The Desmoid Dilemma: Challenges and Opportunities in Assessing Tumor Burden and Therapeutic Response
Abstract
:1. Introduction
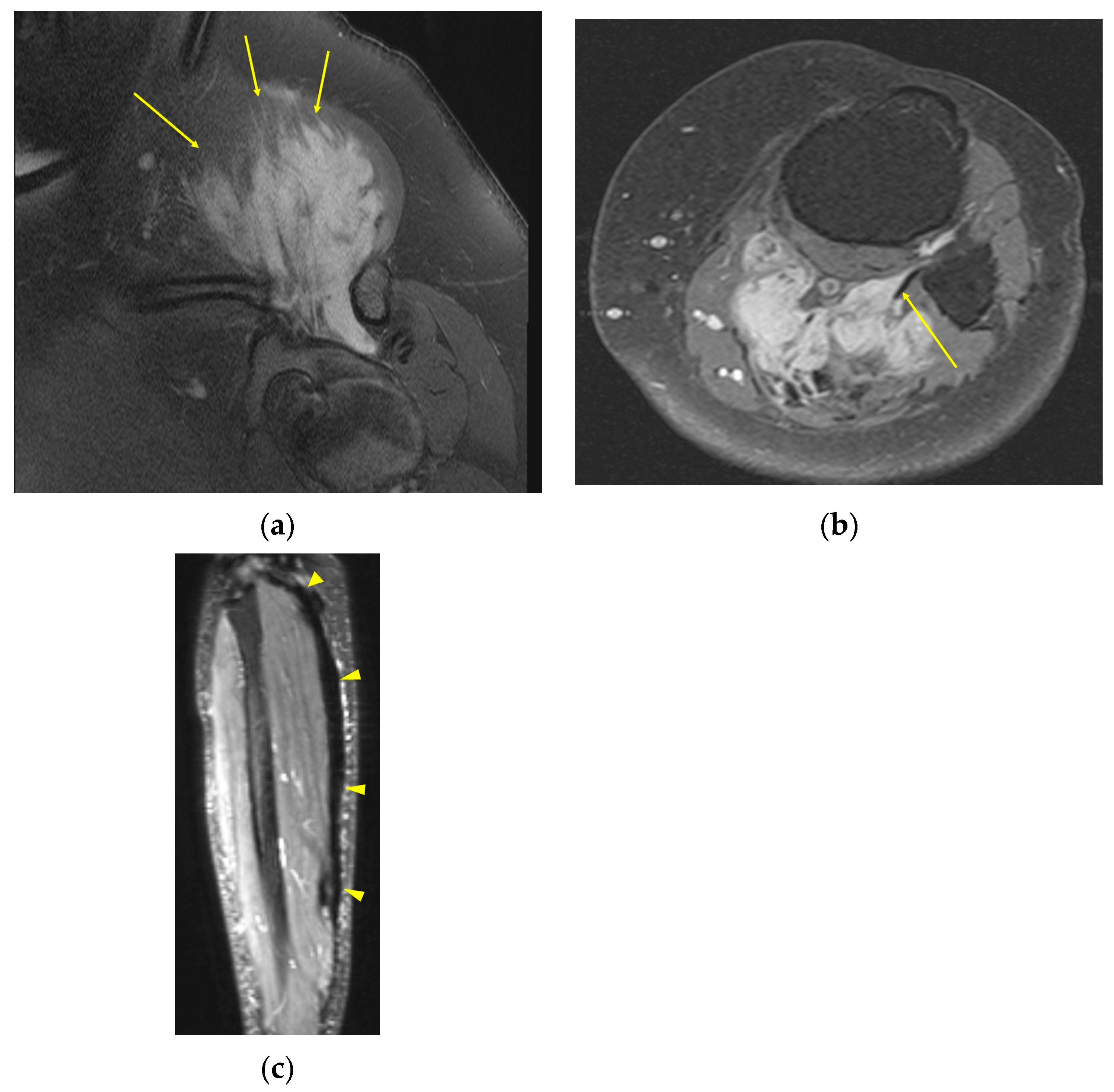
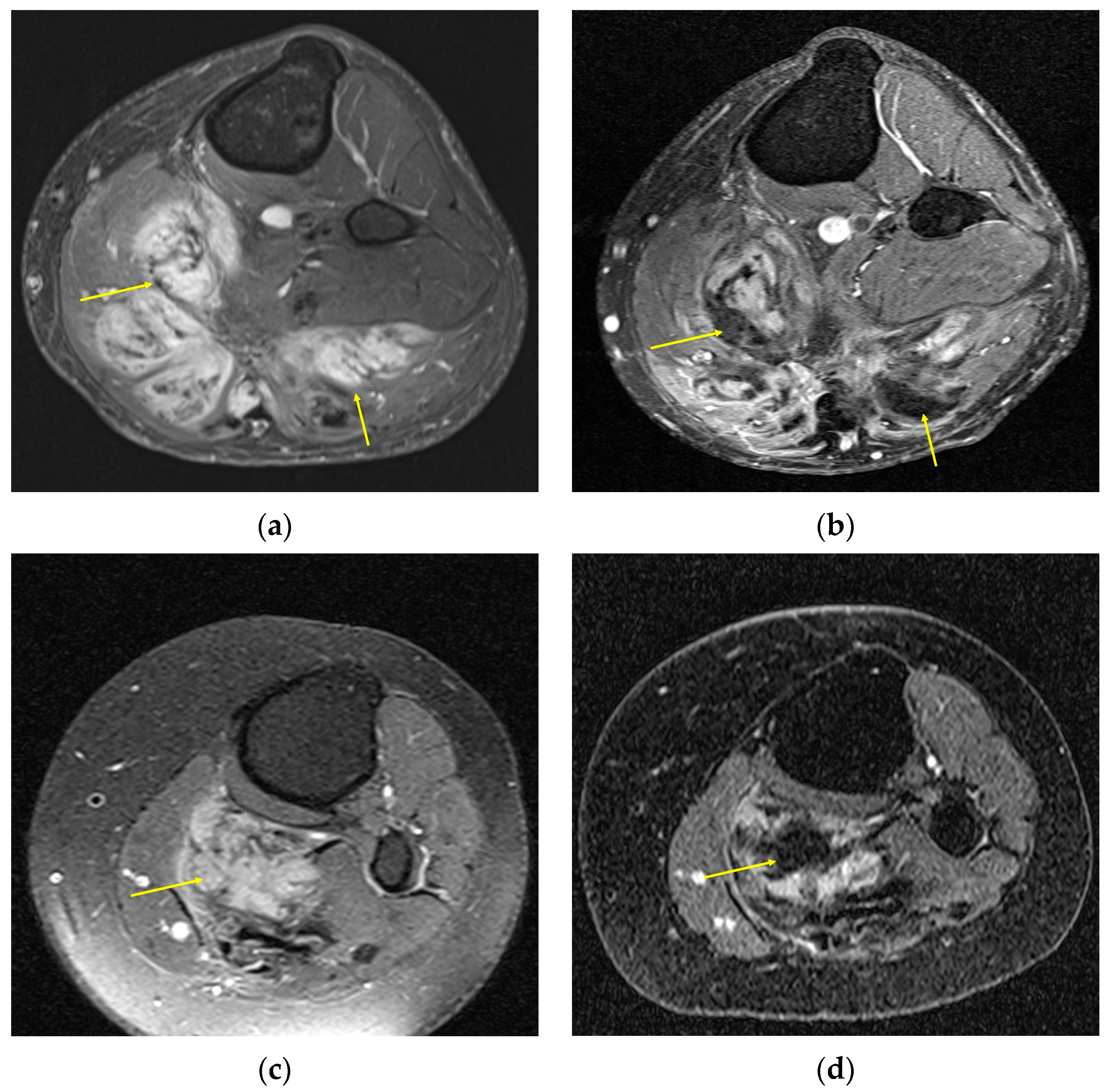
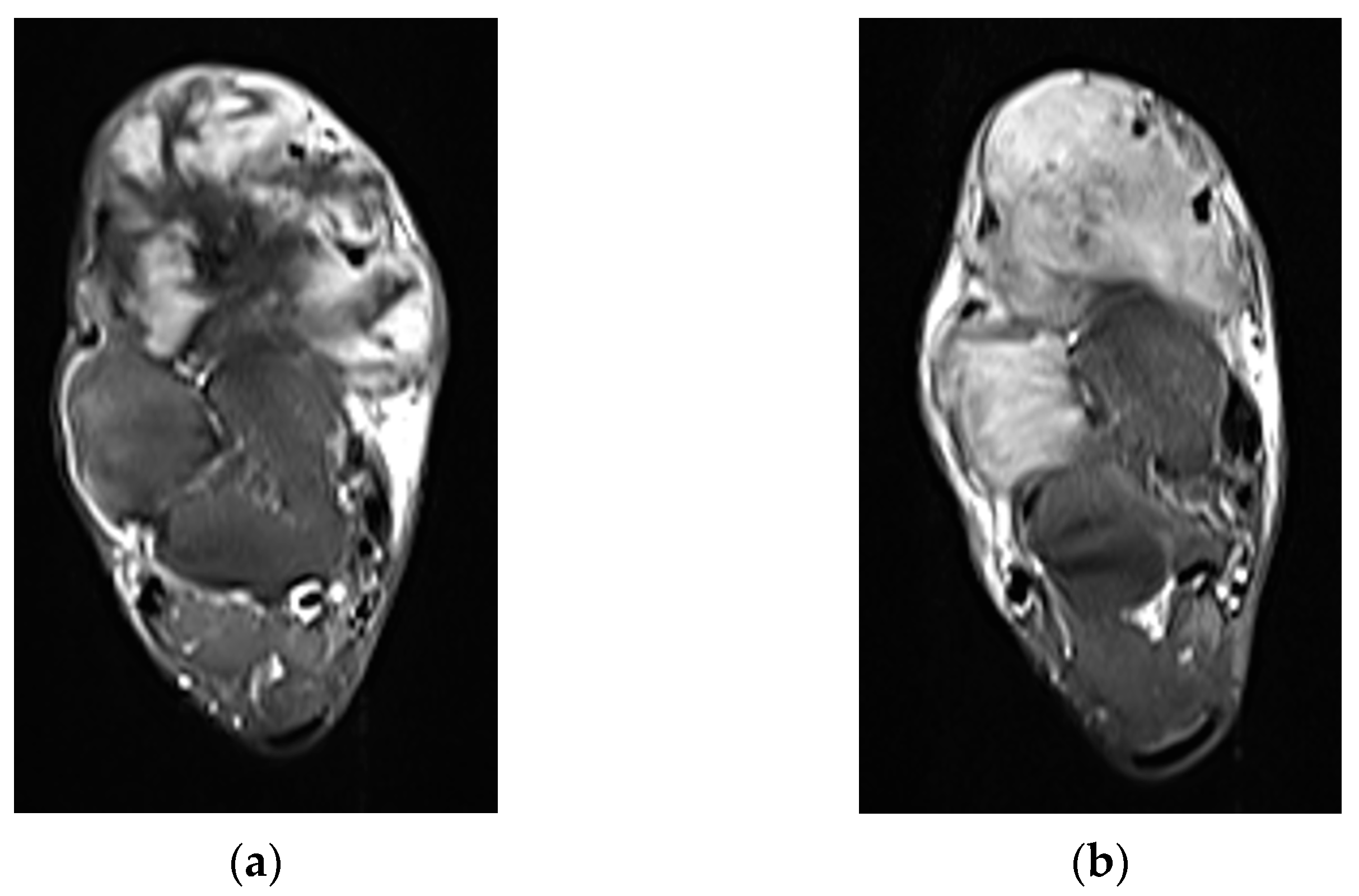
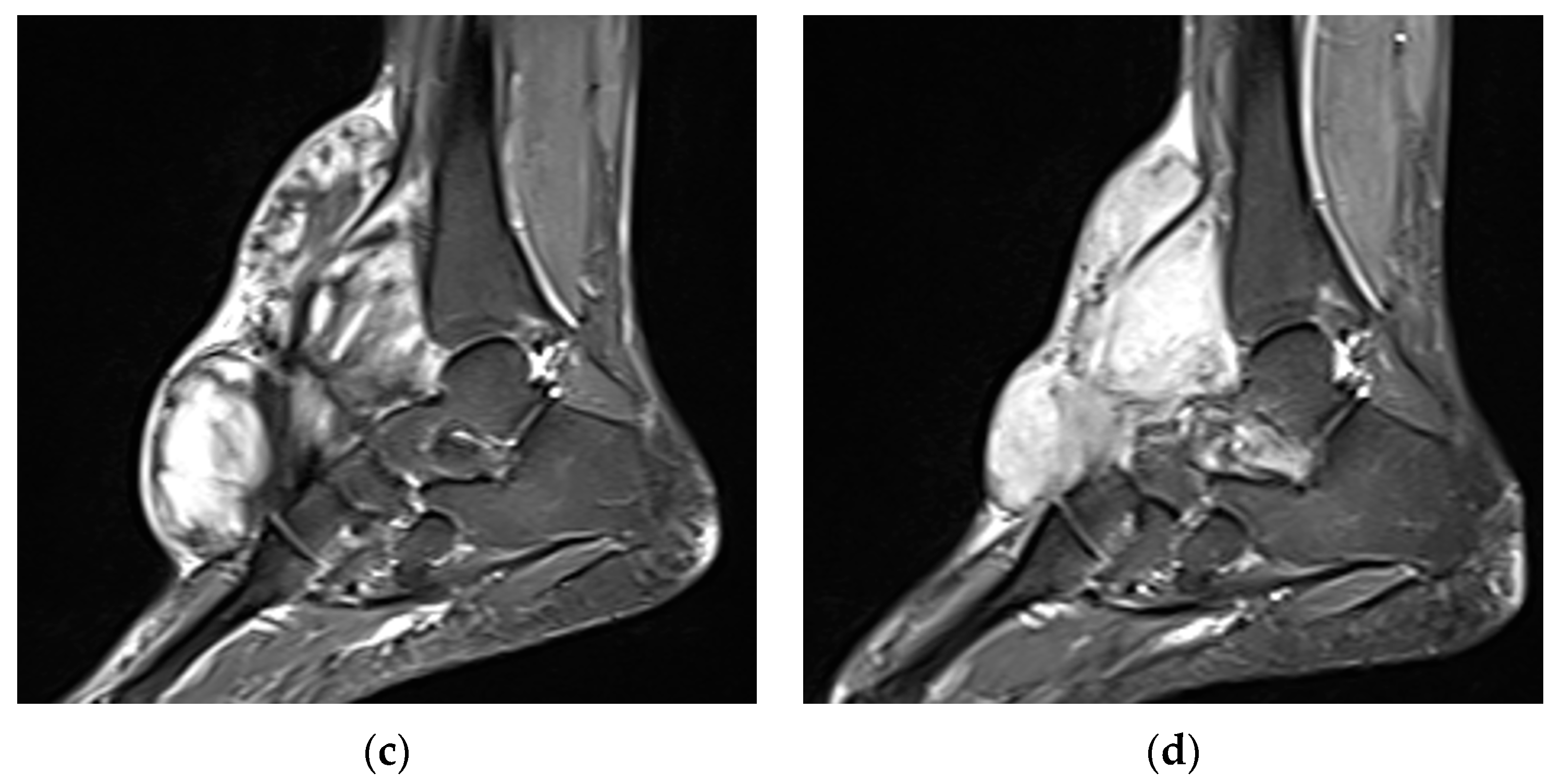
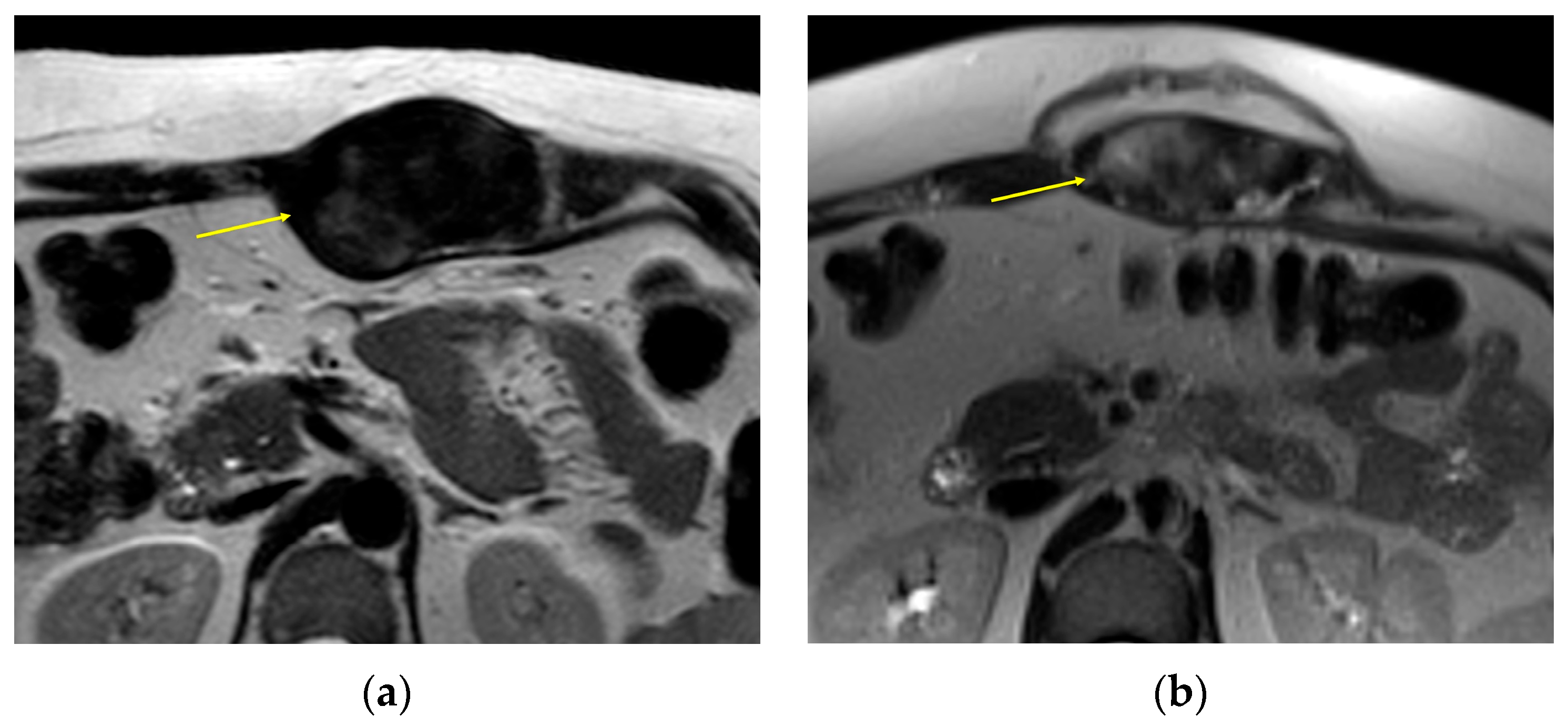
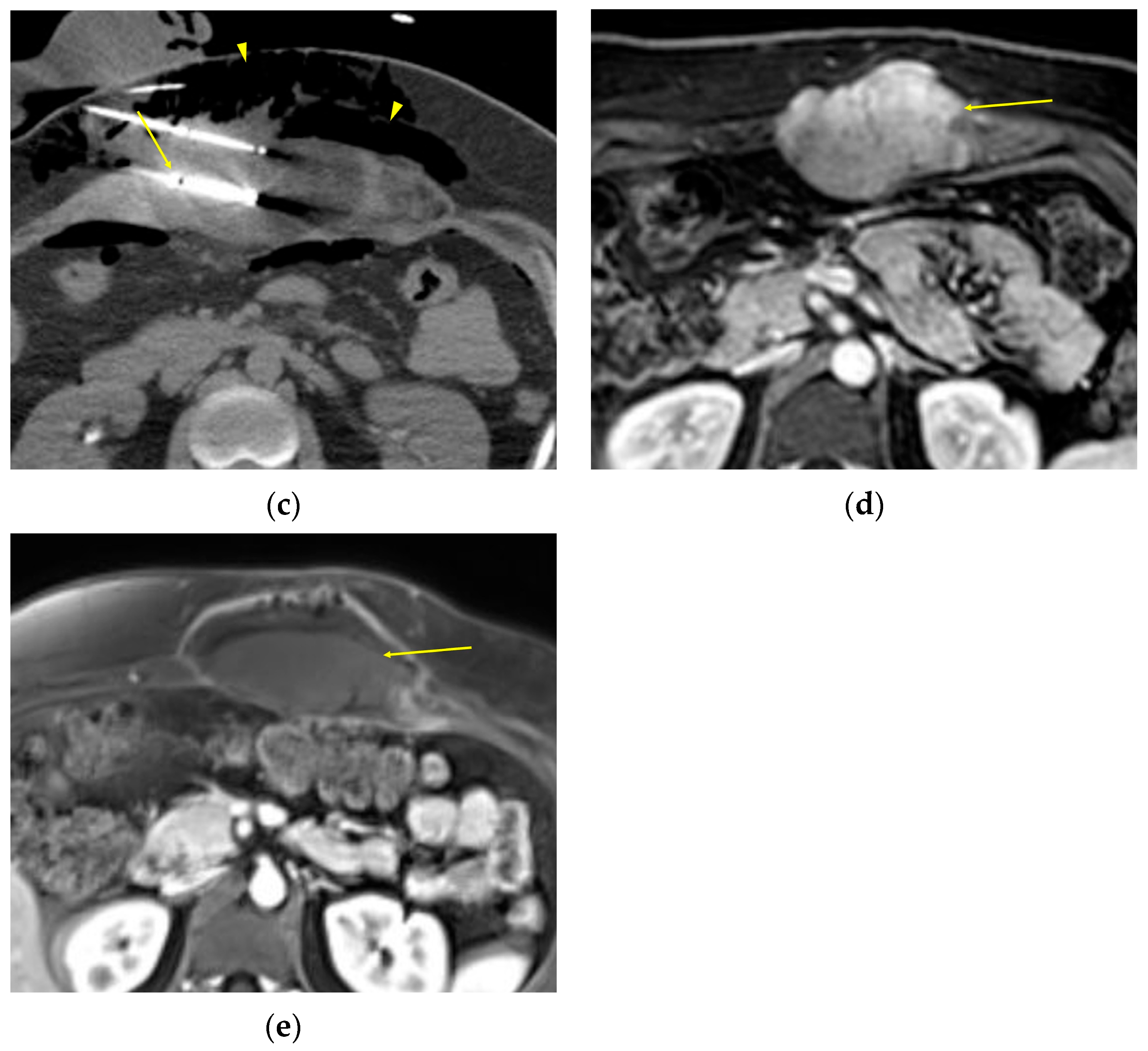
2. Imaging of Desmoid Tumors
| Modality | Appearance | Strengths | Limitations |
|---|---|---|---|
| MRI | Well-circumscribed, heterogenous with nonenhancing T1/T2 hypointense curvilinear areas related to collagen, and T1 intermediate to low/T2 intermediate to high areas related to cellularity or the myxoid matrix. Additional secondary signs (“flame”, “staghorn”, “fascial tail”, etc.) are described in text. |
|
|
| CT | Well-circumscribed, relatively homogenous soft tissue attenuation (generally hypodense to muscle) with variable enhancement. |
|
|
| US | Smooth, well-defined margins with homogenous hypoechoic echogenicity. Echogenicity and vascularity can vary depending on internal composition. Borders can also be ill-defined or irregular. |
|
|
| PET | Mild to moderate uptake with median SUV of 3.1 (range 2.0–7.3) [7]. |
|
|
3. Current State of Evaluating Treatment Response
| Criteria | Partial Response | Progressive Disease |
|---|---|---|
| RECIST 1.1 | ≥30% decrease in sum of longest diameters (SLD) of target lesions without new lesions or progression of non-target lesions | ≥20% increase and ≥5 mm absolute increase in SLD of target lesions or presence of new lesions or progression of non-target lesions |
| modified RECIST | ≥30% decrease in SLD of contrast-enhancing areas of target lesions | ≥20% increase in SLD of contrast-enhancing areas of target lesions |
| WHO | ≥50% decrease in sum of the product of the diameters (SPD) of lesions | ≥25% increase in SPD of at least one of the lesions or presence of new lesions |
