1. Introduction
The space age has unveiled significant insights into how gravity impacts human physiology, particularly for astronauts on long-duration missions who encounter environmental stressors (i.e., acceleration, vibrations, microgravity, and radiation) that can negatively affect both the structure and function of the brain, potentially leading to cognitive deficits [
1] and long-lasting changes [
2,
3,
4]. Research using mice indicates that cosmic radiation may promote amyloid-β accumulation, neuroinflammation, and cognitive dysfunction related to the hippocampus [
5,
6]. Simulated microgravity (MG) disrupts protein clearance, with various ground simulation studies on rhesus macaques and rats revealing pathological changes ([
7] and references therein). Additionally, gravity influences protein aggregation, suggesting a direct role of physical forces in this process [
8,
9]. Furthermore, the literature suggests that spaceflight may accelerate brain aging, like its established effects on cardiovascular and musculoskeletal systems [
6,
10]. These findings raise crucial concerns for astronaut health and also offer insights into neurodegenerative mechanisms that could be relevant for both space and terrestrial biomedicine [
11,
12].
Neurodegenerative diseases, which are more common with age and for which there are no cures [
13], encompass a range of disorders, including tauopathies characterized by the accumulation of Tau protein in neurons [
13,
14,
15]. The hyperphosphorylation of Tau leads to its detachment from microtubules, forming neurofibrillary tangles (NFTs) that disrupt cellular functions and cause neuronal death [
13,
16]. Synthetic forms of NFTs, called Tau seeds (TSs), behave as seeds in promoting neurodegeneration when introduced to cells in vitro, with phosphorylation by kinases like Extracellular signal-Regulated Kinase (ERK) being critical for aggregate formation [
13]. Kinase inhibitors, including those approved for cancer treatment, may also be effective for Tau-related pathologies. One such inhibitor, PD-0325901 (PD-901), has demonstrated promise in reducing Tau aggregate formation in a neurodegenerative model [
17].
Changes in gravitational force affect the cytoskeleton across different cell types ([
18,
19], and therein). For example, human neuroblastoma cells SH-SY5Y exhibit distinct behaviors when exposed to MG versus hypergravity (HG).
This work, although preliminary, explores the possibility of applying centrifuge therapy to the treatment of neurodegeneration at large. Centrifuge therapy has already been in clinical practice for several decades now for a number of human conditions, including peripheral artery disease, coronary artery disease, lymphedema, complex regional pain syndrome, secondary Raynaud’s phenomenon, and systemic sclerosis [
20]. In human research and application, one of the very early predecessors of the current short arm centrifuges was probably Cox’s chair, described more than two centuries ago in his book “Practical Observations on Insanity” [
21]. Also, the later work by Halloran and his application of a circulating swing used in clinical medicine in the treatment of mental health issues was the basis for rotating devices in human medicine [
22]. More recently, a case study reported improvement in mobility and changes in the electro-encephalogram (EEG) of a patient with secondary progressive multiple sclerosis following multiple, mild HG sessions [
23]. Observations from space medicine suggest that artificial gravity training is a promising avenue for physical rehabilitation, especially in counteracting musculoskeletal decline due to microgravity and inactivity. HG has also been explicitly proposed as a potential therapeutic approach specifically against neurodegeneration [
24], although the molecular mechanisms underlying these effects remain largely unexplored.
In our previous gravitational biology studies [
18,
19], we could observe the cytoskeletal adaptation changes induced by HG in a cell model in vitro. Since neurodegenerative pathologies do involve cytoskeletal damage [
25,
26], based on the above, we decided to test the hypothesis whether or not HG had an effect on a neural cell model of neurodegeneration associated with Tau protein deposition and neurofibrillary tangles formation in vitro.
Therefore, in this study, we investigated the effects of HG, also combined with the ERK inhibitor PD-901, on Tau aggregates in the hippocampal neuronal cell line HT22. Our findings indicated that HG does not compromise cell viability; the combination of HG and PD-901 led to a synergistic reduction in the size of Tau aggregates, correlating with decreased phosphorylation at pS262 and pS396, both associated with neurodegeneration progression, while leaving pT231 unaltered. Utilizing the Large Diameter Centrifuge (LDC) of the European Space Agency (ESA, Noordwijk, The Netherlands,
Supplementary Scheme S1), we observed a threshold effect from HG treatments.
Understanding how changes in gravitational forces affect neuronal behavior could provide valuable insights into the mechanisms of the disease and possibly new therapeutic tools.
2. Materials and Methods
2.1. Cell Culture
HT-22 cells (Mouse Hippocampal Neuronal Cell Line, Sigma-Aldrich, St. Louis, MO, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) Low-Glucose (Sigma, St. Louis, MO, USA) complemented with 10% Fetal Bovine Serum (FBS, Gibco, Grand Island, NY, USA), 200 mM L-Glutamine (Sigma-Aldrich), and 1% Penicillin/Streptomycin (Sigma-Aldrich) in ventilated plastic flasks (Sarstedt, Nümbrecht, Germany) at 37 °C, 5% CO2. Cells were split every 48 h, at about 80% confluency, using 1X Trypsin/EDTA (Sigma-Aldrich) in 1X Phosphate Buffer Saline (PBS).
Live cells were shipped from Italy to the LDC facility (
Supplementary Scheme S1) in 1M HEPES, pH 7.4 (Sigma-Aldrich), and added to the complete medium. For HG treatments, 20,000 cells were seeded on day 1 in Nunc Lab-Tek Flask on Slide, and from there on flaskettes with glass bottom, for microscopy analysis (ThermoFisher Scientific, Waltham, MA, USA). To improve cell adhesion, flaskettes were treated with Poly-D-Lysine (Sigma-Aldrich) for one hour at 37 °C, washed with sterile water, and let dry under the laminar-flow hood for at least three hours, at RT. During HG protocols, cells were kept at 37 °C within an incubator placed inside a gondola of the LDC.
