The Intrinsic GDP/GTP Exchange Activities of Cdc42 and Rac1 Are Critical Determinants for Their Specific Effects on Mobilization of the Actin Filament System
Abstract
1. Introduction
2. Experimental
2.1. Mutant Small GTPases
2.2. Antibodies and Reagents
2.3. Cell Cultivation and Transfection
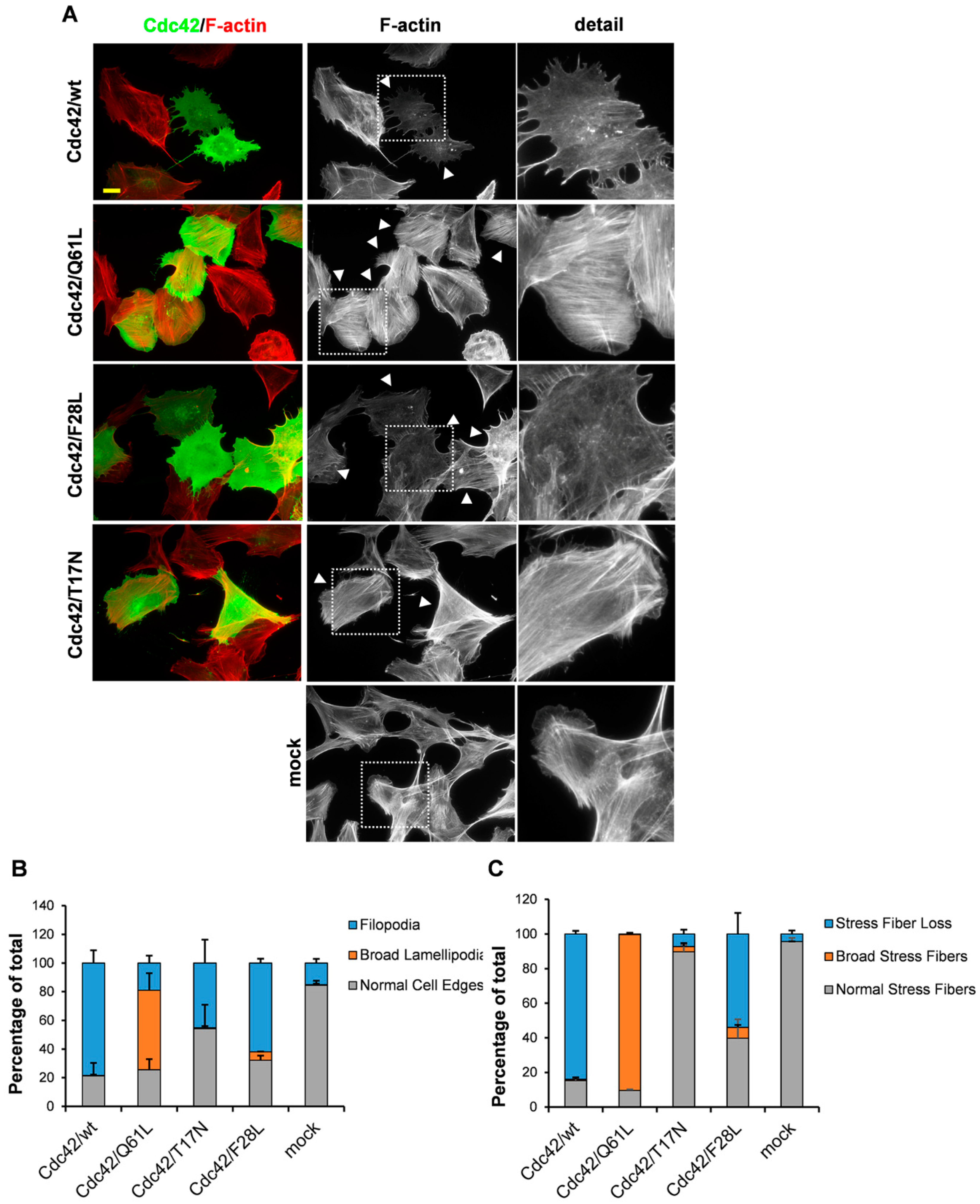
3. Results
3.1. An Intact GDP/GTP Exchange Activity is the Basis for Cdc42-Induced Filopodia Formation
3.2. The Role of the Effector Loop in Cdc42-Induced Filopodia Formation
3.3. RhoGDI Binding Is Not Necessary for Cdc42-Dependent Actin Reorganization
3.4. Membrane Targeting Is Necessary for Cdc42-Dependent Actin Reorganization
3.5. Rac1 Mutants with Elevated Intrinsic GDP/GTP Exchange Activities Induce Filopodia
3.6. The Involvement of Formins and Arp2/3 in Cdc42- and Rac1-Induced Actin Reorganization
4. Discussion
Supplementary Materials
Funding
Conflicts of Interest
References
- Ridley, A.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Aspenström, P.; Fransson, Å.; Saras, J.; Aspenstrm, P. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem. J. 2004, 377, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Colicelli, J. Human RAS Superfamily Proteins and Related GTPases. Sci. STKE 2004, 2004, re13. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Der, C.J. Ras history: The saga continues. Small GTPases 2010, 1, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.R.; Rossman, K.L.; Der, C.J. Rho guanine nucleotide exchange factors: Regulators of Rho GTPase activity in development and disease. Oncogene 2014, 33, 4021–4035. [Google Scholar] [CrossRef]
- Tcherkezian, J.; Lamarche-Vane, N.; Lamarche-Vane, N. Current knowledge of the large RhoGAP family of proteins. Biol. Cell 2007, 99, 67–86. [Google Scholar] [CrossRef]
- Xie, F.; Shao, S.; Aziz, A.U.R.; Zhang, B.; Wang, H.; Liu, B. Role of Rho-specific guanine nucleotide dissociation inhibitor α regulation in cell migration. Acta Histochem. 2017, 119, 183–189. [Google Scholar] [CrossRef]
- Chardin, P. Function and regulation of Rnd proteins. Nat. Rev. Mol. Cell Biol. 2006, 7, 54–62. [Google Scholar] [CrossRef]
- Aspenström, P. Fast-cycling Rho GTPases. Small GTPases 2018, 1–8. Available online: https://www.tandfonline.com/doi/full/10.1080/21541248.2017.1391365 (accessed on 17 July 2019).
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Boureux, A.; Vignal, E.; Faure, S.; Fort, P. Evolution of the Rho family of Ras-like GTPases in eukaryotes. Mol. Biol. Evol. 2007, 24, 203–216. [Google Scholar] [CrossRef]
- Krengel, U.; Schlichting, I.; Scherer, A.; Schümann, R.; Frech, M.; John, J.; Kabsch, W.; Pai, E.F.; Wittinghofer, A. Three-dimensional structures of H-ras p21 mutants: Molecular basis for their inability to function as signal switch molecules. Cell 1990, 62, 539–548. [Google Scholar] [CrossRef]
