Use of a Hybrid Adeno-Associated Viral Vector Transposon System to Deliver the Insulin Gene to Diabetic NOD Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Vector Construction and Production
2.1.1. AAV Vectors
2.1.2. HIV/MSCV Lentiviral Vector
2.2. Transduction of Liver Tissue
2.3. Functional Analysis
2.4. Microscopic Analysis
2.5. Vector Copy Number Analysis
2.6. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) Analysis
2.7. Statistical Analysis
3. Results
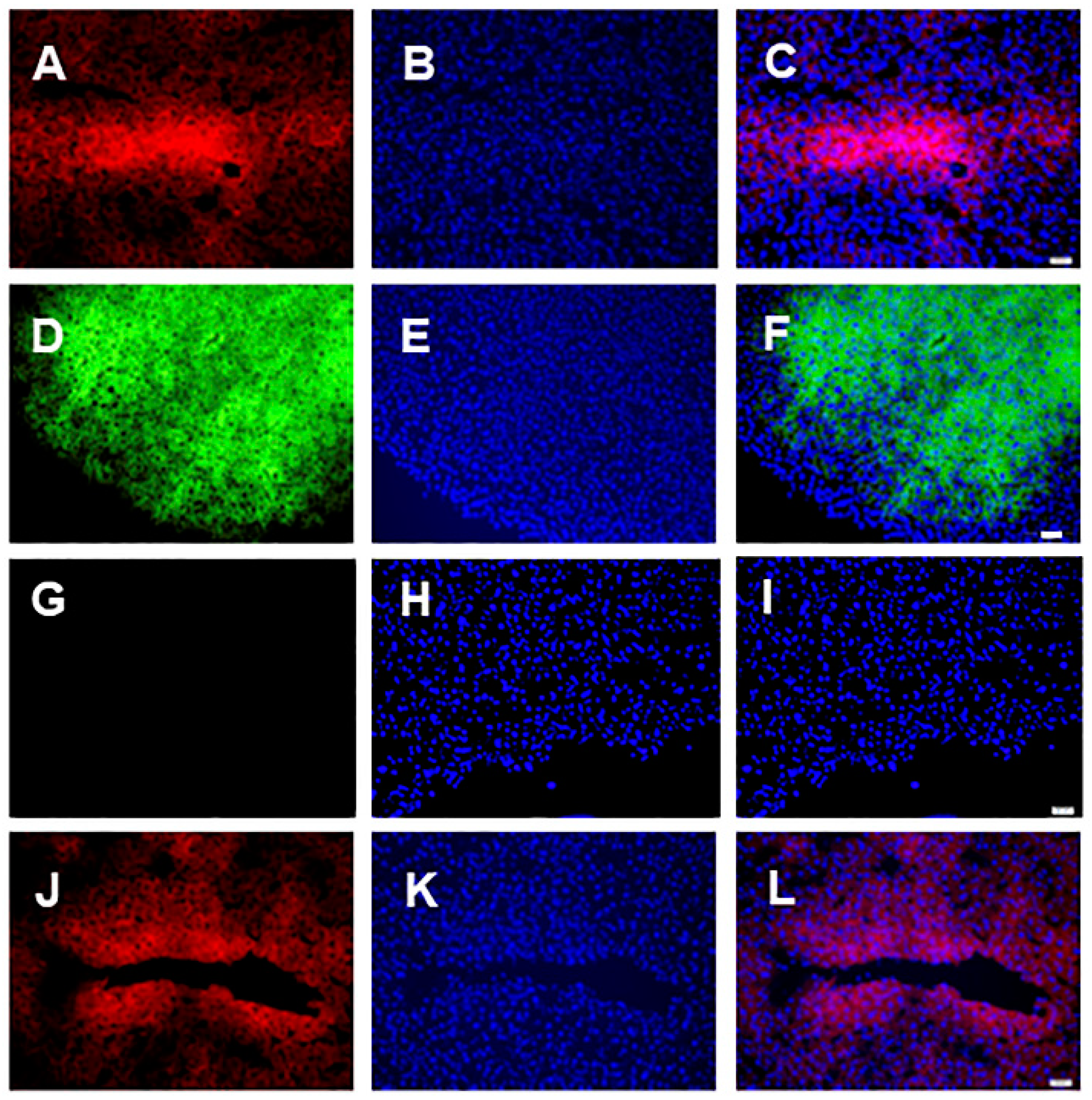
3.1. Microscopic Analysis
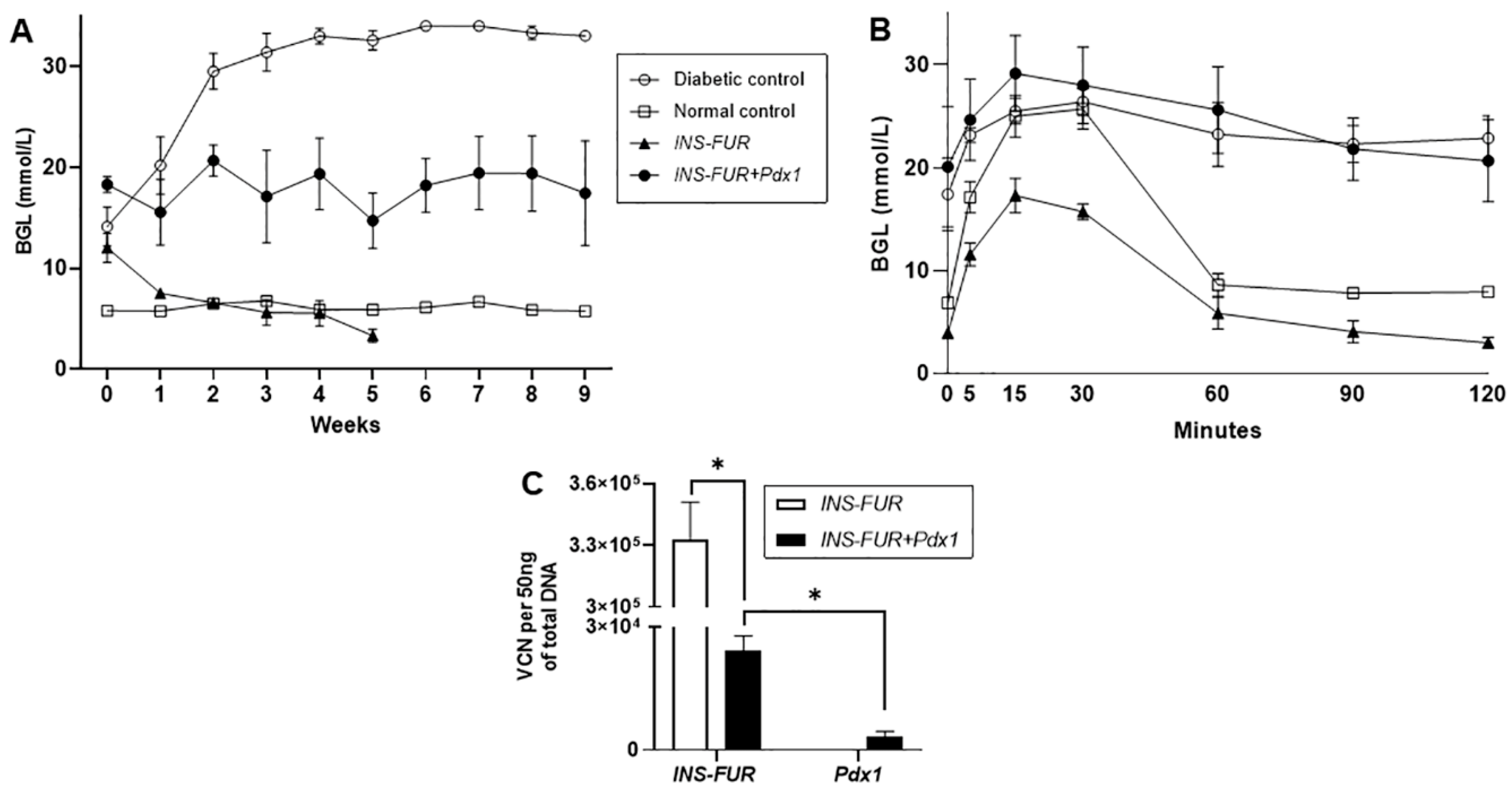
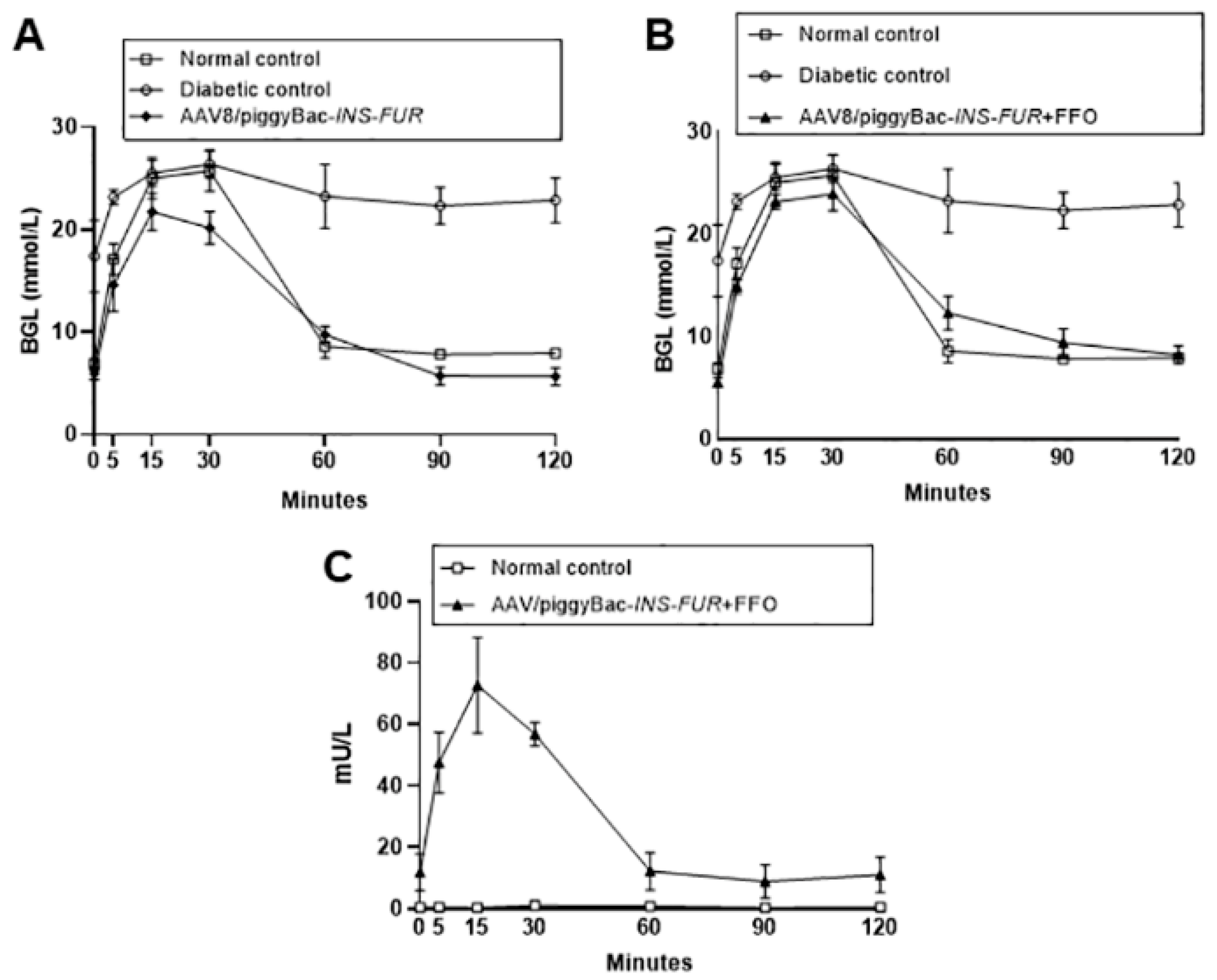
3.2. Delivery of AAV8 Expressing INS-FUR ± Pdx1 Fails to Reverse Diabetes
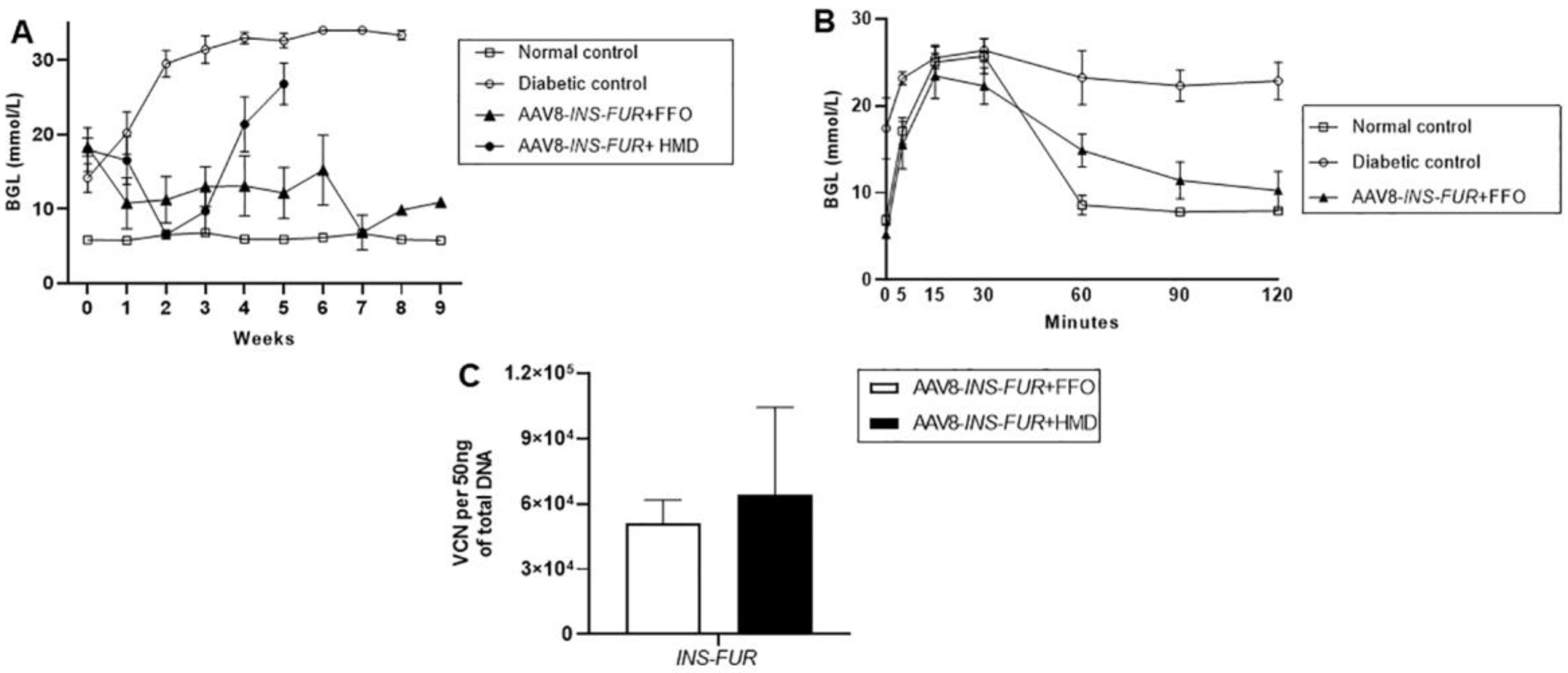
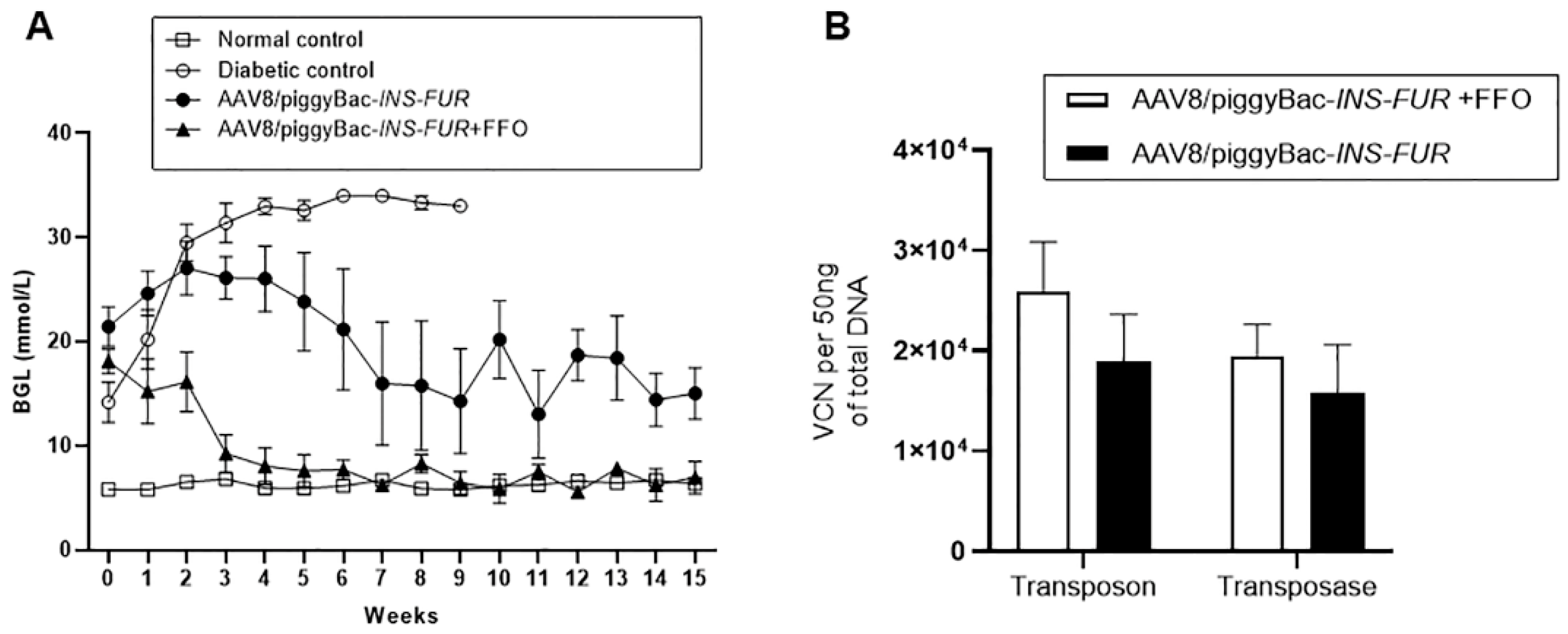
3.3. Reversal of Autoimmune Diabetes Using the AAV8/piggyBac-LSP-INS-FUR Vector System and FFO Surgery
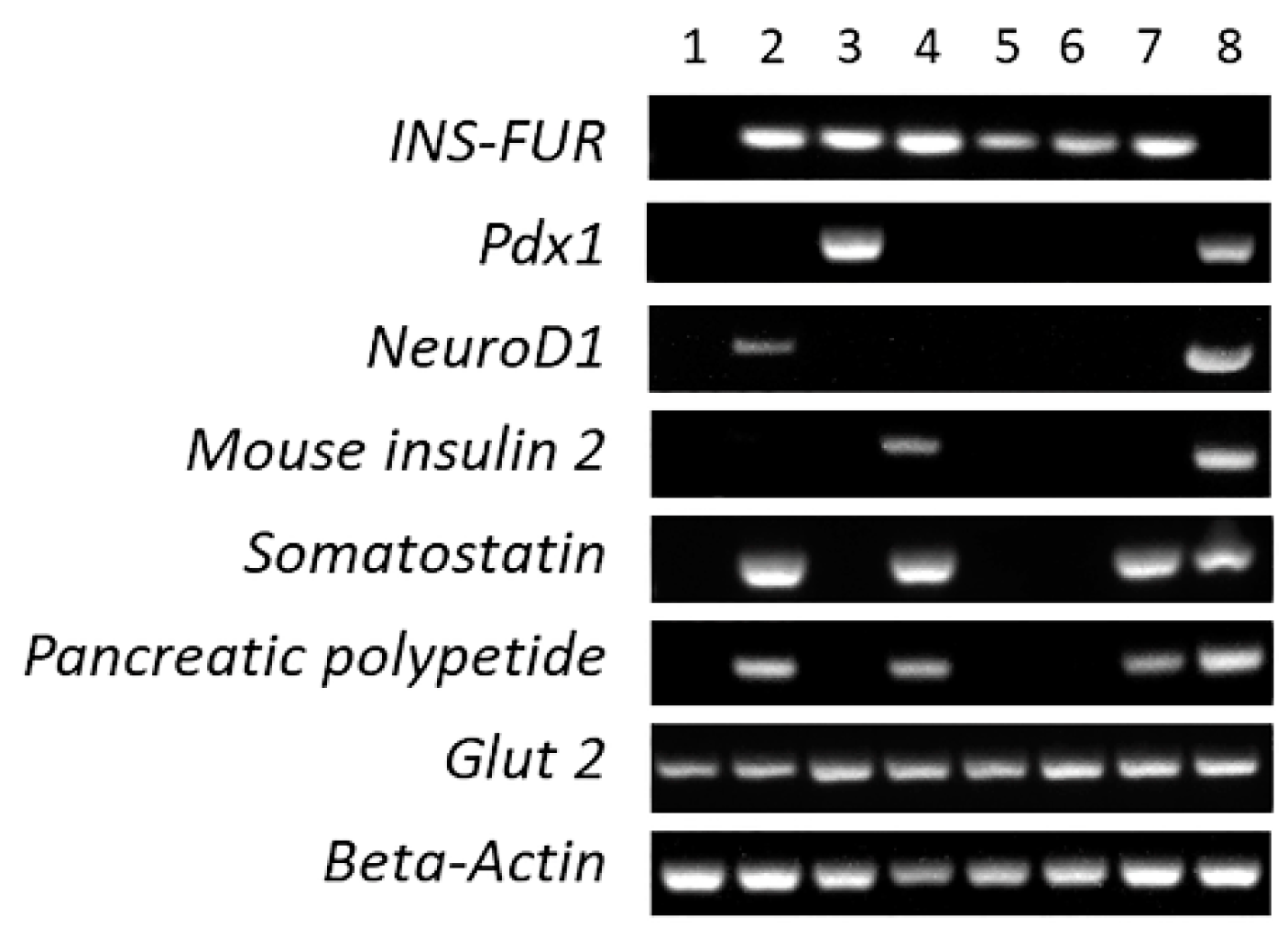
3.4. RT-PCR Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.; Zimmet, P.G. Definition, diagnosis and classification of diabetes mellitus and its complication. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Yeh, H.C.; Brown, T.T.; Maruthur, N.; Ranasinghe, P.; Berger, Z.; Suh, Y.D.; Wilson, L.M.; Haberl, E.B.; Brick, J.; Bass, E.B.; et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: A systematic review and met-analysis. Ann. Intern. Med. 2012, 157, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Franek, E.; Haluzik, M.; Varzic, S.C.; Sargin, M.; Macura, S.; Zacho, J.; Christiansen, J.S. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naïve adults with type 2 diabetes. Diabet. Med. 2016, 33, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, T.L.; Coppieters, K.T.; von Herrath, M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Weisman, A.; Bai, J.W.; Cardinez m Kramer, C.K.; Perkins, B.A. Effect of artificial pancreas systems on glycaemic control in patients with Type 1 duabetes: A systematic review and meta-analysis of outpatient randomised control trials. Lancet Diabetes Endocrinol. 2017, 5, 501–512. [Google Scholar] [CrossRef]