| Choi | ≥10% decrease in SLD of target lesions or ≥15% decrease in attenuation on CT, without new lesions or progression of nonmeasurable disease | ≥10% increase in SLD of target lesions or increase/<15% decrease in attenuation on CT or new lesions or progression of nonmeasurable disease |
| modified Choi | ≥10% decrease in SLD of target lesions AND ≥15% decrease in attenuation on CT, without new lesions or progression of nonmeasurable disease | ≥10% increase in SLD of target lesions AND increase/<15% decrease in attenuation on CT or new lesions or progression of nonmeasurable disease |
| MRI-based modified Choi [25,28] | ≥10% decrease in SLD of target lesions AND ≥30% decrease in volumetric tumor-to-muscle signal ratio from baseline, without new lesions or progression of nonmeasurable disease | ≥10% increase in SLD of target lesions AND >20% increase in volumetric tumor-to-muscle signal ratio from baseline or new lesions or progression of nonmeasurable disease |
| Volumetric [25,28] | >50% decrease in total volumetric size | >25% increase in total volumetric size |
| EASL | ≥50% decrease in total tumor load | >25% increase in size of one or more lesions or presence of new lesions |
| Cheson | ≥50% decrease in SPD from baseline | ≥50% increase in SPD from baseline or ≥50% increase in longest diameter of any lesion or presence of new lesions |
4. Experimental Approaches
5. Persistent Challenges to Evaluation of Treatment Response
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DT | Desmoid tumor |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| T1 | T1-weighted imaging |
| T2 | T2-weighted imaging |
| SLD | Sum of longest diameters |
| SPD | Sum of product of diameters |
| DWI | Diffusion-weighted imaging |
| ADC | Apparent diffusion coefficient |
| CE-SWI | Contrast-enhanced susceptibility-weighted imaging |
| PFS | Progression free survival |
| GODDESS | GOunder/Desmoid tumor research foundation DEsmoid Symptom/impact Scale |
