Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis
Abstract
1. Introduction
2. Materials and Methods
3. Results
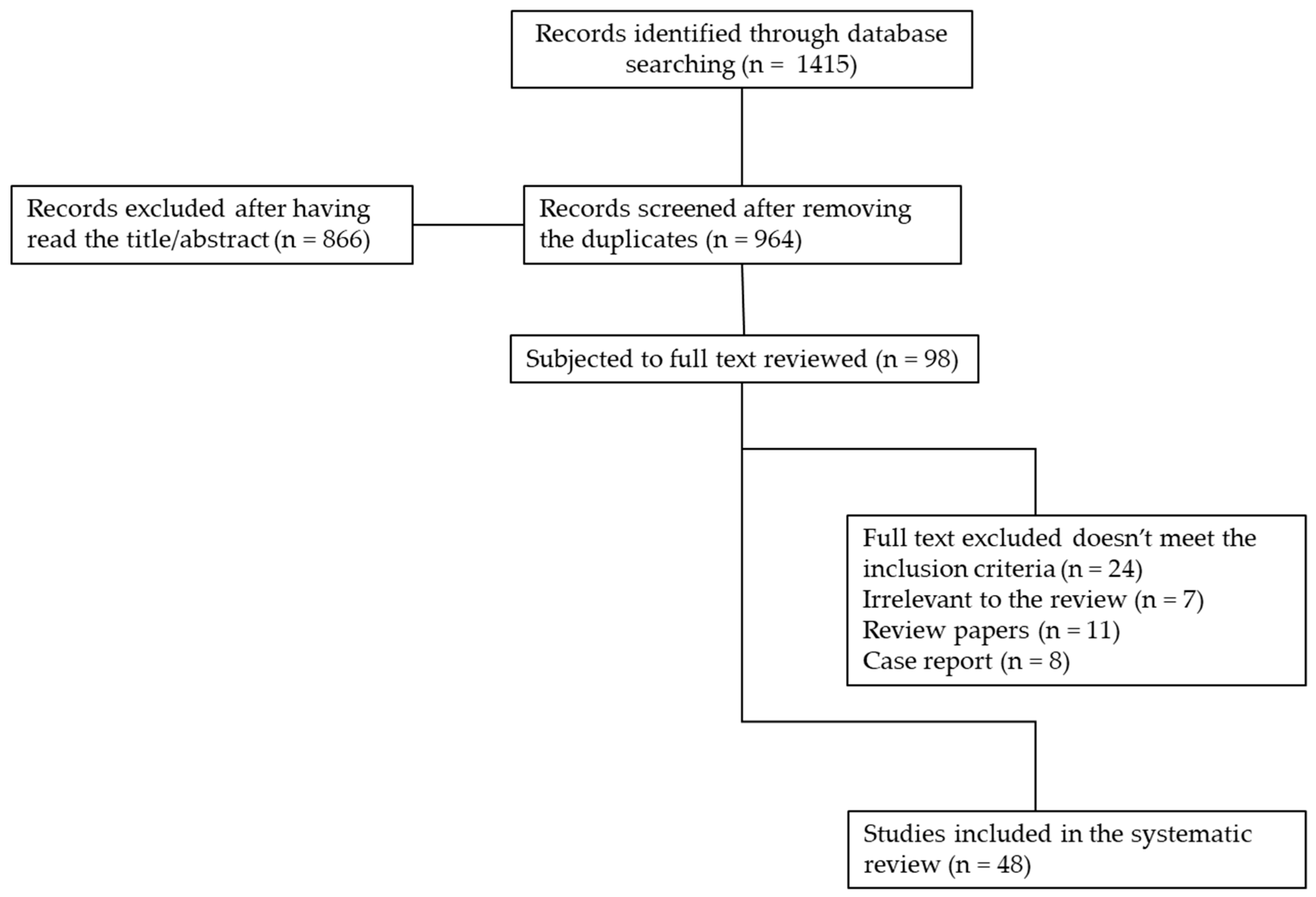
3.1. Systematic Literature Search
3.2. Selection of Literature
4. Results of the Literature Search
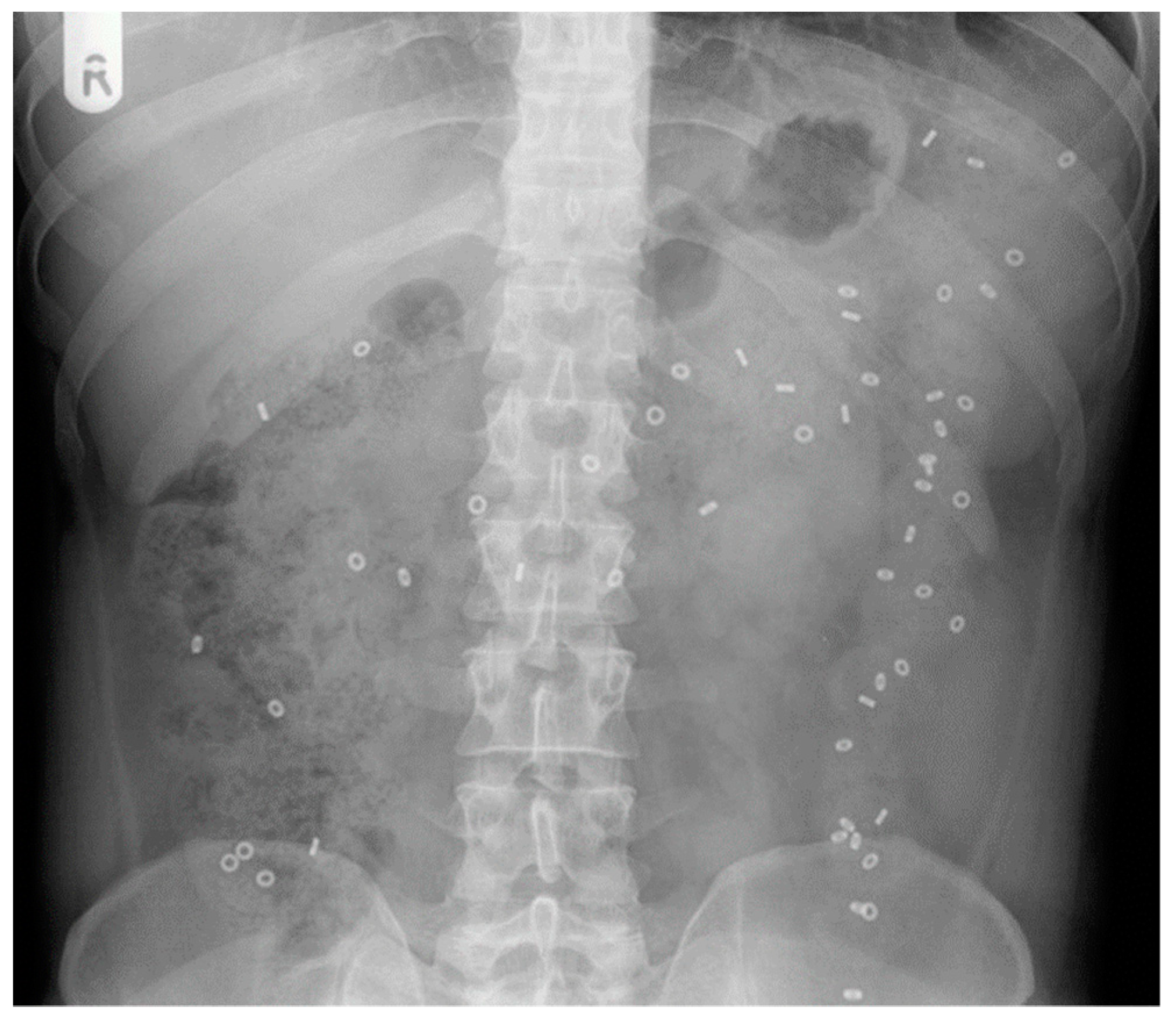
4.1. X-ray Radiopaque Markers
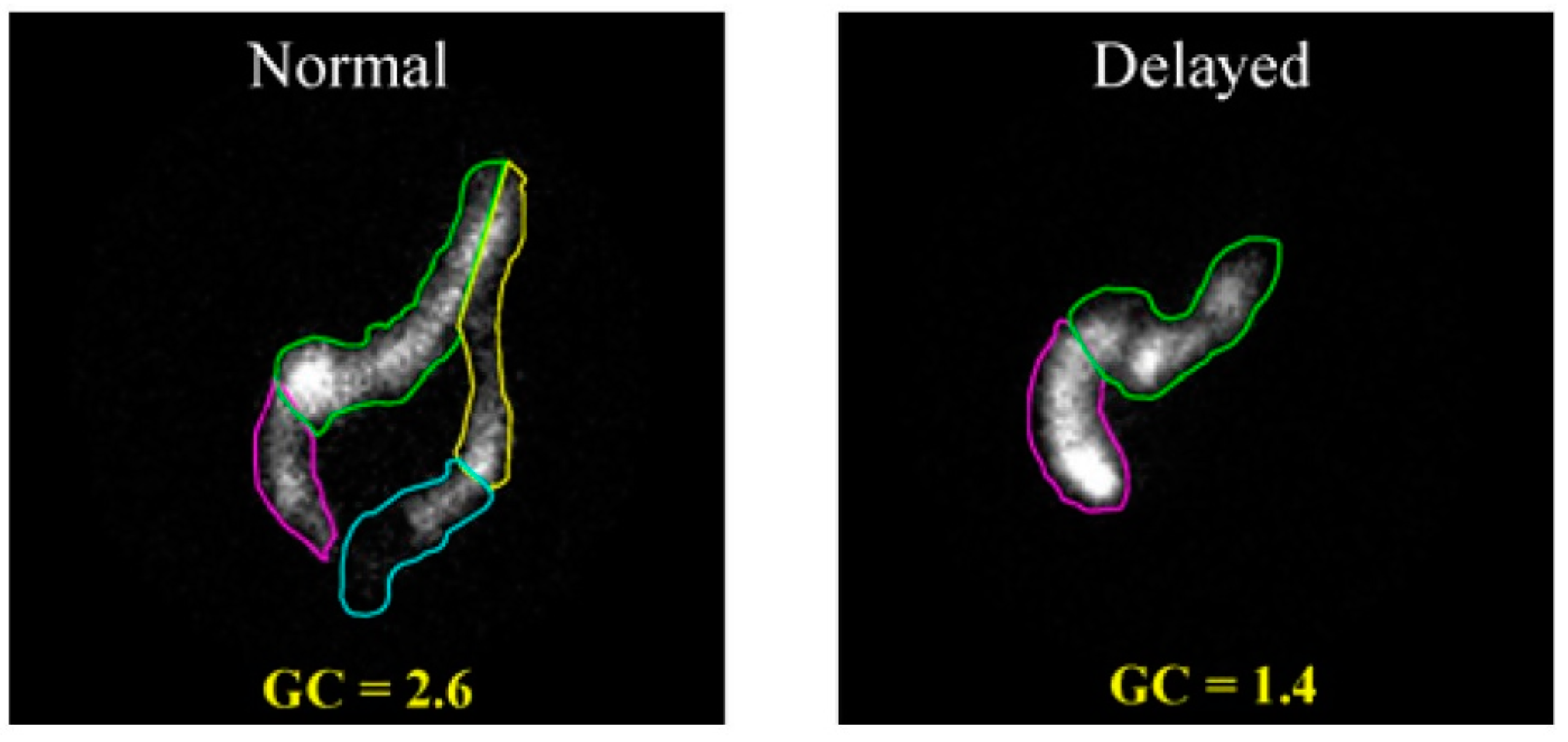
4.2. Gamma Scintigraphy
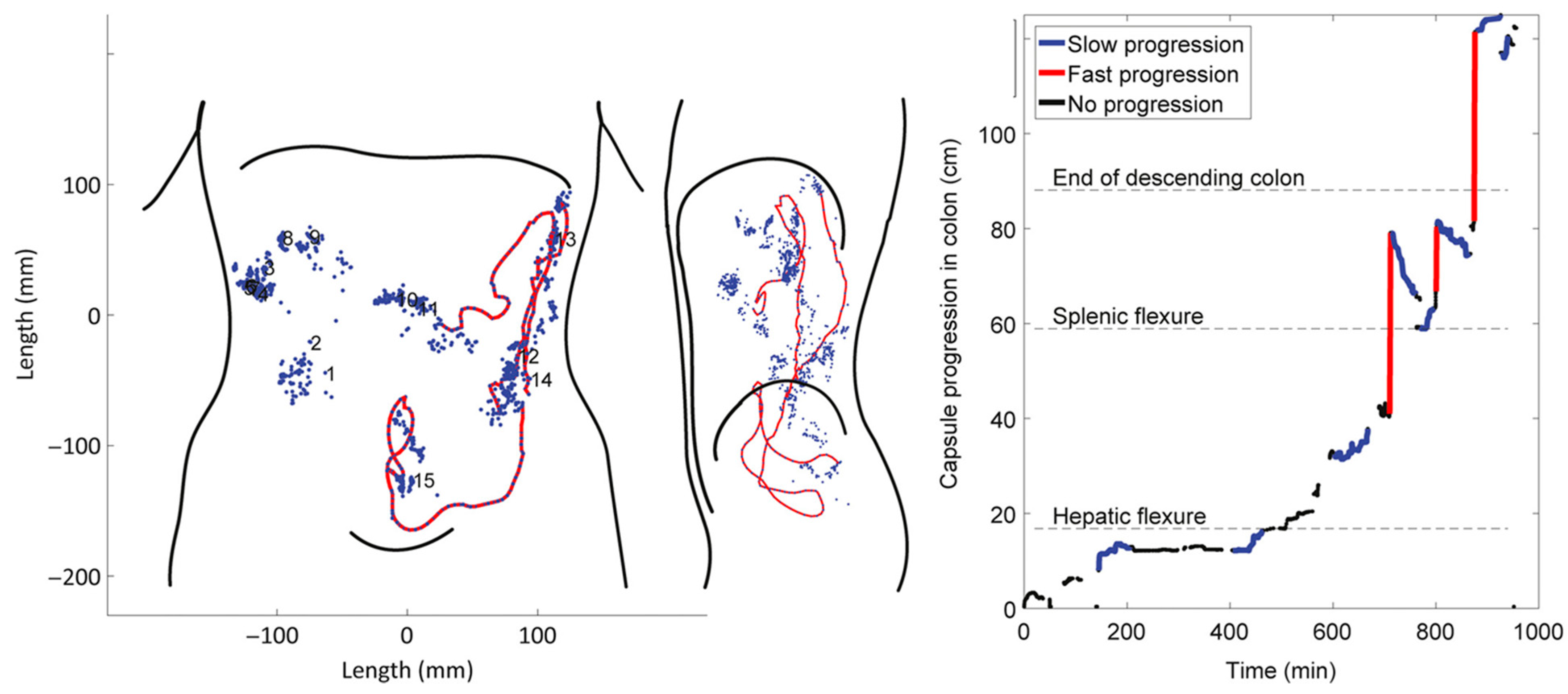
4.3. Tracking Systems
4.4. Magnetic Resonance Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tack, J.; Drossman, D.A. What’s new in Rome IV? Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Camilleri, M.; Hasler, W.L.; Maurer, A.H.; Parkman, H.P.; Saad, R.; Scott, M.S.; Simren, M.; Soffer, E.; Szarka, L. Evaluation of gastrointestinal transit in clinical practice: Position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol. Motil. 2010, 23, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Koppen, I.J.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Benninga, M.A. The pediatric Rome IV criteria: what’s new? Expert Rev. Gastroenterol. Hepatol. 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef]
- Suri, J.; Kataria, R.; Malik, Z.; Parkman, H.P.; Schey, R. Elevated methane levels in small intestinal bacterial overgrowth suggests delayed small bowel and colonic transit. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Basilisco, G.; Coletta, M. Chronic constipation: A critical review. Dig. Liver Dis. 2013, 45, 886–893. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R. American Gastroenterological Association Technical Review on Constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Sommers, T.; Corban, C.; Sengupta, N.; Jones, M.; Cheng, V.; Bollom, A.; Nurko, S.; Kelley, J.; Lembo, A. Emergency Department Burden of Constipation in the United States from 2006 to 2011. Am. J. Gastroenterol. 2015, 110, 572–579. [Google Scholar] [CrossRef]
- Turawa, E.B.; Musekiwa, A.; Rohwer, A.C. Interventions for treating postpartum constipation. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Pritchard, S.E.; Paul, J.; Major, G.; Marciani, L.; Gowland, P.A.; Spiller, R.C.; Hoad, C.L. Assessment of motion of colonic contents in the human colon using MRI tagging. Neurogastroenterol. Motil. 2017, 29, e13091. [Google Scholar] [CrossRef] [PubMed]
- Frattini, J.; Nogueras, J. Slow Transit Constipation: A Review of a Colonic Functional Disorder. Clin. Colon Rectal Surg. 2008, 21, 146–152. [Google Scholar] [CrossRef]