Following data acquisition, cells were fixed with ice-cold methanol at RT for 3 min and then stored in 1X PBS at 4 °C.
2.2. Transfection Protocols
The plasmid encoding the CST
P301S was transfected in an antibiotic-free medium, 24 h after cell seeding. Recombinant heparin-assembled P301S Tau fibrils were prepared as in [
17]. Tau fibrils were delivered to cells at a concentration of 0.3 µg/cm
2. Transfection protocols were performed with Lipofectamine 2000 (ThermoFisher, Waltham, MA, USA) diluted in DMEM-LG without supplements. Two hours after transfection, DMEM-LG complete was added without removing the transfection solution.
2.3. Drug Treatment
Cells were exposed to 1 µM PD-0325901 (PD-901, for brevity) in DMSO (Sigma-Aldrich) or DMSO as the vehicle. Pharmacological treatment was performed 72 h after cell seeding and lasted 72 h without any medium refresh.
2.4. HG Protocols
HG is a circumstance in which samples experience an acceleration higher than gravitational acceleration on the Earth’s surface (1×
g = 9.8 m/s
2). Centrifuges produce HG environments, resulting from the sum of centrifugal acceleration and gravitational acceleration vectors. Two types of centrifuges were used for this study: the Large Diameter Centrifuge (LDC) of the European Space Research and Technology Center (ESTEC) of ESA (Noordwijk, The Netherlands), during the “Spin your Thesis!” (SYT!) 2020 campaign (
Supplementary Scheme S1), where cells were stimulated at 10×
g and 20×
g, for 3 h. In the home laboratory, we used the 5804 bench-top centrifuge (Eppendorf, Hamburg, Germany), a classic bench-top centrifuge that holds the swing-out rotor (A-2-DWP) and allows the insertion of cell plates. In the home lab, cells were exposed to a nominal value of 50×
g. Overall, 50×
g was chosen as the lowest setting of the home centrifuge that allows us to obtain consistent biological results.
We took into account the possible effect of inertial shear forces in the home lab standard centrifuge. To minimize the effect, we used a swing-out rotor, which should ensure that F
g is administered perpendicularly to the surface of the cell culture at regime during the run [
27]. Also, we used relatively small wells (3 cm diameter) to minimize the inertial shear force that increases laterally from the center of centrifugation. To minimize unwanted forces and ensure maximal consistency, we performed all experiments using a single glass-bottom plate (3 cm diameter) fixed to the center of the rotor rack (
Supplementary Scheme S2).
2.5. Viability Assays
To evaluate the effects of chemical compounds and/or HG, cell viability was evaluated either with manual counting (using Trypan blue dye exclusion) or with the MTT Assay (Sigma-Aldrich), according to manufacturer instructions. Overall, 20,000 cells were seeded per one chamber slide (ThermoFisher); the next day, cells were either placed in the centrifuge (50× g, RT, 3 h), or left in the incubator (1× g, 37 °C, 3 h), or placed over the centrifuge lid (1× g, RT, 3 h), as reference samples. Cells were counted 24 and 48 h after HG.
2.6. Immunofluorescence (IF) Assay and Fluorescence Staining
Cells were cultured in chamber slides (ThermoFisher) and in WillCo dishes (Willcowells, Amsterdam The Netherlands). Cells were fixed with either 4% paraformaldehyde (PFA) or ice-cold methanol. After three washes with 1X phosphate buffer solution (PBS), cells were permeabilized with PBS-Triton 0.1% for 10 min at RT, then blocked with 2% bovine serum albumin (BSA) for 60 min. Primary antibodies were added for 60 min at 37 °C, in humid chamber (α-tubulin, 1:500, ThermoFisher). After three washes with 1X PBS-Tween 0.1%, secondary antibody (Anti-mouse, 1:500, Life Technologies, Waltham, MA, USA), or Phalloidin Atto 550 (1:350, Sigma-Aldrich), and DAPI (1:1000, Sigma-Aldrich) were added for 60 min at RT. After three washes with 1X PBS-Tween 0.1%, samples were finally mounted with glass coverslips in Aqua PolyMount (Polysciences, Warrington, PA, USA).
2.7. Western Blot
Total cell extracts were prepared in lysis buffer supplemented with protease and phosphatase inhibitors. For each sample, we loaded 20 μg of each fraction. Proteins were separated by 8% acrylamide gel and electroblotted onto nitrocellulose membranes, Hybond-C-Extra (Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked for 5 min (EveryBlot Blocking Buffer, Biorad, Hercules, CA, USA) and incubated with the primary antibody (overnight, 4 °C) and with horseradish peroxidase (HRP)-conjugated secondary antibodies (1 h, RT). Membranes were incubated for 1 min with Amersham ECL Detection Reagent (Cytiva, Marlborough, MA, USA) or SuperSignal West (ThermoFisher Scientific), and immunoblot images were acquired by ChemiDoc Imaging System (Biorad) (acquisition setup: Auto and Quality exposure). Primary antibodies were as follows: mouse anti-Tau (Tau13) 1:1000 (SantaCruz, Dallas, TX, USA); rabbit anti-pTau S262 1:500 (Thermo Fisher Scientific); rabbit anti-pTau S396 1:500 (Thermo Fisher Scientific); rabbit anti-pTau T231 1:500 (Thermo Fisher Scientific); mouse anti-GAPDH 1:15,000 (Fitzgerald, North Acton, MA, USA).
