A Morphological Evaluation of the Antibiofilm Activity on an Implant Surface Using a New Electric Device: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
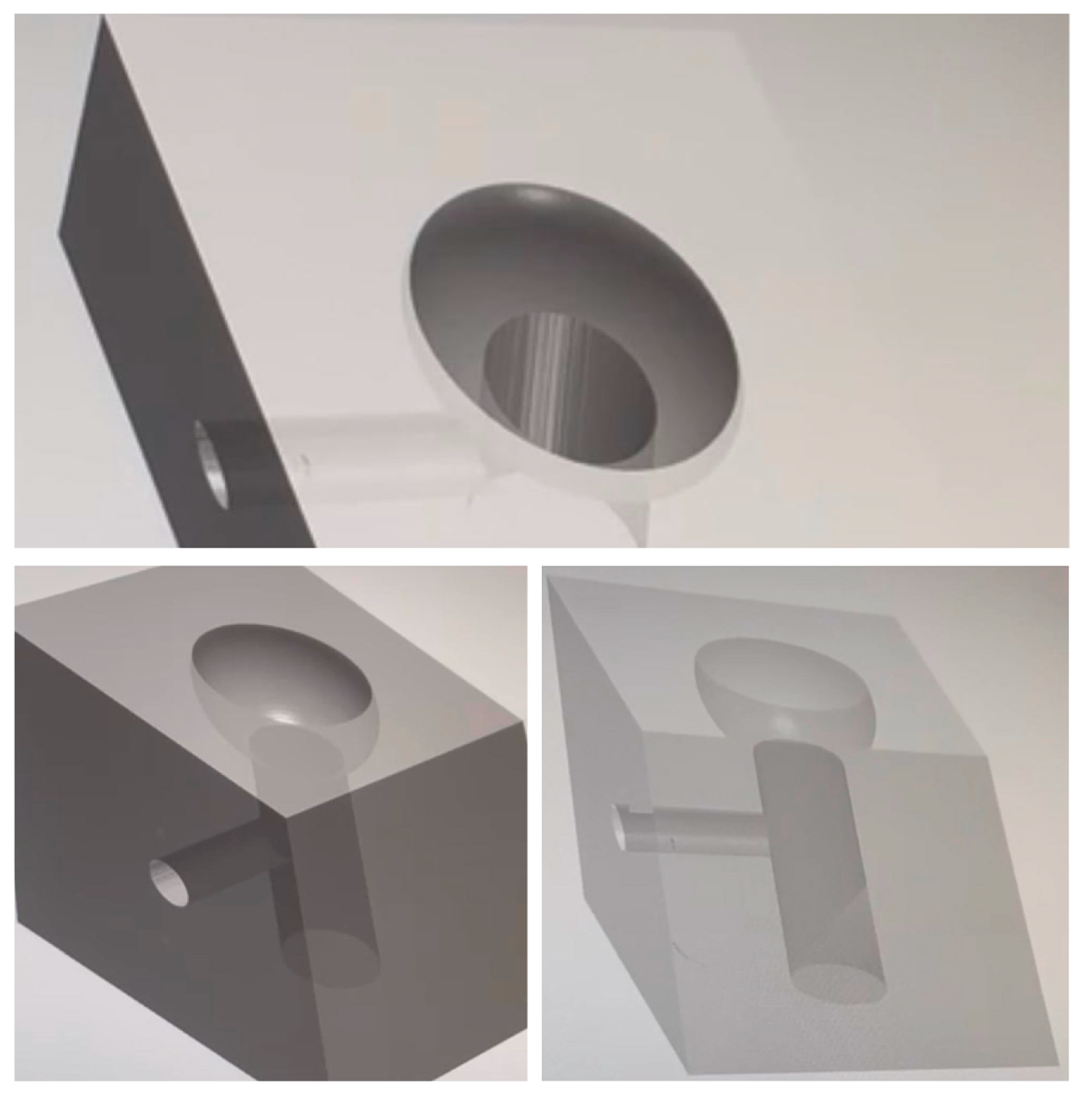
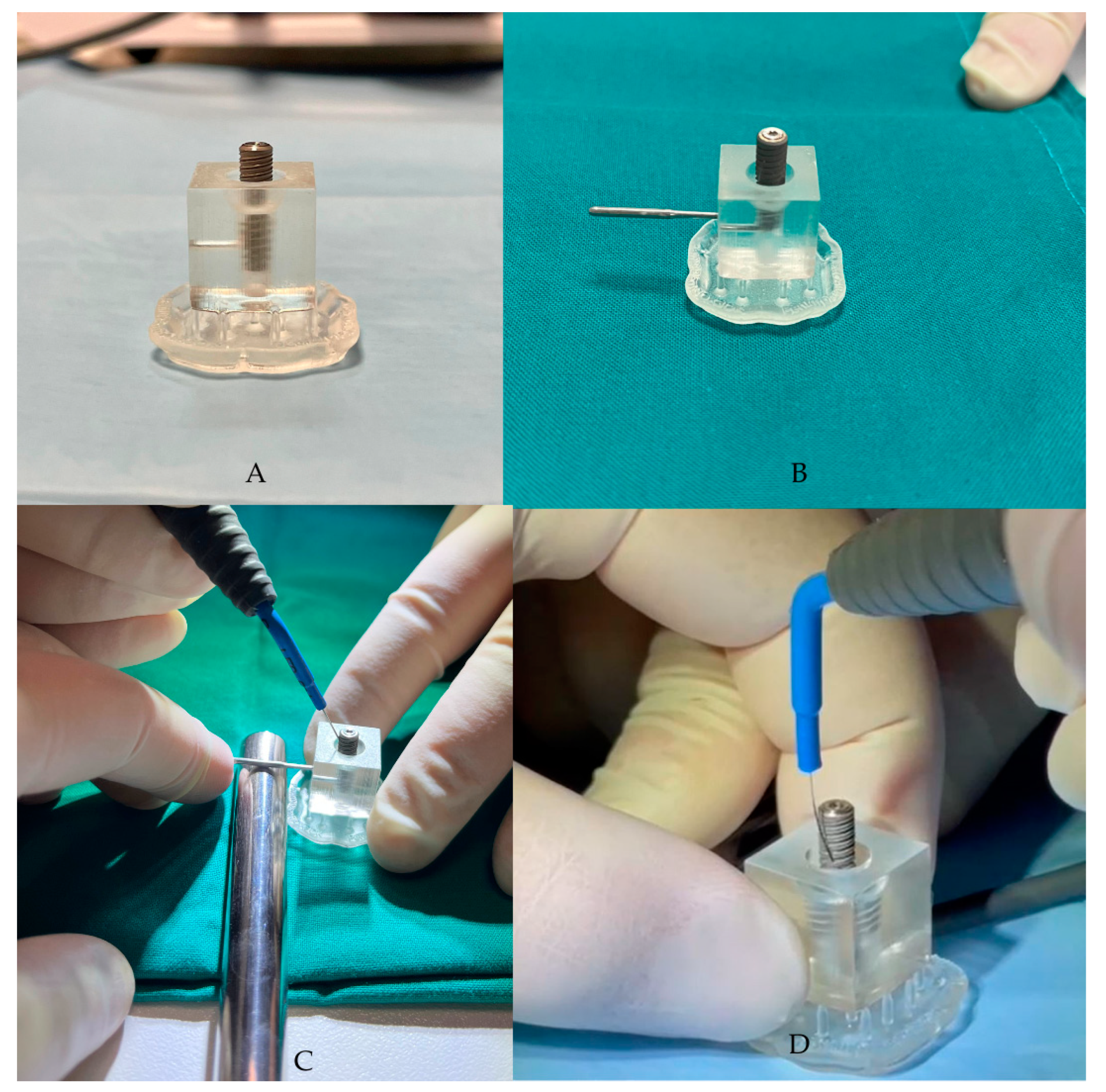
2.3. Implant Fixture Contamination: Assessment of the Microbial Load and Realization of the Peri-Implantitis Site Replica
2.4. Ximplant and Peri-Implantitis Protocol
2.5. Live/Dead Staining, Microscopy Observation, and Quantitative Image Analysis
- Selection of the captured picture;
- Splitting of the colors into the red and green channels to quantify the signal;
- Obtainment of a single channel 8-bit type image, which is a gray-scale type of image;
- Cleaning of noise from the image;
- Segmentation of the image, transforming it into a binary black-and-white image;
- Counting of the segmented cells.
