In-Hospital Mortality Among Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion with and Without Malignancy
Simple Summary
Abstract
1. Introduction
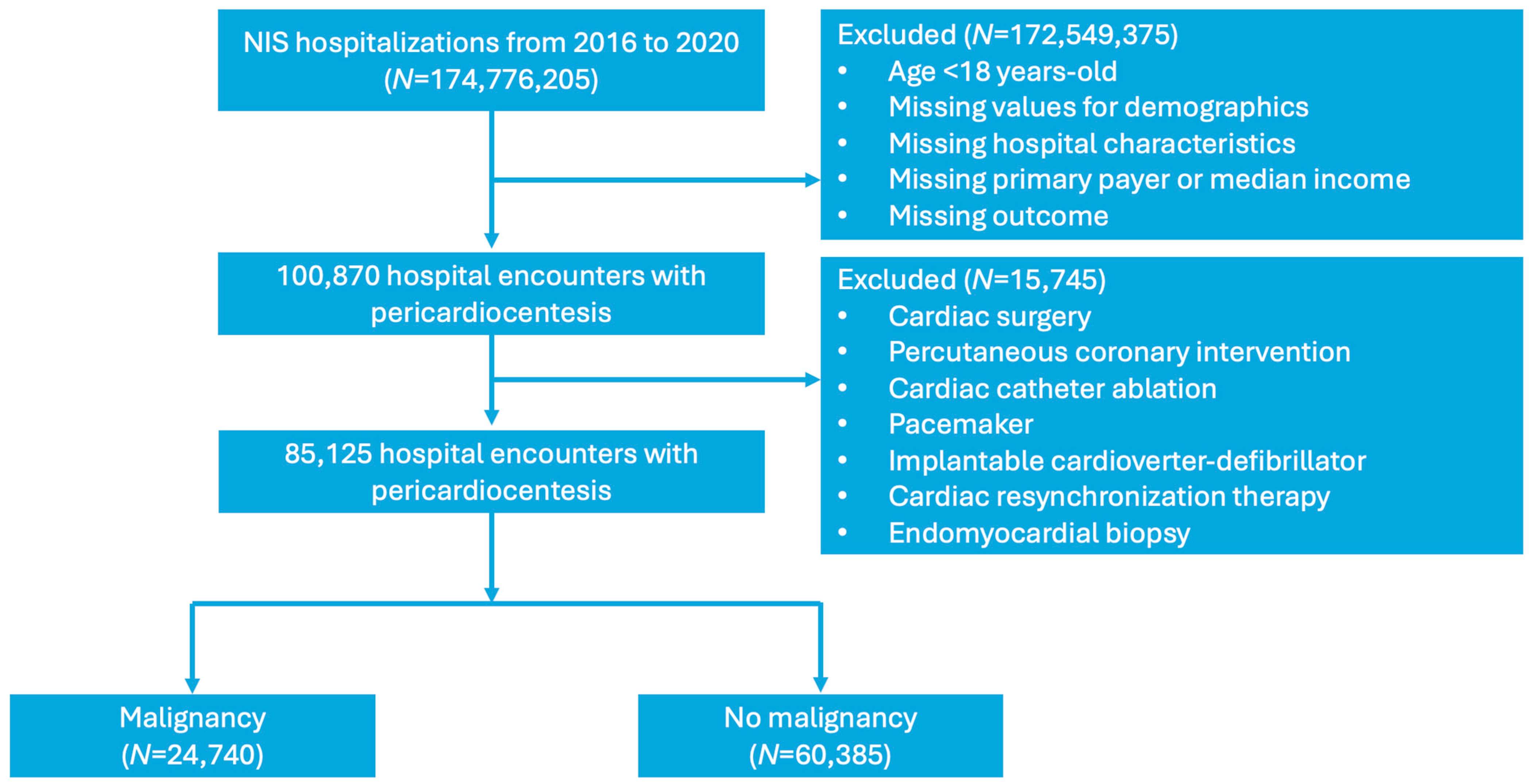
2. Method
2.1. Data Source
2.2. Study Population and Covariates
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics
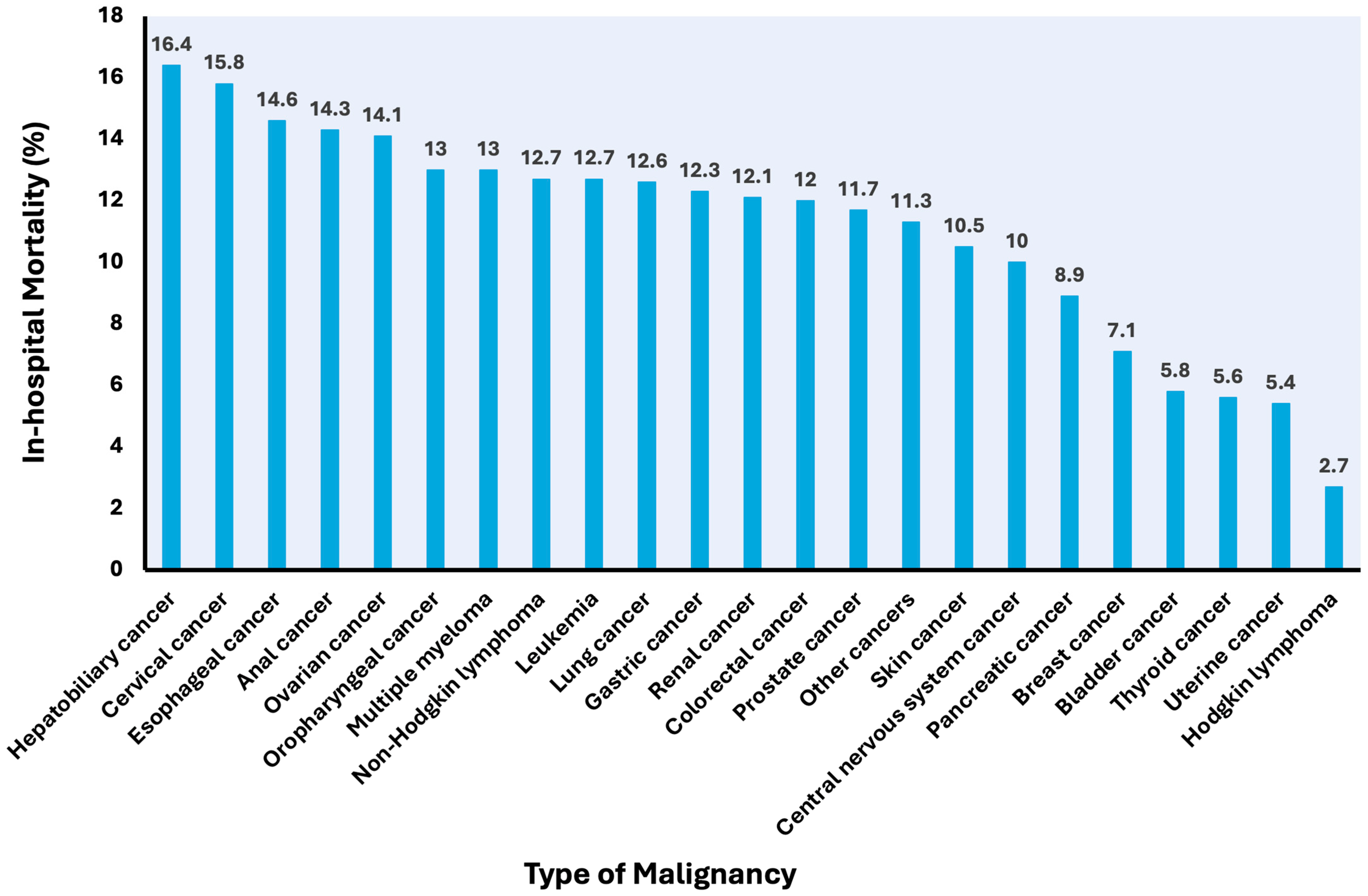
3.2. Mortality
3.3. Secondary Outcomes
3.4. Covariates Associated with In-Hospital Mortality
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Horin, S.; Bank, I.; Guetta, V.; Livneh, A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: A study in 173 consecutive patients undergoing pericardiocentesis. Medicine 2006, 85, 49–53. [Google Scholar] [CrossRef]
- Gornik, H.L.; Gerhard-Herman, M.; Beckman, J.A. Abnormal cytology predicts poor prognosis in cancer pa-tients with pericardial effusion. J. Clin. Oncol. 2005, 23, 5211–5216. [Google Scholar] [CrossRef]
- Quirk, T.; Yao, Y.; Sverdlov, A.; Murch, S. Malignant pericardial effusions: A retrospective look at etiology and prognosis in a tertiary oncological center. Asia-Pac. J. Clin. Oncol. 2022, 19, 468–472. [Google Scholar] [CrossRef]
- Shih, C.-T.; Lee, W.-C.; Fang, H.-Y.; Wu, P.-J.; Fang, Y.-N.; Chong, S.-Z. Outcomes of Patients with and without Malignancy Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. J. Cardiovasc. Dev. Dis. 2021, 8, 150. [Google Scholar] [CrossRef]
- El Haddad, D.; Iliescu, C.; Yusuf, S.W.; William, W.N.; Khair, T.H.; Song, J.; Mouhayar, E.N. Outcomes of Cancer Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. JACC 2015, 66, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- HCUP Databases. Available online: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp (accessed on 12 February 2024).