2.8. Data Acquisition and Analysis
Cell imaging was performed under the Nikon Ti2 microscope (Nikon, Melville, NY, USA), with objective Nikon Plan APO lambda 60X/1.40 Oil. IF assays on fixed cells were analyzed under Nikon Ti2 microscope with the objective Nikon Plan APO lambda 60X/1.40 Oil, equipped with Mono Camera Nikon DS-Fi3.
Images were analyzed with ImageJ 1.53 e. The dimensions and shape of cells or nuclei were obtained by drawing the contour of cells or nuclei. The Area of cells and nuclei was obtained by calculating how many pixels were present within the contour line and then converted to μm2. Circularity was calculated according to the formula 4π × [Area]/[Perimeter]2. Roundness was calculated according to the formula 4 × [Area]/(π × [Major axis]2).
The Plot Profile algorithm (ImageJ suite) was used to analyze the cytoskeleton. A segment of fixed dimension was drawn across a single cell or nucleus, and we calculated the intensity of fluorescence along every single pixel of the segment.
2.9. Statistics
Statistical analyses were performed with GraphPad software v10 (Prism). Specific tests are indicated in the figure legends.
3. Results
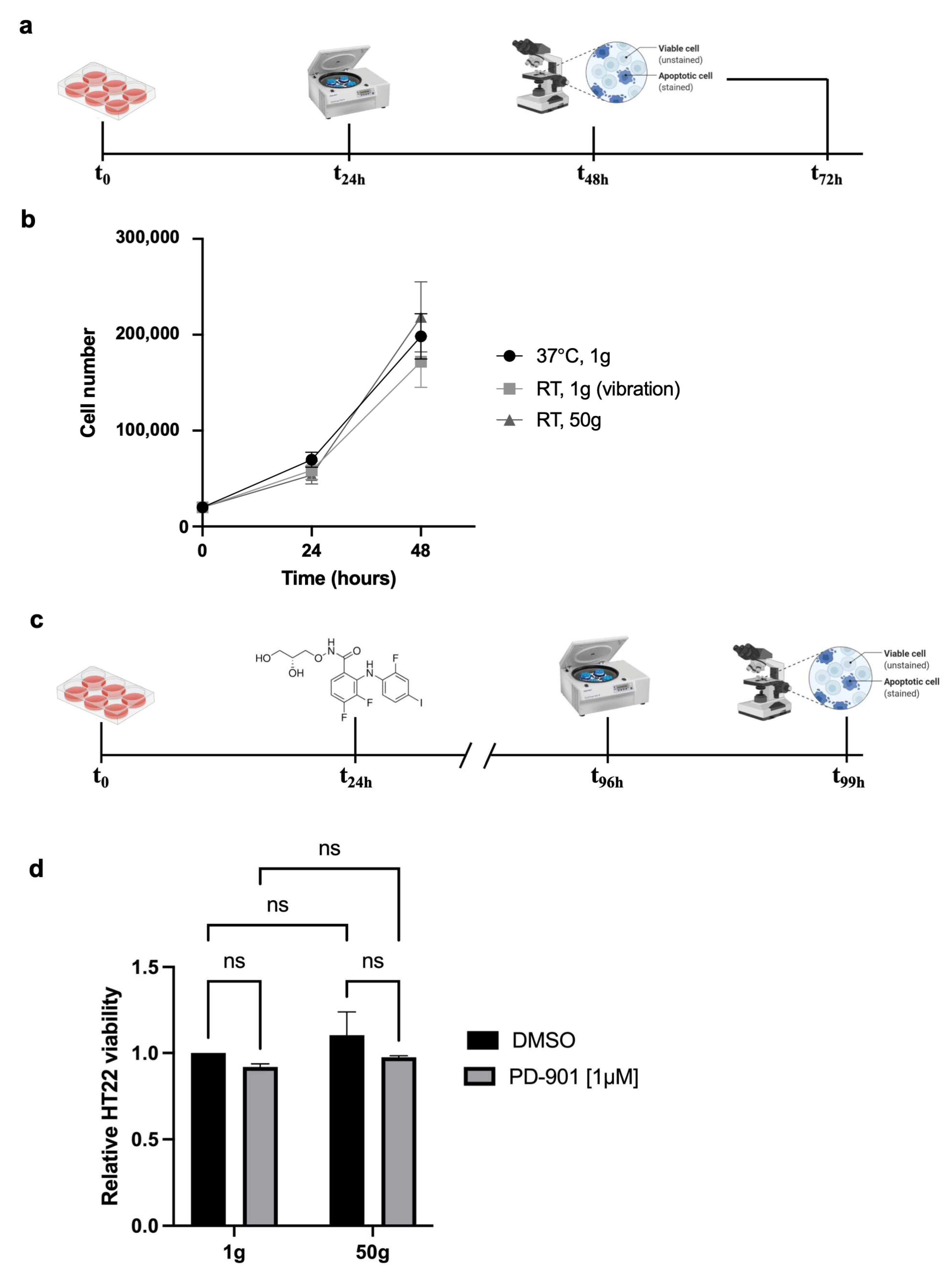
We studied the effects of HG on a neuronal cell model of tauopathy, beginning by testing the feasibility of exposing HT22 cells to 50×
g (
Figure 1a). Throughout the study, we employed two control groups: one stayed under standard culture conditions in the cell incubator at 37 °C, the other was placed on top of the centrifuge lid at room temperature (RT) during the centrifugation, to be subjected to the centrifuge vibrations without exposure to HG.
The results showed no significant differences in cell viability among the conditions tested (
Figure 1b), suggesting the HT22 cells also tolerated 50×
g when combined with the ERK inhibitor PD-901 (experimental design in
Figure 1c). The feasibility of double stimulation (
Figure 1d) allowed us to proceed with further experiments, based on the observation that HG (50×
g) alone or combined with PD-901 does not affect HT22 viability.