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Initial Microbial Population Assessment
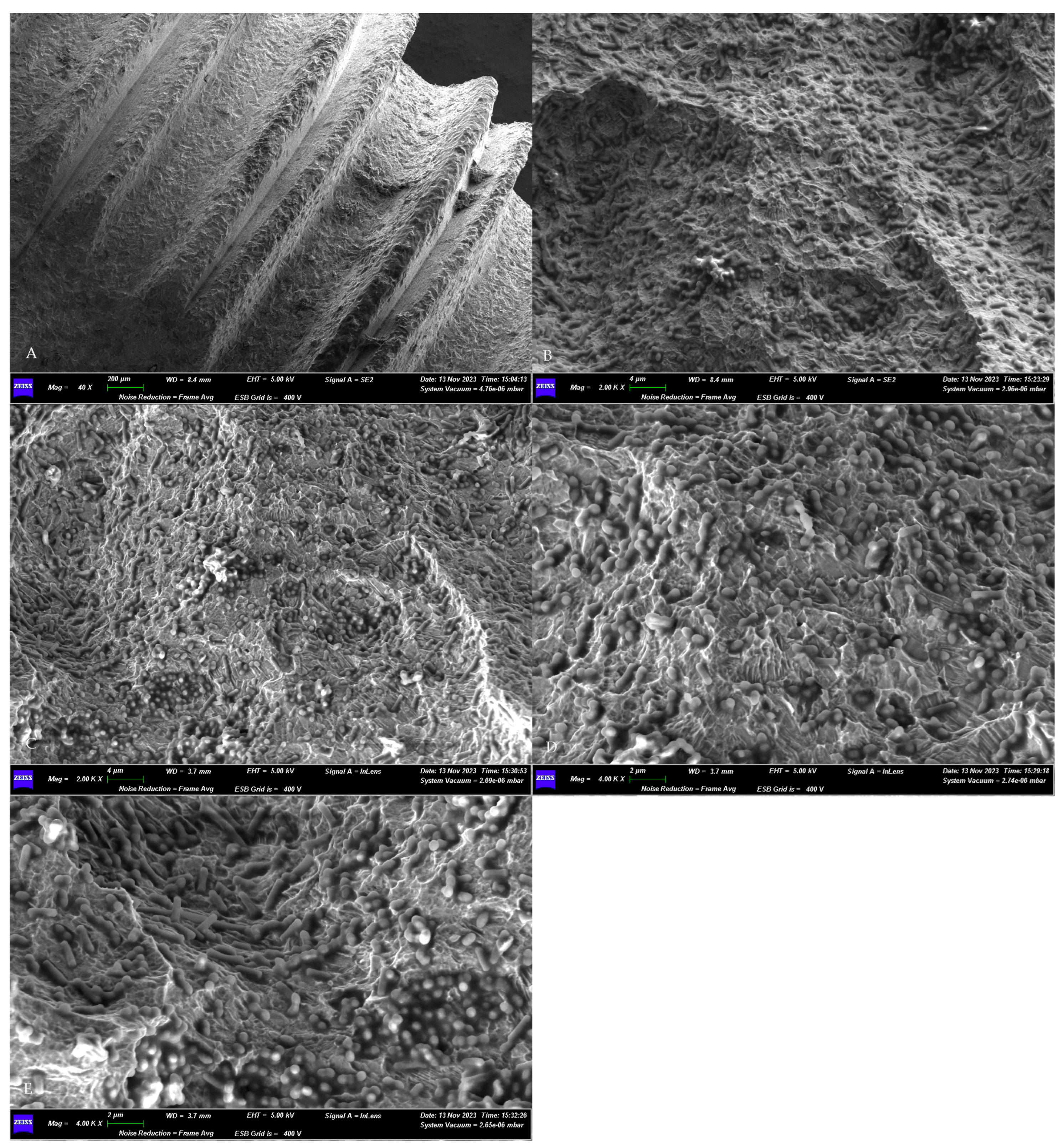
3.2. SEM Assessment of Microbial Growth
3.3. Qualitative Fluorescence Observation
3.4. Quantitative Image Analysis and Statistical Results
4. Discussion
4.1. Peri-Implantitis: The New Emerging Dental Disease That Should Be Avoided as Much as Possible
4.2. The Use of Electric and Electrolytic Effects to Remove Peri-Implant Biofilms as a Reliable Clinical Option
4.3. Strengths and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartoretto, S.C.; Shibli, J.A.; Javid, K.; Cotrim, K.; Canabarro, A.; Louro, R.S.; Lowenstein, A.; Mourão, C.F.; Moraschini, V. Comparing the Long-Term Success Rates of Tooth Preservation and Dental Implants: A Critical Review. J. Funct. Biomater. 2023, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Rösing, C.K.; Fiorini, T.; Haas, A.N.; Muniz, F.W.M.G.; Oppermann, R.V.; Susin, C. The impact of maintenance on peri-implant health. Braz. Oral Res. 2019, 33 (Suppl. S1), e074. [Google Scholar] [CrossRef] [PubMed]
- Santostasi, N.; Gerardi, D.; Rinaldi, F.; Bernardi, S.; Bianchi, I.; Pinchi, V.; Piattelli, M.; Varvara, G. Relationship between interleukin 1 (IL-1) genetic polymorphism and periimplantitis: Systematic literature review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3566–3582. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Wang, H.L. Peri-implant diseases: Current understanding and management. Int. J. Oral Implantol. 2021, 14, 263–282. [Google Scholar] [PubMed]
- Gerardi, D.; Bernardi, S.; Bruni, A.; Falisi, G.; Botticelli, G. Characterization and morphological methods for oral biofilm visualization: Where are we nowadays? AIMS Microbiol. 2024, 10, 391–414. [Google Scholar] [CrossRef]
- Franco, R.; Rosa, A.; Lupi, E.; Capogreco, M. The Influence of Dental Implant Roughness on Biofilm Formation: A Comprehensive Strategy. Dent. Hypotheses 2023, 14, 90–92. [Google Scholar] [CrossRef]
- Verket, A.; Koldsland, O.C.; Bunaes, D.; Lie, S.A.; Romandini, M. Non-surgical therapy of peri-implant mucositis-Mechanical/physical approaches: A systematic review. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed] [PubMed Central]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontology 2000 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Polizzi, A.; Cavalcanti, R.; Ronsivalle, V.; Chaurasia, A.; Spagnuolo, G.; Isola, G. Impact of Laser Therapy on Periodontal and Peri-Implant Diseases. Photobiomodul. Photomed. Laser Surg. 2022, 40, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.A. The role of lasers in the treatment of peri-implant diseases: A review. Saudi Dent. J. 