- Lin, R.; Bagrodia, S.; Cerione, R.; Manor, D. A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol. 1997, 7, 794–797. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Barry, D.C.; Chanock, S.J.; Bokoch, G.M. Guanine Nucleotide Binding Properties of Rac2 Mutant Proteins and Analysis of the Responsiveness to Guanine Nucleotide Dissociation Stimulator. Biochemistry 1997, 36, 626–632. [Google Scholar] [CrossRef]
- Reinstein, J.; Schlichting, I.; Frech, M.; Goody, R.S.; Wittinghofer, A. p21 with a phenylalanine 28—leucine mutation reacts normally with the GTPase activating protein GAP but nevertheless has transforming properties. J. Biol. Chem. 1991, 266, 17700–17706. [Google Scholar]
- Jordan, P.; Brazåo, R.; Boavida, M.G.; Gespach, C.; Chastre, E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene 1999, 18, 6835–6839. [Google Scholar] [CrossRef]
- Schnelzer, A.; Prechtel, D.; Knaus, U.; Dehne, K.; Gerhard, M.; Graeff, H.; Harbeck, N.; Schmitt, M.; Lengyel, E. Rac1 in human breast cancer: Overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 2000, 19, 3013–3020. [Google Scholar] [CrossRef]
- Fiegen, D.; Haeusler, L.C.; Blumenstein, L.; Herbrand, U.; Dvorský, R.; Vetter, I.R.; Ahmadian, M.R. Alternative splicing of Rac1 generates Rac1b, a self-activating GTPase. J. Biol. Chem. 2004, 2004, 4743–4749. [Google Scholar] [CrossRef]
- Matos, P.; Collard, J.G.; Jordan, P. Tumor-related Alternatively Spliced Rac1b Is Not Regulated by Rho-GDP Dissociation Inhibitors and Exhibits Selective Downstream Signaling. J. Biol. Chem. 2003, 278, 50442–50448. [Google Scholar] [CrossRef]
- Singh, A.; Karnoub, A.E.; Palmby, T.R.; Lengyel, E.; Sondek, J.; Der, C.J. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene 2004, 23, 9369–9380. [Google Scholar] [CrossRef]
- Hall, A. Rho family GTPases. Biochem. Soc. Trans. 2012, 40, 1378–1382. [Google Scholar] [CrossRef]
- Kozma, R.; Ahmed, S.; Best, A.; Lim, L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 1995, 15, 1942–1952. [Google Scholar] [CrossRef]
- Tu, S.S.; Wu, W.J.; Yang, W.; Nolbant, P.; Hahn, K.; Cerione, R.A. Antiapoptotic Cdc42 Mutants Are Potent Activators of Cellular Transformation. Biochemistry 2002, 41, 12350–12358. [Google Scholar] [CrossRef]
- Lamarche, N.; Tapon, N.; Stowers, L.; Burbelo, P.D.; Aspenström, P.; Bridges, T.; Chant, J.; Hall, A. Rac and Cdc42 Induce Actin Polymerization and G1 Cell Cycle Progression Independently of p65PAK and the JNK/SAPK MAP Kinase Cascade. Cell 1996, 87, 519–529. [Google Scholar] [CrossRef]
- Abo, A.; Freeman, J.L.; Lambeth, J.D. Rac “Insert Region” Is a Novel Effector Region That Is Implicated in the Activation of NADPH Oxidase, but Not PAK65. J. Biol. Chem. 1996, 271, 19794–19801. [Google Scholar]
