Somatostatin Receptor 2 Overexpression in Hepatocellular Carcinoma: Implications for Cancer Biology and Therapeutic Applications
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
- TNMplot (https://tnmplot.com)—accessed 20 August 2025
- HPA (https://www.proteinatlas.org)—accessed 20 August 2025
- TCGA-LIHC via cBioPortal (https://www.cbioportal.org)—accessed 21 August 2025 [14]
- SRplot (https://www.bioinformatics.com.cn/en)—accessed 22 August 2025 [15]
- KMplot (https://kmplot.com)—accessed 21 August 2025 [16]
2.2. Pan-Cancer and Protein Expression Assessment
2.3. TCGA-LIHC Cohort, Preprocessing, and Group Definition
2.4. Differential Expression and Correlation Analyses
2.5. Functional Enrichment and GSEA
2.6. Proteomics (RPPA)
2.7. Copy-Number Alterations (CNA)
2.8. Survival Analyses
2.9. Statistics, Software, and Reproducibility
2.10. Ethical Considerations
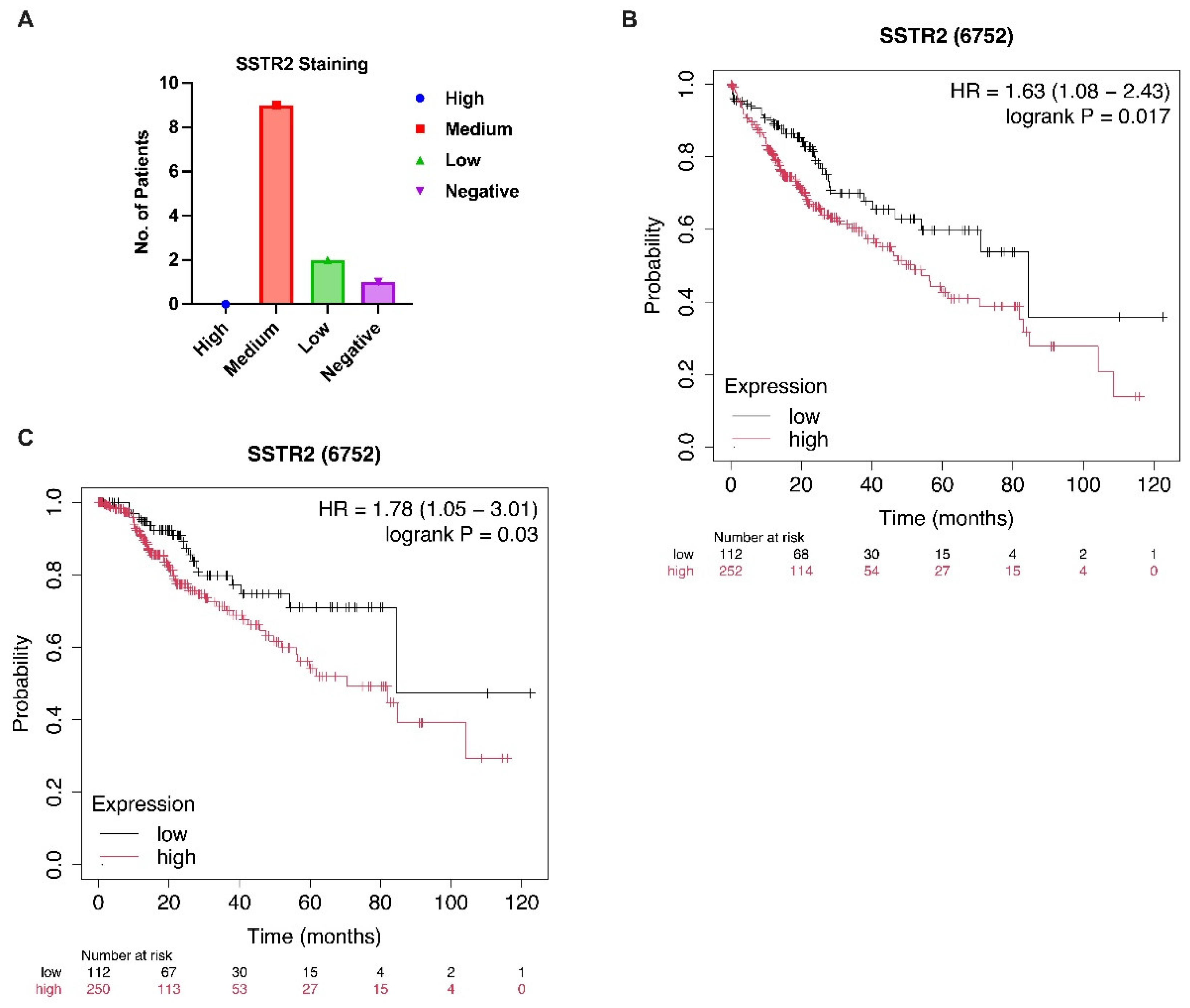
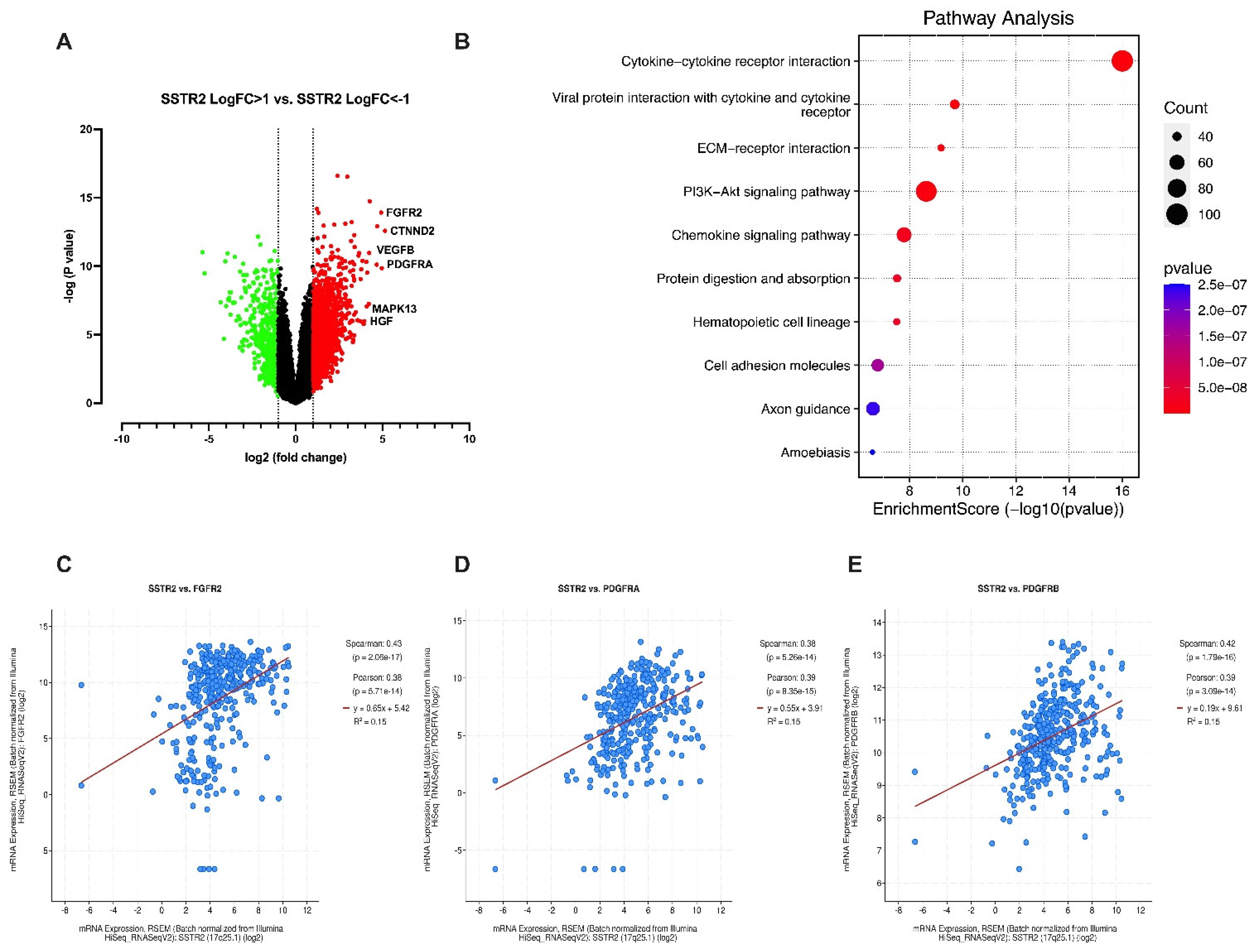
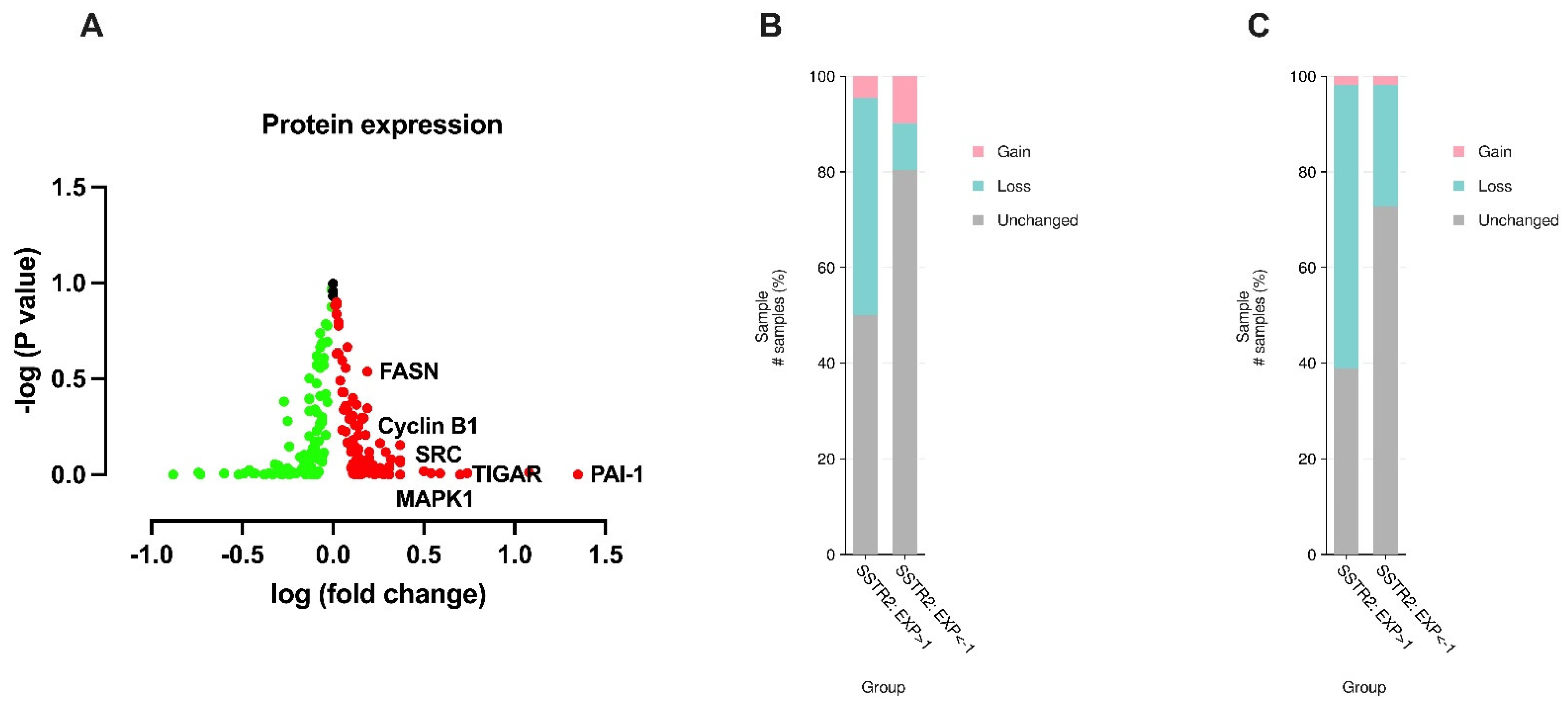
3. Results
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| SSTR2 | Somatostatin receptor 2 |
References
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Safri, F.; Nguyen, R.; Zerehpooshnesfchi, S.; George, J.; Qiao, L. Heterogeneity of hepatocellular carcinoma: From mechanisms to clinical implications. Cancer Gene Ther. 2024, 31, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.G.; Macias, R.I.R.; Monte, M.J.; Romero, M.R.; Asensio, M.; Sanchez-Martin, A.; Cives-Losada, C.; Temprano, A.G.; Espinosa-Escudero, R.; Reviejo, M.; et al. Molecular Bases of Drug Resistance in Hepatocellular Carcinoma. Cancers 2020, 12, 1663. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Guenter, R.; Aweda, T.; Carmona Matos, D.M.; Jang, S.; Whitt, J.; Cheng, Y.Q.; Liu, X.M.; Chen, H.; Lapi, S.E.; Jaskula-Sztul, R. Overexpression of somatostatin receptor type 2 in neuroendocrine tumors for improved Ga68-DOTATATE imaging and treatment. Surgery 2020, 167, 189–196. [Google Scholar] [CrossRef]
