The Use of Vacuum Plasma Surface Treatment to Improve the Hydrophilicity and Wettability of Bone Graft Substitutes and Resorbable Membranes: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Vacuum Plasma Surface Treatment
2.3. SEM Analysis
2.4. Outcome Measures
- -
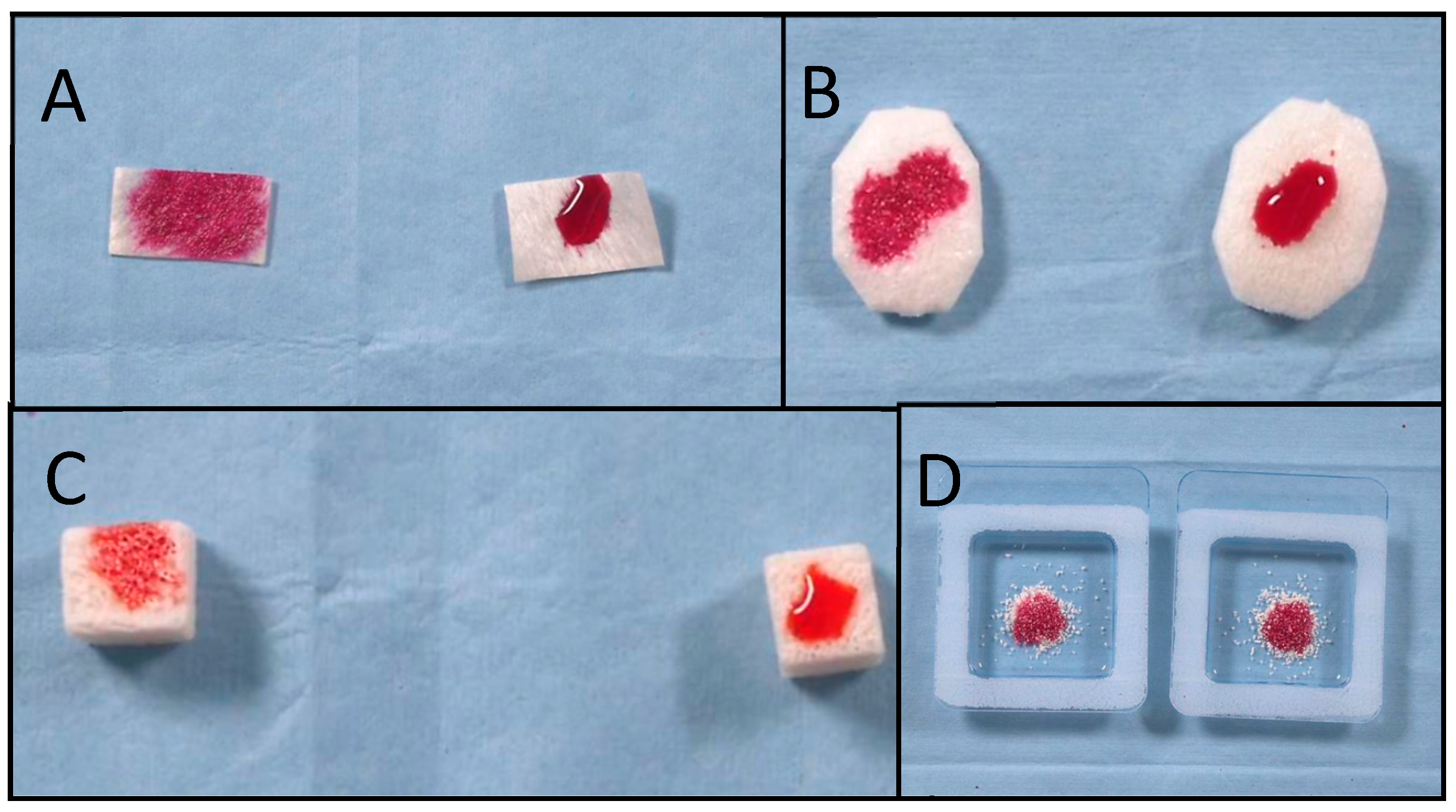
- Absorption time was defined as the interval, in seconds, from the moment that the last drop touched the biomaterial’s surface to the moment when all of the ink drops had been absorbed into the biomaterial. The recorded video was evaluated using a video editing application (iMovie for MacOS), and the absorption time was measured using the expanded timeline. All of the measurements were repeated three times by two different operators (M.T. and M.T.). The mean value and standard deviation (SD) were calculated.