- Wu, W.-J.; Leonard, D.A.; A-Cerione, R.; Manor, D. Interaction between Cdc42Hs and RhoGDI Is Mediated through the Rho Insert Region. J. Biol. Chem. 1997, 272, 26153–26158. [Google Scholar] [CrossRef]
- Lin, Q.; Fuji, R.N.; Yang, W.; Cerione, R.A. RhoGDI Is Required for Cdc42-Mediated Cellular Transformation. Curr. Biol. 2003, 13, 1469–1479. [Google Scholar] [CrossRef]
- Gao, J.; Liao, J.; Yang, G.Y. CAAX-box protein, prenylation process and carcinogenesis. Am. J. Transl. Res. 2009, 1, 312–325. [Google Scholar]
- Krauthammer, M.; Kong, Y.; Ha, B.H.; Evans, P.; Bacchiocchi, A.; McCusker, J.P.; Cheng, E.; Davis, M.J.; Goh, G.; Choi, M.; et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012, 44, 1006–1014. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Neidt, E.M.; Cui, J.; Feiger, Z.; Skau, C.T.; Gardel, M.L.; Kozmin, S.A.; Kovar, D.R. Identification and Characterization of a Small Molecule Inhibitor of Formin-Mediated Actin Assembly. Chem. Biol. 2009, 16, 1158–1168. [Google Scholar] [CrossRef]
- Hetrick, B.; Han, M.S.; Helgeson, L.A.; Nolen, B.J. Small molecules CK-666 and CK-869 inhibit Arp2/3 complex by blocking an activating conformational change. Chem. Biol. 2013, 20, 701–712. [Google Scholar] [CrossRef]
- Czuchra, A.; Wu, X.; Meyer, H.; Van Hengel, J.; Schroeder, T.; Geffers, R.; Rottner, K.; Brakebusch, C.; Ginsberg, M. Cdc42 Is Not Essential for Filopodium Formation, Directed Migration, Cell Polarization, and Mitosis in Fibroblastoid Cells. Mol. Biol. Cell 2005, 16, 4473–4484. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Zheng, Y. Gene Targeting of Cdc42 and Cdc42GAP Affirms the Critical Involvement of Cdc42 in Filopodia Induction, Directed Migration, and Proliferation in Primary Mouse Embryonic Fibroblasts. Mol. Biol. Cell 2006, 17, 4675–4685. [Google Scholar] [CrossRef]
- Gao, Y.; Dickerson, J.B.; Guo, F.; Zheng, J.; Zheng, Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 7618–7623. [Google Scholar] [CrossRef]
- Surviladze, Z.; Waller, A.; Strouse, J.J.; Bologa, C.; Ursu, O.; Salas, V.; Parkinson, J.F.; Phillips, G.K.; Romero, E.; Wandinger-Ness, A.; et al. A Potent and Selective Inhibitor of Cdc42 GTPase. In Probe Reports from the NIH Molecular Libraries Program [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Owen, D.; Mott, H.R.; Laue, E.D.; Lowe, P.N. Residues in Cdc42 That Specify Binding to Individual CRIB Effector Proteins. Biochemistry 2000, 39, 1243–1250. [Google Scholar] [CrossRef]
- Spoerner, M.; Herrmann, C.; Wittinghofer, A.; Vetter, I.R.; Kalbitzer, H.R. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc. Natl. Acad. Sci. USA 2001, 98, 4944–4949. [Google Scholar] [CrossRef]
- McCallum, S.J.; Wu, W.J.; Cerione, R.A. Identification of a Putative Effector for Cdc42Hs with High Sequence Similarity to the RasGAP-related Protein IQGAP1 and a Cdc42Hs Binding Partner with Similarity to IQGAP2. J. Biol. Chem. 1996, 271, 18825–18830. [Google Scholar] [CrossRef]