- Lehman, J.M.; Hoeksema, M.D.; Staub, J.; Qian, J.; Harris, B.; Callison, J.C.; Miao, J.; Shi, C.; Eisenberg, R.; Chen, H.; et al. Somatostatin receptor 2 signaling promotes growth and tumor survival in small-cell lung cancer. Int. J. Cancer 2019, 144, 1104–1114. [Google Scholar] [CrossRef]
- He, J.H.; Wang, J.; Yang, Y.Z.; Chen, Q.X.; Liu, L.L.; Sun, L.; Hu, W.M.; Zeng, J. SSTR2 is a prognostic factor and a promising therapeutic target in glioma. Am. J. Transl. Res. 2021, 13, 11223–11234. [Google Scholar] [PubMed]
- Lequoy, M.; Desbois-Mouthon, C.; Wendum, D.; Gupta, V.; Blachon, J.L.; Scatton, O.; Dumont, S.; Bonnemaire, M.; Schmidlin, F.; Rosmorduc, O.; et al. Somatostatin receptors in resected hepatocellular carcinoma: Status and correlation with markers of poor prognosis. Histopathology 2017, 70, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.; Kim, Y.I.; Yang, D.H.; Yoo, C.; Park, I.J.; Ryoo, B.Y.; Ryu, J.S.; Hong, S.M. Somatostatin receptor 2 (SSTR2) expression is associated with better clinical outcome and prognosis in rectal neuroendocrine tumors. Sci. Rep. 2024, 14, 4047. [Google Scholar] [CrossRef]
- Xu, Y.; Quan, Z.; Zhan, Y.; Wang, H.; Luo, J.; Wang, W.; Fan, S. SSTR2 positively associates with EGFR and predicts poor prognosis in nasopharyngeal carcinoma. J. Clin. Pathol. 2024, 77, 829–834. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Lanczky, A.; Gyorffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, B. Hepatocellular carcinoma: Molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023, 42, 629–652. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: Signaling pathways and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 35. [Google Scholar] [CrossRef]