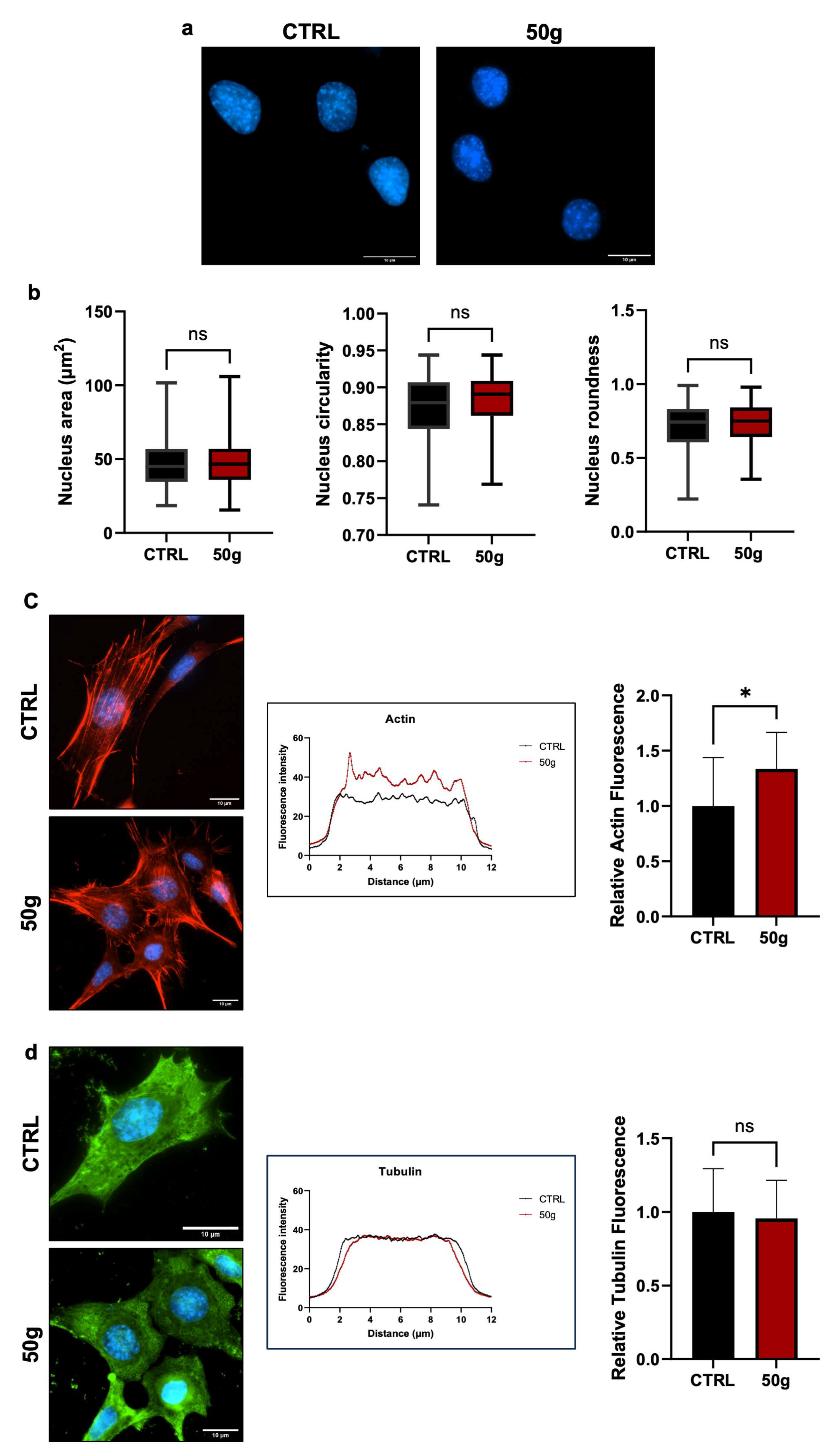
Changes in gravitational loading create mechanical stress that may affect nuclear and cell morphology. To assess that, we measured the Area, Circularity, and Roundness of nuclei of HT22 cells treated as in
Figure 1a. The results revealed no significant differences in any of the parameters (
Figure 2a,b), indicating that three hours at 50×
g did not affect such morphological parameters in centrifuged cells. Also, 50×
g increased the fluorescence intensity of F-actin and changed the shape of the fluorescence curves (
Figure 2c), suggesting a macroscopic rearrangement of microfilaments. The areas under the red and black curves are different, indicating a weaker fluorescence intensity of the control cells (black curve) compared to the centrifuged cells (red curve). The quantitative analysis in the histogram on the right side confirms the interpretation of the plot: the relative fluorescence measurement shows a statistically significant increase (* =
p < 0.05, Student
t-test for unpaired samples) in centrifuged cells over control cells. The different indentation of the two curves indicates a different occurrence of stress fiber thickness [
28].
No changes were found for microtubules (
Figure 2d). We concluded that HG does not affect nuclear morphology and microtubule patterns but affects the F-actin dynamics in HT22 cells.
Next, we transfected HT22 cells with the Conformational Sensitive Tau (CST) sensor to study adaptive changes of Tau aggregation to HG. CST allows for the real-time visualization of Tau in living cells due to the presence of a Fluorescence Resonance Energy Transfer (FRET) couple at the N-terminus and C-terminus of the Tau protein [
29] (
Figure 3a). We measured the Area and morphology of CST-positive cells before and after three hours of centrifugation (
Figure 3b). The results showed no significant changes in Circularity, Roundness, or cell Area between the CST-positive cells exposed to 50×
g and controls (
Figure 3c), confirming that the expression of the CST sensor did not alter the morphological parameters in HG and control cells.