- Yong, D.; Beattie, R.M. Normal bowel habit and prevalence of constipation in primary school children. Ambul. Child Health 1998, 4, 277–282. [Google Scholar]
- Afzal, N.A.; Tighe, M.P.; Thomson, M.A. Constipation in children. Ital. J. Pediatr. 2011, 37. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of childhood constipation: A systematic review. Am. J. Gastroenterol. 2006, 101, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.G.; Keller, C.; Zwiener, R.; Hyman, P.E.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Shulman, R.J.; Hyams, J.S.; Palsson, O.; et al. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria. J. Pediatr. 2018, 195, 134–139. [Google Scholar] [CrossRef]
- Scarpato, E.; Kolacek, S.; Jojkic-Pavkov, D.; Konjik, V.; Zivkovic, N.; Roman, E.; Kostovski, A.; Zdraveska, N.; Altamimi, E.; Papadopoulou, A.; et al. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents in the Mediterranean Region of Europe. Clin. Gastroenterol. Hepatol. 2018, 16, 870–876. [Google Scholar] [CrossRef]
- Colombo, J.M.; Wassom, M.C.; Rosen, J.M. Constipation and Encopresis in Childhood. Pediatr. Rev. 2015, 36, 392–402. [Google Scholar] [CrossRef]
- Yik, Y.I.; Hutson, J.; Southwell, B. Home-Based Transabdominal Interferential Electrical Stimulation for Six Months Improves Paediatric Slow Transit Constipation (STC). Neuromodulation Technol. Neural Interface 2017, 21, 676–681. [Google Scholar] [CrossRef]
- Benninga, M.A.; Nurko, S.; Faure, C.; Hyman, P.E.; Roberts, I.S.; Schechter, N.L. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, N.; Lee, Z.E.; Olden, K.W. Diagnostic Approach to Chronic Constipation in Adults. Am. Fam. Physician 2011, 84, 299–306. [Google Scholar] [PubMed]
- Lee, Y.Y.; Erdogan, A.; Rao, S.S.C. How to Assess Regional and Whole Gut Transit Time With Wireless Motility Capsule. J. Neurogastroenterol. Motil. 2014, 20, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ Br. Med. J. 2016, 355. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ Br. Med. J. 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Grp, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef]
- Hinton, J.M.; Lennardj, J.; Young, A.C. A new method for studying gut transit times using radiopaque markers. Gut 1969, 10, 842–847. [Google Scholar] [CrossRef]
- Hwan Bae, S. Value of 24-hour delayed film of barium enema for colon transit function in young children with constipation. Am. J. Gastroenterol. 2014, 109, S594. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Son, J.S.; Park, H.W.; Kwak, B.O.; Kim, H.S.; Bae, S.H. Value of 24-hour Delayed Film of Barium Enema for Evaluation of Colon Transit Function in Young Children with Constipation. J. Neurogastroenterol. Motil. 2016, 22, 483–489. [Google Scholar] [CrossRef][Green Version]