2016, 28, 103–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komatsu, Y.; Kubota, T.; Yasuda, T.; Takahashi, T.; Yamamoto, A.; Kono, T.; Tabata, H.; Nohno, K.; Shibutani, T.; Umeda, M.; et al. Effectiveness of an erbium-doped: Yttrium, aluminum and garnet laser for treatment of peri-implant disease: Clinical, microbiological, and biochemical marker analyses. J. Clin. Exp. Dent. 2018, 10, e970–e978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atieh, M.A.; Fadhul, I.; Shah, M.; Hannawi, H.; Alsabeeha, N.H.M. Diode Laser as an Adjunctive Treatment for Peri-implant Mucositis: A Systematic Review and Meta-analysis. Int. Dent. J. 2022, 72, 735–745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abduljabbar, T.; Javed, F.; Kellesarian, S.V.; Vohra, F.; Romanos, G.E. Effect of Nd:YAG laser-assisted non-surgical mechanical debridement on clinical and radiographic peri-implant inflammatory parameters in patients with peri-implant disease. J. Photochem. Photobiol. B Biol. 2017, 168, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Gerardi, D.; Bartsch, S.; Macchiarelli, G.; Hellwig, E.; Al-Ahmad, A. Antimicrobial therapy using VIS plus water-filtered infrared-A as an alternative method to treat oral diseases. Futur. Microbiol. 2024, 19, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Qorri, E.; Botticelli, G.; Scarano, A.; Marzo, G.; Gatto, R.; Greco Lucchina, A.; Mortellaro, C.; Lupi, E.; Rastelli, C.; et al. Use of electrical field for biofilm implant removal. Eur. Rev. Med. Pharmacol. Sci. 2023, 27 (Suppl. S3), 114–121. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz-Zochniak, A.; Strom, K.; Jarzynka, S.; Iwańczyk, B.; Koryszewska-Bagińska, A.; Olędzka, G. Effect of Low Amperage Electric Current on Staphylococcus Aureus-Strategy for Combating Bacterial Biofilms Formation on Dental Implants in Cystic Fibrosis Patients, In Vitro Study. Materials 2021, 14, 6117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahrmann, P.; Zehnder, M.; Mohn, D.; Meier, A.; Imfeld, T.; Thurnheer, T. Effect of low direct current on anaerobic multispecies biofilm adhering to a titanium implant surface. Clin. Implant. Dent. Relat. Res. 2014, 16, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Memè, L.; Bambini, F.; Pizzolante, T.; Principi, M.; Sampalmieri, F.; Mummolo, S. Microscopic Analysis and Evaluation of Thermal Elevation and Wear of Drills for Implant Site Preparation: An In Vitro Study. Materials 2024, 17, 5524. [Google Scholar] [CrossRef]
- Memè, L.; Strappa, E.M.; Monterubbianesi, R.; Bambini, F.; Mummolo, S. SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Appl. Sci. 2022, 12, 1480. [Google Scholar] [CrossRef]
