Unsupervised Clustering of Patients Undergoing Thoracoscopic Ablation Identifies Relevant Phenotypes for Advanced Atrial Fibrillation
Abstract
:1. Introduction
- Clusters of patients with different outcomes of thoracoscopic ablation;
- Clusters of patients with differences beyond AF type and cardiovascular risk profiles (CHA2DS2-VASc score).
2. Materials and Methods
2.1. Data Collection
2.2. Data Preprocessing
2.3. Unsupervised Machine Learning
2.4. Validation
2.5. Statistical Analysis
3. Results
3.1. Data Collection and Preprocessing
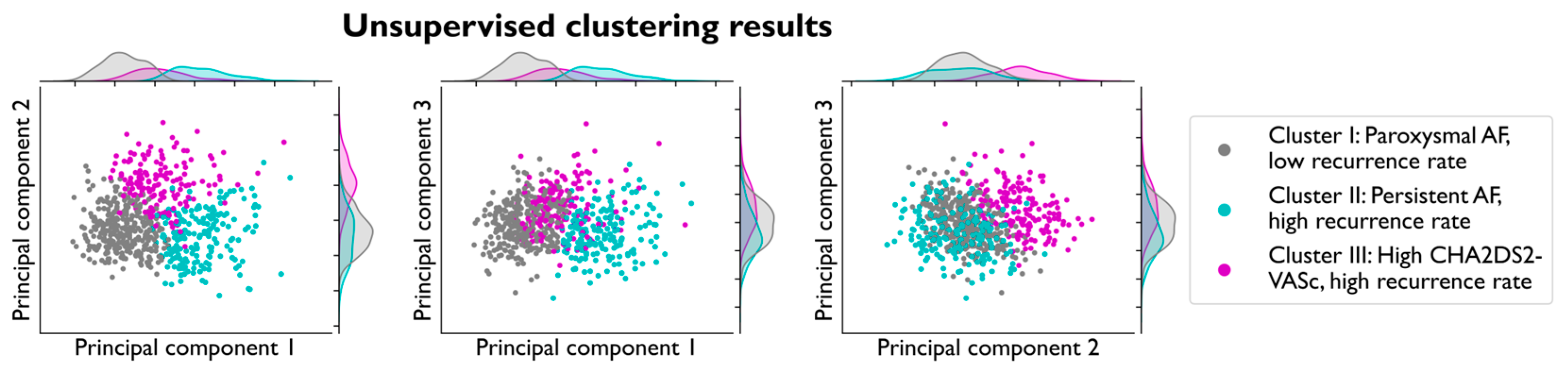
3.2. Cluster Creation and Validation
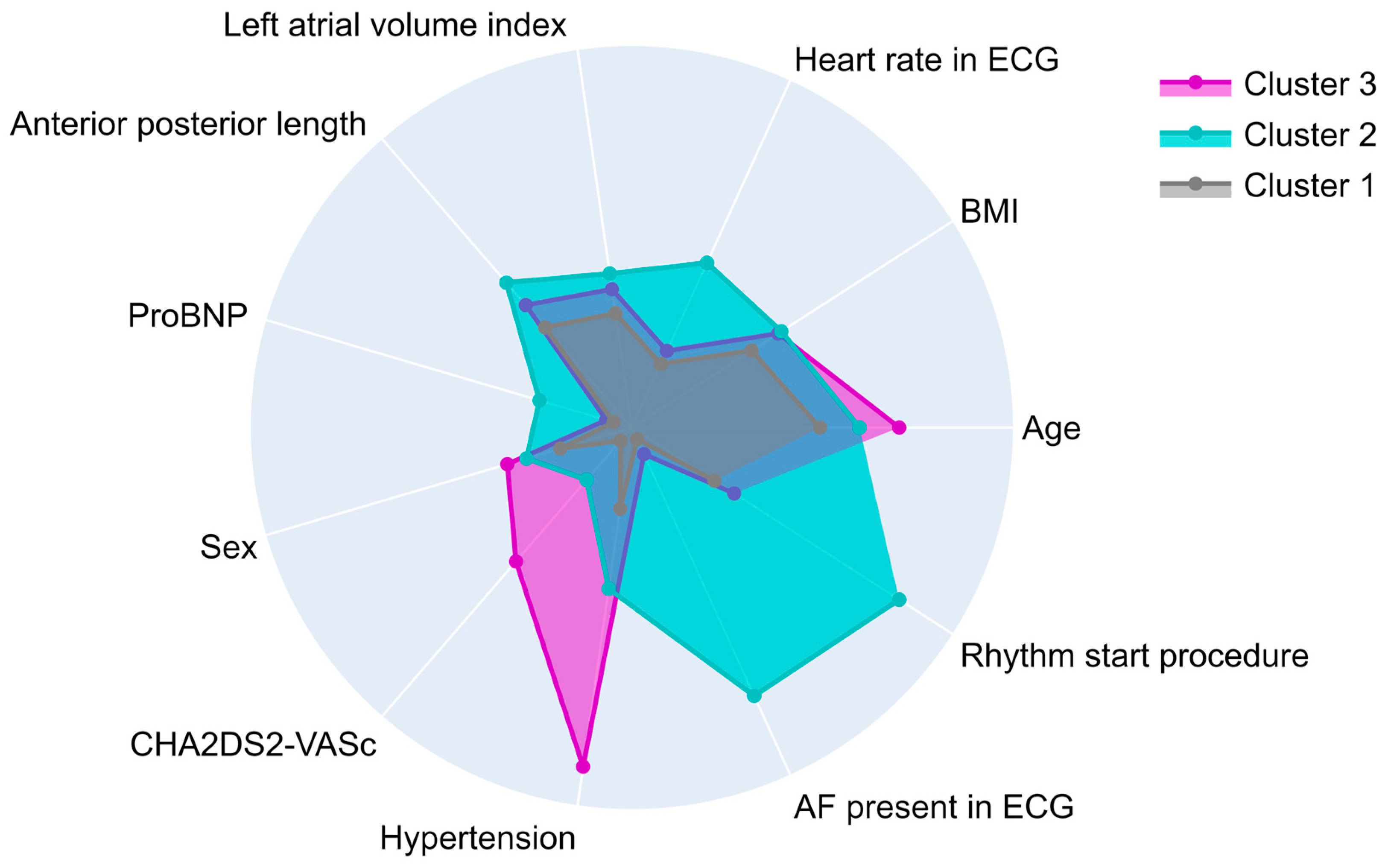
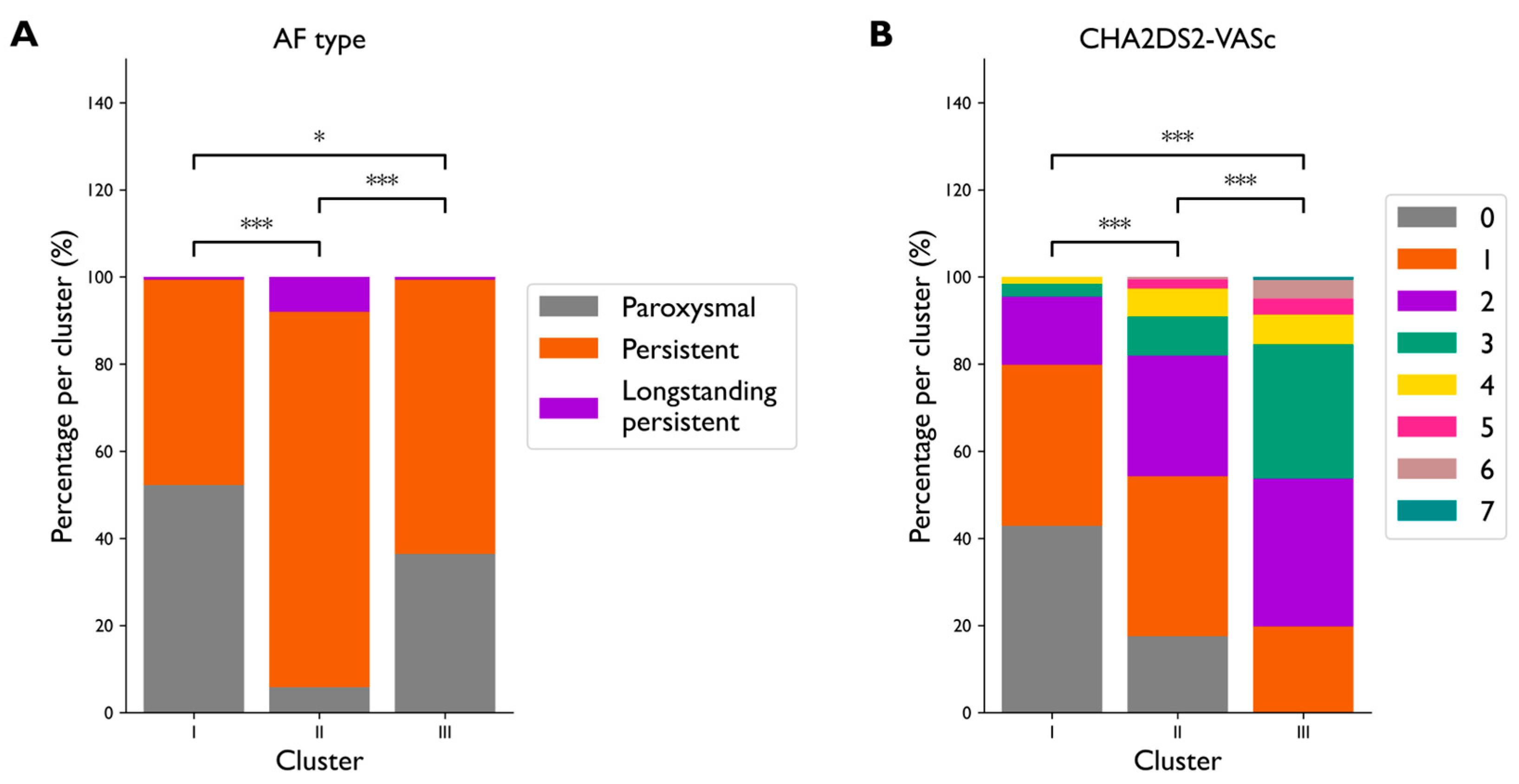
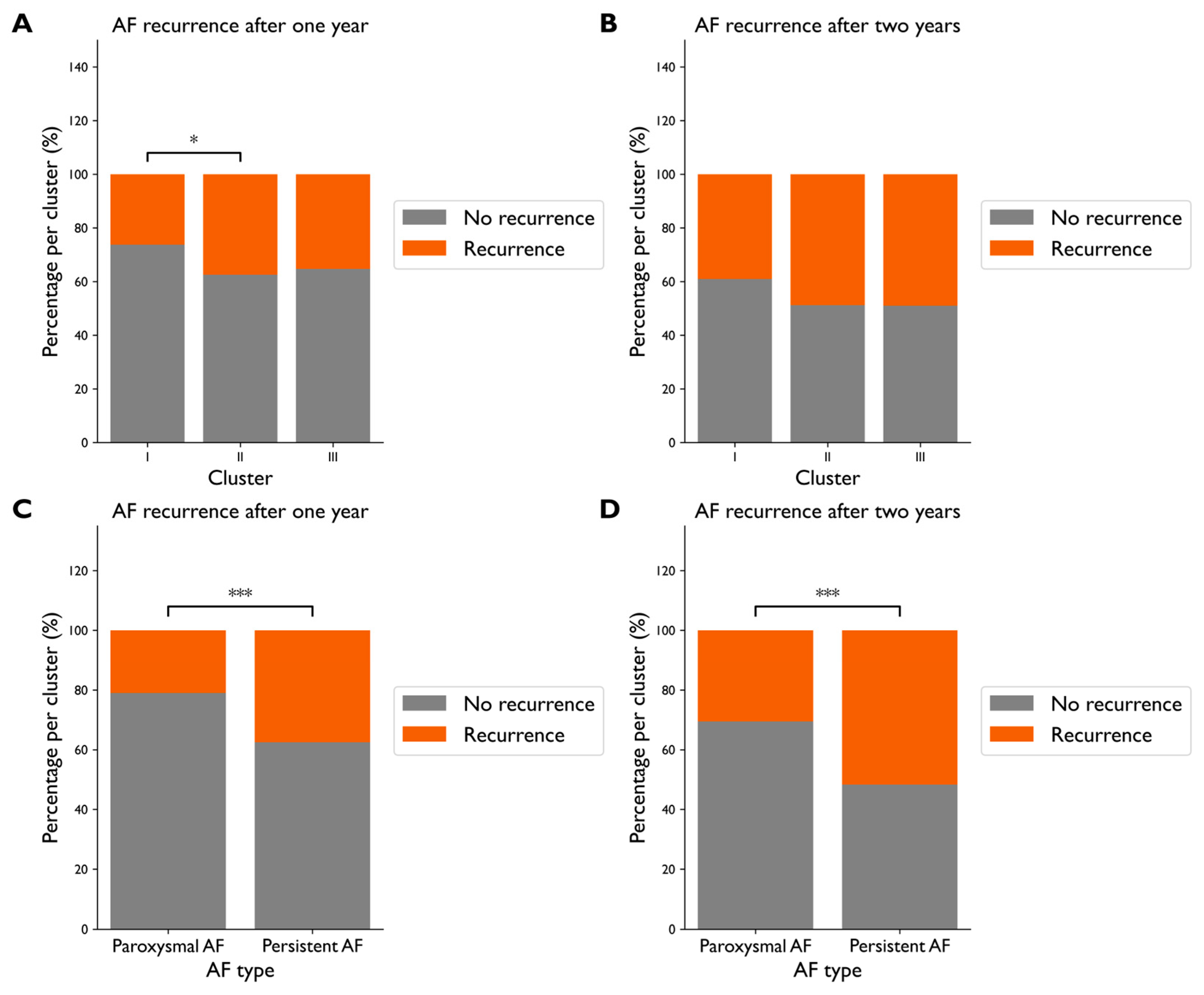
3.3. Clustering Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| BMI | body mass index |
| PCA | principal component analysis |
| LAVI | left atrial volume index |
| ProBNP | prohormone of brain natriuretic peptide |
| MRI | magnetic resonance imaging |
| CT | computed tomography |
References
- Zathar, Z.; Karunatilleke, A.; Fawzy, A.M.; Lip, G.Y.H. Atrial Fibrillation in Older People: Concepts and Controversies. Front. Med. 2019, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Gandjbakhch, E.; Mandel, F.; Dagher, Y.; Hidden-Lucet, F.; Rollin, A.; Maury, P. Incidence, epidemiology, diagnosis and prognosis of atrio-oesophageal fistula following percutaneous catheter ablation: A French nationwide survey. EP Eur. 2021, 23, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.A.; Castella, M.; Van