Artificial Intelligence in Coronary Artery Calcium Scoring Detection and Quantification
Abstract
:1. Introduction
1.1. Artificial Intelligence in Medical Imaging
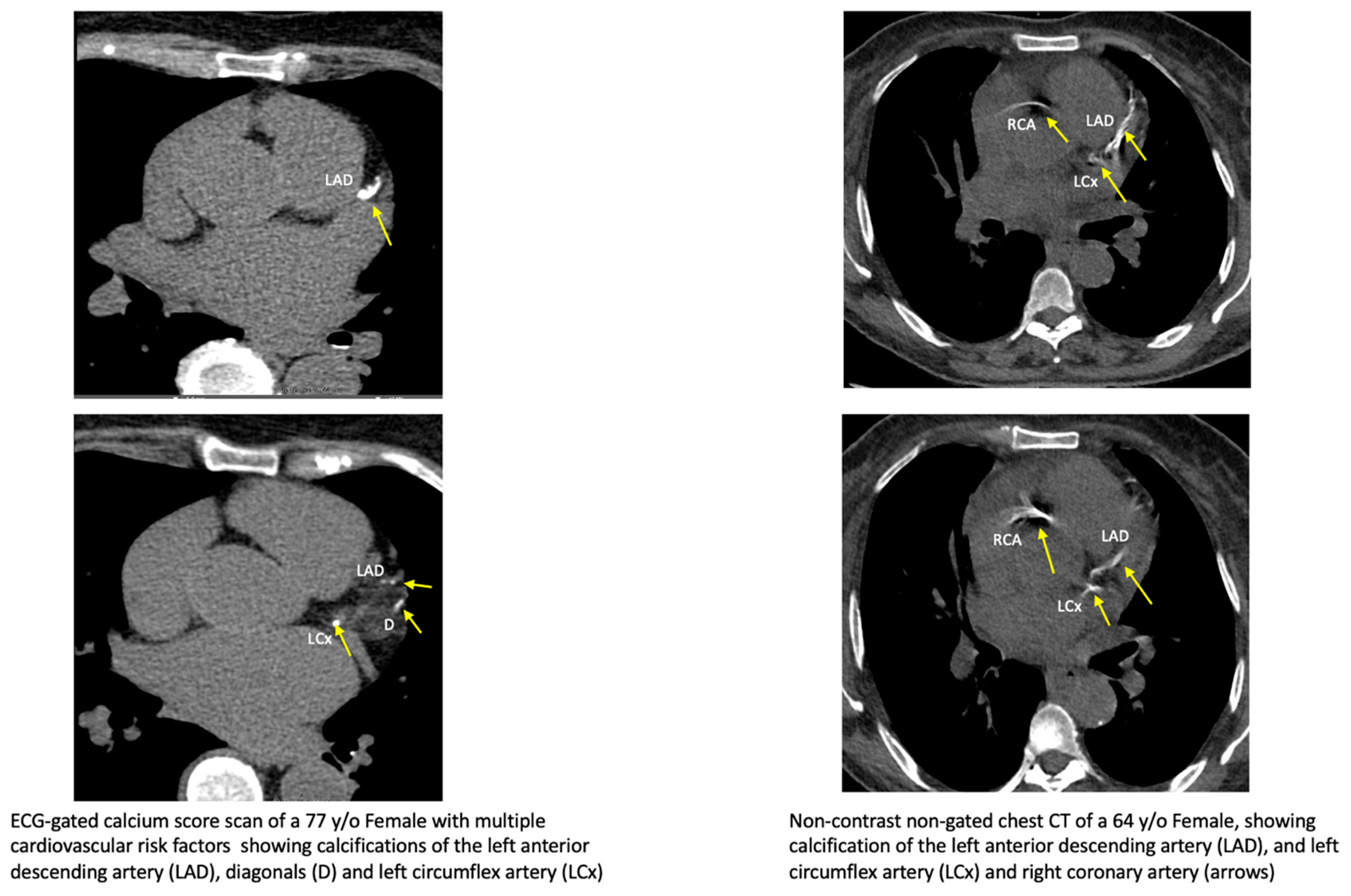
1.2. Coronary Artery Calcium Assessment and Interpretation of Non-Cardiac Exams
1.3. Artificial Intelligence and CACS
2. AI Applications for CAC Scoring from Cardiac/Gated Scans
2.1. Early ML-Based Approaches
2.2. Deep Learning and Convolutional Neural Networks
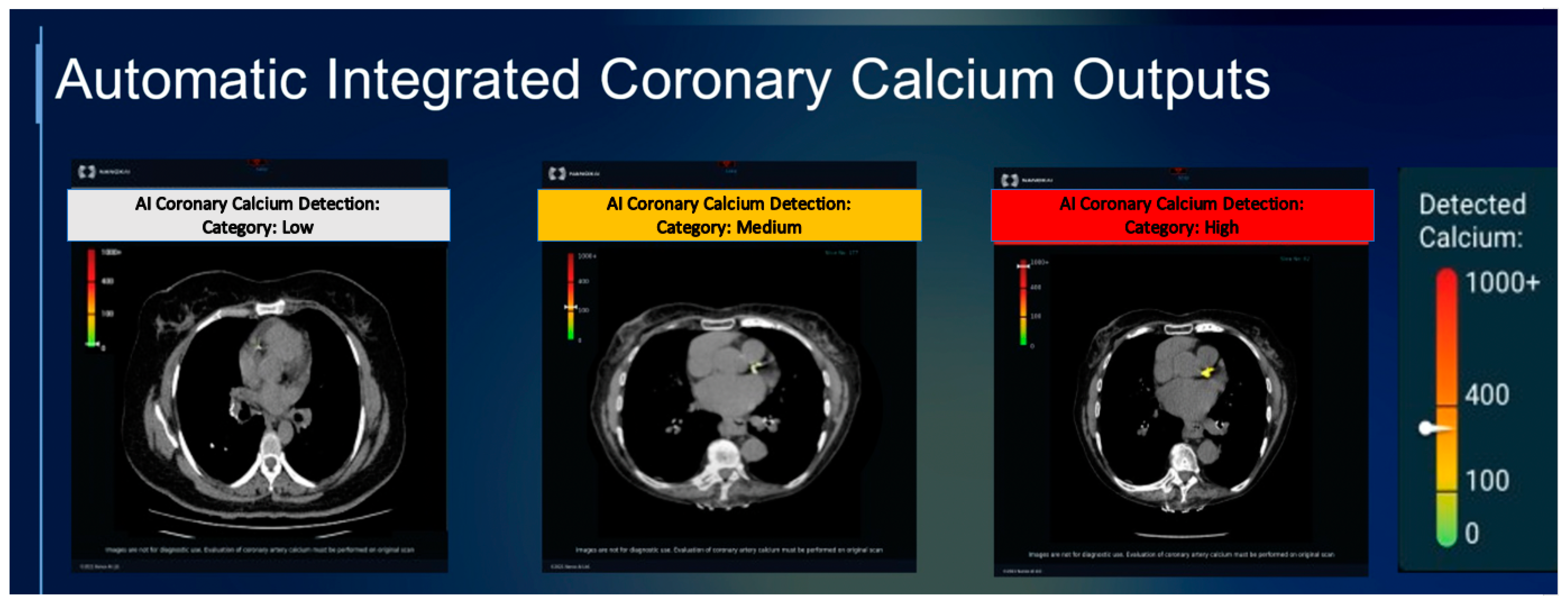
3. AI for CAC Analysis on Non-Gated CT Scans
- •
- Improve ability to detect presence and burden of CAC
- •
- Improve reproducibility and accuracy of CAD detection
- •
- Enhance risk assessment, thereby guiding need & intensity of preventive therapies
- •
- Improve population-based preventive efforts
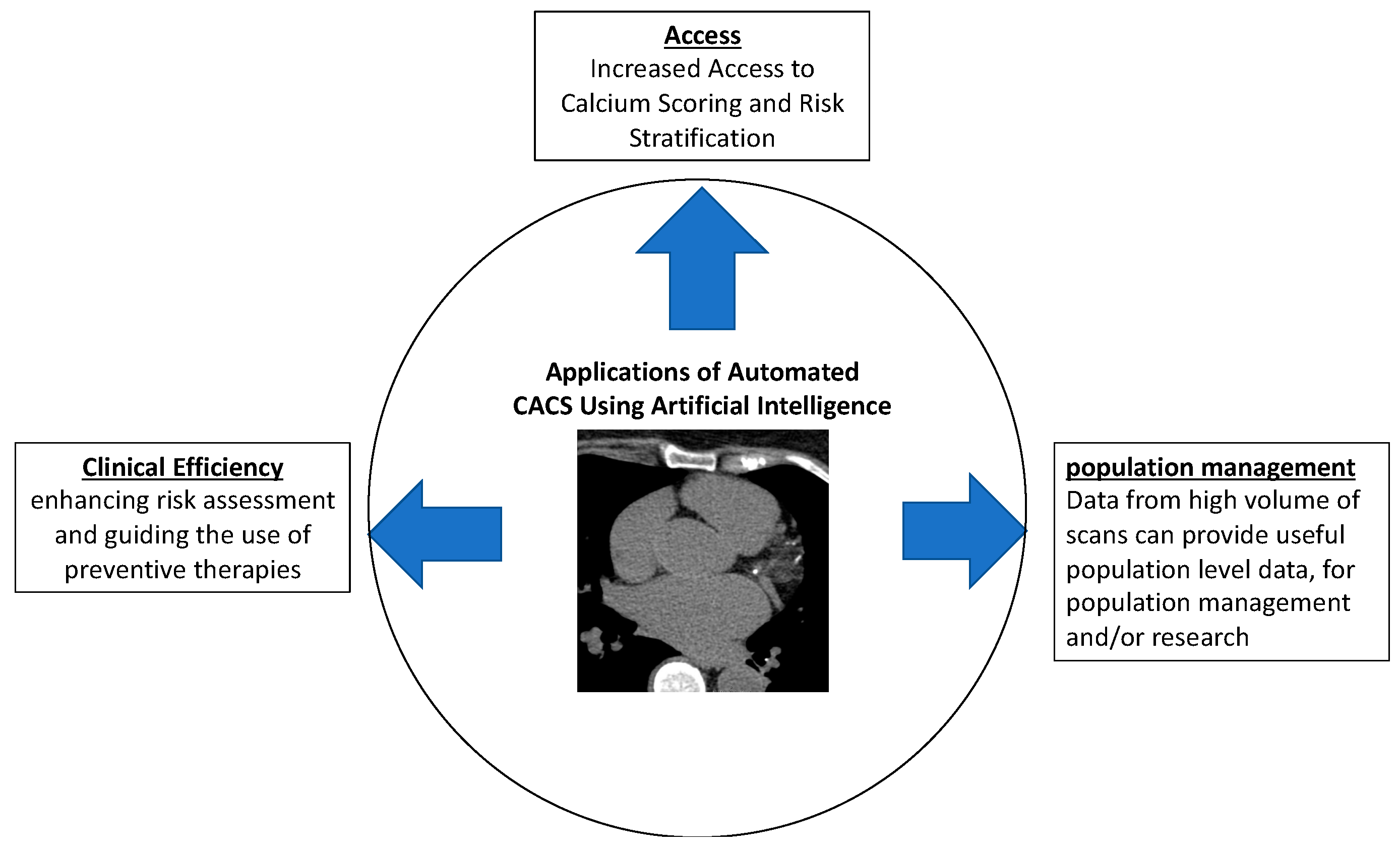
4. Additional Applications
5. Future Directions for AI in CAC Scoring
6. Potential Challenges to Automated CAC Scoring
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, M.A.; Aikawa, E. Cardiovascular calcification: Artificial intelligence and big data accelerate mechanistic discovery. Nat. Rev. Cardiol. 2019, 16, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.J.; Tison, G.H.; Delling, F.N. Artificial Intelligence in Cardiovascular Imaging. Methodist. Debakey Cardiovasc. J. 2020, 16, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.L. Artificial Intelligence in Imaging: The Radiologist’s Role. J. Am. Coll. Radiol. 2019, 16, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, K.; Wu, T.; Weidman, D.; Lure, F.; Li, J. Use of multimodality imaging and artificial intelligence for diagnosis and prognosis of early stages of Alzheimer’s disease. Transl. Res. 2018, 194, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef]
- Lessmann, N.; van Ginneken, B.; Zreik, M.; de Jong, P.A.; de Vos, B.D.; Viergever, M.A.; Isgum, I. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks With Dilated Convolutions. IEEE Trans. Med. Imaging 2018, 37, 615–625. [Google Scholar] [CrossRef]
- Wolterink, J.M.; Leiner, T.; de Vos, B.D.; Coatrieux, J.-L.; Kelm, B.M.; Kondo, S.; Salgado, R.A.; Shahzad, R.; Shu, H.; Snoeren, M.; et al. An evaluation of automatic coronary artery calcium scoring methods with cardiac CT using the orCaScore framework. Med. Phys. 2016, 43, 2361. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaur, I. Artificial intelligence, machine learning and deep learning: Definitions and differences. Clin. Exp. Dermatol. 2020, 45, 131–132. [Google Scholar] [CrossRef]
- Majaj, N.J.; Pelli, D.G. Deep learning—Using machine learning to study biological vision. J. Vis. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Young, R.; Burke, G.; Jeffrey Carr, J.; Detrano, R.C.; Folsom, A.R.; Kronmal, R.; Lima, J.A.C.; Liu, K.J.; McClelland, R.L.; et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: The multi-ethnic study of atherosclerosis (MESA). Eur. Heart J. 2018, 39, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Bittencourt, M.S.; Blaha, M.J.; Blankstein, R.; Agatson, A.S.; Rivera, J.J.; Miedema, M.D.; Miemdema, M.D.; Sibley, C.T.; Shaw, L.J.; et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2015, 66, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Orringer, C.E.; Blaha, M.J.; Blankstein, R.; Budoff, M.J.; Goldberg, R.B.; Gill, E.A.; Maki, K.C.; Mehta, L.; Jacobson, T.A. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J. Clin. Lipidol. 2021, 15, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Blaha, M.J.; Berman, D.S.; Nasir, K.; Budoff, M.; Leipsic, J.; Blankstein, R.; Narula, J.; Rumberger, J.; Shaw, L.J. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, G.R.; Mirbolouk, M.; Dardari, Z.A.; Al-Mallah, M.H.; Rumberger, J.A.; Shaw, L.J.; Blankstein, R.; Miedema, M.D.; Berman, D.S.; Budoff, M.J.; et al. Interplay of Coronary Artery Calcium and Risk Factors for Predicting CVD/CHD Mortality: The CAC Consortium. JACC Cardiovasc. Imaging 2020, 13, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Gupta, A.; Rana, J.S.; Nasir, K. The Implication of Coronary Artery Calcium Testing for Cardiovascular Disease Prevention and Diabetes. Endocrinol. Metab. 2017, 32, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Elkeles, R.S.; Godsland, I.F.; Feher, M.D.; Rubens, M.B.; Roughton, M.; Nugara, F.; Humphries, S.E.; Richmond, W.; Flather, M.D. PREDICT Study Group Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: The PREDICT study. Eur. Heart J. 2008, 29, 2244–2251. [Google Scholar] [CrossRef]
- Cardoso, R.; Dudum, R.; Ferraro, R.A.; Bittencourt, M.; Blankstein, R.; Blaha, M.J.; Nasir, K.; Rajagopalan, S.; Michos, E.D.; Blumenthal, R.S.; et al. Cardiac Computed Tomography for Personalized Management of Patients with Type 2 Diabetes Mellitus. Circ. Cardiovasc. Imaging 2020, 13, e011365. [Google Scholar] [CrossRef]
- Berrington de González, A.; Mahesh, M.; Kim, K.-P.; Bhargavan, M.; Lewis, R.; Mettler, F.; Land, C. Projected Cancer Risks from Computed Tomographic Scans Performed in the United States in 2007. Arch. Intern. Med. 2009, 169, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Cè, M.; Irmici, G.; Ascenti, V.; Khenkina, N.; Toto-Brocchi, M.; Martinenghi, C.; Papa, S.; Carrafiello, G. Artificial Intelligence in Lung Cancer Imaging: Unfolding the Future. Diagnostics 2022, 12, 2644. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Austin, J.M.; Dominguez, A.; Allison, M.A.; Wassel, C.L.; Rifkin, D.E.; Morgan, C.G.; Daniels, M.R.; Ikram, U.; Knox, J.B.; Wright, C.M.; et al. Relationship of Coronary Calcium on Standard Chest CT Scans with Mortality. JACC Cardiovasc. Imaging 2016, 9, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.; Khalaili, L.; Naoum, I.; Abedalghani, A.; Zoubi, R.; Haber, C.C.; Kassem, S. Does incidental calcium deposition in non-cardiac CT scans predict cardiovascular morbidity and mortality in young adults? A retrospective study. Eur. Heart J. 2021, 42, ehab724.2526. [Google Scholar] [CrossRef]