- -
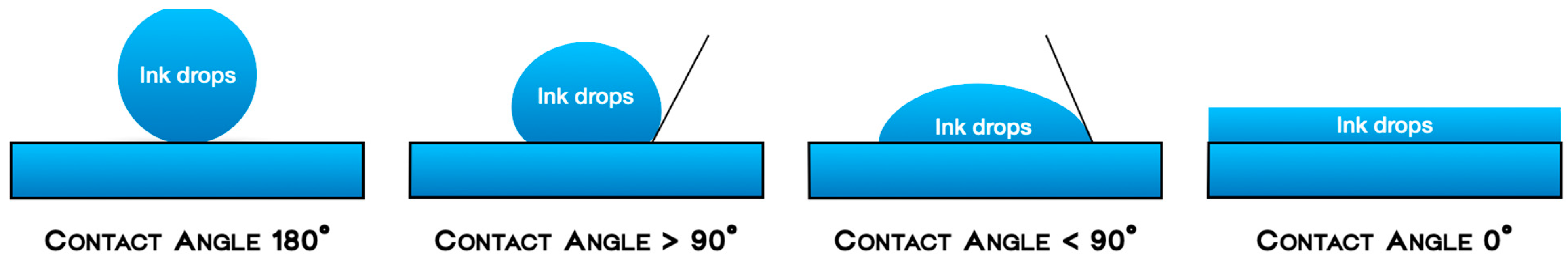
- Wettability (generally referred to as hydrophilicity) was defined as the spreads of the ink drops over the biomaterials’ surfaces, measured by the flatness of a droplet on the solid surface. The four grades of wettability were defined as follows:
- -
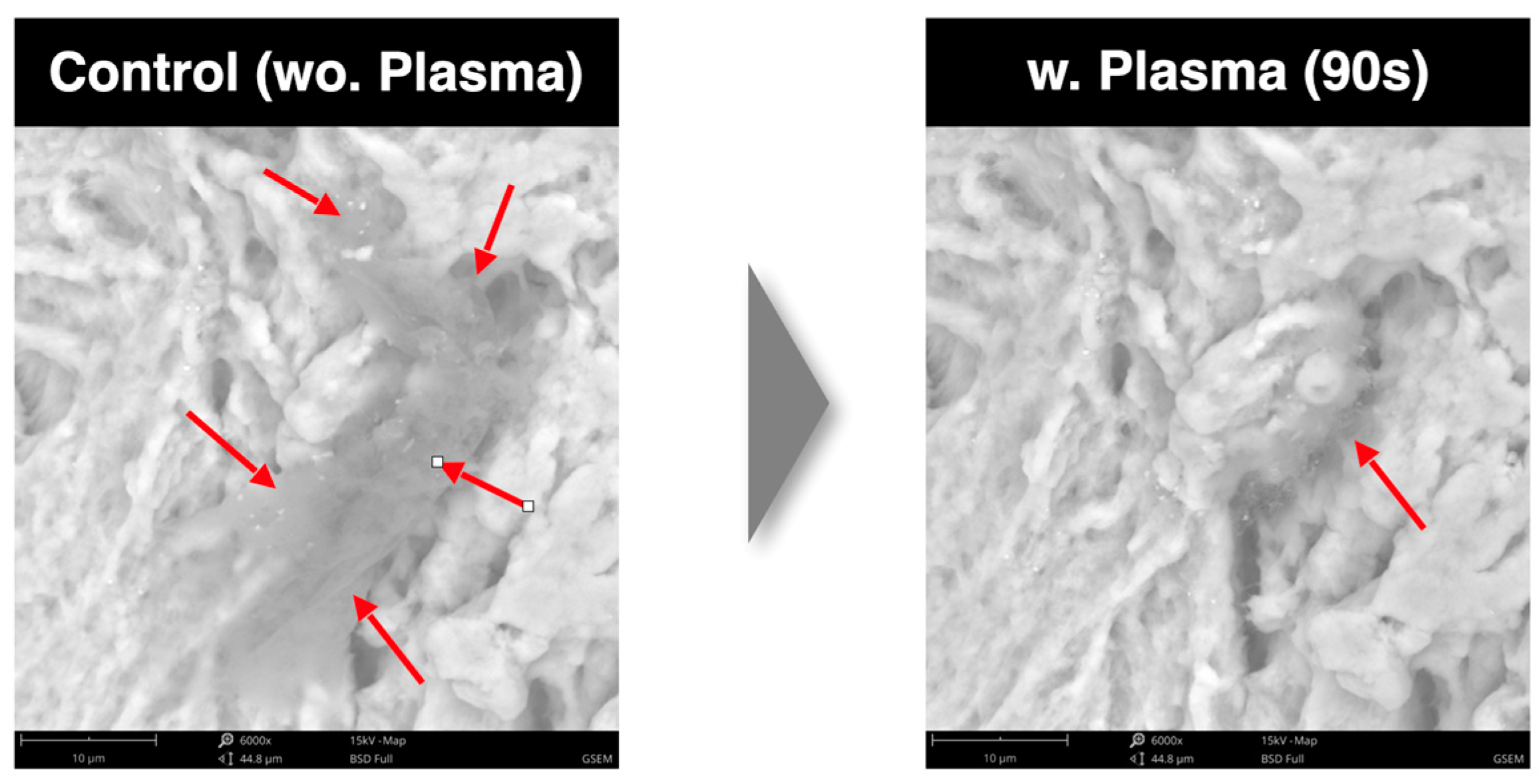
- SEM analysis. Scanning electron microscopy was employed to visualize high-resolution images of the sample surfaces. Using the SEM, the surface topography in the images was analyzed. In particular, the SEM images were used to evaluate the reduction rate of carbon impurities with three different cycle times. The energy-dispersive X-ray spectroscope (EDS) detector was used to measure the energy of the emitted photons in the X-ray electromagnetic spectrum and to obtain chemical information (the atomic percentage).
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Busenlechner, D.; Fürhauser, R.; Haas, R.; Watzek, G.; Mailath, G.; Pommer, B. Long-term implant success at the Academy for Oral Implantology: 8-year follow-up and risk factor analysis. J. Periodontal Implant. Sci. 2014, 44, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-I. A Tribute to Dr. Per-Ingvar Brånemark. J. Periodontal Implant. Sci. 2014, 44, 265. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of Implant Osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Peñarrocha, D.M.; Cavani, U.; Cuadrado, L. Atlas of Immediate Dental Implant Loading; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 9783030055462, 3030055469. [Google Scholar]
- Wong, M.; Eulenberger, J.; Schenk, R.; Hunziker, E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J. Biomed. Mater. Res. 1995, 29, 1567–1575. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7, S93–S105. [Google Scholar] [CrossRef]
- Sun, X.D.; Liu, T.T.; Wang, Q.Q.; Zhang, J.; Cao, M.S. Surface Modification and Functionalities for Titanium Dental Implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Tallarico, M.; Peñarrocha, M.; Corrente, G.; Fiorellini, J.; Peñarrocha, D. Plasma of Argon Cleaning Treatment on Implant Abutments in Periodontally Healthy Patients: Six Years Postloading Results of a Randomized Controlled Trial. Int. J. Periodontics Restor. Dent. 2017, 37, 683–690. [Google Scholar] [CrossRef]