- Yuan, W.; Zhang, Z.; Liu, J.; Li, Z.; Song, J.; Wu, C.; Wang, G. Simplified assessment of segmental gastrointestinal transit time with orally small amount of barium. Eur. J. Radiol. 2012, 81, 1986–1989. [Google Scholar] [CrossRef]
- Metcalf, A.M.; Phillips, S.F.; Zinsmeister, A.R.; Maccarty, R.L.; Beart, R.W.; Wolff, B.G. Simplified assessment of segmental colonic transit. Gastroenterology 1987, 92, 40–47. [Google Scholar] [CrossRef]
- Ansari, R.; Sohrabi, S.; Ghanaie, O.; Amjadi, H.; Merat, S.; Vahedi, H.; Khatibian, M. Comparison of colonic transit time between patients with constipation-predominant irritable bowel syndrome and functional constipation. Indian J. Gastroenterol. 2010, 29, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Buller, H.A.; Staalman, C.R.; Gubler, F.M.; Bossuyt, P.M.; Van der Plas, R.N.; Taminiau, J.A.J.M. Defaecation disorders in children, colonic transit time versus the Barr-score. Eur. J. Pediatr. 1995, 154, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, H.; Antov, S.; Bosaeus, I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal X-ray in healthy subjects and constipated patients. Scand. J. Gastroenterol. Suppl. 1988, 23, 72–80. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Devroede, G.; Arhan, P.; Strom, B.; Weber, J.; Cugnenc, P.H.; Denis, P.; Barbier, J.P. What is the meaning of colorectal transit time measurement? Dis. Colon Rectum 1992, 35, 773–782. [Google Scholar] [CrossRef]
- Enqiang, L.; Chunying, Z. Colonic transit time study in patients with chronic constipation using a radiopaque marker. Am. J. Gastroenterol. 2017, 112, S1457. [Google Scholar] [CrossRef]
- Evans, R.C.; Kamm, M.A.; Hinton, J.M.; Lennardjones, J.E. The normal range and simple diagram for recording whole gut transit time. Int. J. Colorectal Dis. 1992, 7, 15–17. [Google Scholar] [CrossRef]
- Bhate, P.A.; Patel, J.A.; Parikh, P.; Ingle, M.A.; Phadke, A.; Sawant, P.D. Total and Segmental Colon Transit Time Study in Functional Constipation: Comparison with Healthy Subjects. Gasteroenterol. Res. 2015, 8, 157–159. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Kuo, B.; McCallum, R.W.; Chey, W.D.; DiBaise, J.K.; Hasler, W.L.; Koch, K.L.; Lackner, J.M.; Miller, C.; Saad, R.; et al. Investigation of Colonic and Whole-Gut Transit with Wireless Motility Capsule and Radiopaque Markers in Constipation. Clin. Gastroenterol. Hepatol. 2009, 7, 537–544. [Google Scholar] [CrossRef]