| PD | Proton density-weighted imaging |
| EASL | European Association for the Study of the Liver |
References
- Alman, B.; Attia, S.; Baumgarten, C.; Benson, C.; Blay, J.Y.; Bonvalot, S.; Breuing, J.; Cardona, K.; Casali, P.G.; van Coevorden, F.; et al. The Management of Desmoid Tumours: A Joint Global Consensus-Based Guideline Approach for Adult and Paediatric Patients. Eur. J. Cancer 2020, 127, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.; Ratan, R.; Alcindor, T.; Schöffski, P.; van der Graaf, W.T.; Wilky, B.A.; Riedel, R.F.; Lim, A.; Smith, L.M.; Moody, S.; et al. Nirogacestat, a γ-Secretase Inhibitor for Desmoid Tumors. N. Engl. J. Med. 2023, 388, 898–912. [Google Scholar] [CrossRef]
- Gounder, M.M.; Mahoney, M.R.; Van Tine, B.A.; Ravi, V.; Attia, S.; Deshpande, H.A.; Gupta, A.A.; Milhem, M.M.; Conry, R.M.; Movva, S.; et al. Sorafenib for Advanced and Refractory Desmoid Tumors. N. Engl. J. Med. 2018, 379, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.E.; Buy, X.; Deschamps, F.; Sauleau, E.; Bouhamama, A.; Toulmonde, M.; Honoré, C.; Bertucci, F.; Brahmi, M.; Chevreau, C.; et al. CRYODESMO-O1: A Prospective, Open Phase II Study of Cryoablation in Desmoid Tumour Patients Progressing after Medical Treatment. Eur. J. Cancer 2021, 143, 78–87. [Google Scholar] [CrossRef]
- Ratain, M.J.; Eckhardt, S.G. Phase II Studies of Modern Drugs Directed against New Targets: If You Are Fazed, Too, Then Resist RECIST. J. Clin. Oncol. 2004, 22, 4442–4445. [Google Scholar] [CrossRef]
- Braschi-Amirfarzan, M.; Keraliya, A.R.; Krajewski, K.M.; Tirumani, S.H.; Shinagare, A.B.; Hornick, J.L.; Baldini, E.H.; George, S.; Ramaiya, N.H.; Jagannathan, J.P. Role of Imaging in Management of Desmoid-Type Fibromatosis: A Primer for Radiologists. Radiographics 2016, 36, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Koo, H.J.; Lim, S.; Lee, J.W.; Lee, H.N.; Kim, D.K.; Song, J.S.; Kim, M.Y. Desmoid-Type Fibromatosis of the Thorax: CT, MRI, and FDG PET Characteristics in a Large Series from a Tertiary Referral Center. Medicine 2015, 94, e1547. [Google Scholar] [CrossRef]