- Bekiari, E.; Kitsios, K.; Thabit, H.; Tauschmann, M.; Athanaasiadou, E.; Karagiannis, T.; Haidich, A.-B.; Hovorka, R.; Tsapas, A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018, 361, k1310. [Google Scholar] [CrossRef]
- Shafiee, A.; Patel, J.; Lee, J.S.; Hutmacher, D.W.; Fisk, N.M.; Khosrotehrani, K. Mesenchymal stem/stromal cells enhance engraftment, vasculogenic and pro-angiogenic activities of endothelial colony forming cells in immunocompetent hosts. Sci. Rep. 2017, 7, 13558. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Pathak, N.M.; O’neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for type 1 diabetes: Current scenario and future perspectives. Clin. Med. Insights 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Ren, B.; O’Brien, B.A.; Swan, M.A.; Kiona, M.E.; Nassif, N.T.; Wei, M.Q.; Simpson, A.M. Long-term correction of diabetes in rats following lentiviral hepatic insulin gene therapy. Diabetologia 2007, 50, 1910–1920. [Google Scholar] [CrossRef]
- Ren, B.; O’Brien, B.A.; Byrne, M.R.; Ch’ng, E.; Gatt, P.N.; Swan, M.A.; Nassif, N.T.; Wei, M.Q.; Gijsbers, R.; Debyser, Z.; et al. Long term reversal of diabetes in non obese diabetic mice by liver-directed gene therapy. J. Gene Med. 2013, 15, 28–41. [Google Scholar] [CrossRef]
- Gerace, D.; Ren, B.; Hawthorne, W.J.; Byrne, M.R.; Phillips, P.M.; O’Brien, B.A.; Nassif, N.T.; Alexander, I.E.; Simpson, A.M. Pancreatic transdifferentiation in porcine liver following lentiviral delivery of human furin-cleavable insulin. Trans. Proc. 2013, 45, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Elsner, M.; Terbish, T.; Jorns, A.; Naujok, O.; Wedekind, D.; Hedrich, H.J.; Lenzen, S. Reversal of diabetes through gene therapy of diabetic rats by hepatic insulin expression via lentiviral transduction. Mol. Ther. 2012, 20, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Ber, I.; Shternhall, K.; Perl, S.; Ohanuna, Z.; Goldberg, I.; Barshack, I.; Benvenisti-Zarum, L.; Meivar-Levy, I.; Ferber, S. Functional, Persistent, and Extended liver to pancreas transdifferentiation. J. Biol. Chem. 2003, 278, 31950–31957. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Lam, K.S.L.; Tam, S.; Xiao, W.; Xu, R. Adeno-associated virus-mediated pancreatic and duodenal homeobox gene-1 expression enhanced differentiation of hepatic oval stem cells to insulin-producing cells in diabetic rats. J. Biomed. Sci. 2008, 15, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Tuch, B.E.; Szymanska, B.; Yao, M.; Tabiin, M.T.; Gross, D.J.; Holman, S.; Swan, M.A.; Humphrey, R.K.B.; Marshall, G.M.; Simpson, A.M. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther. 2003, 10, 490–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferber, S.; Cohen, H.; Ber, I.; Einav, Y.; Goldberg, I.; Barshack, I.; Seijffers, R.; Kopolovic, J.; Kaiser, N.; Karasik, A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycaemia. Nat. Med. 2000, 6, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Matsummura, K.; Younan, P.; Imaeda, H.; Maeda, M.; Chan, L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 2003, 9, 596–603. [Google Scholar] [CrossRef]
- Tang, D.-Q.; Shun, L.; Koya, V.; Sun, Y.; Wang, Q.; Wang, H.; Li, S.-W.; Sun, Y.; Purich, D.L.; Zhang, C.; et al. Genetically reprogrammed, liver-derived insulin-producing cells are glucose-responsive, but susceptible to autoimmune destruction in settings of murine model of type 1 diabetes. Am. J. Transl. Res. 2013, 5, 184–199. [Google Scholar] [PubMed]