- Canullo, L.; Tallarico, M.; Botticelli, D.; Alccayhuaman, K.A.A.; Neto, E.C.M.; Xavier, S.P. Hard and Soft Tissue Changes around Implants Activated Using Plasma of Argon: A Histomorphometric Study in Dog. Clin. Oral Implant. Res. 2018, 29, 389–395. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, B.; Shin, C.; You, B.; Oh, H.S.; Lee, J.; Lee, J.; Oh, S.K.; Oh, S.H. Improvement in Biocompatibility and Biointegration of Human Acellular Dermal Matrix through Vacuum Plasma Surface Treatment. Bioengineering 2024, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Shibata, Y.; Miyazaki, T. Glow Discharge Plasma Pretreatment Enhances Osteoclast Differentiation and Survival on Titanium Plates. Biomaterials 2004, 25, 1805–1811. [Google Scholar] [CrossRef]
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow Discharge Plasma Treatment of Titanium Plates Enhances Adhesion of Osteoblast-like Cells to the Plates through the Integrin-Mediated Mechanism. Int. J. Oral Maxillofac. Implant. 2002, 17, 771–777. [Google Scholar] [PubMed]
- Canullo, L.; Genova, T.; Mandracci, P.; Mussano, F.; Abundo, R.; Fiorellini, J.P. Morphometric Changes Induced by Cold Argon Plasma Treatment on Osteoblasts Grown on Different Dental Implant Surfaces. Int. J. Periodontics Restor. Dent. 2017, 37, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of Surface Topography and Surface Physicochemistry on Wettability of Zirconia (Tetragonal Zirconia Polycrystal). J. Biomed. Mater. Res. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef]
- Canullo, L.; Tallarico, M.; Peñarrocha-Oltra, D.; Monje, A.; Wang, H.; Peñarrocha-Diago, M. Implant Abutment Cleaning by Plasma of Argon: 5-Year Follow-Up of a Randomized Controlled Trial. J. Periodontol. 2016, 87, 434–442. [Google Scholar] [CrossRef]
- Krithikadatta, J.; Gopikrishna, V.; Datta, M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J. Conserv. Dent. 2014, 17, 301–304. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Chinigò, G.; Iacono, R.; Pesce, P.; Menini, M.; Mussano, F. Vacuum Plasma Treatment Device for Enhancing Fibroblast Activity on Machined and Rough Titanium Surfaces. Dent. J. 2024, 12, 71. [Google Scholar] [CrossRef]