- Cost-to-Charge Ratio for Inpatient Files. Available online: https://hcup-us.ahrq.gov/db/ccr/ip-ccr/ip-ccr.jsp (accessed on 12 February 2024).
- Matetic, A.; Ky, B.; Yang, E.H.; Myint, P.K.; Rashid, M.; Zieroth, S.; Paul, T.K.; Elbadawi, A.; Mamas, M.A. Prevalence, characteristics and mortality of cancer patients undergoing pericardiocentesis in the United States between 2004 and 2017. Cancer Med. 2022, 12, 5471–5484. [Google Scholar] [CrossRef]
- Imazio, M.; Demichelis, B.; Parrini, I.; Favro, E.; Beqaraj, F.; Cecchi, E.; Pomari, F.; Demarie, D.; Ghisio, A.; Belli, R.; et al. Relation of Acute Pericardial Disease to Malignancy. Am. J. Cardiol. 2005, 95, 1393–1394. [Google Scholar] [CrossRef] [PubMed]
- Girardi, L.N.; Ginsberg, R.J.; Burt, M.E. Pericardiocentesis and Intrapericardial Sclerosis: Effective Therapy for Malignant Pericardial Effusions. Ann. Thorac. Surg. 1997, 64, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Strobbe, A.; Adriaenssens, T.; Bennett, J.; Dubois, C.; Desmet, W.; McCutcheon, K.; Van Cleemput, J.; Sinnaeve, P.R. Etiology and Long-Term Outcome of Patients Undergoing Pericardiocentesis. J. Am. Hear. Assoc. 2017, 6, e007598. [Google Scholar] [CrossRef]
- Schusler, R.; Meyerson, S.L. Pericardial Disease Associated with Malignancy. Curr. Cardiol. Rep. 2018, 20, 92. [Google Scholar] [CrossRef]
- Nguyen, O.; Ouellette, D. Survival Post Surgery for Malignant Pericardial Effusion. Clin. Pract. 2011, 1, e38. [Google Scholar] [CrossRef] [PubMed]
- Dequanter, D.; Lothaire, P.; Berghmans, T.; Sculier, J.P. Severe Pericardial Effusion in Patients with Concurrent Malignancy: A Retrospective Analysis of Prognostic Factors Influencing Survival. Ann. Surg. Oncol. 2008, 15, 3268–3271. [Google Scholar] [CrossRef]
- Lekhakul, A.; Assawakawintip, C.; Fenstad, E.R.; Pislaru, S.V.; Thaden, J.J.; Sinak, L.J.; Kane, G.C. Safety and Outcome of Percutaneous Drainage of Pericardial Effusions in Patients with Cancer. Am. J. Cardiol. 2018, 122, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Ilerhunmwuwa, N.; Sedeta, E.; Wasifuddin, M.; Hakobyan, N.; Aiwuyo, H.O.; Perry, J.C.; Uche, I.; Okhawere, K.; Torere, B.E.; Burak, E.; et al. Cardiac Tamponade in Patients With Breast Cancer: A Systematic Review. Cureus 2022, 14, e33123. [Google Scholar] [CrossRef]
- Castro, J.A.F.; Rasha, A.; Pandu, A.; Mushiyev, S. Cardiac Tamponade as the Initial Presentation of Metastatic Esophageal Adenocarcinoma. Cureus 2021, 13, e16863. [Google Scholar] [CrossRef]
- Earle, C.C.; Park, E.R.; Lai, B.; Weeks, J.C.; Ayanian, J.Z.; Block, S. Identifying Potential Indicators of the Quality of End-of-Life Cancer Care From Administrative Data. J. Clin. Oncol. 2003, 21, 1133–1138. [Google Scholar] [CrossRef]
- Baqi, A.; Ahmed, I.; Shams, P. Best management of patients with malignant pericardial effusion: A comparative study between imaging-guided pericardiocentesis and surgical pericardial window. J. Clin. Transl. Res. 2023, 9, 206–211. [Google Scholar]