- Camilleri, M.; Thorne, N.K.; Ringel, Y.; Hasler, W.L.; Kuo, B.; Esfandyari, T.; Gupta, A.; Scott, S.M.; McCallum, R.W.; Parkman, H.P.; et al. Wireless pH-motility capsule for colonic transit: Prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol. Motil. 2010, 22, 874. [Google Scholar] [CrossRef]
- Kuo, B.; Maneerattanaporn, M.; Lee, A.A.; Baker, J.R.; Wiener, S.M.; Chey, W.D.; Wilding, G.E.; Hasler, W.L. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig. Dis. Sci. 2011, 56, 2928–2938. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, E.S.; Maurer, A.H.; Davidoff, S.; Krevsky, B.; Fisher, R.S.; Parkman, H.P. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am. J. Gastroenterol. 2000, 95, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Charles, F.; Camilleri, M.; Phillips, S.F.; Thomforde, G.M.; Forstrom, L.A. Scintigraphy of the whole gut: Clinical evaluation of transit disorders. Mayo Clin. Proc. 1995, 70, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Carmo, R.L.M.L.; Oliveira, R.P.M.; Ribeiro, A.E.A.; Lima, M.C.L.; Amorim, B.J.; Ribeiro, A.F.; Ramos, C.D.; Bustorff-Silva, J.M.; Lomazi, E.A. Colonic transit in children and adolescents with chronic constipation. J. Pediatr. 2015, 91, 386–391. [Google Scholar] [CrossRef][Green Version]
- Vlajkovic, M.; Rajic, M.; Stevic, M.; Zivkovic, V.; Dordevic, I.; Matovic, M. Assessment of functional constipation in children by means of Tc-99m-DTPA-activated carbon. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, S588. [Google Scholar] [CrossRef]
- Krevsky, B.; Maurer, A.H.; Fisher, R.S. Patterns of colonic transit in chronic idiopathic constipation. Am. J. Gastroenterol. 1989, 84, 127–132. [Google Scholar]
- van der Sijp, J.R.; Kamm, M.A.; Nightingale, J.M.; Britton, K.E.; Mather, S.J.; Morris, G.P.; Akkermans, L.M.; Lennard-Jones, J.E. Radioisotope determination of regional colonic transit in severe constipation: Comparison with radio opaque markers. Gut 1993, 34, 402–408. [Google Scholar] [CrossRef]
- Bartholomeusz, D.; Chatterton, B.E.; Bellen, J.C.; Gaffney, R.; Hunter, A. Segmental colonic transit after oral 67Ga-citrate in healthy subjects and those with chronic idiopathic constipation. J. Nucl. Med. 1999, 40, 277–282. [Google Scholar]
- Cheng, K.-Y.; Tsai, S.-C.; Lin, W.-Y. Gallium-67 activated charcoal: A new method for preparation of radioactive capsules for colonic transit study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 907–911. [Google Scholar] [CrossRef]
