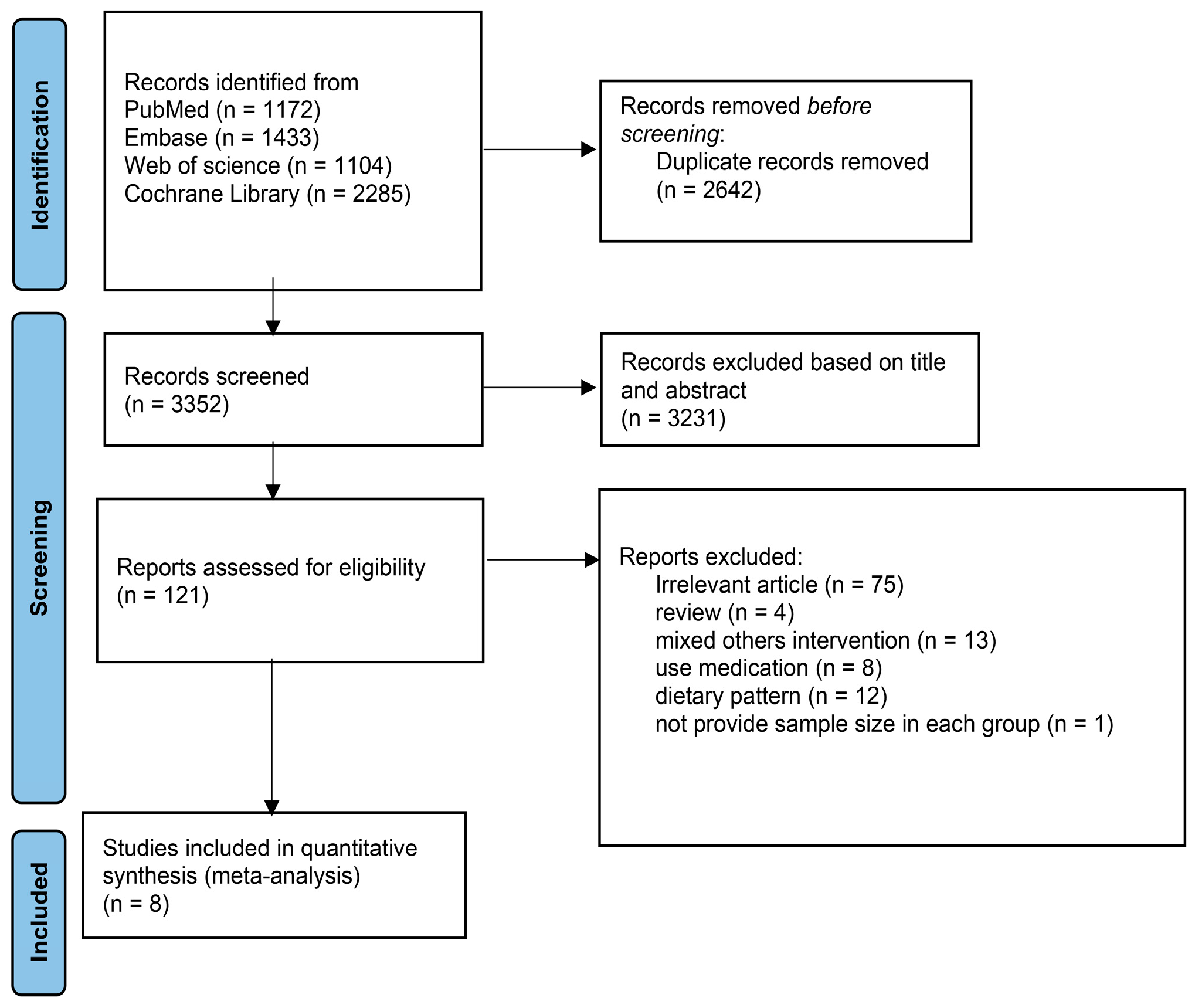
3. Results
3.1. Description of Selected Trials
3.2. Study Characteristics
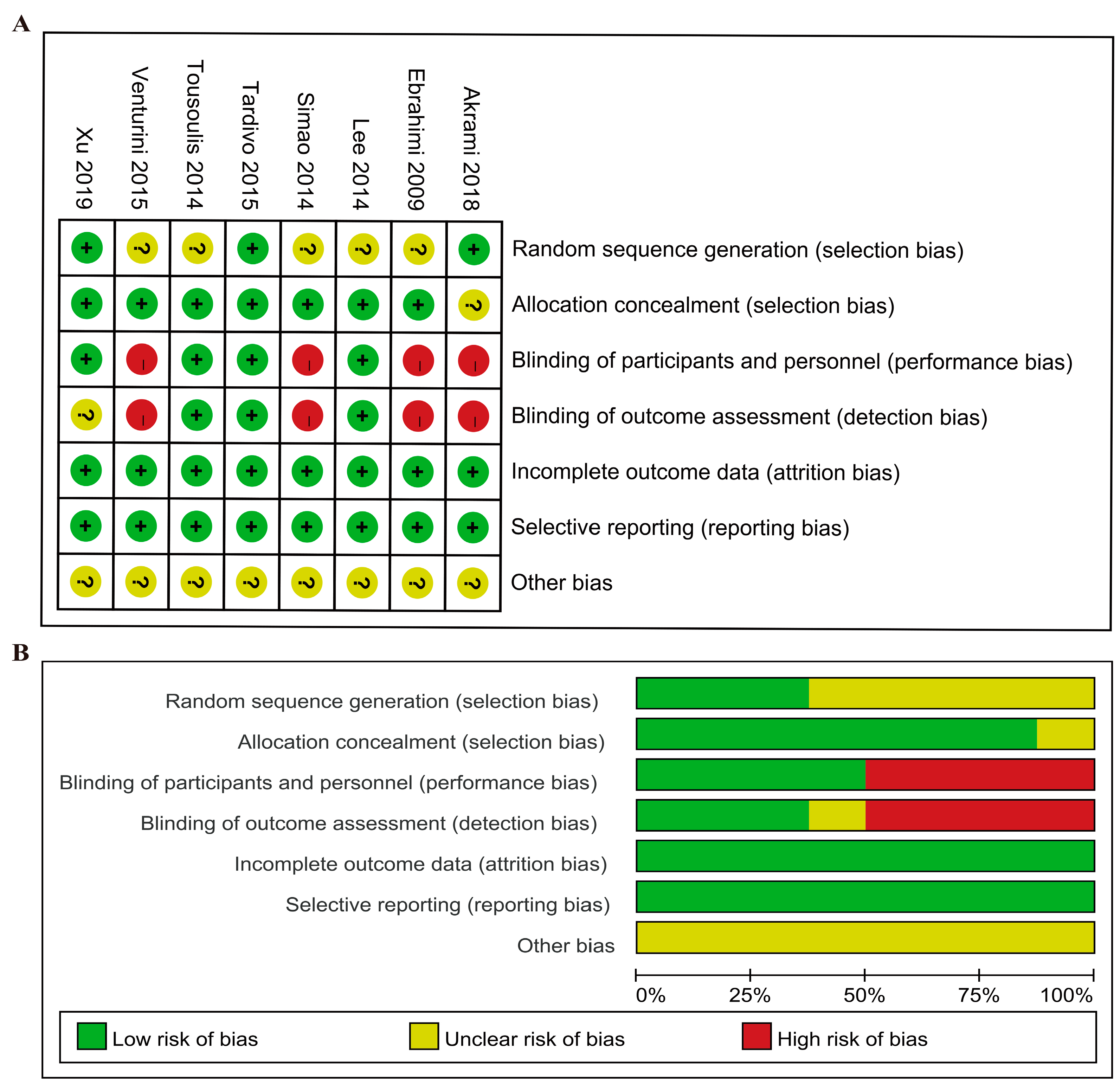
3.3. Risk of Bias Assessment
3.4. Low-Density Lipoprotein Cholesterol
3.5. High-Density Lipoprotein Cholesterol
3.6. Total Cholesterol
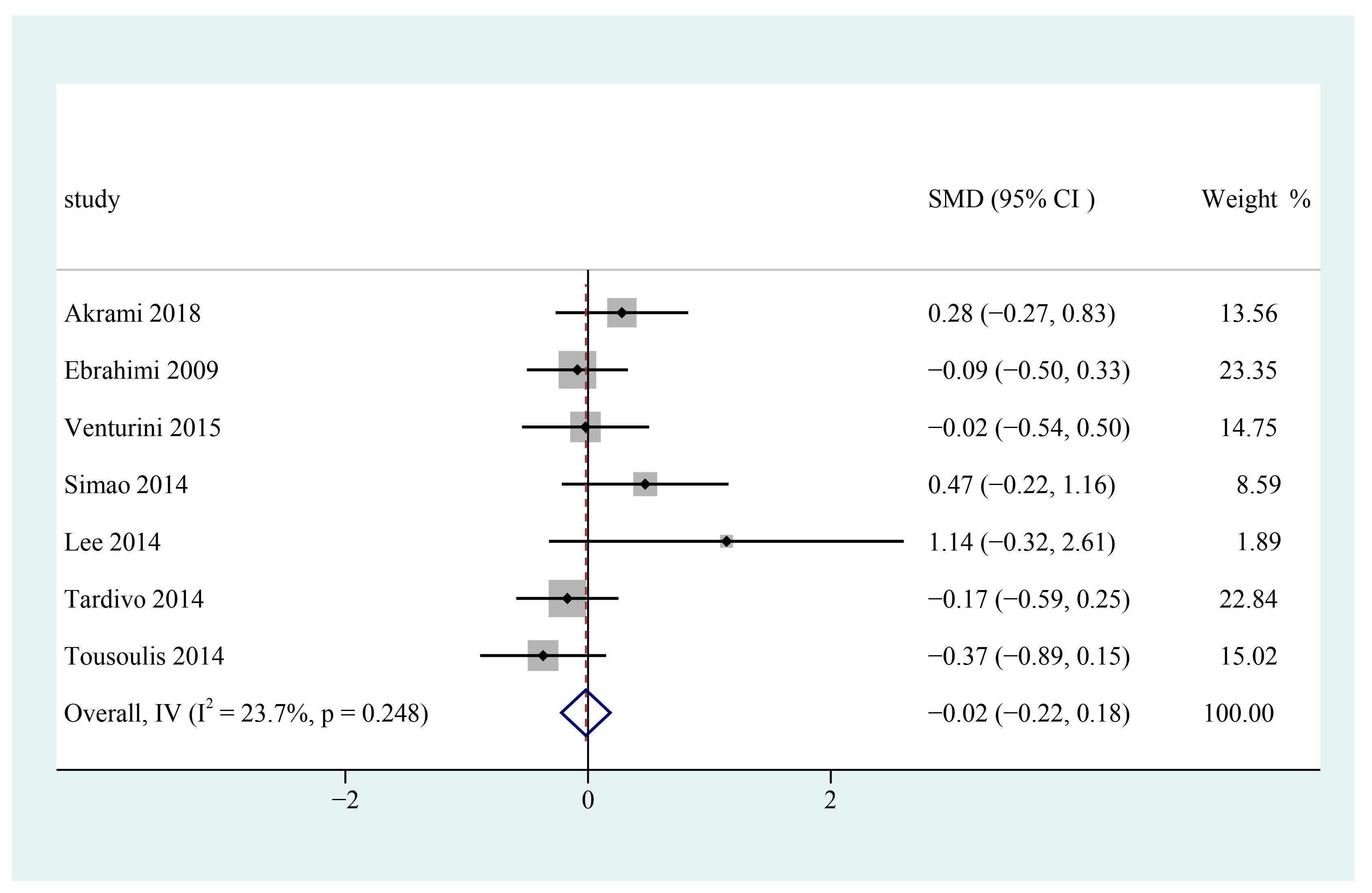
3.7. Triglycerides
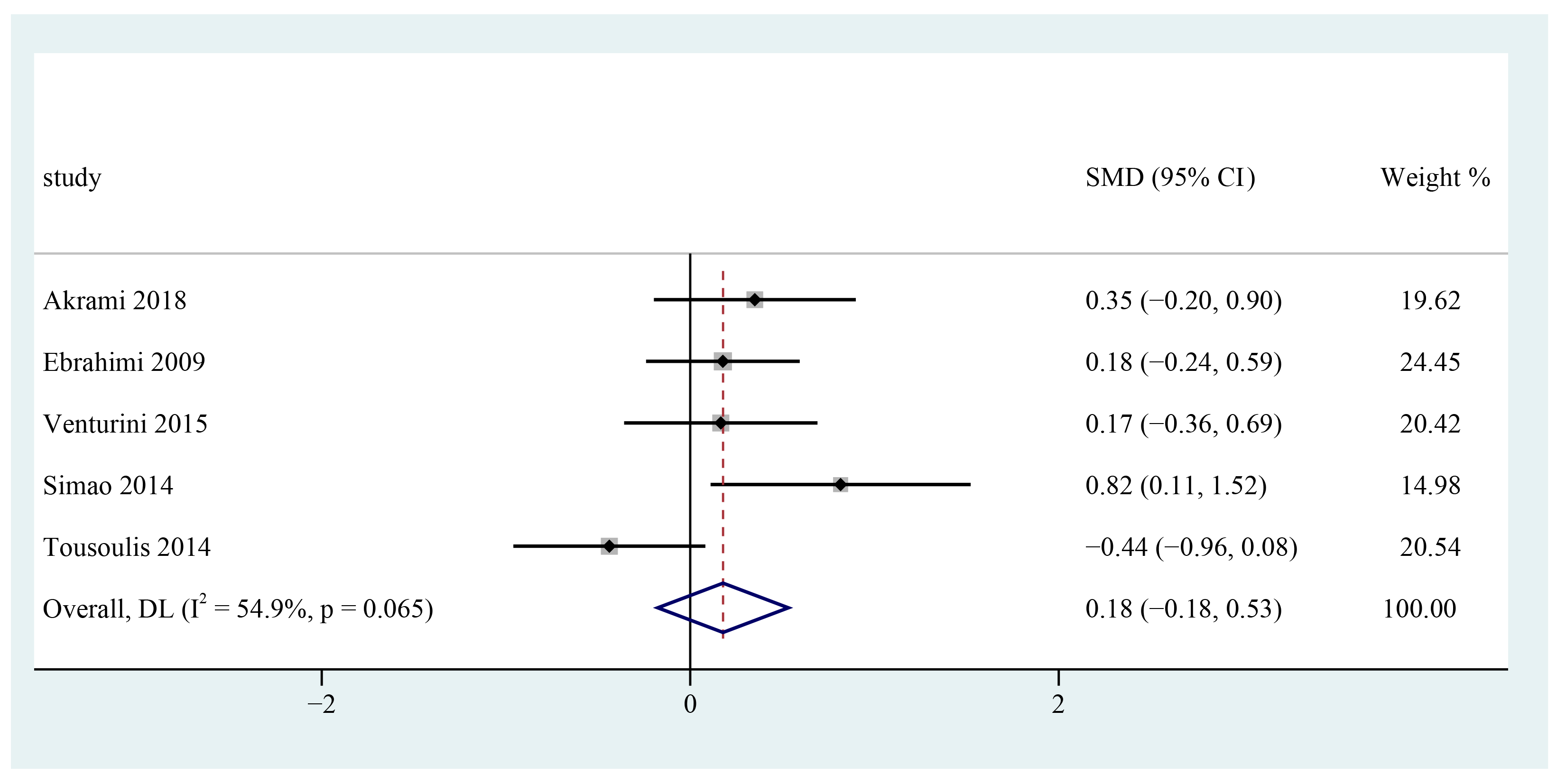
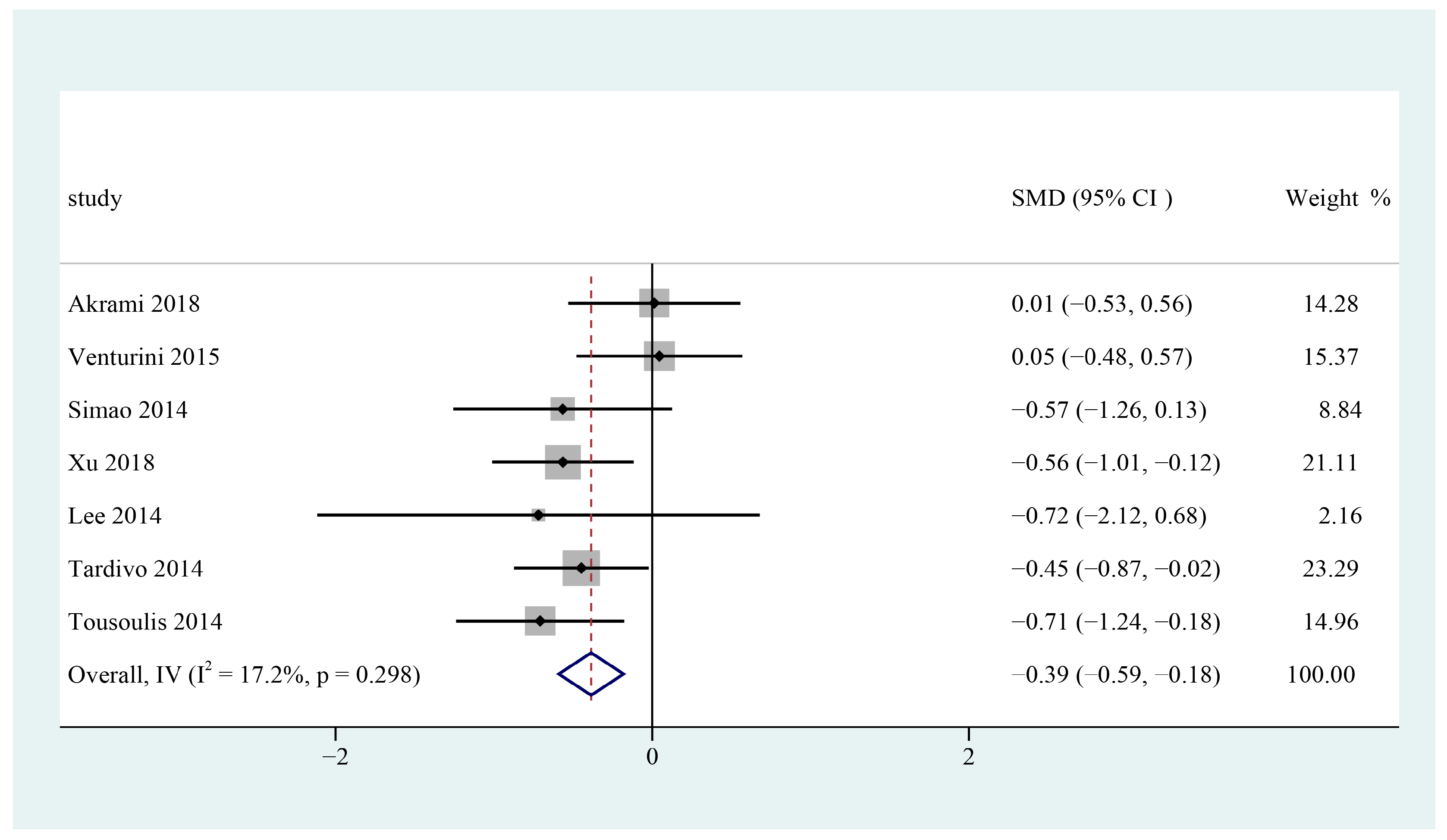
3.8. Systolic Blood Pressure
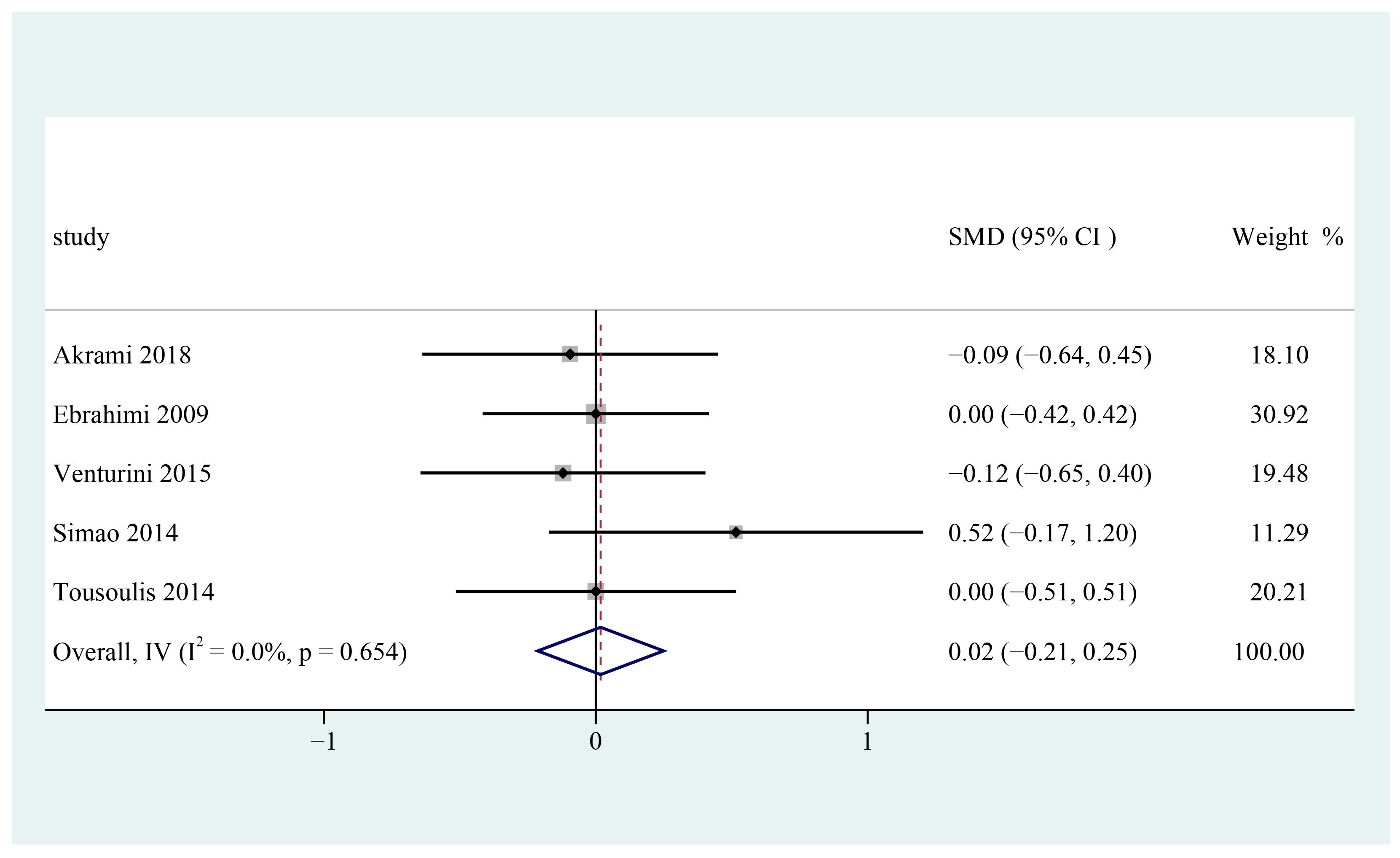
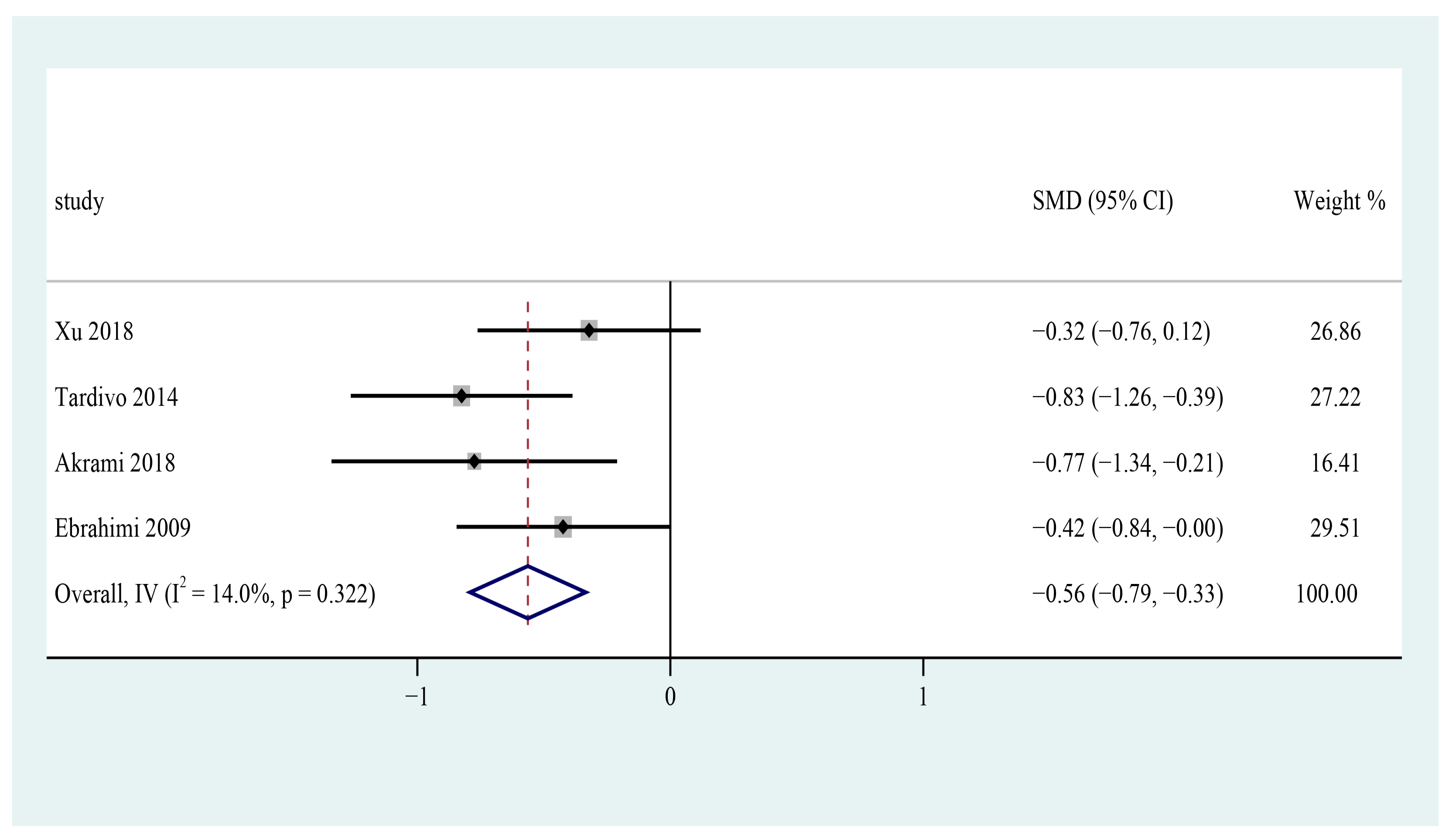
3.9. Diastolic Blood Pressure
3.10. Subgroup Analysis and Sensitivity Analysis
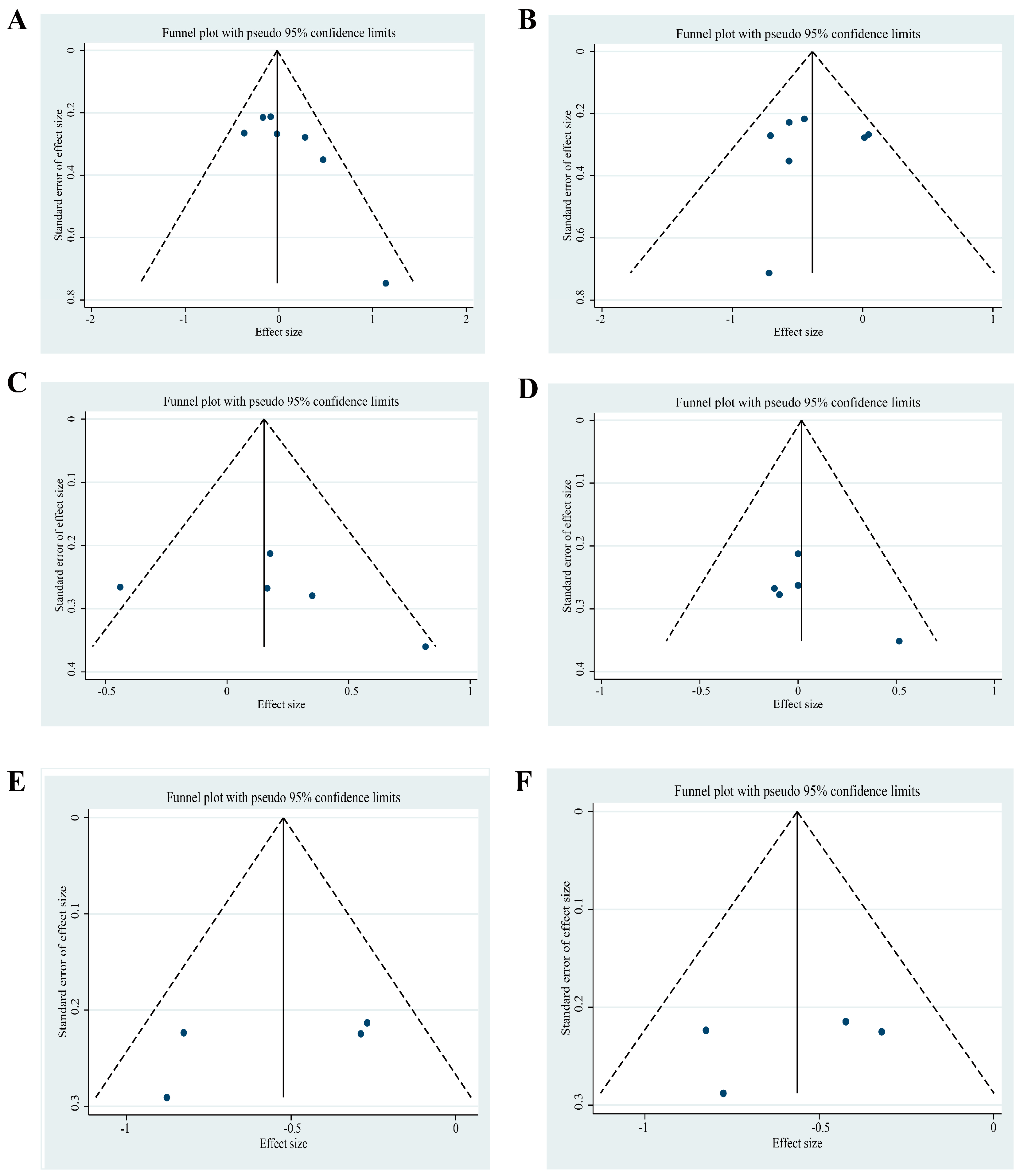
3.11. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Search Strategy
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- de la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martinez, J.A.; Reglero, G.; Ramirez de Molina, A. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Abete, I.; Astrup, A.; Martínez, J.A.; Thorsdottir, I.; Zulet, M.A. Obesity and the metabolic syndrome: Role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr. Rev. 2010, 68, 214–231. [Google Scholar] [CrossRef]