- Jacobs, P.C.; Gondrie, M.J.; Mali, W.P.; Oen, A.L.; Prokop, M.; Grobbee, D.E.; van der Graaf, Y. Unrequested information from routine diagnostic chest CT predicts future cardiovascular events. Eur. Radiol. 2011, 21, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-T.; Yang, P.; Huang, Y.-L.; Chen, J.-S.; Chuo, C.-C.; Yeh, C.; Chang, R.-S. Coronary Arterial Calcification on Low-Dose Ungated MDCT for Lung Cancer Screening: Concordance Study with Dedicated Cardiac CT. Am. J. Roentgenol. 2008, 190, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.; Buitrago, I.; Mohammed, T.-L.H.; Gao, T.; Asher, C.R.; Novaro, G.M. Detection of coronary calcium during standard chest computed tomography correlates with multi-detector computed tomography coronary artery calcium score. Int. J. Cardiovasc. Imaging 2012, 28, 1249–1256. [Google Scholar] [CrossRef]
- Budoff, M.J.; Nasir, K.; Kinney, G.L.; Hokanson, J.E.; Barr, R.G.; Steiner, R.; Nath, H.; Lopez-Garcia, C.; Black-Shinn, J.; Casaburi, R. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: Comparison of ungated and gated examinations in patients from the COPD Gene cohort. J. Cardiovasc. Comput. Tomogr. 2011, 5, 113–118. [Google Scholar] [CrossRef]
- Hecht, H.S.; Cronin, P.; Blaha, M.J.; Budoff, M.J.; Kazerooni, E.A.; Narula, J.; Yankelevitz, D.; Abbara, S. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J. Cardiovasc. Comput. Tomogr. 2017, 11, 74–84. [Google Scholar] [CrossRef]
- Velangi, P.S.; Kenny, B.; Hooks, M.; Kanda, A.; Schertz, K.; Kharoud, H.; Sandhu, G.S.; Kalra, R.; Allen, T.; Begnaud, A.; et al. Impact of 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncardiac chest CT scans on lung cancer screening CT reporting. Int. J. Cardiovasc. Imaging 2021, 37, 2777–2784. [Google Scholar] [CrossRef]
- Cellina, M.; Cacioppa, L.M.; Cè, M.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Pais, D.; Bausano, M.V.; Rossini, N.; Bruno, A.; et al. Artificial Intelligence in Lung Cancer Screening: The Future Is Now. Cancers 2023, 15, 4344. [Google Scholar] [CrossRef] [PubMed]
- Salehi, N.; Janjani, P.; Tadbiri, H.; Rozbahani, M.; Jalilian, M. Effect of cigarette smoking on coronary arteries and pattern and severity of coronary artery disease: A review. J. Int. Med. Res. 2021, 49, 3000605211059893. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Kim, J.T.; Holohan, K.M. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J. Cardiovasc. Comput. Tomogr. 2013, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Uretsky, S.; Chokshi, N.; Kobrinski, T.; Agarwal, S.K.; Po, J.R.; Awan, H.; Jagarlamudi, A.; Gudiwada, S.P.; D’Avino, R.C.; Rozanski, A. The Interplay of Physician Awareness and Reporting of Incidentally Found Coronary Artery Calcium on the Clinical Management of Patients Who Underwent Noncontrast Chest Computed Tomography. Am. J. Cardiol. 2015, 115, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lau, E.; Varshney, R.; Hulten, E.A.; Cheezum, M.; Bittencourt, M.S.; Blaha, M.J.; Wong, N.D.; Blumenthal, R.S.; Budoff, M.J.; et al. The Identification of Calcified Coronary Plaque Is Associated With Initiation and Continuation of Pharmacological and Lifestyle Preventive Therapies. JACC Cardiovasc. Imaging 2017, 10, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Hong, J.-S.; Tzeng, Y.-H.; Yin, W.-H.; Wu, K.-T.; Hsu, H.-Y.; Lu, C.-F.; Liu, H.-R.; Wu, Y.-T. Automated coronary artery calcium scoring using nested U-Net and focal loss. Comput. Struct. Biotechnol. J. 2022, 20, 1681–1690. [Google Scholar] [CrossRef]