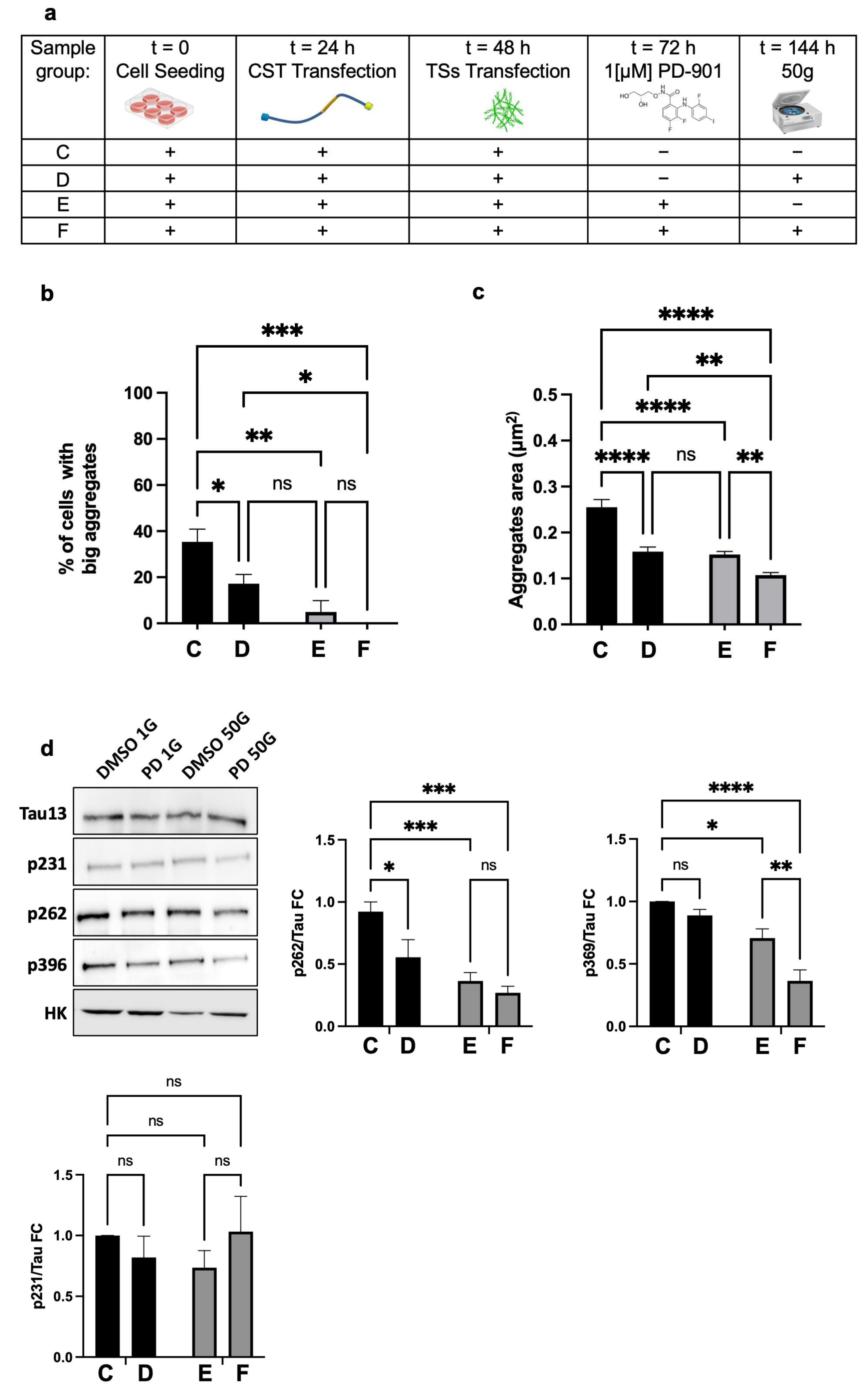
Next, we induced a neurodegenerative phenotype characterized by Tau aggregation by transfecting CST-positive HT22 cells with synthetic Tau neurofibrillary tangle seeds (TSs, experimental design in
Figure 4a). The treatment produced phenotypes that, with a phenotypic screening approach, we divided into Classes 1 to 3 (
Figure 4b). Class 3 was considered the most degenerated phenotype.
To determine whether 50×
g could prevent the precipitation of aggregates induced by TS transfection, we centrifuged HT22 cells before the transfection of TSs (referred to as Pre-TSs HG). We then counted the percentage of CST-positive cells exhibiting Tau aggregates (experimental design in
Figure 4c; samples are labeled with letters for clarity) and found that 50×
g did not affect the percentage of cells with aggregates of any size (Classes 2 and 3 combined,
Figure 4d left). Next, we asked whether Pre-TSs HG would influence the proportion of Class 3 cells (highly degenerated phenotype) relative to cells with Tau aggregates of any size, and found this is not the case (
Figure 4d center). Additionally, since the size of pathological inclusions of Tau can vary significantly (
Figure 4b), we assessed whether Pre-TSs HG could modulate the Area of Tau aggregates in HT22 CST-positive, TS-transfected cells. We found that the treatment (
Figure 4c) did not change the percentage of cells with aggregate sizes exceeding the 75th percentile of the reference group (Group A) (
Figure 4d, right). Overall, we concluded that Pre-TS HG does not change the percentage of cells with Tau aggregates, their numerosity, or Area.
Thereafter, to determine whether the combination of HG and PD-901 could rescue the degenerated phenotype, we combined centrifugation at 50×
g with the administration of 1µM PD-901, on NFT-positive cells (experimental design in
Figure 5a).