- Vanden Bogaerde, L. A proposal for the classification of bony defects adjacent to dental implants. Int. J. Periodontics Restor. Dent. 2004, 24, 264–271. [Google Scholar] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Allevi Protocols. Live/Dead Quantification Using Fiji—Step-by-Step Guide. Updated on 4 October 2022. Available online: https://www.allevi3d.com/livedead-assay-quantification-fiji/ (accessed on 12 December 2024).
- Falisi, G.; Di Paolo, C.; Rastelli, C.; Franceschini, C.; Rastelli, S.; Gatto, R.; Botticelli, G. Ultrashort Implants, Alternative Prosthetic Rehabilitation in Mandibular Atrophies in Fragile Subjects: A Retrospective Study. Healthcare 2021, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Botticelli, G.; Quinzi, V.; Giuca, G.; Mancini, L.; Marzo, G. Implant-safe test in patients with peri-implantitis. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S1), 147–153. [Google Scholar]
- Ozan, O.; Şeker, E.; Çakmak, G.; Guo, X.; Yilmaz, B. Effect of guide sleeve material, region, diameter, and number of times drills were used on the material loss from sleeves and drills used for surgical guides: An in vitro study. J. Prosthet. Dent. 2022, 128, 746–753. [Google Scholar] [CrossRef]
- Falisi, G.; Foffo, G.; Severino, M.; Di Paolo, C.; Bianchi, S.; Bernardi, S.; Pietropaoli, D.; Rastelli, S.; Gatto, R.; Botticelli, G. SEM-EDX Analysis of Metal Particles Deposition from Surgical Burs after Implant Guided Surgery Procedures. Coating 2022, 12, 240. [Google Scholar] [CrossRef]
- Saleh, M.H.A.; Dias, D.R.; Kumar, P. The economic and societal impact of periodontal and peri-implant diseases. Periodontol. 2000 2024. [Google Scholar] [CrossRef]
- Vernazza, C.; Heasman, P.; Gaunt, F.; Pennington, M. How to measure the cost-effectiveness of periodontal treatments. Periodontology 2012, 60, 138–146. [Google Scholar]
- Matarazzo, F.; Sabóia-Gomes, R.; Alves, B.E.S.; de Oliveira, R.P.; Araújo, M.G. Prevalence, extent and severity of peri-implant diseases. A cross-sectional study based on a university setting in Brazil. J. Periodontal Res. 2018, 53, 910–915. [Google Scholar]
- Shimchuk, A.A.; Weinstein, B.F.; Daubert, D.M. The impact of a change in classification criteria on the prevalence of peri-implantitis: A cross-sectional analysis. J. Periodontol. 2021, 92, 1339–1346. [Google Scholar]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990-2019: An analysis of the global burden of disease study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [PubMed]
- Zhang, Y.; Li, Y.; Yang, Y.; Wang, Y.; Cao, X.; Jin, Y.; Xu, Y.; Li, S.C.; Zhou, Q. Periodontal and Peri-Implant Microbiome Dysbiosis Is Associated With Alterations in the Microbial Community Structure and Local Stability. Front. Microbiol. 2022, 12, 785191. [Google Scholar] [CrossRef]