- Eng, D.; Chute, C.; Khandwala, N.; Rajpurkar, P.; Long, J.; Shleifer, S.; Khalaf, M.H.; Sandhu, A.T.; Rodriguez, F.; Maron, D.J.; et al. Automated coronary calcium scoring using deep learning with multicenter external validation. npj Digit. Med. 2021, 4, 88. [Google Scholar] [CrossRef]
- Ihdayhid, A.R.; Lan, N.S.R.; Williams, M.; Newby, D.; Flack, J.; Kwok, S.; Joyner, J.; Gera, S.; Dembo, L.; Adler, B.; et al. Evaluation of an artificial intelligence coronary artery calcium scoring model from computed tomography. Eur. Radiol. 2022, 33, 321–329. [Google Scholar] [CrossRef]
- Hampe, N.; Wolterink, J.M.; van Velzen, S.G.M.; Leiner, T.; Išgum, I. Machine Learning for Assessment of Coronary Artery Disease in Cardiac CT: A Survey. Front. Cardiovasc. Med. 2019, 6, 172. [Google Scholar] [CrossRef]
- Išgum, I.; Rutten, A.; Prokop, M.; van Ginneken, B. Detection of coronary calcifications from computed tomography scans for automated risk assessment of coronary artery disease. Med. Phys. 2007, 34, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Kurkure, U.; Chittajallu, D.R.; Brunner, G.; Le, Y.H.; Kakadiaris, I.A. A supervised classification-based method for coronary calcium detection in non-contrast CT. Int. J. Cardiovasc. Imaging 2010, 26, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; van Walsum, T.; Schaap, M.; Rossi, A.; Klein, S.; Weustink, A.C.; de Feyter, P.J.; van Vliet, L.J.; Niessen, W.J. Vessel Specific Coronary Artery Calcium Scoring: An Automatic System. Acad. Radiol. 2013, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Slomka, P.J.; Diaz-Zamudio, M.; Germano, G.; Berman, D.S.; Terzopoulos, D.; Dey, D. Automated coronary artery calcium scoring from non-contrast CT using a patient-specific algorithm. In Proceedings of the Medical Imaging 2015: Image Processing, Orlando, FL, USA, 24–26 February 2015; Volume 9413, pp. 767–772. [Google Scholar]
- Gogin, N.; Viti, M.; Nicodème, L.; Ohana, M.; Talbot, H.; Gencer, U.; Mekukosokeng, M.; Caramella, T.; Diascorn, Y.; Airaud, J.-Y.; et al. Automatic coronary artery calcium scoring from unenhanced-ECG-gated CT using deep learning. Diagn. Interv. Imaging 2021, 102, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. In Proceedings of the Advances in Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012. [Google Scholar]
- Lee, H.; Martin, S.; Burt, J.R.; Bagherzadeh, P.S.; Rapaka, S.; Gray, H.N.; Leonard, T.J.; Schwemmer, C.; Schoepf, U.J. Machine Learning and Coronary Artery Calcium Scoring. Curr. Cardiol. Rep. 2020, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Measures of Interrater Agreement. J. Thorac. Oncol. 2011, 6, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, M. Weighted kappa statistic for clustered matched-pair ordinal data. Comput. Stat. Data Anal. 2015, 82, 1–18. [Google Scholar] [CrossRef]