- Casillas, J.; Sais, G.J.; Greve, J.L.; Iparraguirre, M.C.; Morillo, G. Imaging of Intra- and Extraabdominal Desmoid Tumors. Radiographics 1991, 11, 959–968. [Google Scholar] [CrossRef]
- Johner, A.; Tiwari, P.; Zetler, P.; Wiseman, S.M. Abdominal Wall Desmoid Tumors Associated with Pregnancy: Current Concepts. Expert Rev. Anticancer Ther. 2009, 9, 1675–1682. [Google Scholar] [CrossRef]
- Zreik, R.T.; Fritchie, K.J. Morphologic Spectrum of Desmoid-Type Fibromatosis. Am. J. Clin. Pathol. 2016, 145, 332–340. [Google Scholar] [CrossRef]
- Sedaghat, S.; Surov, A.; Krohn, S.; Sedaghat, M.; Reichardt, B.; Nicolas, V. Configuration of Primary and Recurrent Aggressive Fibromatosis on Contrast-Enhanced MRI with an Evaluation of Potential Risk Factors for Recurrences in MRI Follow-Up. RoFo Fortschritte Geb. Röntgenstrahlen Bildgeb. Verfahr. 2020, 192, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Dheeksha, D.S.; Dhamija, E.; Mani, K.; Rastogi, S.; Pushpam, D.; Bakhshi, S.; Chandrashekhara, S.H.; Barwad, A. Contrast Enhanced Ultrasound versus MRI for Response Assessment of Extra-Abdominal Desmoid Fibromatosis- A Feasibility Study. Eur. J. Radiol. 2025, 187, 112071. [Google Scholar] [CrossRef]
- Huang, C.C.; Ko, S.F.; Yeh, M.C.; Ng, S.H.; Huang, H.Y.; Lee, C.C.; Lee, T.Y. Aggressive Fibromatosis of the Chest Wall: Sonographic Appearance of the Fascial Tail and Staghorn Patterns. J. Ultrasound Med. 2009, 28, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Kamali, F.; Wang, W.L.; Guadagnolo, B.A.; Fox, P.S.; Lewis, V.O.; Lazar, A.J.; Conley, A.P.; Ravi, V.; Toliyat, M.; Ladha, H.S.; et al. MRI May Be Used as a Prognostic Indicator in Patients with Extra-Abdominal Desmoid Tumours. Br. J. Radiol. 2016, 89, 20150308. [Google Scholar] [CrossRef]