Boven, W.; Berruezo, A.; Yilmaz, A.; Nadal, M.; Sandoval, E.; Calvo, N.; Brugada, J.; Kelder, J.; et al. Atrial Fibrillation Catheter Ablation Versus Surgical Ablation Treatment (FAST). Circulation 2012, 125, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Krul, S.P.J.; Driessen, A.H.G.; Van Boven, W.J.; Linnenbank, A.C.; Geuzebroek, G.S.C.; Jackman, W.M.; Wilde, A.A.M.; De Bakker, J.M.T.; De Groot, J.R. Thoracoscopic Video-Assisted Pulmonary Vein Antrum Isolation, Ganglionated Plexus Ablation, and Periprocedural Confirmation of Ablation Lesions. Circ. Arrhythm Electrophysiol. 2011, 4, 262–270. [Google Scholar] [CrossRef]
- Pison, L.; La Meir, M.; van Opstal, J.; Blaauw, Y.; Maessen, J.; Crijns, H.J. Hybrid Thoracoscopic Surgical and Transvenous Catheter Ablation of Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 54–61. [Google Scholar] [CrossRef]
- Berger, W.R.; Meulendijks, E.R.; Limpens, J.; Van Den Berg, N.W.E.; Neefs, J.; Driessen, A.H.G.; Krul, S.P.J.; Van Boven, W.J.P.; De Groot, J.R. Persistent atrial fibrillation: A systematic review and meta-analysis of invasive strategies. Int. J. Cardiol. 2019, 278, 137–143. [Google Scholar] [CrossRef]
- Phan, K.; Phan, S.; Thiagalingam, A.; Medi, C.; Yan, T.D. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur. J. Cardiothorac. Surg. 2016, 49, 1044–1051. [Google Scholar] [CrossRef]
- Haldar, S.; Khan, H.R.; Boyalla, V.; Kralj-Hans, I.; Jones, S.; Lord, J.; Onyimadu, O.; Satishkumar, A.; Bahrami, T.; De Souza, A.; et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur. Heart J. 2020, 41, 4471–4480. [Google Scholar] [CrossRef]