We assessed the percentage of cells with larger aggregates, setting the threshold at the 75th percentile of the reference group (Group C,
Figure 5a). We found that Group D (50×
g) or Group E (PD-901) had significantly fewer cells exceeding the threshold and, interestingly, without significant difference between the two groups (D and E). However, cells that underwent the combined treatments (Group F) showed no aggregates above the threshold (
Figure 5b). These data strongly suggested that 50×
g or PD-901 are almost equally effective in reducing the formation of larger Tau aggregates.
Regarding the Area of CST aggregates, the mean Area in Groups C and D was significantly lower compared to controls. Notably, the combination of HG and PD-901 (Group F) led to a further significant reduction in aggregate Area against controls (
Figure 5c). We concluded that 50×
g alone significantly reduced the number of cells with larger Tau aggregates and decreased the overall Tau aggregate Area. Furthermore, the inhibition of ERK activity and HG worked synergistically to reduce the formation of Tau intracellular inclusions.
The rearrangement of F-actin caused by HG likely influences the availability of ERK. Since Tau phosphorylation strongly associates with its pathological aggregation, we used Western blot to analyze in HT22 cells the phosphorylation of residues that are relevant to Tau destabilization/aggregation. As expected [
17], we found that PD-901 alone significantly reduced the phosphorylation of S262 and S396 (
Figure 5d), both of which are key residues located in the microtubule-binding domain and C-terminal regions that facilitate the formation of pathological inclusions. Interestingly, 50×
g alone caused a notable decrease in pS262 but did not significantly affect pS396, indicating a lower efficacy in preventing the phosphorylation of this critical residue. However, the combination of PD-901 and HG led to a substantial reduction in both pS262 and pS396 (about 65–75% compared to control cells), which was significantly greater than what was observed with either treatment alone. This suggests that the two treatments work synergistically to prevent Tau phosphorylation, thereby reducing aggregation. Noticeably, the experimental conditions above did not affect the phosphorylation of Tau at pT231, suggesting they target specific pathological residues. We concluded that HG and ERK inhibition combined significantly reduce pathological Tau phosphorylation at specific, critical residues.
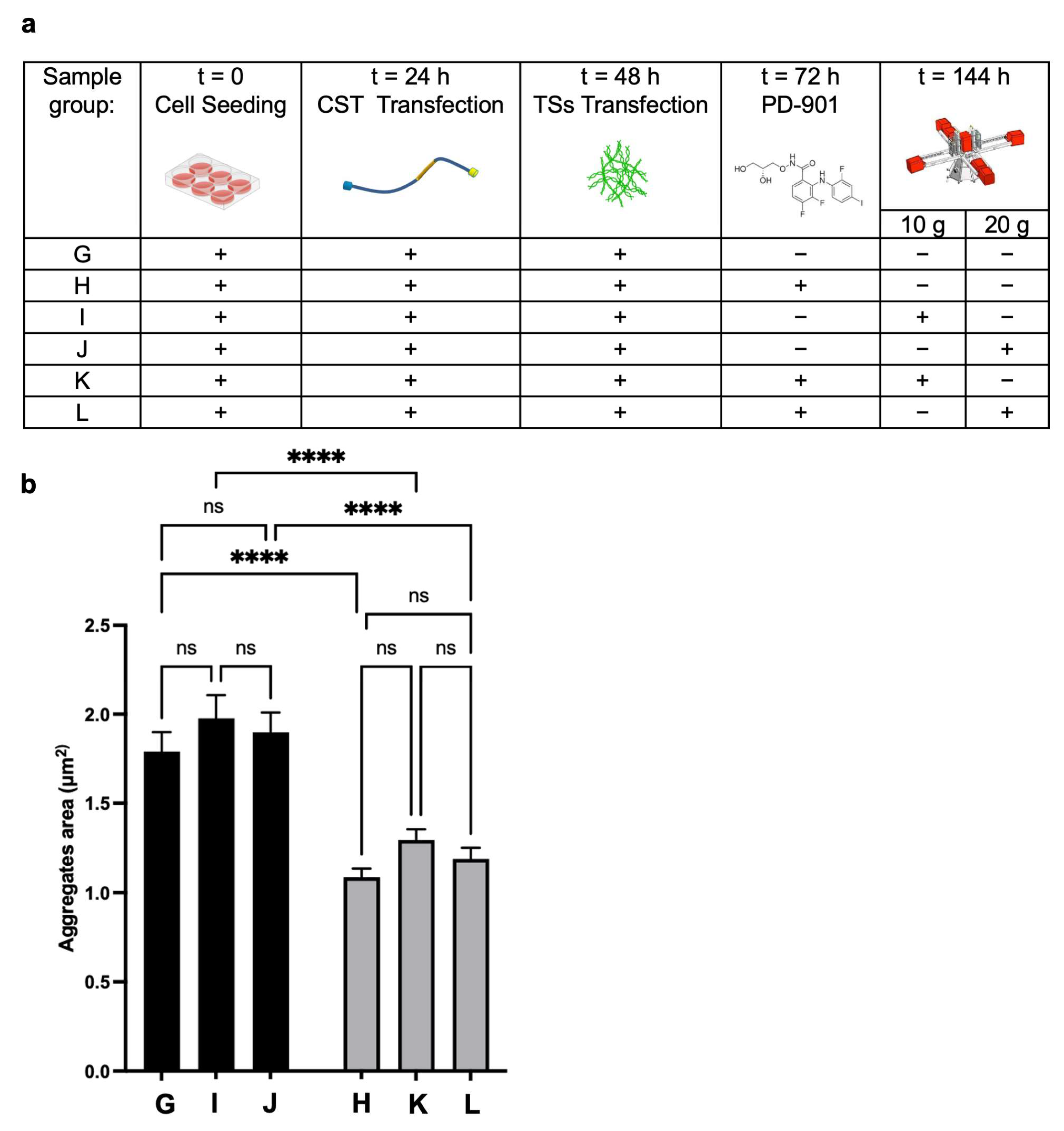
To study the effects of lower g-values, we performed an experimental campaign at the LDC, testing 10×
g and 20×
g that cannot be reliably produced with standard lab centrifuges, along with other characteristics of the run, such as minimal shear stress [
27]. We evaluated the impact of lower HG alone and combined with PD-901 (experimental design in
Figure 6a) on the Area of CST aggregates.