- Greenland, P.; Bonow, R.O.; Brundage, B.H.; Budoff, M.J.; Eisenberg, M.J.; Grundy, S.M.; Lauer, M.S.; Post, W.S.; Raggi, P.; Redberg, R.F.; et al. ACCF/AHA 2007 Clinical Expert Consensus Document on Coronary Artery Calcium Scoring by Computed Tomography in Global Cardiovascular Risk Assessment and in Evaluation of Patients with Chest Pain: A Report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Developed in Collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J. Am. Coll. Cardiol. 2007, 49, 378–402. [Google Scholar] [CrossRef]
- Bobak, C.A.; Barr, P.J.; O’Malley, A.J. Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med. Res. Methodol. 2018, 18, 93. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Sandstedt, M.; Henriksson, L.; Janzon, M.; Nyberg, G.; Engvall, J.; De Geer, J.; Alfredsson, J.; Persson, A. Evaluation of an AI-based, automatic coronary artery calcium scoring software. Eur. Radiol. 2020, 30, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; van Assen, A.M.; Rapaka, S.; Hudson, H.T.; Fischer, A.M.; Varga-Szemes, A.; Sahbaee, P.; Schwemmer, C.; Gulsun, M.A.; Cimen, S.; et al. Evaluation of a Deep Learning–Based Automated CT Coronary Artery Calcium Scoring Algorithm. JACC Cardiovasc. Imaging 2020, 13, 524–526. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. arXiv 2015, arXiv:1512.03385. [Google Scholar]
- Winkel, D.J.; Suryanarayana, V.R.; Ali, A.M.; Görich, J.; Buß, S.J.; Mendoza, A.; Schwemmer, C.; Sharma, P.; Schoepf, U.J.; Rapaka, S. Deep learning for vessel-specific coronary artery calcium scoring: Validation on a multi-centre dataset. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, G.; Zhang, W.; Wang, W.; Zhou, Z.; Zhang, H.; Xu, L.; Chen, Y. Fully automatic framework for comprehensive coronary artery calcium scores analysis on non-contrast cardiac-gated CT scan: Total and vessel-specific quantifications. Eur. J. Radiol. 2021, 134, 109420. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Chen, Q.; Zhou, Z.; Wang, R.; Wang, H.; Zhang, N.; Chen, Y.; Sun, Z.; Xu, L. Coronary artery calcium score quantification using a deep-learning algorithm. Clin. Radiol. 2020, 75, 237.e11–237.e16. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.P.; de Jong, P.A.; Leiner, T.; Oudkerk, M.; de Koning, H.J.; Mol, C.P.; Viergever, M.A.; Išgum, I. Automated Coronary Artery Calcification Scoring in Non-Gated Chest CT: Agreement and Reliability. PLoS ONE 2014, 9, e91239. [Google Scholar] [CrossRef]
- Lessmann, N.; Išgum, I.; Setio, A.A.A.; de Vos, B.D.; Ciompi, F.; de Jong, P.A.; Oudkerk, M.; Mali, W.P.T.M.; Viergever, M.A.; Ginneken, B. van Deep convolutional neural networks for automatic coronary calcium scoring in a screening study with low-dose chest CT. In Proceedings of the Medical Imaging 2016: Computer-Aided Diagnosis, San Diego, CA, USA, 27 February–3 March 2016; Volume 9785, pp. 255–260. [Google Scholar]
- Cano-Espinosa, C.; González, G.; Washko, G.R.; Cazorla, M.; Estépar, R.S.J. Automated Agatston score computation in non-ECG gated CT scans using deep learning. In Proceedings of the Medical Imaging 2018: Image Processing, Houston, TX, USA, 10–15 February 2018; Volume 10574, pp. 673–678. [Google Scholar]