- Deb, B.; Rogers, A.J.; Bhatia, N.K.; Baykaner, T.; Turakhia, M.; Clopton, P.L.; Chang, H.J.; Brodt, C.; Narayan, S.M.; Wang, P.J.; et al. Machine learned clusters explain heterogeneity in outcomes from map-guided ablation of Atrial Fibrillation results from the large PROspective STanford AF Registry (ProSTAR). Eur. Heart J. 2022, 43, 474. [Google Scholar] [CrossRef]
- Bisson, A.; Fawzy, A.M.; El-Bouri, W.; Angoulvant, D.; Lip, G.Y.H.; Fauchier, L.; Clementy, N. Clinical Phenotypes and Atrial Fibrillation Recurrences After Catheter Ablation: An Unsupervised Cluster Analysis. Curr. Probl. Cardiol. 2023, 48, 101732. [Google Scholar] [CrossRef] [PubMed]
- Bisson, A.; Fawzy, A.M.; Romiti, G.F.; Proietti, M.; Angoulvant, D.; El-Bouri, W.; Lip, G.Y.H.; Fauchier, L. Phenotypes and outcomes in non-anticoagulated patients with atrial fibrillation: An unsupervised cluster analysis. Arch. Cardiovasc. Dis. 2023, 116, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Piccini, J.P.; Mahaffey, K.W.; Kimura, T.; Katsumata, Y.; Tanimoto, K.; Inagawa, K.; Ikemura, N.; Ueda, I.; Fukuda, K.; et al. A Cluster Analysis of the Japanese Multicenter Outpatient Registry of Patients With Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 871–878. [Google Scholar] [CrossRef]
- Inohara, T.; Shrader, P.; Pieper, K.; Blanco, R.; Thomas, L.; Singer, D.; Freeman, J.; Allen, L.; Fonarow, G.; Gersh, B.; et al. Association of of Atrial Fibrillation Clinical Phenotypes With Treatment Patterns and Outcomes: A Multicenter Registry Study. JAMA Cardiol. 2018, 3, 54–63. [Google Scholar] [CrossRef]