- Luo, X.; He, X.; Zhang, X.; Zhao, X.; Zhang, Y.; Shi, Y.; Hua, S. Hepatocellular carcinoma: Signaling pathways, targeted therapy, and immunotherapy. MedComm 2024, 5, e474. [Google Scholar] [CrossRef]
- Dimri, M.; Satyanarayana, A. Molecular Signaling Pathways and Therapeutic Targets in Hepatocellular Carcinoma. Cancers 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, S.D.; Zhang, D.; Liu, D.; Zhang, F.Y.; Zheng, T.Y.; Cui, M.M.; Dai, C.L. Delta-catenin promotes the proliferation and invasion of colorectal cancer cells by binding to E-cadherin in a competitive manner with p120 catenin. Target. Oncol. 2014, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Tang, Z.Y.; Xue, Q.; Shi, D.R.; Song, H.Y.; Tang, H.B. Invasion and metastasis of hepatocellular carcinoma in relation to urokinase-type plasminogen activator, its receptor and inhibitor. J. Cancer Res. Clin. Oncol. 2000, 126, 641–646. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Lu, J.; Qian, G.; Zheng, J.C.; Ge, S. Knockdown of TIGAR by RNA interference induces apoptosis and autophagy in HepG2 hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2013, 437, 300–306. [Google Scholar] [CrossRef]
- Hong, J.; Yuan, Y.; Wang, J.; Liao, Y.; Zou, R.; Zhu, C.; Li, B.; Liang, Y.; Huang, P.; Wang, Z.; et al. Expression of variant isoforms of the tyrosine kinase SYK determines the prognosis of hepatocellular carcinoma. Cancer Res. 2014, 74, 1845–1856. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Xing, Z.; Lou, C.; Fang, J.; Wang, Z.; Li, M.; He, H.; Bai, H. Fibronectin 1 derived from tumor-associated macrophages and fibroblasts promotes metastasis through the JUN pathway in hepatocellular carcinoma. Int. Immunopharmacol. 2022, 113, 109420. [Google Scholar] [CrossRef]