- Sørensen, A.; Keller, J.; Nielsen, O.S.; Jensen, O.M. Treatment of Aggressive Fibromatosis: A Retrospective Study of 72 Patients Followed for 1-27 Years. Acta Orthop. Scand. 2002, 73, 213–219. [Google Scholar] [CrossRef]
- Gondim Teixeira, P.A.; Chanson, A.; Verhaeghe, J.L.; Lecocq, S.; Louis, M.; Hossu, G.; Blum, A. Correlation between Tumor Growth and Hormonal Therapy with MR Signal Characteristics of Desmoid-Type Fibromatosis: A Preliminary Study. Diagn. Interv. Imaging 2018, 100, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Colombo, C.; Le Péchoux, C.; Dei Tos, A.P.; Le Cesne, A.; Marrari, A.; Penel, N.; Grignani, G.; Blay, J.Y.; Casali, P.G.; et al. Sporadic Desmoid-Type Fibromatosis: A Stepwise Approach to a Non-Metastasising Neoplasm-A Position Paper from the Italian and the French Sarcoma Group. Ann. Oncol. 2013, 25, 578–583. [Google Scholar] [CrossRef]
- Mangla, A.; Agarwal, N.; Schwartz, G. Desmoid Tumors: Current Perspective and Treatment. Curr. Treat. Options Oncol. 2024, 25, 161–175. [Google Scholar] [CrossRef]
- Kotiligam, D.; Lazar, A.J.F.; Pollock, R.E.; Lev, D. Desmoid Tumor: A Disease Opportune for Molecular Insights. Histol. Histopathol. 2008, 23, 117–126. [Google Scholar]
- Bhattacharya, B.; Dilworth, H.P.; Iacobuzio-Donahue, C.; Ricci, F.; Weber, K.; Furlong, M.A.; Fisher, C.; Montgomery, E. Nuclear β-Catenin Expression Distinguishes Deep Fibromatosis from Other Benign and Malignant Fibroblastic and Myofibroblastic Lesions. Am. J. Surg. Pathol. 2005, 29, 653–659. [Google Scholar] [CrossRef]
- Bektas, M.; Bell, T.; Khan, S.; Tumminello, B.; Fernandez, M.M.; Heyes, C.; Oton, A.B. Desmoid Tumors: A Comprehensive Review. Adv. Ther. 2023, 40, 3697–3722. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Gounder, M.M.; Lefkowitz, R.A.; Keohan, M.L.; D’Adamo, D.R.; Hameed, M.; Antonescu, C.R.; Singer, S.; Stout, K.; Ahn, L.; Maki, R.G. Activity of Sorafenib against Desmoid Tumor/Deep Fibromatosis. Clin. Cancer Res. 2011, 17, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Gondim Teixeira, P.A.; Biouichi, H.; Abou Arab, W.; Rios, M.; Sirveaux, F.; Hossu, G.; Blum, A. Evidence-Based MR Imaging Follow-up Strategy for Desmoid-Type Fibromatosis. Eur. Radiol. 2020, 30, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Subhawong, T.K.; Feister, K.; Sweet, K.; Alperin, N.; Kwon, D.; Rosenberg, A.; Trent, J.; Wilky, B.A. MRI Volumetrics and Image Texture Analysis in Assessing Systemic Treatment Response in Extra-Abdominal Desmoid Fibromatosis. Radiol. Imaging Cancer 2021, 3, e210016. [Google Scholar] [CrossRef] [PubMed]
- Libertini, M.; Mitra, I.; van der Graaf, W.T.A.; Miah, A.B.; Judson, I.; Jones, R.L.; Thomas, K.; Moskovic, E.; Szucs, Z.; Benson, C.; et al. Aggressive Fibromatosis Response to Tamoxifen: Lack of Correlation between MRI and Symptomatic Response. Clin. Sarcoma Res. 2018, 8, 13. [Google Scholar] [CrossRef]
- Zhu, H.C.; Li, X.T.; Ji, W.Y.; Li, S.; Sun, Y.S. Desmoid-Type Fibromatosis: Tumour Response Assessment Using Magnetic Resonance Imaging Signal and Size Criteria. Clin. Imaging 2020, 68, 111–120. [Google Scholar] [CrossRef]
- Valenzuela, R.F.; Sierra, E.D.; Canjirathinkal, M.A.; Costelloe, C.M.; Madewell, J.E.; Murphy, W.A., Jr.; Amini, B. Early Results in the Novel Use of Contrast-Enhanced Susceptibility-Weighted Imaging in the Assessment of Response and Progression in Desmoid Fibromatosis: A Pilot Study in a Specialized Cancer Institution. Tumor Discov. 2023, 2, 1414. [Google Scholar] [CrossRef]
- Zanchetta, E.; Ciniselli, C.M.; Bardelli, A.; Colombo, C.; Stacchiotti, S.; Baldi, G.G.; Provenzano, S.; Bertulli, R.; Bini, F.; Casale, A.; et al. Magnetic Resonance Imaging Patterns of Tumor Response to Chemotherapy in Desmoid-Type Fibromatosis. Cancer Med. 2021, 10, 4356–4365. [Google Scholar] [CrossRef]