- Wang, A.Y.; Ehrhardt, A.; Xu, H.; Kay, M.A. Adenovirus transduction is required for the correction of diabetes using Pdx1 of Neurogenin 3 in the liver. Am. Soc. Ggene Ther. 2007, 15, 255–263. [Google Scholar] [CrossRef]
- Ren, B.; Tao, C.; Swan, M.A.; Joachim, N.; Martiniello-Wilks, R.; Nassif, N.T.; O’Brien, B.A.; Simpson, A.M. Pancreatic transdiffereniation and glucose-regulated production of human insulin in the H4 IIE rat liver cell line. Int. J. Mol. Sci. 2016, 17, 534. [Google Scholar] [CrossRef]
- Alam, T.; Wai, P.; Held, D.; Vakill, S.T.T.; Forsberg, E.; Sollinger, H. Correction of diabetic hyperglycaemia and amelioration of metabolic anomalies by minicircle DNA mediated glucose-dependent hepatic insulin production. PLoS ONE 2013, 8, e67515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashimoto, H.; Mizushima, T.; Ogura, T.; Kagawa, T.; Tomiyama, K.; Takahashi, R.; Yagoto, M.; Kawai, K.; Chijiwa, T.; Nakamuar, M.; et al. Study on AAV-mediated gene therapy for diabetes in humanized liver mouse to predict efficacy in humans. Biochem. Biophys. Res. Commun. 2016, 478, 1254–1260. [Google Scholar] [CrossRef]
- Gan, S.U.; Fu, Z.; Sia, K.C.; Kon, O.L.; Calne, R.; Lee, K.O. development of a liver-specific Tet-off AAV8 vector for improved safety of isnulin gene therapy for diabetes. J. Gene Med. 2018, 21, e3067. [Google Scholar] [CrossRef] [PubMed]
- Recino, A.; Gan, S.U.; Sia, K.C.; Sawyer, Y.; Trendell, J.; Kay, R.; Gribble, F.M.; Reimann, F.; Foale, R.; Notaridou, M. Immunosuppression overcomes insulin- and vector-specific immune responses that limit efficacy of AAV2/ 8-mediated insulin gene therapy in NOD mice. Gene Ther. 2019, 26, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; La, Q.T.; O’Brien, B.A.; Nassif, N.T.; Yan, Y.; Gerace, D.; Martiniello-Wilks, R.; Torpy, F.; Dane, A.P.; Alexander, I.E.; et al. Partial pancreatic transdifferentiation of primary human hepatocytes in the livers of an humanized mouse model. J. Gene Med. 2018, 20, e3017. [Google Scholar] [CrossRef]
- Azuma, H.; Paulk, N.; Ranade, A.; Dorrell, C.; Al-Dhalimy, M.; Ellis, E.; Strom, S.; Kay, M.A.; Finegold, M.; Grompe, M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Ilrg−/− mice. Nat. Biotech. 2007, 25, 903–910. [Google Scholar] [CrossRef]
- Bissig, K.-D.; Le, T.; Verma, I.M. Repopulation of adult and neonatal mice with human hepatocytes: A chimeric animal model. Proc. Natl. Acad. Sci. USA 2007, 104, 20507–20511. [Google Scholar] [CrossRef]
- DePolo, N.J.; Reed, J.D.; Sheridan, P.L.; Townsend, K.; Sauter, S.L.; Jolly, D.J.; Dubensky, T.W. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000, 2, 218–222. [Google Scholar] [CrossRef]
- Lisowski, L.; Dane, A.P.; Chu, K.; Cunningham, S.C.; Wilson, E.M.; Nygaard, S.; Grompe, M.; Alexander, I.E.; Kay, M.A. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 2014, 506, 382–386. [Google Scholar] [CrossRef]
- Cabanes-Cres, M.; Westhaus, A.; Navarro, R.G.; Baltazar, G.; Zhu, E.; Amaya, A.K.; Liao, S.H.Y.; Scott, S.; Sallard, E.; Dilworth, K.L.; et al. Attentuation of heparin sulfate proteoglycan binding enhances in vivo transduction of human primary hepatocytes with AAV2. Mol. Ther. Meth. Clin. Dev. 2020, 17, 1139–1154. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Tuddenham, E.G.D.; Rangarajan, S.; McIntosh, J.; Linch, D.C.; Chir, B.; Chowdary, P.; Ridell, A.; Jaquilmac, A.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in haemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Dane, A.P.; Wowro, S.J.; Cunningham, S.C.; Alexander, I.E. Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotrophic AAV pseudo-serotypes. Gene Ther. 2013, 20, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Cary, L.C.; Goebel, M.; Corsaro, B.G.; Wang, H.-G.; Rosen, E.; Fraser, M.J. Transposon mutagenesis of baculoviruses: Analysis of Trichopulsia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 1989, 172, 156–169. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Siew, S.M.; Hallwirth, C.V.; Bolitho, C.; Garg, G.; Michael, I.P.; Hetherington, N.A.; Carpenter, K.; de Alencastro, G.; Nagy, A.; et al. Modeling correction of severe urea cycle defects in the growing murine liver using a hybrid recombinant adeno-associated virus/piggyback transposase gene delivery system. Hepatology 2015, 62, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Higuchi y Kawakami, S.; Yamashita, F.; Hashida, S. piggyBack transposon-mediated long-term gene expression in mice. Mol. Ther. 2010, 18, 707–714. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Dane, A.P.; Spinoulos, A.; Alexander, I.E. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol. Ther. 2008, 16, 1081–1088. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Kok, C.Y.; Dane, A.P.; Carpenter, K.; Kizana, E.; Kuchel, P.W.; Alexander, I.E. Induction and prevention of severe hyperammonemia in the spfash mouse model of ornithine transcarbamylase deficiency using shRNA and rAAV-mediated gene delivery. Mol. Ther. 2011, 19, 854–859. [Google Scholar] [CrossRef]
- Choi, J.K.; Hoang, N.; Vilardi, A.M.; Conrad, P.; Emerson, S.G.; Gewirtz, A.M. Hybrid HIV/MSCV LTR enhances transgene expression of lentiviral vectors in human CD34+ hematopoetic cells. Stem Cells 2001, 19, 236–246. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Spinoulas, A.; Carpenter, K.H.; Wicken, B.; Kuchel, P.W.; Alexander, I.E. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spfash mice. Mol. Ther. 2009, 17, 1340–1346. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Sivam, N.S.; Xiang, T.K.; Pan, L.W.; Fui, T.Z.; Kien, C.; Khoo, N.; Yi, F.J.; Chellian, J.; Cheng, L.L.; et al. Gene therapy for type 1 diabetes mellitus. Biomed. Pharmacother. 2018, 108, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Volpers, C.; Kochanek, S. Adenoviral vectors for gene transfer and theropy. J. Gene Med. 2004, 6, S164–S171. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Hacein-bey, S.; Basile, C.D.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.-L.; et al. Gene therapy of severe combined immunodeficiency (SCID)-XI disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Whittaker, R.; Gandere, C.; Stoll, E.A. A guide to approaching regulatory considerations for lentiviral-mediated gene transfer. Hum. Gene Ther. 2007, 28, 136–176. [Google Scholar]
- Wang, L.; Wang, H.; Bell, P.; McMenamin, D.; Wilson, J.M. Hepatic gene transfer in neonatal mice by adeno-associated virus serotype 8 vector. Hum. Gene Ther. 2012, 23, 533–539. [Google Scholar] [CrossRef]
- Shanmukhappa, K.; Mourya, R.; Sabla, G.E.; Degen, J.L.; Bezerra, J.A. Hepatic to pancreatic switch defines a role for hemostatic factors in cellular plasticity in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 10182–10187. [Google Scholar] [CrossRef]
- Yang, L.; Shiwu, L.; Hatch, H.; Ahrens, K.; Cornelius, J.G.; Petersen, B.E.; Ammon, A.B. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8078–8083. [Google Scholar] [CrossRef]
- Cerda-Esteban, N.; Naumann, H.; Ruzittu, S.; Mah, N.; Pongrac, I.M.; Cozzitorto, C.; Hommel, A.; Andrade-Navarro, M.A.; Bonifacio, E.; Spagnoli, F.M. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Dorrell, C.; Naugler, W.E.; Heskett, M.; Spellman, P.; Li, B.; Gavivo, F.; Haft, A.; Wakefield, L.; Grompe, M. Long-term correction of diabetes in mice by in vivo reprogramming of pancreatic ducts. Mol. Ther. 2018, 26, 1327–1342. [Google Scholar] [CrossRef]