- Asli, I.N.; Ehsani, M.J.; Javadi, H.; Jallalat, S.; Assadi, M. Colon transit scintigraphy in the idiopathic constipated patients by 67Ga-citrate. Iran. J. Nucl. Med. 2010, 18, 64. [Google Scholar]
- Roberts, J.P.; Newell, M.S.; Deeks, J.J.; Waldron, D.W.; Garvie, N.W.; Williams, N.S. Oral [111In]DTPA scintigraphic assessment of colonic transit in constipated subjects. Dig. Dis. Sci. 1993, 38, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.B.; Valenzuela, G.A.; Stubbs, C.C.; Croft, B.Y.; Teates, C.D.; Plankey, M.W.; McCallum, R.W. A noninvasive scintigraphic assessment of the colonic transit of nondigestible solids in man. J. Nucl. Med. 1991, 32, 1375–1381. [Google Scholar] [PubMed]
- McLean, R.G.; Smart, R.C.; Gaston-Parry, D.; Barbagallo, S.; Baker, J.; Lyons, N.R.; Bruck, C.E.; King, D.W.; Lubowski, D.Z.; Talley, N.A. Colon transit scintigraphy in health and constipation using oral iodine-131-cellulose. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1990, 31, 985–989. [Google Scholar]
- Smart, R.C.; McLean, R.G.; Gaston-Parry, D.; Barbagallo, S.; Bruck, C.E.; King, D.W.; Lubowski, D.Z.; Talley, N.A. Comparison of oral iodine-131-cellulose and indium-111-DTPA as tracers for colon transit scintigraphy: Analysis by colon activity profiles. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1991, 32, 1668–1674. [Google Scholar]
- Kekilli, E.; Yagmur, C.; Isik, B.; Aydin, O.M. Calculating colon transit time with radionuclide-filled capsules in constipated patients: A new method for colon transit study. Abdom. Imaging 2005, 30, 593–597. [Google Scholar] [CrossRef]
- Wagner, R.H.; Savir-Baruch, B.; Halama, J.R.; Venu, M.; Gabriel, M.S.; Bova, D. Proof of concept: Design and initial evaluation of a device to measure gastrointestinal transit time. J. Nucl. Med. Technol. 2017, 45, 230–235. [Google Scholar] [CrossRef]
- Wagner, R.; Halama, J.; Venu, M.; Bova, D.; Savir-Baruch, B.; Gabriel, M. A point source device to determine small and large bowel transit time-proof of concept and initial results. J. Nucl. Med. 2016, 57, 649. [Google Scholar]
- Eising, E.G.; von der Ohe, M.R. Differentiation of prolonged colonic transit using scintigraphy with indium-111-labeled polystyrene pellets. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1998, 39, 1062–1066. [Google Scholar]
- Deiteren, A.; Camilleri, M.; Bharucha, A.E.; Burton, D.; McKinzie, S.; Rao, A.S.; Zinsmeister, A.R. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22, 415-e95. [Google Scholar] [CrossRef]