- Derivery, E.; Gautreau, A. Generation of branched actin networks: Assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays 2010, 32, 119–131. [Google Scholar] [CrossRef]
- Kuhn, S.; Geyer, M. Formins as effector proteins of Rho GTPases. Small GTPases 2014, 5, e983876. [Google Scholar] [CrossRef]




| A. Cdc42 and Rac1 variants used in the study | ||
| Small GTPase | Mutant | Phenotype |
| Cdc42 | Q61L | GTPase defective |
| G12V | GTPase defective | |
| T17N | Dominant negative (nucleotide binding-defective) | |
| F28L | Fast-cycling (increased GDP/GTP exchange) | |
| D118N | Elevated GDP/GTP exchange | |
| R66A | RhoGDI binding-defective | |
| T35A | Effector loop mutant | |
| F37A | Effector loop mutant | |
| Y40C | Effector loop mutant | |
| Δins | Insert domain mutant | |
| SAAX | CAAX box mutant | |
| Rac1 | Q61L | GTPase defective |
| T17N | Dominant negative (nucleotide binding- defective) | |
| F28L | Fast-cycling (increased GDP/GTP exchange) | |
| P29S | Fast-cycling (increased GDP/GTP exchange). Cancer mutation | |
| Rac1B | Fast-cycling (increased GDP/GTP exchange). Cancer mutation | |
| B. Inhibitors used in the study | ||
| Inhibitor | Concentration Used | Targeted Pathway |
| GGTI298 | 10 μM | Inhibitor of geranylgeranylation |
| FFT277 | 10 μM | Inhibitor of farnesylation |
| 2-bromopalmitate (2-BP) | 100 μM | Inhibitor of palmitoylation |
| SU6656 | 2 μM | Inhibitor of Src family kinases |
| LY294002 | 10 μM | Inhibitor of PI3 kinases |
| Y27632 | 10 μM | Inhibitor of Rho kinase (ROCK) |
| NSC23766 | 30 μM | Inhibitor of Rac |
| ML-141 | 10 μM | Inhibitor of Cdc42 |
| SMIFH2 | 30 μM | Inhibitor of formins |
| CK-666 | 100 μM | Inhibitor of Arp2/3 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aspenström, P. The Intrinsic GDP/GTP Exchange Activities of Cdc42 and Rac1 Are Critical Determinants for Their Specific Effects on Mobilization of the Actin Filament System. Cells 2019, 8, 759. https://doi.org/10.3390/cells8070759
Aspenström P. The Intrinsic GDP/GTP Exchange Activities of Cdc42 and Rac1 Are Critical Determinants for Their Specific Effects on Mobilization of the Actin Filament System. Cells. 2019; 8(7):759. https://doi.org/10.3390/cells8070759
Chicago/Turabian StyleAspenström, Pontus. 2019. "The Intrinsic GDP/GTP Exchange Activities of Cdc42 and Rac1 Are Critical Determinants for Their Specific Effects on Mobilization of the Actin Filament System" Cells 8, no. 7: 759. https://doi.org/10.3390/cells8070759
APA StyleAspenström, P. (2019). The Intrinsic GDP/GTP Exchange Activities of Cdc42 and Rac1 Are Critical Determinants for Their Specific Effects on Mobilization of the Actin Filament System. Cells, 8(7), 759. https://doi.org/10.3390/cells8070759