Tau inclusions were present in CST-positive reference cells (Group G). As expected, the aggregate Area was significantly reduced in cells treated with the drug (Group H) compared to the control (
Figure 6b); however, it was not smaller in cells from Group I and Group J (exposed to 10×
g and 20×
g). Coherently, cells exposed to the combined treatments (PD-901 and 10×
g or 20×
g) did not exhibit significant differences in aggregate area compared to cells treated with the drug alone, suggesting that 10×
g and 20×
g do not have an independent effect. We propose that there is a potential threshold effect of gravitational force in modulating CST aggregate formation and Area in the in vitro model, even when combined with the ERK inhibitor.
4. Discussion
Here, we present critical insights into the impact of HG on neuronal cell behavior in the context of tauopathy. We show that hippocampal neuronal cells can withstand HG without compromising cell viability or altering cell/nuclear morphology, which lays the groundwork for further investigations into the effects of gravitational changes on neuronal pathophysiology. Our results with the CST sensor further elucidate the cellular response to HG. The combined analyses of Tau aggregation post-TS transfection indicated that pre-exposure to HG does not mitigate Tau aggregation, which may counter some expectations regarding gravitational force acting as a protective mechanistic factor against neurodegeneration [
30]. Despite the lack of an observable preventive effect of HG on Tau aggregates, our subsequent experiments demonstrated that the combination of 50×
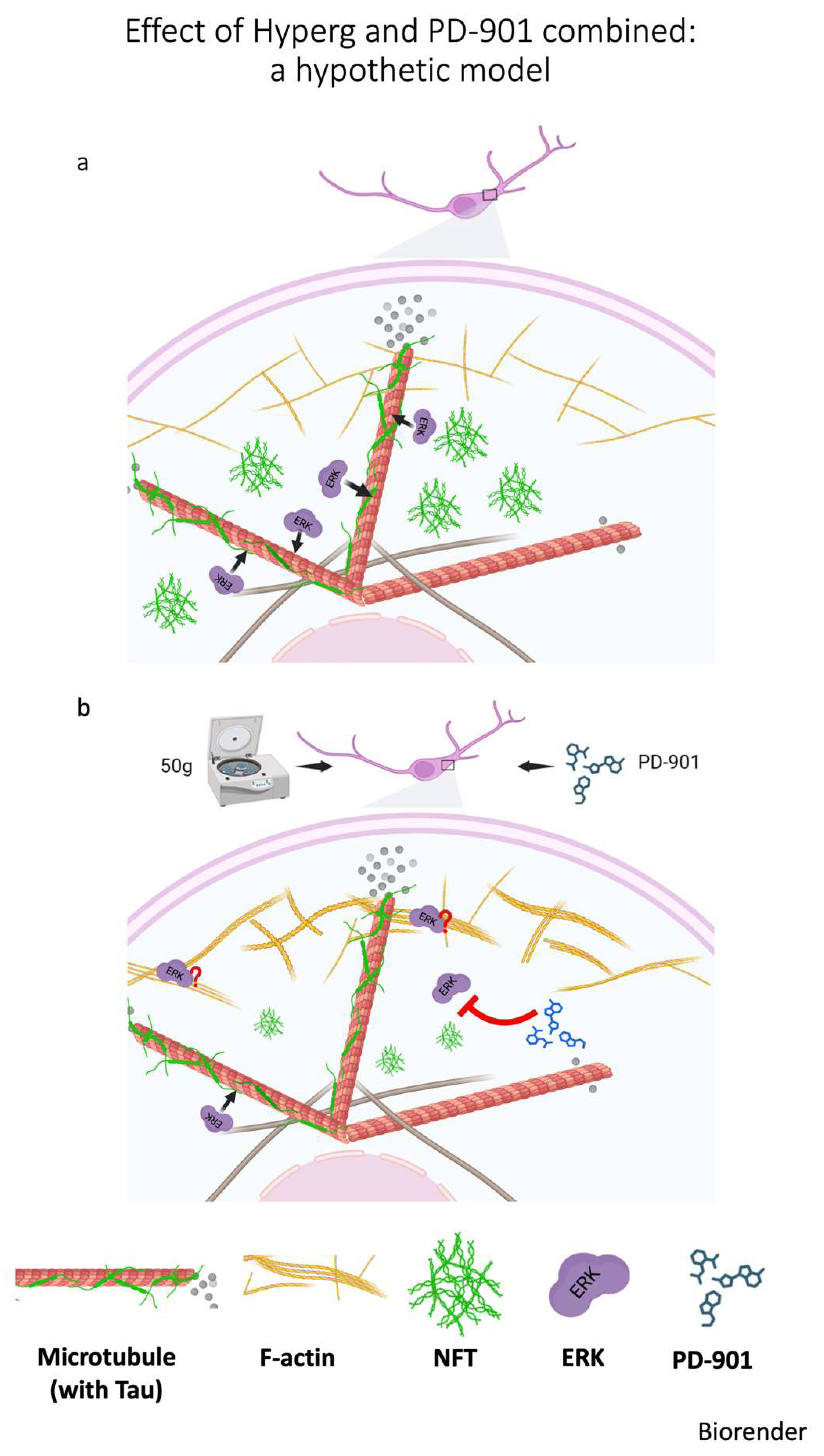
g and the ERK inhibitor PD-901 significantly reduces the formation of larger Tau aggregates while concurrently decreasing the pathological phosphorylation of Tau at key residues. This finding presents a compelling case for the synergistic action of HG and pharmacological intervention to mitigate Tau-related pathology. A hypothetical model is proposed in
Figure 7. It is known that ERK localizes in distinct regions within the cell, also associating with F-actin that can serve as a scaffold protein, thereby affecting ERK availability and activity [
31]. Thicker actin bundles, as a consequence of HG, likely hinder the accessibility of ERK to its substrates, among which Tau protein. The hypothesis relates to the spatial organization and density of actin: Thicker actin bundles may sequester ERK away from its target substrates, leading to reduced phosphorylation of Tau. This sequestration could be due to the physical barrier that the dense actin structures create, limiting ERK movement within the cytoplasm and its interaction with Tau. Or, thick bundles might contribute to altered mechanical properties and cell signaling dynamics, which in turn could influence ERK distribution. Consequently, specific cellular stressors that promote thicker actin filaments may create conditions that downregulate ERK’s ability to phosphorylate Tau. Noticeably, activation of ERK on stress fibers increases with the magnitude of the tensile forces acting on the fibers [
32]; furthermore, HG causes a rearrangement of F-actin, producing thicker stress fibers [
18]. So, HG could potentially impact ERK/Tau interaction, which is critical in neurodegeneration [
33]. Therefore, understanding the interplay between ERK and actin architecture in HG may provide insights into cellular signaling and conditions affecting Tau hyperphosphorylation in neurodegeneration.
The observation that pre-TSs do not protect against degeneration induced by TSs suggests that, in this experimental setting, centrifugation before Tau aggregation might not effectively mimic the cellular or molecular conditions present during and after the initiation of Tau-related pathology.
The interplay between mechanical forces, cell stress-response, and Tau aggregation mechanisms is complex and highlights the significance of timing in addition to the specific cell context [
34,
35]. Further investigations will elucidate why the timing of HG exposure yields different effects on Tau pathology.