- Cremonini, F.; Mullan, B.P.; Camilleri, M.; Burton, D.D.; Rank, M.R. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment. Pharmacol. Ther. 2002, 16, 1781–1790. [Google Scholar] [CrossRef]
- Lin, H.C.; Prather, C.; Fisher, R.S.; Meyer, J.H.; Summers, R.W.; Pimentel, M.; McCallum, R.W.; Akkermans, L.M.A.; Loening-Baucke, V.; AMS Task Force Committee on Gastrointestinal Transit. Measurement of gastrointestinal transit. Dig. Dis. Sci. 2005, 50, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, S.; Parkman, H.P.; Friedenberg, F.K. Wireless capsule motility: Comparison of the smartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig. Dis. Sci. 2009, 54, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.; Neri, M.; Carriero, A.; Casciardi, S.; Comani, S.; Delgratta, C.; Didonato, L.; Diluzio, S.; Macri, M.A.; Pasquarelli, A.; et al. Measurement of segmental transit through the gut in man. A novel approach by the biomagnetic method. Dig. Dis. Sci. 1992, 37, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Worsoe, J.; Fynne, L.; Gregersen, T.; Schlageter, V.; Christensen, L.A.; Dahlerup, J.F.; Rijkhoff, N.J.M.; Laurberg, S.; Krogh, K. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. Bmc Gastroenterol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Weitschies, W.; Blume, H.; Mönnikes, H. Magnetic Marker Monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hiroz, P.; Schlageter, V.; Givel, J.C.; Kucera, P. Colonic movements in healthy subjects as monitored by a Magnet Tracking System. Neurogastroenterol. Motil. 2009, 21, 838-e57. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.M.; Fallet, S.; Otto, M.; Scott, S.M.; Schlageter, V.; Krogh, K. Gastrointestinal motility during sleep assessed by tracking of telemetric capsules combined with polysomnography—a pilot study. Clin. Exp. Gastroenterol. 2015, 8, 327–332. [Google Scholar] [CrossRef]
- Haase, A.M.; Gregersen, T.; Schlageter, V.; Scott, M.S.; Demierre, M.; Kucera, P.; Dahlerup, J.F.; Krogh, K. Pilot study trialling a new ambulatory method for the clinical assessment of regional gastrointestinal transit using multiple electromagnetic capsules. Neurogastroenterol. Motil. 2014, 26, 1783–1791. [Google Scholar] [CrossRef]
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Frokjaer, J.B.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Assessment of colorectal length using the electromagnetic capsule tracking system: A comparative validation study in healthy subjects. Colorectal Dis. 2017, 19, O350–O357. [Google Scholar] [CrossRef]