- Pan, C.S.; Mabeza, R.M.; Tran, Z.; Lee, C.; Hadaya, J.; Sanaiha, Y.; Benharash, P.; Wang, M.-S. Pericardiocentesis or surgical drainage: A national comparison of clinical outcomes and resource use. PLoS ONE 2022, 17, e0267152. [Google Scholar] [CrossRef]
- Wen, F.-H.; Chen, J.-S.; Chang, W.-C.; Chou, W.-C.; Hsieh, C.-H.; Tang, S.T. Accurate prognostic awareness and preference states influence the concordance between terminally ill cancer patients’ states of preferred and received life-sustaining treatments in the last 6 months of life. Palliat. Med. 2019, 33, 1069–1079. [Google Scholar] [CrossRef]
- Balasubramanian, I.; Malhotra, C.; The COMPASS Study Group. Why is end-of-life inpatient cost high among cancer patients? A prospective cohort study. Cancer Med. 2024, 13, e7057. [Google Scholar] [CrossRef]
- Mudra, S.E.; Rayes, D.L.; Agrawal, A.; Kumar, A.K.; Li, J.Z.; Njus, M.; McGowan, K.; Kalam, K.A.; Charalampous, C.; Schleicher, M.; et al. Immune checkpoint inhibitors and pericardial disease: A systematic review. Cardio-Oncology 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Nardin, S.; Ruffilli, B.; Costantini, P.; Mollace, R.; Taglialatela, I.; Pagnesi, M.; Chiarito, M.; Soldato, D.; Cao, D.; Conte, B.; et al. Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management. J. Cardiovasc. Dev. Dis. 2025, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef]


| Malignancy (+) | Malignancy (−) | p-Value | |
|---|---|---|---|
| Number of hospitalizations | 24,740 | 60,385 | <0.001 |
| Male sex (%) | 49.3 | 50.9 | 0.053 |
| Age, mean (SE) | 62.1 (14.2) | 62.8 (17.1) | 0.005 |
| Race (%) | <0.001 | ||
| White | 70.1 | 67.3 | |
| Black | 12.8 | 16.0 | |
| Hispanic | 8.1 | 10.5 | |
| Asian | 5.1 | 2.9 | |
| AI/AN | 0.4 | 0.4 | |
| Other | 3.5 | 2.9 | |
| Relevant medical conditions (%) | |||
| Hypothyroidism | 12.9 | 15.4 | <0.001 |
| End-stage renal disease | 2.6 | 11.4 | <0.001 |
| Acute pericarditis | 7.5 | 14.4 | <0.001 |
| Infective endocarditis | 0.3 | 1.0 | <0.001 |
| History of irradiation | 11.1 | 2.1 | <0.001 |
| Systemic lupus erythematosus | 0.5 | 3.1 | <0.001 |
| Rheumatoid arthritis | 1.7 | 3.6 | <0.001 |
| Systemic sclerosis | 0.3 | 0.9 | <0.001 |
| Inflammatory myopathy | 0.0 | 0.1 | 0.292 |
| Sjogren’s disease | 0.2 | 0.5 | 0.037 |
| Sarcoidosis | 0.2 | 0.6 | <0.001 |
| Comorbidities (%) | |||
| Smoking | 45.6 | 34.9 | <0.001 |
| Hypertension | 30.8 | 27.4 | <0.001 |
| Diabetes mellitus | 19.4 | 31.0 | <0.001 |
| Hyperlipidemia | 31.1 | 43.3 | <0.001 |
| Obesity | 9.5 | 20.7 | <0.001 |
| Congestive heart failure | 19.2 | 38.1 | <.0001 |
| Chronic ischemic heart disease | 14.2 | 18.8 | <0.001 |
| Atrial fibrillation | 30.0 | 37.9 | <0.001 |
| COPD | 23.9 | 16.2 | <0.001 |
| Pulmonary hypertension | 4.0 | 9.4 | <0.001 |
| Chronic kidney disease | 15.2 | 31.9 | <0.001 |
| Liver cirrhosis | 3.1 | 4.3 | <0.001 |
| Dementia | 1.8 | 3.5 | <0.001 |
| Anemia | 34.0 | 34.0 | 0.974 |
| Malnutrition | 18.8 | 9.3 | <0.001 |
| Coagulopathy | 13.3 | 13.1 | 0.776 |
| Thrombocytopenia | 7.7 | 6.6 | 0.011 |