Furthermore, the observation that HG affects F-actin without significantly impacting microtubule organization or nuclear morphology is intriguing. It suggests a selective mechanical response of the cytoskeleton, potentially modulating cellular functions crucial for maintaining neuronal health, thereby paving the way for studies into the mechanotransductive pathways involved.
Moreover, the characterization of the different classes of Tau aggregates provides valuable information on the cell state in terms of disease progression. The classification system based on aggregate “presence” and “morphology” enables a more nuanced discussion regarding stages of tauopathy and responses to therapeutic modalities. The negligible difference in aggregation between the groups treated with PD-901 and those subjected to HG alone underscores the need for further exploration into the molecular mechanisms driving Tau phosphorylation and aggregation.
Interestingly, the experimental results from the lower gravitational conditions (10× g and 20× g) suggest a threshold effect of gravitational forces on Tau pathophysiology, where higher gravitational forces may be necessary to instigate significant changes in Tau behavior in vitro.
On this basis, it is essential to consider the broader implications for therapeutic strategies targeting tauopathies. Our results suggest that innovative approaches may integrate mechanical forces, such as HG, with pharmacological agents to enhance therapeutic efficacy. Additionally, studies exploring more extensive ranges of HG values and their impact on neuronal plasticity and integrity could potentially lead to new therapeutic modalities compatible with treatments for human subjects. In fact, it was proposed that HG exerts a hormetic effect, meaning it is a stressor that, in low amounts or for a short period of time, might exert beneficial/protective effects [
36]. However, the underpinning molecular mechanisms are still completely unknown.
5. Conclusions and Study Limitations
This study, for the first time, sheds light on the complex interplay between mechanical forces and intracellular signaling pathways that underpin Tau phosphorylation and aggregation. The demonstrated protective effect of combining HG with ERK inhibition offers a promising avenue for developing novel interventions for tauopathies. Further in vivo studies and research using humanized models are warranted to elucidate the underlying molecular mechanisms. In fact, our hypothesis (in
Figure 7)—that combined treatments with HG and drug may cause denser or thicker F-actin structures to physically interfere with the movement or distribution of ERK—has not been directly demonstrated in this study, and this represents a limitation. However, this hypothesis is supported by substantial evidence from the literature indicating that, in various systems, ERK and F-actin physically interact [
37,
38,
39]. F-actin is required for the formation of signaling complexes where pERK co-localizes with F-actin and other signaling components; disrupting actin polymerization prevents pERK stabilization [
40]. Collectively, evidences support a model whereby F-actin plays dual roles depending on its stabilization status: it can serve as a scaffold and localize ERK, facilitating or restricting its phosphorylation, depending on the context; or, when disrupted, it can release ERK, altering both the level and localization of its activation [
40]. Supporting this, in a smooth muscle cell line, the association of ERK1/2 with the actin cytoskeleton influences ERK1/2 signaling outcome, indicating that this interaction plays a regulatory role [
41].
Furthermore, treatments with actin-disrupting agents such as cytochalasin D [
41] or latrunculin B [
39] significantly attenuated ERK phosphorylation, suggesting that an intact actin cytoskeleton is essential for optimal ERK activation. Future experiments will explore whether combining HG and PD-901 treatment with actin polymerization inhibitors can reverse HG-induced effects on Tau phosphorylation. Additionally, the co-immunoprecipitation of actin and ERK proteins following HG treatment could help confirm a direct physical interaction in this context.
Addressing this gap will be a focus of future research efforts.