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Espersen, M.; Gregersen, T.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterol. Motil. 2019, 31. [Google Scholar] [CrossRef]
- Chaddock, G.; Lam, C.; Hoad, C.L.; Costigan, C.; Cox, E.F.; Placidi, E.; Thexton, I.; Wright, J.; Blackshaw, P.E.; Perkins, A.C.; et al. Novel MRI tests of orocecal transit time and whole gut transit time: Studies in normal subjects. Neurogastroenterol. Motil. 2014, 26, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Chaddock, G.; Hoad, C.L.; Costigan, C.; Cox, E.; Marciani, L.; Gowland, P.A.; Spiller, R.C. A new validated MRI method for measuring whole gut transit time. Gastroenterology 2013, 144, S920. [Google Scholar] [CrossRef]
- Lam, C.; Chaddock, G.; Marciani, L.; Costigan, C.; Paul, J.; Cox, E.; Hoad, C.; Menys, A.; Pritchard, S.; Garsed, K.; et al. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol. Motil. 2016, 28, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Chaddock, G.; Marciani Laurea, L.; Costigan, C.; Cox, E.; Hoad, C.; Pritchard, S.; Gowland, P.; Spiller, R. Distinct Abnormalities of Small Bowel and Regional Colonic Volumes in Subtypes of Irritable Bowel Syndrome Revealed by MRI. Am. J. Gastroenterol. 2017, 112, 346–355. [Google Scholar] [CrossRef]
- Buhmann, S.; Kirchhoff, C.; Ladurner, R.; Mussack, T.; Reiser, M.F.; Lienemann, A. Assessment of colonic transit time using MRI: A feasibility study. Eur. Radiol. 2007, 17, 669–674. [Google Scholar] [CrossRef]
- Zhi, M.; Zhou, Z.; Chen, H.; Xiong, F.; Huang, J.; He, H.; Zhang, M.; Su, M.; Gao, X.; Hu, P. Clinical application of a gadolinium-based capsule as an MRI contrast agent in slow transit constipation diagnostics. Neurogastroenterol. Motil. 2017, 29, e13020. [Google Scholar] [CrossRef]
- Patak, M.A.; Weishaupt, D.; Frohlich, J.M.; Debatin, J.F. Sequential fast 3D MRI following oral ingestion of Gd-DOTA: A new means to assess intestinal transit time. J. Magn. Reson. Imaging 1999, 10, 474–476. [Google Scholar] [CrossRef]
- Hahn, T.; Kozerke, S.; Schwizer, W.; Fried, M.; Boesiger, P.; Steingoetter, A. Visualization and quantification of intestinal transit and motor function by real-time tracking of 19F labeled capsules in humans. Magn. Reson. Med. 2011, 66, 812–820. [Google Scholar] [CrossRef]
- Yeong, C.H.; Abdullah, B.J.J.; Ng, K.H.; Chung, L.Y.; Goh, K.L.; Perkins, A.C. Fusion of gamma scintigraphic and magnetic resonance images improves the anatomical delineation of radiotracer for the assessment of gastrointestinal transit. Nucl. Med. Commun. 2013, 34, 645–651. [Google Scholar] [CrossRef]