- Jeon, H.J.; Jung, A.; Kim, H.J.; Seo, J.S.; Kim, J.Y.; Yum, M.S.; Gweon, B.; Lim, Y. Enhanced Osteoblast Adhesion and Proliferation on Vacuum Plasma-Treated Implant Surface. Appl. Sci. 2022, 12, 9884. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Santori, G.; Giovanni, E.D.; Bagnasco, F.; Canullo, L. Photo and Plasma Activation of Dental Implant Titanium Surfaces. A Systematic Review with Meta-Analysis of Pre-Clinical Studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, H.J.; Jung, A.; Kim, J.; Kim, J.Y.; Lee, S.H.; Kim, H.; Yeom, M.S.; Choe, W.; Gweon, B.; et al. Improvement of osseointegration efficacy of titanium implant through plasma surface treatment. Biomed. Eng. Lett. 2022, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Azarbayjani, A.; Jouyban, A. Surface tension in human pathophysiology and its application as a medical diagnostic tool. Bioimpacts 2015, 5, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Hrncír, E.; Rosina, J. Surface tension of blood. Physiol. Res. 1997, 46, 319–321. [Google Scholar] [PubMed]
- Baklanov, M.R.; Gismatulin, A.A.; Naumov, S.; Perevalov, T.V.; Gritsenko, V.A.; Vishnevskiy, A.S.; Rakhimova, T.V.; Vorotilov, K.A. Comprehensive Review on the Impact of Chemical Composition, Plasma Treatment, and Vacuum Ultraviolet (VUV) Irradiation on the Electrical Properties of Organosilicate Films. Polymers 2024, 16, 2230. [Google Scholar] [CrossRef]
- Primc, G. Strategies for Improved Wettability of Polyetheretherketone (PEEK) Polymers by Non-Equilibrium Plasma Treatment. Polymers 2022, 14, 5319. [Google Scholar] [CrossRef]


| Test (Plasma) | Control (No-Treatment) | ||||
|---|---|---|---|---|---|
| Absorption Time 2 | Wettability Grade | Absorption Time | Wettability Grade | p Value 3 | |
| RE-BONE Blocks 10 × 10 × 20 mm (n = 2) 56 mN/m ink 1 | 0.19 ± 0.03 s | Medium | 23.27 ± 0.10 s | Low | 0.0048 |
| RE-BONE Blocks 10 × 10 × 10 mm (n = 6) 72 mN/m ink 1 | 0.3 ± 0.02 s | Medium | 7.37 ± 0.06 s | Low | 0.0048 |
| HEART Pericardium Membranes (n = 4) 56 mN/m ink 1 | 15.37 ± 0.16 s | High | ∼30 min | Null | 0.0048 |
| Cancellous Granules, OSTEOXENON (n = 2) 56 mN/m ink 1 | 1.72 ± 0.10 s | Medium | 3.19 ± 0.12 s | Medium | 0.0043 |
| Cancellous Granules, BIO-GEN (n = 2) 56 mN/m ink 1 | 2.36 ± 0.18 s | High | 1.19 ± 0.05 s | Medium | 0.0048 |
| Cancellous Granules, BIO-GEN (n = 2) 72 mN/m ink 1 | 2.72 ± 0.10 s | High | 1.90 ± 0.04 s | Medium | 0.0047 |
| XC Collagen Xenomatrix (n = 2) 56 mN/m ink 1 | 3.88 ± 0.14 s | Medium | ∼30 min | Null | 0.0048 |