- Lv, S.; Ning, H.; Li, Y.; Wang, J.; Jia, Q.; Wen, H. Inhibition of cyclinB1 Suppressed the Proliferation, Invasion, and Epithelial Mesenchymal Transition of Hepatocellular Carcinoma Cells and Enhanced the Sensitivity to TRAIL-Induced Apoptosis. Onco Targets Ther. 2020, 13, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.W.; Zhang, J.W.; Liu, Y.; Liu, Y.; Liu, J.W.; Huang, J.Z. SRC-1 and Twist1 are prognostic indicators of liver cancer and are associated with cell viability, invasion, migration and epithelial-mesenchymal transformation of hepatocellular carcinoma cells. Transl. Cancer Res. 2020, 9, 603–612. [Google Scholar] [CrossRef]
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef]
- Che, L.; Paliogiannis, P.; Cigliano, A.; Pilo, M.G.; Chen, X.; Calvisi, D.F. Corrigendum: Pathogenetic, Prognostic, and Therapeutic Role of Fatty Acid Synthase in Human Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 874053. [Google Scholar] [CrossRef]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Tanaka, S.; Shiraha, H.; Nakanishi, Y.; Nishina, S.; Matsubara, M.; Horiguchi, S.; Takaoka, N.; Iwamuro, M.; Kataoka, J.; Kuwaki, K.; et al. Runt-related transcription factor 3 reverses epithelial-mesenchymal transition in hepatocellular carcinoma. Int. J. Cancer 2012, 131, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Krajnovic, M.; Kozik, B.; Bozovic, A.; Jovanovic-Cupic, S. Multiple Roles of the RUNX Gene Family in Hepatocellular Carcinoma and Their Potential Clinical Implications. Cells 2023, 12, 2303. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Stock, P.; Monga, D.; Tan, X.; Micsenyi, A.; Loizos, N.; Monga, S.P. Platelet-derived growth factor receptor-alpha: A novel therapeutic target in human hepatocellular cancer. Mol. Cancer Ther. 2007, 6, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.C.; Xu, Y.; Zhang, K.Z.; Wang, L.; Qin, L.X.; Wu, W.Z.; Liu, Y.K.; Ye, S.L.; Tang, Z.Y. Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clin. Cancer Res. 2005, 11, 8557–8563. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Li, Y.; Xia, J.; Shao, F.; Zhou, Y.; Yu, J.; Wu, H.; Du, J.; Ren, X. Sorafenib induces mitochondrial dysfunction and exhibits synergistic effect with cysteine depletion by promoting HCC cells ferroptosis. Biochem. Biophys. Res. Commun. 2021, 534, 877–884. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Lin, L.C.; Kuo, Y.C.; Liang, J.A.; Kuo, C.C.; Chiou, J.F. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Momeny, M.; AghaAmiri, S.; Hernandez Vargas, S.; Acidi, B.; Ghosh, S.C.; Bateman, T.M.; Adams, J.T.; Khalaj, V.; Kaseb, A.O.; Tran Cao, H.S. SSTR2-targeted theranostics in hepatocellular carcinoma. Cancers 2025, 17, 162. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Ueno, S.; Sakoda, M.; Iino, S.; Hiwatashi, K.; Minami, K.; Ando, K.; Mataki, Y.; Maemura, K.; Shinchi, H.; et al. Clinical implication of ZEB-1 and E-cadherin expression in hepatocellular carcinoma (HCC). BMC Cancer 2013, 13, 572. [Google Scholar] [CrossRef]
- Zhao, S.; Li, H.; Wang, Q.; Su, C.; Wang, G.; Song, H.; Zhao, L.; Luan, Z.; Su, R. The role of c-Src in the invasion and metastasis of hepatocellular carcinoma cells induced by association of cell surface GRP78 with activated alpha2M. BMC Cancer 2015, 15, 389. [Google Scholar] [CrossRef]
- Mo, S.; Fang, D.; Zhao, S.; Thai Hoa, P.T.; Zhou, C.; Liang, T.; He, Y.; Yu, T.; Chen, Y.; Qin, W.; et al. Down regulated oncogene KIF2C inhibits growth, invasion, and metastasis of hepatocellular carcinoma through the Ras/MAPK signaling pathway and epithelial-to-mesenchymal transition. Ann. Transl. Med. 2022, 10, 151. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Deppen, S.A.; Liu, E.; Blume, J.D.; Clanton, J.; Shi, C.; Jones-Jackson, L.B.; Lakhani, V.; Baum, R.P.; Berlin, J.; Smith, G.T.; et al. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J. Nucl. Med. 2016, 57, 708–714. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef]