- Saito, Y.; Omae, Y.; Nagashima, K.; Miyauchi, K.; Nishizaki, Y.; Miyazaki, S.; Hayashi, H.; Nojiri, S.; Daida, H.; Minamino, T.; et al. Phenotyping of atrial fibrillation with cluster analysis and external validation. Heart 2023, 109, 1751–1758. [Google Scholar] [CrossRef]
- Vitolo, M.; Proietti, M.; Shantsila, A.; Boriani, G.; Lip, G.Y.H. Clinical Phenotype Classification of Atrial Fibrillation Patients Using Cluster Analysis and Associations with Trial-Adjudicated Outcomes. Biomedicines 2021, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Assent, I. Clustering high dimensional data. WIREs Data Min. Knowl. Discov. 2012, 2, 340–350. [Google Scholar] [CrossRef]
- Driessen, A.H.G.; Berger, W.R.; Krul, S.P.J.; van den Berg, N.W.E.; Neefs, J.; Piersma, F.R.; Chan Pin Yin, D.; de Jong, J.; van Boven, W.P.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef]
- Edgerton, J.R.; Jackman, W.M.; Mahoney, C.; Mack, M.J. Totally thorascopic surgical ablation of persistent AF and long-standing persistent atrial fibrillation using the “Dallas” lesion set. Heart Rhythm. 2009, 6, S64–S70. [Google Scholar] [CrossRef]
- Gómez-Méndez, I.; Joly, E. Regression with missing data, a comparison study of techniques based on random forests. J. Stat. Comput. Simul. 2023, 93, 1924–1949. [Google Scholar] [CrossRef]
- Alsaber, A.R.; Pan, J.; Al-Hurban, A. Handling Complex Missing Data Using Random Forest Approach for an Air Quality Monitoring Dataset: A Case Study of Kuwait Environmental Data (2012 to 2018). Int. J. Environ. Res. Public. Health 2021, 18, 1333. [Google Scholar] [CrossRef] [PubMed]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson Correlation Coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Stekhoven, D.J.; Bühlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhu, J.; Ma, Y.; Zhou, X. Accuracy, robustness and scalability of dimensionality reduction methods for single-cell RNA-seq analysis. Genome Biol. 2019, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A K-Means Clustering Algorithm. J. R. Stat. Soc. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Ding, C.; He, X. K-means Clustering via Principal Component Analysis. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 29. [Google Scholar]
- Makiewicz, A.; Ratajczak, W. Principal Component Analysis (PCA). Comput. Geosci. 1993, 19, 303–342. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Jackson, D.A. Stopping Rules in Principal Components Analysis: A Comparison of Heuristical and Statistical Approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- Schölkopf, B.; Smola, A.; Müller, K.-R. Kernel principal component analysis. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 1997; pp. 583–588. [Google Scholar]
| Cluster | Risk of AF Recurrence | Relative Risk | |
|---|---|---|---|
| Paroxysmal AF | Persistent AF | Persistent AF/Paroxysmal AF | |
| I | 0.30 (45/151) | 0.49 (67/136) | 1.65 |
| II | 0.13 (1/8) | 0.51 (80/158) | 4.05 |
| III | 0.35 (19/54) | 0.57 (53/93) | 1.62 |
| Male | Female | Female/Male | |
| I | 0.37 (85/228) | 0.46 (27/59) | 1.22 |
| II | 0.44 (53/121) | 0.62 (28/45) | 1.42 |
| III | 0.39 (38/97) | 0.68 (34/50) | 1.74 |
| LAVI ≤ 40 | LAVI > 40 | (LAVI > 40)/(LAVI ≤ 40) | |
| I | 0.34 (60/174) | 0.49 (50/102) | 1.42 |