- Crombé, A.; Kind, M.; Ray-Coquard, I.; Isambert, N.; Chevreau, C.; André, T.; Lebbe, C.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; et al. Progressive Desmoid Tumor: Radiomics Compared with Conventional Response Criteria for Predicting Progression during Systemic Therapy—A Multicenter Study by the French Sarcoma Group. Am. J. Roentgenol. 2020, 215, 1539–1548. [Google Scholar] [CrossRef]
- Crombé, A.; Périer, C.; Kind, M.; De Senneville, B.D.; Le Loarer, F.; Italiano, A.; Buy, X.; Saut, O. T2-Based MRI Delta-Radiomics Improve Response Prediction in Soft-Tissue Sarcomas Treated by Neoadjuvant Chemotherapy. J. Magn. Reson. Imaging 2019, 50, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Crombe; Kind, M.; Ray-Coquard, I.L.; Isambert, N.; Chevreau, C.M.; Andre, T.; Lebbe, C.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; et al. Radiomics Improves Response Evaluation for Desmoid Tumours Treated with Chemotherapy. Ann. Oncol. 2019, 30, v699. [Google Scholar] [CrossRef]
- Kasper, B.; Gounder, M.; Hernandez, L.; Baumgarten, C.; Ratan, R. Capturing Patient Voice to Improve Outcomes That Matter to Patients with Desmoid Tumor. Cancer Manag. Res. 2024, 16, 617–628. [Google Scholar] [CrossRef] [PubMed]
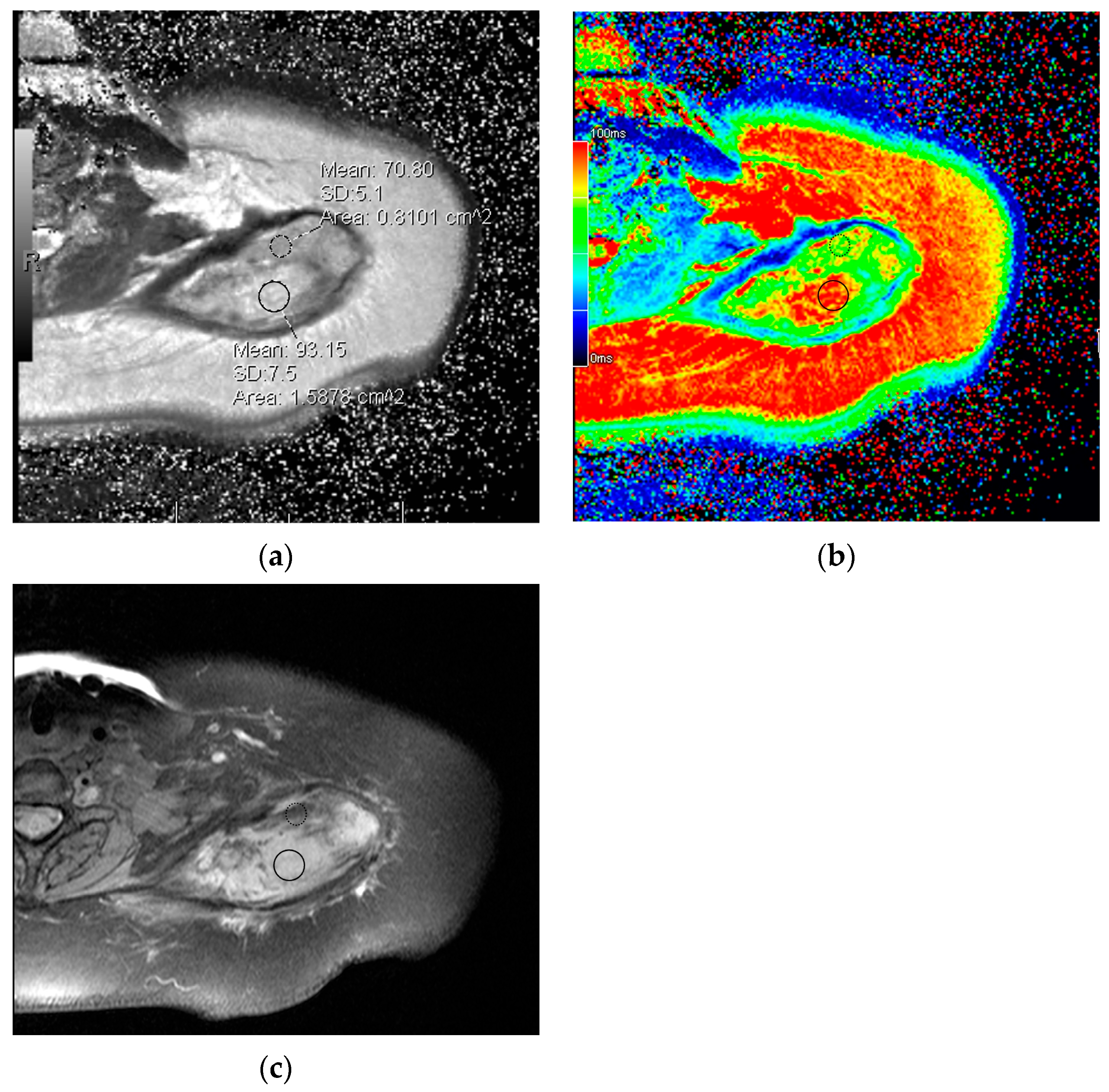
- Souza, F.F.; D’Amato, G.; Jonczak, E.E.; Costa, P.; Trent, J.C.; Rosenberg, A.E.; Yechieli, R.; Temple, H.T.; Pattany, P.; Subhawong, T.K. MRI T2 Mapping Assessment of T2 Relaxation Time in Desmoid Tumors as a Quantitative Imaging Biomarker of Tumor Response: Preliminary Results. Front. Oncol. 2023, 13, 1286807. [Google Scholar] [CrossRef]
- Morochnik, S.; Ozhinsky, E.; Rieke, V.; Bucknor, M.D. T2 Mapping as a Predictor of Nonperfused Volume in MRgFUS Treatment of Desmoid Tumors. Int. J. Hyperth. 2019, 36, 1271–1276. [Google Scholar] [CrossRef]
- Hancock, C.; Bernal, B.; Medina, C.; Medina, S. Cost Analysis of Diffusion Tensor Imaging and MR Tractography of the Brain. Open J. Radiol. 2014, 4, 260–269. [Google Scholar] [CrossRef]