| XC Collagen Xenomatrix (n = 2) 72 mN/m ink 1 | 3.80 ± 0.14 s | High | 13.49 ± 0.18 s | Medium | 0.0048 |
| Plasma 30 s | Plasma 90 s | |||||
|---|---|---|---|---|---|---|
| No Plasma (at.%) | Plasma (at.%) | Reduction Rate (%) | No Plasma (at.%) | Plasma (at.%) | Reduction Rate (%) | |
| 1. | 74.98 | 70.29 | 4.69 | 42.17 | 20.92 | 50.39 |
| 2. | 70.12 | 62.93 | 10.25 | 24.73 | 24.05 | 2.75 |
| 3. | 61.99 | 59.34 | 4.27 | 84.68 | 47.30 | 44.14 |
| Avg. | 69.03 | 64.2 | 6.4 | 50.5 | 30.8 | 32.4 |
| Test (Plasma) 30′ | Test (Plasma) 60′ | Test (Plasma) 90′ | ||||
|---|---|---|---|---|---|---|
| Absorption Time 2 | Wettability Grade | Absorption Time 2 | Wettability Grade | Absorption Time 2 | Wettability Grade | |
| RE-BONE Blocks 10 × 10 × 20 mm 56 mN/m ink 1 | 0.19 ± 0.03 s (n = 20) | Medium | 0.08 ± 0.02 s (n = 20) | High | 0.07 ± 0.04 s (n = 20) | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallarico, M.; Meloni, S.M.; Troia, M.; Cacciò, C.; Lumbau, A.I.; Gendviliene, I.; Ceruso, F.M.; Pisano, M. The Use of Vacuum Plasma Surface Treatment to Improve the Hydrophilicity and Wettability of Bone Graft Substitutes and Resorbable Membranes: An In Vitro Study. Dent. J. 2025, 13, 141. https://doi.org/10.3390/dj13040141
Tallarico M, Meloni SM, Troia M, Cacciò C, Lumbau AI, Gendviliene I, Ceruso FM, Pisano M. The Use of Vacuum Plasma Surface Treatment to Improve the Hydrophilicity and Wettability of Bone Graft Substitutes and Resorbable Membranes: An In Vitro Study. Dentistry Journal. 2025; 13(4):141. https://doi.org/10.3390/dj13040141
Chicago/Turabian StyleTallarico, Marco, Silvio Mario Meloni, Michele Troia, Carlotta Cacciò, Aurea Immacolata Lumbau, Ieva Gendviliene, Francesco Mattia Ceruso, and Milena Pisano. 2025. "The Use of Vacuum Plasma Surface Treatment to Improve the Hydrophilicity and Wettability of Bone Graft Substitutes and Resorbable Membranes: An In Vitro Study" Dentistry Journal 13, no. 4: 141. https://doi.org/10.3390/dj13040141
APA StyleTallarico, M., Meloni, S. M., Troia, M., Cacciò, C., Lumbau, A. I., Gendviliene, I., Ceruso, F. M., & Pisano, M. (2025). The Use of Vacuum Plasma Surface Treatment to Improve the Hydrophilicity and Wettability of Bone Graft Substitutes and Resorbable Membranes: An In Vitro Study. Dentistry Journal, 13(4), 141. https://doi.org/10.3390/dj13040141