- van Assen, M.; Martin, S.S.; Varga-Szemes, A.; Rapaka, S.; Cimen, S.; Sharma, P.; Sahbaee, P.; De Cecco, C.N.; Vliegenthart, R.; Leonard, T.J.; et al. Automatic coronary calcium scoring in chest CT using a deep neural network in direct comparison with non-contrast cardiac CT: A validation study. Eur. J. Radiol. 2021, 134, 109428. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Guo, N.; Chen, L.; Song, W.; Guo, D.; Zhang, Y.; Fang, Z. Performance of artificial intelligence-based coronary artery calcium scoring in non-gated chest CT. Eur. J. Radiol. 2021, 145, 110034. [Google Scholar] [CrossRef]
- van Velzen, S.G.M.; Lessmann, N.; Velthuis, B.K.; Bank, I.E.M.; van den Bongard, D.H.J.G.; Leiner, T.; de Jong, P.A.; Veldhuis, W.B.; Correa, A.; Terry, J.G.; et al. Deep Learning for Automatic Calcium Scoring in CT: Validation Using Multiple Cardiac CT and Chest CT Protocols. Radiology 2020, 295, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, R.; Foldyna, B.; Eslami, P.; Weiss, J.; Alexander, I.; Taron, J.; Parmar, C.; Alvi, R.M.; Banerji, D.; Uno, M.; et al. Deep convolutional neural networks to predict cardiovascular risk from computed tomography. Nat. Commun. 2021, 12, 715. [Google Scholar] [CrossRef]
- Kamel, P.I.; Yi, P.H.; Sair, H.I.; Lin, C.T. Prediction of Coronary Artery Calcium and Cardiovascular Risk on Chest Radiographs Using Deep Learning. Radiol. Cardiothorac. Imaging 2021, 3, e200486. [Google Scholar] [CrossRef] [PubMed]
- Pieszko, K.; Shanbhag, A.; Killekar, A.; Miller, R.J.H.; Lemley, M.; Otaki, Y.; Singh, A.; Kwiecinski, J.; Gransar, H.; Van, K.S.D.; et al. Deep Learning of Coronary Calcium Scores From PET/CT Attenuation Maps Accurately Predicts Adverse Cardiovascular Events. JACC Cardiovasc. Imaging 2023, 16, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Morf, C.; Sartoretti, T.; Gennari, A.G.; Maurer, A.; Skawran, S.; Giannopoulos, A.A.; Sartoretti, E.; Schwyzer, M.; Curioni-Fontecedro, A.; Gebhard, C.; et al. Diagnostic Value of Fully Automated Artificial Intelligence Powered Coronary Artery Calcium Scoring from 18F-FDG PET/CT. Diagnostics 2022, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Rim, T.H.; Lee, C.J.; Tham, Y.-C.; Cheung, N.; Yu, M.; Lee, G.; Kim, Y.; Ting, D.S.W.; Chong, C.C.Y.; Choi, Y.S.; et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit. Health 2021, 3, e306–e316. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; van der Bijl, P.; Bax, J.J. Using a deep learning algorithm to score coronary artery calcium in myocardial perfusion imaging: A real opportunity or just a new hype? J. Nucl. Cardiol. 2022, 30, 251–253. [Google Scholar] [CrossRef]
- Mu, D.; Bai, J.; Chen, W.; Yu, H.; Liang, J.; Yin, K.; Li, H.; Qing, Z.; He, K.; Yang, H.-Y.; et al. Calcium Scoring at Coronary CT Angiography Using Deep Learning. Radiology 2022, 302, 309–316. [Google Scholar] [CrossRef]