- Banu Raza, F.; Vijayaragavalu, S.; Kandasamy, R.; Krishnaswami, V.; Kumar, V.A. Microbiome and the inflammatory pathway in peri-implant health and disease with an updated review on treatment strategies. J. Oral Biol. Craniofacial Res. 2023, 13, 84–91. [Google Scholar] [CrossRef]
- Markowska, K.; Szymanek-Majchrzak, K.; Pituch, H.; Majewska, A. Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. Int. J. Mol. Sci. 2024, 25, 12808. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Sager, M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 202–213. [Google Scholar]
- Tastepe, C.S.; van Waas, R.; Liu, Y.; Wismeijer, D. Air powder abrasive treatment as an implant surface cleaning method: A literature review. Int. J. Oral Maxillofac. Implant. 2012, 27, 1461–1473. [Google Scholar]
- Giok, K.C.; Veettil, S.K.; Menon, R.K. Comparative effectiveness of interventions for the treatment of peri-implantitis: A systematic review with network meta-analysis. J. Prosthet. Dent. 2024; in press. [Google Scholar] [CrossRef]
- Sen, C.K.; Mathew-Steiner, S.S.; Das, A.; Sundaresan, V.B.; Roy, S. Electroceutical Management of Bacterial Biofilms and Surgical Infection. Antioxid. Redox Signal. 2020, 33, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Rodrigues da Silva, M.; Silva, F.S.; Madeira, S.; Carvalho, Ó. Electric Current Application on Dental Implant Biofilms: Review. J. Funct. Biomater. 2024, 15, 197. [Google Scholar] [CrossRef]
- Schlee, M.; Wang, H.-L.; Stumpf, T.; Brodbeck, U.; Bosshardt, D.; Rathe, F. Treatment of Periimplantitis with Electrolytic Cleaning versus Mechanical and Electrolytic Cleaning: 18-MonthResults from a Randomized Controlled Clinical Trial. J. Clin. Med. 2021, 10, 3475. [Google Scholar] [CrossRef]
- Ratka, C.; Weigl, P.; Henrich, D.; Koch, F.; Schlee, M.; Zipprich, H. The Effect of In Vitro Electrolytic Cleaning on Biofilm-Contaminated Implant Surfaces. J. Clin. Med. 2019, 8, 1397. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Brodbeck, U.R.; Rathe, F.; Stumpf, T.; Imber, J.C.; Weigl, P.; Schlee, M. Evidence of re-osseointegration after electrolytic cleaning and regenerative therapy of peri-implantitis in humans: A case report with four implants. Clin. Oral Investig. 2022, 26, 3735–3746. [Google Scholar] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar]
- Tawakoli, P.N.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 2013, 17, 841–850. [Google Scholar] [CrossRef]
- Wojcik, K.; Dobrucki, J.W. Interaction of a DNA intercalator DRAQ5, and a minor groove binder SYTO17, with chromatin in live cells--influence on chromatin organization and histone-DNA interactions. Cytometry A 2008, 73, 555–562. [Google Scholar] [CrossRef]
