Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies
Abstract
1. Introduction
2. The Interrelationship Between Tumor Heterogeneity and Hypoxia
2.1. The Concept of Tumor Heterogeneity: Origins and Effects
2.2. Hypoxia as Mediator of Intratumoral Heterogeneity
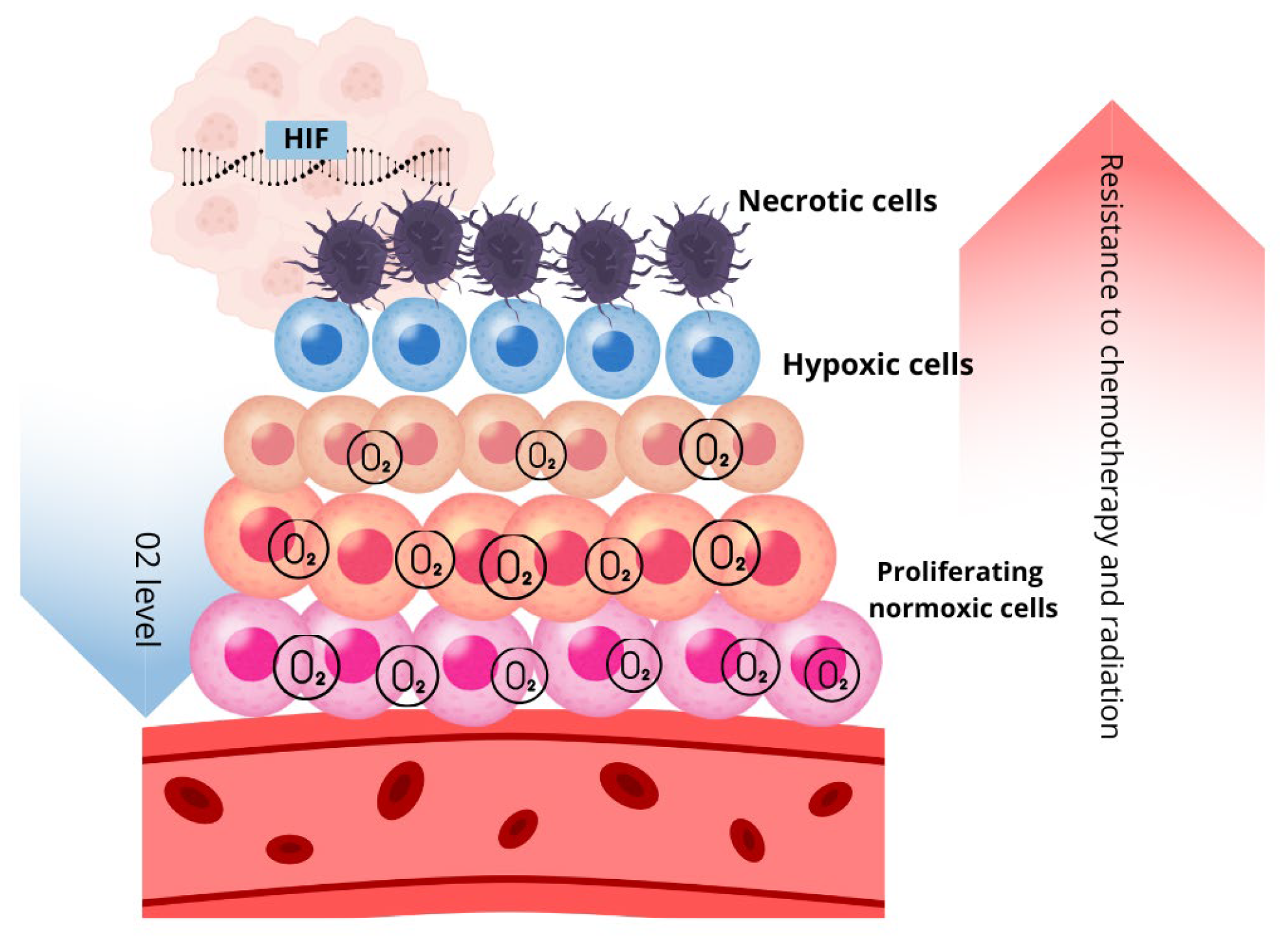
2.2.1. Spatial and Temporal Distribution of Oxygen
2.2.2. Hypoxia and Metabolic Heterogeneity
2.2.3. Hypoxia and Different Cancer Cell Phenotypes
- Cancer Stem Cells