- Wolterink, J.M.; Leiner, T.; de Vos, B.D.; van Hamersvelt, R.W.; Viergever, M.A.; Išgum, I. Automatic coronary artery calcium scoring in cardiac CT angiography using paired convolutional neural networks. Med. Image Anal. 2016, 34, 123–136. [Google Scholar] [CrossRef]
- Atkins, K.M.; Weiss, J.; Zeleznik, R.; Bitterman, D.S.; Chaunzwa, T.L.; Huynh, E.; Guthier, C.; Kozono, D.E.; Lewis, J.H.; Tamarappoo, B.K.; et al. Elevated Coronary Artery Calcium Quantified by a Validated Deep Learning Model from Lung Cancer Radiotherapy Planning Scans Predicts Mortality. JCO Clin. Cancer Inform. 2022, 6, e2100095. [Google Scholar] [CrossRef]
- Peng, A.W.; Dudum, R.; Jain, S.S.; Maron, D.J.; Patel, B.N.; Khandwala, N.; Eng, D.; Chaudhari, A.S.; Sandhu, A.T.; Rodriguez, F. Association of Coronary Artery Calcium Detected by Routine Ungated CT Imaging with Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2023, 82, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, A.R.; Narula, J.; Lin, F.Y.; van den Hoogen, I.J.; Gianni, U.; Al Hussein Alawamlh, O.; Dunham, P.C.; Peña, J.M.; Lee, S.-E.; Andreini, D.; et al. Association of High-Density Calcified 1K Plaque with Risk of Acute Coronary Syndrome. JAMA Cardiol. 2020, 5, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kianoush, S.; Rifai, M.A.; Cainzos-Achirica, M.; Al-Mallah, M.H.; Tison, G.H.; Yeboah, J.; Miedema, M.D.; Allison, M.A.; Wong, N.D.; DeFilippis, A.P.; et al. Thoracic extra-coronary calcification for the prediction of stroke: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2017, 267, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular Events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Mortensen, M.B.; Kianoush, S.; Tota-Maharaj, R.; Cainzos-Achirica, M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc. Imaging 2017, 10, 923–937. [Google Scholar] [CrossRef]
- Eisen, A.; Tenenbaum, A.; Koren-Morag, N.; Tanne, D.; Shemesh, J.; Imazio, M.; Fisman, E.Z.; Motro, M.; Schwammenthal, E.; Adler, Y. Calcification of the Thoracic Aorta as Detected by Spiral Computed Tomography among Stable Angina Pectoris Patients. Circulation 2008, 118, 1328–1334. [Google Scholar] [CrossRef]
- Iribarren, C.; Sidney, S.; Sternfeld, B.; Browner, W.S. Calcification of the Aortic ArchRisk Factors and Association with Coronary Heart Disease, Stroke, and Peripheral Vascular Disease. JAMA 2000, 283, 2810–2815. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Zhang, Y.; Wang, H.; Tan, Y.; Liu, Y.; Huang, L.; Zhang, H.; Ma, Y.; Cong, H. Epicardial Fat Volume Improves the Prediction of Obstructive Coronary Artery Disease Above Traditional Risk Factors and Coronary Calcium Score. Circ. Cardiovasc. Imaging 2019, 12, e008002. [Google Scholar] [CrossRef]
- Chhabra, R.; O’Keefe, J.H.; Patil, H.; O’Keefe, E.; Thompson, R.C.; Ansari, S.; Kennedy, K.F.; Lee, L.W.; Helzberg, J.H. Association of coronary artery calcification with hepatic steatosis in asymptomatic individuals. Mayo Clin. Proc. 2013, 88, 1259–1265. [Google Scholar] [CrossRef]
| Study | Year Published | Study Size (Testing) | Algorithm Type | Risk Categories | Accuracy | Conclusions |
|---|---|---|---|---|---|---|