- Park, S.-W.; Kim, D.-Y.; Bak, G.-T.; Hyun, D.-S.; Kim, S.-K. Relation of Dietary n-3 and n-6 Fatty Acid Intakes to Metabolic Syndrome in Middle-Aged People Depending on the Level of HbA1c: A Review of National Health and Nutrition Survey Data from 2014 to 2016. Medicina 2022, 58, 1017. [Google Scholar] [CrossRef]
- Lai, Y.H.; Petrone, A.B.; Pankow, J.S.; Arnett, D.K.; North, K.E.; Ellison, R.C.; Hunt, S.C.; Djoussé, L. Association of dietary omega-3 fatty acids with prevalence of metabolic syndrome: The National Heart, Lung, and Blood Institute Family Heart Study. Clin. Nutr. 2013, 32, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.M.; Galli, C. Fat and Fatty Acid Terminology, Methods of Analysis and Fat Digestion and Metabolism: A Background Review Paper. Ann. Nutr. Metab. 2009, 55, 8–43. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health Benefits of n-3 Polyunsaturated Fatty Acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n−3 Fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499S–1504S. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Xun, P.; Iribarren, C.; Van Horn, L.; Steffen, L.; Daviglus, M.L.; Siscovick, D.; Liu, K.; He, K. Intake of fish and long-chain omega-3 polyunsaturated fatty acids and incidence of metabolic syndrome among American young adults: A 25-year follow-up study. Eur. J. Nutr. 2016, 55, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Gao, X.; Guo, X.