- EMT as a Process Leading to Phenotypic Heterogeneity
- Chemoresistant Cancer Cells
2.3. Heterogeneity in DNA Repair and the Role of Hypoxia
3. Hypoxia-Induced Modulation of DNA Repair Pathways
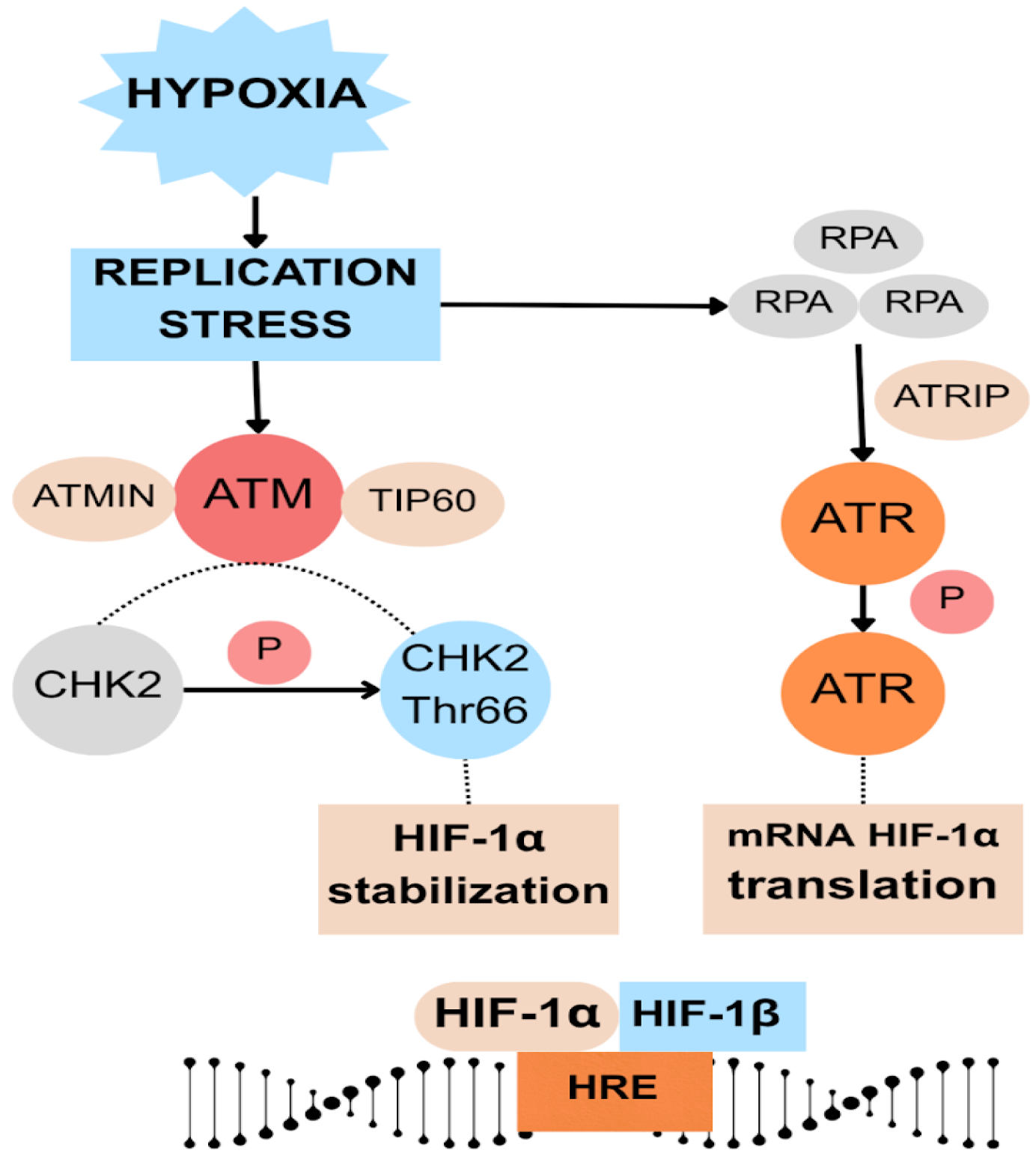