- Wang, M.; Ateia, M.; Hatano, Y.; Miyanaga, K.; Yoshimura, C. Novel fluorescence-based method for rapid quantification of live bacteria in river water and treated wastewater. Environ. Sci. Adv. 2022, 1, 30–36. [Google Scholar] [CrossRef]


| Imaging Sample | Area Squadre cm | Green Particles | Red Particles |
|---|---|---|---|
| A1 | 0.14 | 992 | 993 |
| A2 | 0.13 | 960 | 8574 |
| A3 | 0.16 | 4741 | 18,470 |
| A4 | 0.18 | 4546 | 6176 |
| B1 | 0.14 | 132 | 726 |
| B2 | 0.14 | 972 | 1342 |
| B3 | 0.13 | 946 | 1837 |
| C1 | 0.15 | 841 | 1693 |
| C2 | 0.16 | 1320 | 1893 |
| C3 | 0.14 | 58 | 731 |
| C4 | 0.16 | 1643 | 6043 |
| D1 | 0.16 | 2614 | 4862 |
| D2 | 0.16 | 948 | 3650 |
| D3 | 0.15 | 756 | 4526 |
| MEAN | 0.15 | 1533.5 | 4394 |
| STANDARD DEVIATION | 0.01 | 1452.8 | 4724.2 |
| Wilcoxon Matched-Pairs Signed Rank Test | |
|---|---|
| p value | 0.0001 |
| Exact or approximate p value? | Exact |
| pvalue summary | *** |
| Significantly different (p < 0.05)? | Yes |
| One- or two-tailed p value? | Two-tailed |
| Sum of positive and negative ranks | 105.0, 0.000 |
| Sum of signed ranks (W) | 105 |
| Number of pairs | 14 |
| Number of ties (ignored) | 0 |
| Median of differences | |
| Median | 1261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botticelli, G.; Falisi, G.; Rastelli, S.; Iacomino, E.; Bruni, A.; Gerardi, D.; Di Fabio, G.; Severino, M.; Bernardi, S. A Morphological Evaluation of the Antibiofilm Activity on an Implant Surface Using a New Electric Device: An In Vitro Study. Dent. J. 2025, 13, 140. https://doi.org/10.3390/dj13040140
Botticelli G, Falisi G, Rastelli S, Iacomino E, Bruni A, Gerardi D, Di Fabio G, Severino M, Bernardi S. A Morphological Evaluation of the Antibiofilm Activity on an Implant Surface Using a New Electric Device: An In Vitro Study. Dentistry Journal. 2025; 13(4):140. https://doi.org/10.3390/dj13040140
Chicago/Turabian StyleBotticelli, Gianluca, Giovanni Falisi, Sofia Rastelli, Enzo Iacomino, Angelo Bruni, Davide Gerardi, Giuseppe Di Fabio, Marco Severino, and Sara Bernardi. 2025. "A Morphological Evaluation of the Antibiofilm Activity on an Implant Surface Using a New Electric Device: An In Vitro Study" Dentistry Journal 13, no. 4: 140. https://doi.org/10.3390/dj13040140
APA StyleBotticelli, G., Falisi, G., Rastelli, S., Iacomino, E., Bruni, A., Gerardi, D., Di Fabio, G., Severino, M., & Bernardi, S. (2025). A Morphological Evaluation of the Antibiofilm Activity on an Implant Surface Using a New Electric Device: An In Vitro Study. Dentistry Journal, 13(4), 140. https://doi.org/10.3390/dj13040140