-F.; Li, K.-L.; Li, S.; Sinclair, A.J.; Li, D. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin. Nutr. 2021, 40, 4538–4550. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Rafraf, M.; Farzadi, L.; Asghari-Jafarabadi, M.; Sabour, S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some meta-bolic risk factors in women with polycystic ovary syndrome. Asia Pac. J. Clin. Nutr. 2012, 21, 511–518. [Google Scholar]
- Ngo Njembe, M.T.; Pachikian, B.; Lobysheva, I.; Van Overstraeten, N.; Dejonghe, L.; Verstraelen, E.; Buchet, M.; Rasse, C.; Gardin, C.; Mignolet, E.; et al. A Three-Month Consumption of Eggs Enriched with ω-3, ω-5 and ω-7 Polyunsaturated Fatty Acids Significantly Decreases the Waist Circumference of Subjects at Risk of Developing Metabolic Syndrome: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 663. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Ghayour-Mobarhan, M.; Rezaiean, S.; Hoseini, M.; Parizade, S.M.; Farhoudi, F.; Hosseininezhad, S.J.; Tavallaei, S.; Vejdani, A.; Azimi-Nezhad, M.; et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009, 64, 321–327. [Google Scholar] [CrossRef]
- Venturini, D.; Simão, A.N.C.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Simao, A.N.C.; Lozovoy, M.A.B.; Dichi, I. Effect of soy product kinako and fish oil on serum lipids and glucose metabolism in women with metabolic syndrome. Nutrition 2014, 30, 112–115. [Google Scholar] [CrossRef]
- Tousoulis, D.; Plastiras, A.; Siasos, G.; Oikonomou, E.; Verveniotis, A.; Kokkou, E.; Maniatis, K.; Gouliopoulos, N.; Miliou, A.; Paraskevopoulos, T.; et al. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 2014, 232, 10–16. [Google Scholar] [CrossRef]
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; Kouba, E.O.; Chilton, F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014, 13, 196. [Google Scholar] [CrossRef]
- Tardivo, A.P.; Nahas-Neto, J.; Orsatti, C.L.; Dias, F.B.; Poloni, P.F.; Schmitt, E.B.; Nahas, E.A.P. Effects of omega-3 on metabolic markers in postmenopausal women with metabolic syndrome. Climacteric J. Int. Menopause Soc. 2015, 18, 290–298. [Google Scholar] [CrossRef]
- Xu, F.; Fan, W.; Wang, W.; Tang, W.; Yang, F.; Zhang, Y.; Cai, J.; Song, L.; Zhang, C. Effects of omega-3 fatty acids on metabolic syndrome in patients with schizophrenia: A 12-week randomized placebo-controlled trial. Psychopharmacology 2019, 236, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Chisholm, W.