| II | 0.48 (31/64) | 0.51 (49/97) | 1.04 |
| III | 0.43 (25/58) | 0.54 (45/84) | 1.24 |
| BMI ≤ 27 | BMI > 27 | (BMI > 27)/(BMI ≤ 27) | |
| I | 0.43 (68/159) | 0.35 (44/127) | 0.81 |
| II | 0.54 (33/61) | 0.46 (48/105) | 0.85 |
| III | 0.41 (26/63) | 0.55 (46/83) | 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijer, I.; Terpstra, M.M.; Camara, O.; Marquering, H.A.; Arrarte Terreros, N.; de Groot, J.R. Unsupervised Clustering of Patients Undergoing Thoracoscopic Ablation Identifies Relevant Phenotypes for Advanced Atrial Fibrillation. Diagnostics 2025, 15, 1269. https://doi.org/10.3390/diagnostics15101269
Meijer I, Terpstra MM, Camara O, Marquering HA, Arrarte Terreros N, de Groot JR. Unsupervised Clustering of Patients Undergoing Thoracoscopic Ablation Identifies Relevant Phenotypes for Advanced Atrial Fibrillation. Diagnostics. 2025; 15(10):1269. https://doi.org/10.3390/diagnostics15101269
Chicago/Turabian StyleMeijer, Ilse, Marc M. Terpstra, Oscar Camara, Henk A. Marquering, Nerea Arrarte Terreros, and Joris R. de Groot. 2025. "Unsupervised Clustering of Patients Undergoing Thoracoscopic Ablation Identifies Relevant Phenotypes for Advanced Atrial Fibrillation" Diagnostics 15, no. 10: 1269. https://doi.org/10.3390/diagnostics15101269
APA StyleMeijer, I., Terpstra, M. M., Camara, O., Marquering, H. A., Arrarte Terreros, N., & de Groot, J. R. (2025). Unsupervised Clustering of Patients Undergoing Thoracoscopic Ablation Identifies Relevant Phenotypes for Advanced Atrial Fibrillation. Diagnostics, 15(10), 1269. https://doi.org/10.3390/diagnostics15101269