- Pritchard, S.E.; Marciani, L.; Garsed, K.C.; Hoad, C.L.; Thongborisute, W.; Roberts, E.; Gowland, P.A.; Spiller, R.C. Fasting and postprandial volumes of the undisturbed colon: Normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol. Motil. 2013, 26, 124–130. [Google Scholar] [CrossRef]
- Nilsson, M.; Sandberg, T.H.; Poulsen, J.L.; Gram, M.; Frøkjaer, J.B.; Østergaard, L.R.; Krogh, K.; Brock, C.; Drewes, A.M. Quantification and variability in colonic volume with a novel magnetic resonance imaging method. Neurogastroenterol. Motil. 2015, 27, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Rhee, P.-L. How to Interpret a Functional or Motility Test—Colon Transit Study. J. Neurogastroenterol. Motil. 2012, 18, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Meduri, K. What is necessary to diagnose constipation? Best Pract. Res. Clin. Gastroenterol. 2011, 25, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Bassotti, G.; Clarke, J.; Dinning, P.; Fox, M.; Grover, M.; Hellstrom, P.M.; Ke, M.Y.; Layer, P.; Malagelada, C.; et al. Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 291–308. [Google Scholar] [CrossRef] [PubMed]
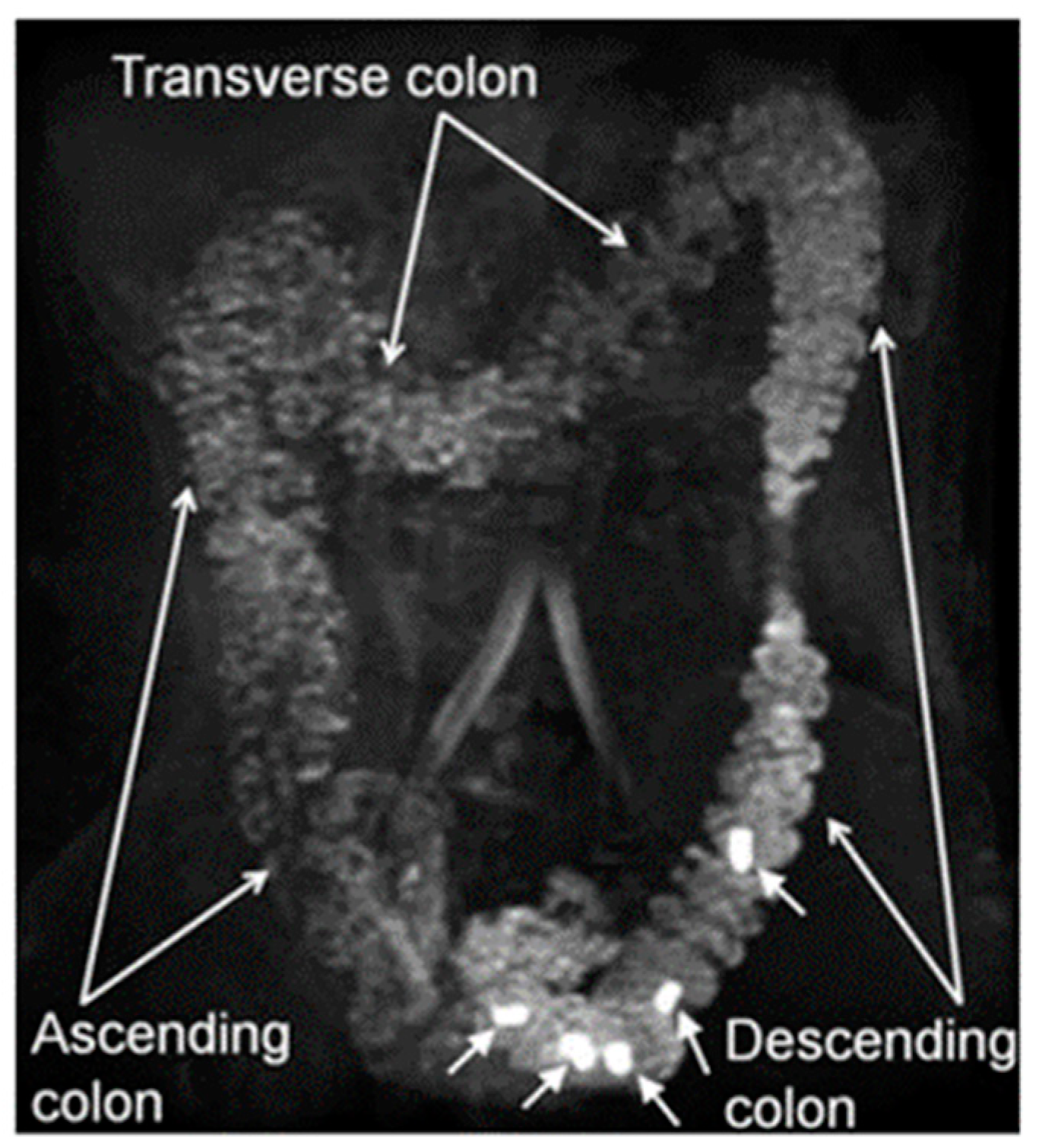
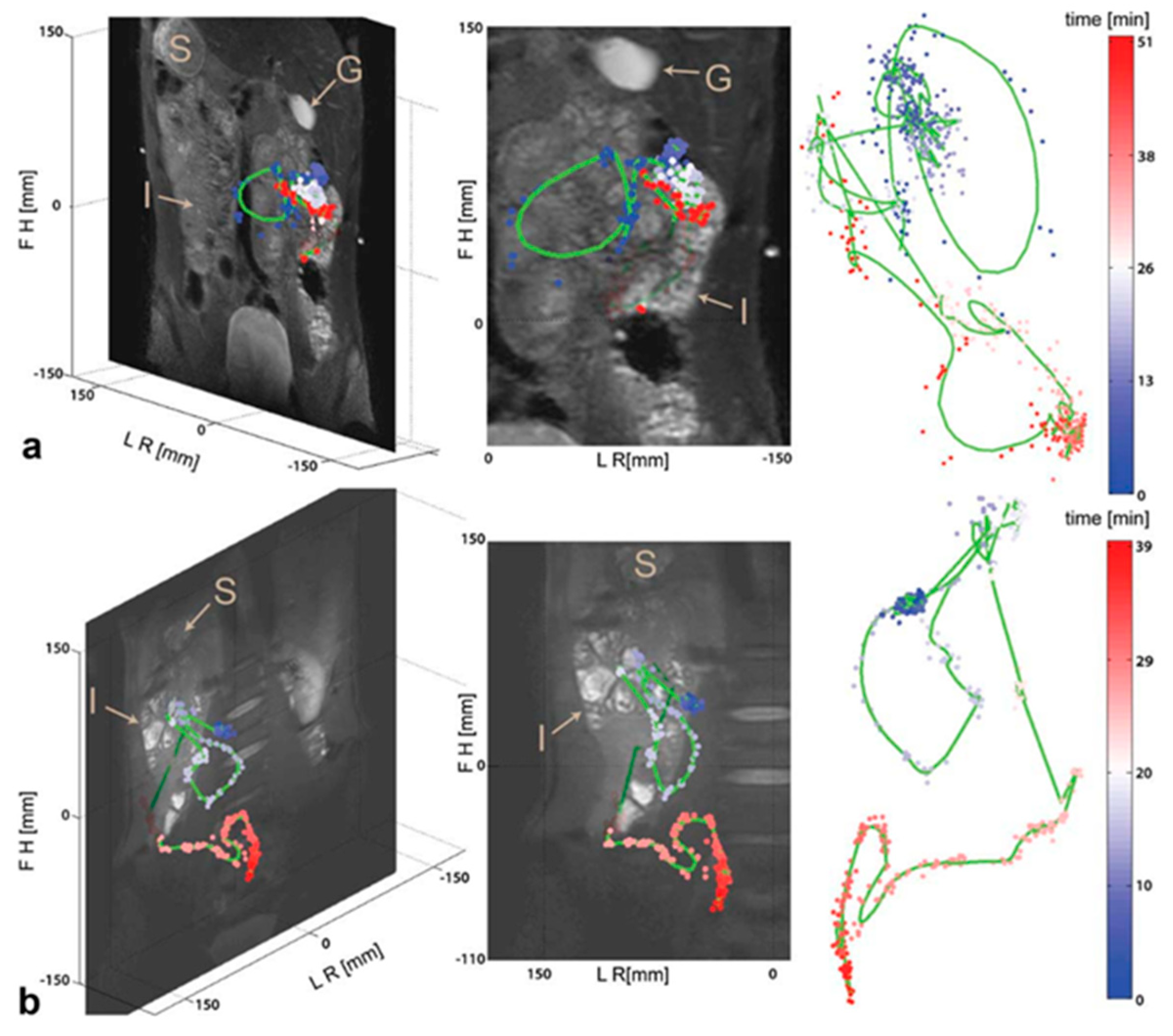
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Participants |
|
|
| Intervention |
|
|
| Comparator |
|
|
| Outcomes |
|
|
| Study design |
|
|
| Diagnostic Approaches | Advantages | Disadvantages |
|---|---|---|
| X-ray Radiopaque Markers |
|
|
| Gamma scintigraphy |
|
|
| Tracking systems |
|
|
| Magnetic Resonance Imaging |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, H.; Devadason, D.; Abrehart, N.; Stevenson, R.; Marciani, L. Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis. Diagnostics 2019, 9, 221. https://doi.org/10.3390/diagnostics9040221
Sharif H, Devadason D, Abrehart N, Stevenson R, Marciani L. Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis. Diagnostics. 2019; 9(4):221. https://doi.org/10.3390/diagnostics9040221
Chicago/Turabian StyleSharif, Hayfa, David Devadason, Nichola Abrehart, Rebecca Stevenson, and Luca Marciani. 2019. "Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis" Diagnostics 9, no. 4: 221. https://doi.org/10.3390/diagnostics9040221
APA StyleSharif, H., Devadason, D., Abrehart, N., Stevenson, R., & Marciani, L. (2019). Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis. Diagnostics, 9(4), 221. https://doi.org/10.3390/diagnostics9040221