-A. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur. J. Clin. Nutr. 2001, 55, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Mater, M.K.; Pan, D.; Bergen, W.G.; Jump, D.B. Arachidonic acid inhibits lipogenic gene expression in 3T3-L1 adipocytes through a prostanoid pathway. J. Lipid Res. 1998, 39, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R. 15-Deoxy-delta 12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Mateș, L.; Popa, D.-S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef]
- Guo, X.-F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Ogawa, A.; Suzuki, Y.; Aoyama, T.; Takeuchi, H. Dietary Alpha-Linolenic Acid Inhibits Angiotensin-Converting Enzyme Activity and mRNA Expression Levels in the Aorta of Spontaneously Hypertensive Rats. J. Oleo Sci. 2009, 58, 355–360. [Google Scholar] [CrossRef]
- Belchior, T.; Paschoal, V.A.; Magdalon, J.; Chimin, P.; Farias, T.M.; Chaves-Filho, A.B.; Gorjão, R.; St.-Pierre, P.; Miyamoto, S.; Kang, J.X.; et al. Omega-3 fatty acids protect from diet-induced obesity, glucose intolerance, and adipose tissue inflammation through PPARγ-dependent and PPARγ-independent actions. Mol. Nutr. Food Res. 2015, 59, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, J.; Bellamy, M.F.; Ramsey, M.W.; Jones, C.J.; Lewis, M.J. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 35, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ghasemifard, S.; Hermon, K.; Turchini, G.M.; Sinclair, A.J. Metabolic fate (absorption, β-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil) in the rat. Br. J. Nutr. 2015, 114, 684–692. [Google Scholar] [CrossRef]
- Sato, A.; Kawano, H.; Notsu, T.; Ohta, M.; Nakakuki, M.; Mizuguchi, K.; Itoh, M.; Suganami, T.; Ogawa, Y. Antiobesity Effect of Eicosapentaenoic Acid in High-Fat/High-Sucrose Diet–Induced Obesity: Importance of hepatic lipogenesis. Diabetes 2010, 59, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Dickinson, H.O.; Critchley, J.A.; Ford, G.A.; Bradburn, M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur. J. Prev. Cardiol. 2013, 20, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Zhu, Y.; Zhang, X. Omega-3 Polyunsaturated Fatty Acids: Versatile Roles in Blood Pressure Regulation. Antioxid. Redox Signal. 2021, 34, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]

| Author | Country | Age, Range (Mean or Median), y | Participants | Study Design | Intervention | Control Group | Intervention Duration | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Akrami 2018 [25] | Iran | 30–60 (48.6) | 52 Participants with metabolic syndrome (26 Experimental group, 26 Control group) | parallel | 25 mL flaxseed oil (10.25 mL ALA) | sunflower seed oil | 7 weeks | TC, TG, HDL-c, LDL-c, SBP, DBP |
| Ebrahimi 2009 [26] | Iran | 40–70 (42.9) | 60 Participants with metabolic syndrome (47 Experimental group, 42 Control group) | parallel | 1 g fish oil (180 mg EPA and 120 mg DHA) | blank control | 6 months | TC, TG, HDL-c, LDL-c, SBP, DBP |
| Venturini 2015 [27] | Brazil | NA (51.45) | 63 Participants with metabolic syndrome (21 Experimental group, 42 Control group) | parallel | 3 g fish oil (1800 mg EPA and 1200 mg DHA) | blank control | 90 days | TC, TG, HDL-c, LDL-c |
| Simao 2014 [28] | Brazil | NA (47.9) | 34 women with metabolic syndrome (19 intervention, 15 control) | parallel | 3 g fish oil (1200 mg DHA + 1800 mg EPA) | blank control | 90 days | TC, TG, HDL-c, LDL-c |
| Tousoulis 2014 [29] | Greece | NA (44) | 29 men with metabolic syndrome | crossover | 2 g n-3 PUFAs (46% EPA, 38% DHA) | placebo | 12 weeks | TC, TG, HDL-c, LDL-c |
| Lee 2014 [30] | USA | NA (33.9) | 14 metabolic syndromes (7 control, 3 fish oil, 4 Botanical oil) | parallel | 9 daily fish oil capsules | corn oil | 8 weeks | TC, TG, HDL-c, LDL-c |