| Palliative care consult (%) | 16.6 | 3.7 | <0.001 |
| Pericardial tamponade (%) | 55.8 | 44.5 | <0.001 |
| Surgical pericardial drainage (%) | 8.8 | 6.2 | <0.001 |
| Mechanical ventilation (%) | 11.8 | 13.8 | <0.001 |
| Need for vasopressor support (%) | 4.4 | 4.5 | 0.825 |
| Hospital characteristics (%) | |||
| Hospital region | 0.064 | ||
| Northwest | 22.8 | 21.1 | |
| Midwest | 23.2 | 22.5 | |
| South | 34.1 | 36.2 | |
| West | 19.9 | 20.2 | |
| Hospital bed size | <0.001 | ||
| Small | 10.5 | 11.8 | |
| Medium | 23.1 | 25.5 | |
| Large | 66.4 | 62.7 | |
| Urban location | 0.068 | ||
| Rural | 2.4 | 2.9 | |
| Urban non-teaching | 12.8 | 13.6 | |
| Urban teaching | 84.8 | 83.6 | |
| Primary payer (%) | <0.001 | ||
| Medicare | 46.4 | 56.3 | |
| Medicaid | 14.2 | 12.1 | |
| Private insurance | 34.5 | 25.9 | |
| Self-pay | 2.5 | 3.2 | |
| No charge | 0.2 | 0.3 | |
| Others | 2.2 | 2.2 | |
| Median income (%) | 0.001 | ||
| Quartile 1 | 25.2 | 27.5 | |
| Quartile 2 | 24.5 | 25.3 | |
| Quartile 3 | 24.2 | 24.3 | |
| Quartile 4 | 26.1 | 22.8 |
| Outcome | Malignancy (+) | Malignancy (−) | Crude Odds Ratio | p-Value | Adjusted Odds Ratio | p-Value |
|---|---|---|---|---|---|---|
| In-hospital mortality (%) | 11.8 | 8.2 | 1.49 (1.34–1.66) | <0.001 | 1.50 (1.34–1.68) | <0.001 |
| Non-home discharge (%) | 53.1 | 45.7 | 1.34 (1.26–1.44) | <0.001 | 1.40 (1.30–1.51) | <0.001 |
| Length of stay (days ± SE) | 9.6 ± 10.4 | 9.1 ± 11.5 | 0.56 (0.19–0.93) a | 0.001 | 0.43 (0.07–0.79) | 0.019 |
| Total hospital cost ($ ± SE) | 34,057 ± 46,335 | 33,404 ± 51,955 | 654 ((−1013)–2321) a | <0.001 | 546 ((−1088)–2180) | 0.512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.Y.; Park, D.Y.; Banna, S.; Hu, J.-R.; Saleh, A.; Mamas, M.A.; McNamara, R.L.; Nanna, M.G.; Setaro, J.F.; Kim, L.K.; et al. In-Hospital Mortality Among Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion with and Without Malignancy. Curr. Oncol. 2025, 32, 514. https://doi.org/10.3390/curroncol32090514
Bae JY, Park DY, Banna S, Hu J-R, Saleh A, Mamas MA, McNamara RL, Nanna MG, Setaro JF, Kim LK, et al. In-Hospital Mortality Among Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion with and Without Malignancy. Current Oncology. 2025; 32(9):514. https://doi.org/10.3390/curroncol32090514
Chicago/Turabian StyleBae, Ju Young, Dae Yong Park, Soumya Banna, Jiun-Ruey Hu, Amr Saleh, Mamas A. Mamas, Robert L. McNamara, Michael G. Nanna, John F. Setaro, Luke K. Kim, and et al. 2025. "In-Hospital Mortality Among Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion with and Without Malignancy" Current Oncology 32, no. 9: 514. https://doi.org/10.3390/curroncol32090514
APA StyleBae, J. Y., Park, D. Y., Banna, S., Hu, J.-R., Saleh, A., Mamas, M. A., McNamara, R. L., Nanna, M. G., Setaro, J. F., Kim, L. K., & Altin, S. E. (2025). In-Hospital Mortality Among Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion with and Without Malignancy. Current Oncology, 32(9), 514. https://doi.org/10.3390/curroncol32090514