- Coleman, M.A.; Jessup, C.F.; Bridge, J.A.; Overgaard, N.H.; Penko, D.; Walters, S.; Borg, D.J.; Galea, R.; Forbes, J.M.; Thomas, R.; et al. Antigen-encoding bone marrow terminates islet-directed memory CD+ T-cell responses to alleviate islet transplant rejection. Diabetes 2016, 65, 1328–1340. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, Q.T.; Ren, B.; Logan, G.J.; Cunningham, S.C.; Khandekar, N.; Nassif, N.T.; O’Brien, B.A.; Alexander, I.E.; Simpson, A.M. Use of a Hybrid Adeno-Associated Viral Vector Transposon System to Deliver the Insulin Gene to Diabetic NOD Mice. Cells 2020, 9, 2227. https://doi.org/10.3390/cells9102227
La QT, Ren B, Logan GJ, Cunningham SC, Khandekar N, Nassif NT, O’Brien BA, Alexander IE, Simpson AM. Use of a Hybrid Adeno-Associated Viral Vector Transposon System to Deliver the Insulin Gene to Diabetic NOD Mice. Cells. 2020; 9(10):2227. https://doi.org/10.3390/cells9102227
Chicago/Turabian StyleLa, Que T., Binhai Ren, Grant J. Logan, Sharon C. Cunningham, Neeta Khandekar, Najah T. Nassif, Bronwyn A. O’Brien, Ian E. Alexander, and Ann M. Simpson. 2020. "Use of a Hybrid Adeno-Associated Viral Vector Transposon System to Deliver the Insulin Gene to Diabetic NOD Mice" Cells 9, no. 10: 2227. https://doi.org/10.3390/cells9102227
APA StyleLa, Q. T., Ren, B., Logan, G. J., Cunningham, S. C., Khandekar, N., Nassif, N. T., O’Brien, B. A., Alexander, I. E., & Simpson, A. M. (2020). Use of a Hybrid Adeno-Associated Viral Vector Transposon System to Deliver the Insulin Gene to Diabetic NOD Mice. Cells, 9(10), 2227. https://doi.org/10.3390/cells9102227