- Delbart, W.; Karabet, J.; Marin, G.; Penninckx, S.; Derrien, J.; Ghanem, G.E.; Flamen, P.; Wimana, Z. Understanding the Radiobiological Mechanisms Induced by (177)Lu-DOTATATE in Comparison to External Beam Radiation Therapy. Int. J. Mol. Sci. 2022, 23, 12369. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez Vargas, S.; Aghaamiri, S.; Adams, J.T.; Bateman, T.M.; Acidi, B.; Ghosh, S.C.; Khalaj, V.; Kaseb, A.O.; Tran Cao, H.S.; Momeny, M.; et al. Somatostatin Receptor 2 Overexpression in Hepatocellular Carcinoma: Implications for Cancer Biology and Therapeutic Applications. Curr. Oncol. 2025, 32, 512. https://doi.org/10.3390/curroncol32090512
Hernandez Vargas S, Aghaamiri S, Adams JT, Bateman TM, Acidi B, Ghosh SC, Khalaj V, Kaseb AO, Tran Cao HS, Momeny M, et al. Somatostatin Receptor 2 Overexpression in Hepatocellular Carcinoma: Implications for Cancer Biology and Therapeutic Applications. Current Oncology. 2025; 32(9):512. https://doi.org/10.3390/curroncol32090512
Chicago/Turabian StyleHernandez Vargas, Servando, Solmaz Aghaamiri, Jack T. Adams, Tyler M. Bateman, Belkacem Acidi, Sukhen C. Ghosh, Vahid Khalaj, Ahmed O. Kaseb, Hop S. Tran Cao, Majid Momeny, and et al. 2025. "Somatostatin Receptor 2 Overexpression in Hepatocellular Carcinoma: Implications for Cancer Biology and Therapeutic Applications" Current Oncology 32, no. 9: 512. https://doi.org/10.3390/curroncol32090512
APA StyleHernandez Vargas, S., Aghaamiri, S., Adams, J. T., Bateman, T. M., Acidi, B., Ghosh, S. C., Khalaj, V., Kaseb, A. O., Tran Cao, H. S., Momeny, M., & Azhdarinia, A. (2025). Somatostatin Receptor 2 Overexpression in Hepatocellular Carcinoma: Implications for Cancer Biology and Therapeutic Applications. Current Oncology, 32(9), 512. https://doi.org/10.3390/curroncol32090512