| Tardivo 2015 [31] | Brazil | 45–75 (55.1) | 63 postmenopausal women with metabolic syndrome (33 intervention, 30 control) | parallel | 900 mg n-3 PUFAs (180 mg EPA, 120 mg DHA) | blank control | 6 months | TC, TG, SBP, DBP |
| Xu 2019 [32] | China | 18–45 (28.4) | 72 metabolic syndromes in patients with schizophrenia (37 intervention, 35 control) | parallel | 720 mg EPA and 480 mg DHA | 100 mg vitamin E (α-tocopherol) | 12 weeks | TG, HDL-c, SBP, DBP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-X.; Yu, J.-H.; Sun, J.-H.; Ma, W.-Q.; Wang, J.-J.; Sun, G.-J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725. https://doi.org/10.3390/foods12040725
Liu Y-X, Yu J-H, Sun J-H, Ma W-Q, Wang J-J, Sun G-J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods. 2023; 12(4):725. https://doi.org/10.3390/foods12040725
Chicago/Turabian StyleLiu, Yin-Xiu, Jun-Hui Yu, Ji-Han Sun, Wen-Qin Ma, Jin-Jing Wang, and Gui-Ju Sun. 2023. "Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Foods 12, no. 4: 725. https://doi.org/10.3390/foods12040725
APA StyleLiu, Y.-X., Yu, J.-H., Sun, J.-H., Ma, W.-Q., Wang, J.-J., & Sun, G.-J. (2023). Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods, 12(4), 725. https://doi.org/10.3390/foods12040725