- Choi, D.L.; Gupta, M.K.; Rebello, R.; Archibald, J.D. Cost-Comparison Analysis of Diffusion Weighted Magnetic Resonance Imaging (DWMRI) versus Second Look Surgery for the Detection of Residual and Recurrent Cholesteatoma. J. Otolaryngol.-Head Neck Surg. 2019, 48, 58. [Google Scholar] [CrossRef]
- Royce, T.J.; Punglia, R.S.; Chen, A.B.; Patel, S.A.; Thornton, K.A.; Raut, C.P.; Baldini, E.H. Cost–Effectiveness of Surveillance for Distant Recurrence in Extremity Soft Tissue Sarcoma. Ann. Surg. Oncol. 2017, 24, 3264–3270. [Google Scholar] [CrossRef]
- Ko, C.C.; Yeh, L.R.; Kuo, Y.T.; Chen, J.H. Imaging Biomarkers for Evaluating Tumor Response: RECIST and Beyond. Biomark. Res. 2021, 9, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Nixon, B.; Souza, F.; Cardoso, F.N.; Dayan, E.; Geiger, E.J.; Rosenberg, A.; D’Amato, G.; Subhawong, T. The Desmoid Dilemma: Challenges and Opportunities in Assessing Tumor Burden and Therapeutic Response. Curr. Oncol. 2025, 32, 288. https://doi.org/10.3390/curroncol32050288
Chang Y-C, Nixon B, Souza F, Cardoso FN, Dayan E, Geiger EJ, Rosenberg A, D’Amato G, Subhawong T. The Desmoid Dilemma: Challenges and Opportunities in Assessing Tumor Burden and Therapeutic Response. Current Oncology. 2025; 32(5):288. https://doi.org/10.3390/curroncol32050288
Chicago/Turabian StyleChang, Yu-Cherng, Bryan Nixon, Felipe Souza, Fabiano Nassar Cardoso, Etan Dayan, Erik J. Geiger, Andrew Rosenberg, Gina D’Amato, and Ty Subhawong. 2025. "The Desmoid Dilemma: Challenges and Opportunities in Assessing Tumor Burden and Therapeutic Response" Current Oncology 32, no. 5: 288. https://doi.org/10.3390/curroncol32050288
APA StyleChang, Y.-C., Nixon, B., Souza, F., Cardoso, F. N., Dayan, E., Geiger, E. J., Rosenberg, A., D’Amato, G., & Subhawong, T. (2025). The Desmoid Dilemma: Challenges and Opportunities in Assessing Tumor Burden and Therapeutic Response. Current Oncology, 32(5), 288. https://doi.org/10.3390/curroncol32050288