| Eng et al. [38] | 2021 | 79 | CNN | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | Mean difference scores: −2.86, Kappa = 0.89, p < 0.0001 | Demonstrated near-perfect agreement with the reference standard and with improved computational speed. |
| Hong et al. [37] | 2022 | 959 | U-Net (CNN) | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | ICC = 1.00, Kappa = 0.931 | Demonstrated excellent agreement with the reference standard and detected mild calcifications not detected by reference. |
| Gogin et al. [45] | 2021 | 98 | U-Net (CNN) | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | Concordance-index = 0.951 | With an ensemble of 5 CNN models, there is high concordance with the standard reference. |
| Zhang et al. [58] | 2021 | 46 | U-Net (CNN) | Risk categorization not compared | ICC = 0.988, mean difference scores: −6.7, p = 0.993 | High-speed and accurate automated quantification of total and vessel-specific CAC in a single-center study. |
| Sandstedt et al. [54] | 2020 | 315 | CNN | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | Mean difference scores: −8.2, ICC = 0.996, Kappa = 0.919 | Single-center study demonstrating near-perfect agreement, including Agatson assessment, volume score, mass score, and number of calcified lesions. |
| Wang et al. [59] | 2019 | 140 | 3D CNN | Categories for Agatson scores of 0, 1–99, 100–299, and >300 | ICC = 0.94, Kappa = 0.77 | Single-center study with near-perfect agreement of Agatson, volume, and mass scores and a reclassification rate of 13%. |
| Martin et al. [55] | 2020 | 511 | ResNet CNN | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | ICC = 0.985, Kappa = 0.932 | Demonstrated outstanding agreement of total Agatson score with the reference standard trained on a dataset of 2000 patients. |
| Winkel et al. [57] | 2022 | 1171 | CNN | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | ICC = 0.84, Kappa = 0.91 | Large, multicenter study demonstrating excellent accuracy on a total and per-vessel basis. |
| Idhayid et al. [39] | 2022 | 1849 | 3D CNN | Categories for Agatston scores of 0, 1–10, 11–100, 101–400, >400 | ICC = 0.98, Kappa = 0.90, p < 0.001 | Large study with scans obtained from multiple vendors demonstrated excellent agreement and efficiency. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, K.; Shiyovich, A.; Huck, D.M.; Berman, A.N.; Weber, B.; Gupta, S.; Cardoso, R.; Blankstein, R. Artificial Intelligence in Coronary Artery Calcium Scoring Detection and Quantification. Diagnostics 2024, 14, 125. https://doi.org/10.3390/diagnostics14020125
Abdelrahman K, Shiyovich A, Huck DM, Berman AN, Weber B, Gupta S, Cardoso R, Blankstein R. Artificial Intelligence in Coronary Artery Calcium Scoring Detection and Quantification. Diagnostics. 2024; 14(2):125. https://doi.org/10.3390/diagnostics14020125
Chicago/Turabian StyleAbdelrahman, Khaled, Arthur Shiyovich, Daniel M. Huck, Adam N. Berman, Brittany Weber, Sumit Gupta, Rhanderson Cardoso, and Ron Blankstein. 2024. "Artificial Intelligence in Coronary Artery Calcium Scoring Detection and Quantification" Diagnostics 14, no. 2: 125. https://doi.org/10.3390/diagnostics14020125