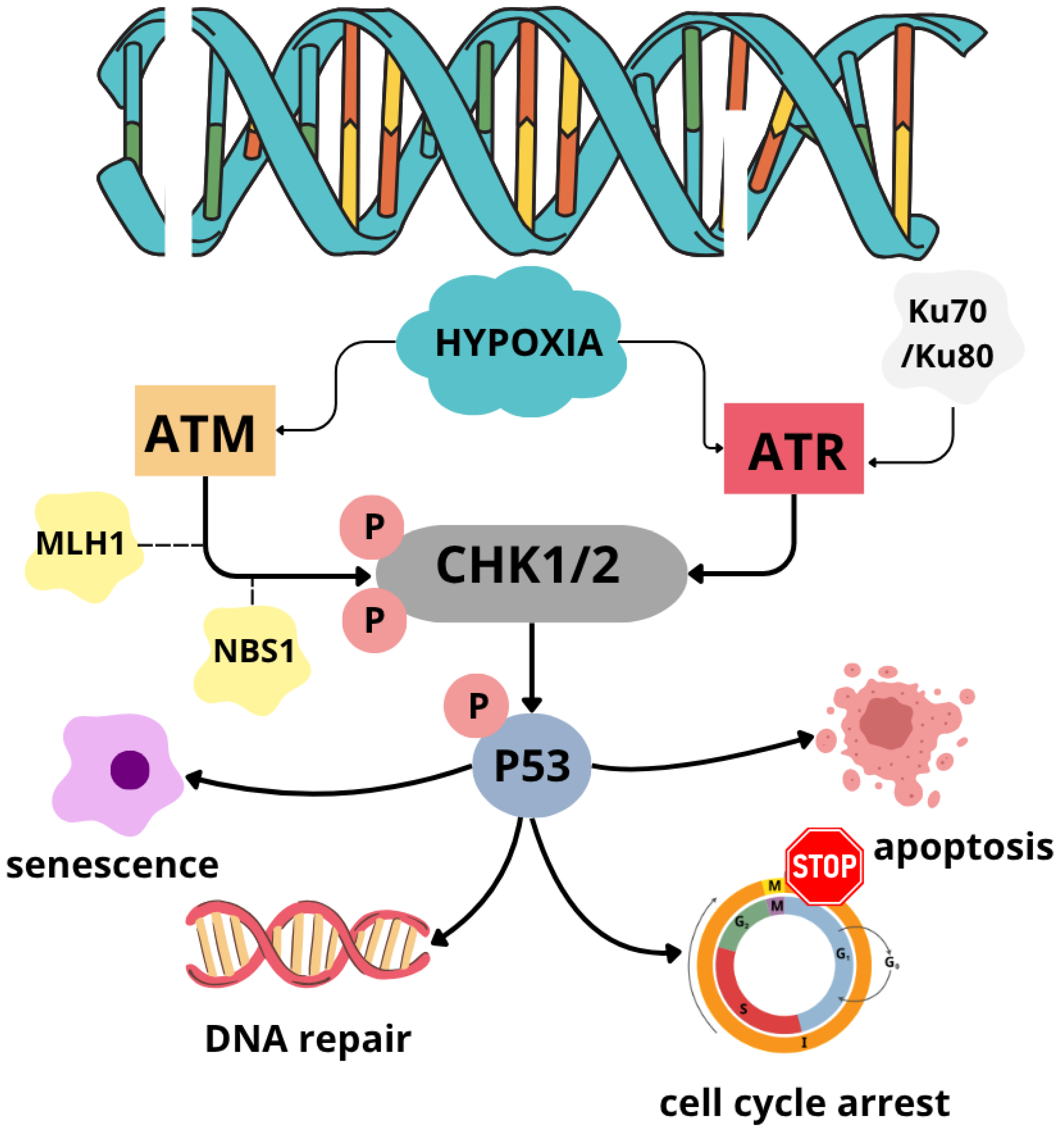
3.1. DNA Damage Response Pathway
3.2. Homologous Recombination
3.3. Non-Homologous End Joining
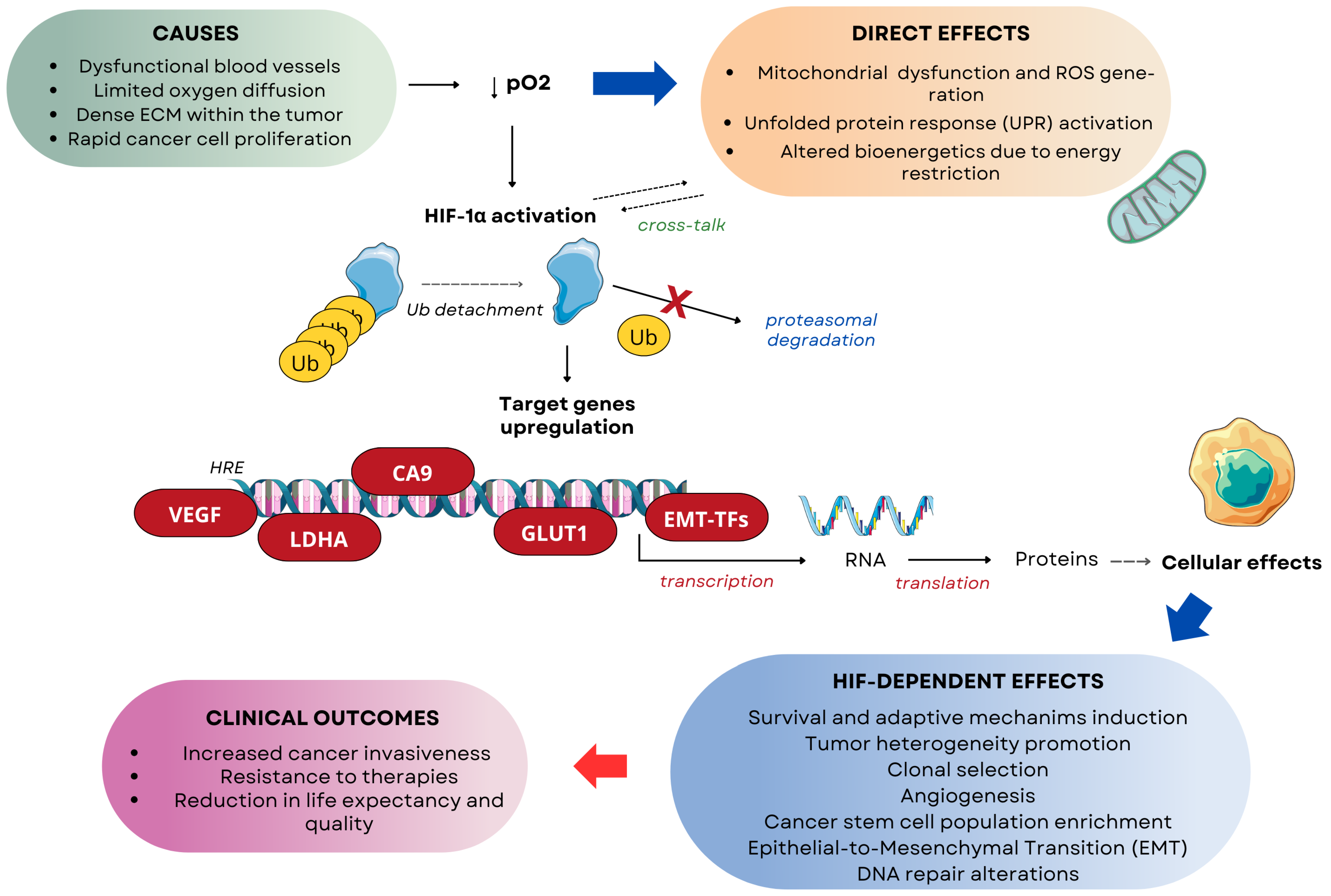
3.4. HIF-1α
3.5. Biomarkers of Hypoxia
4. Impact of Hypoxia on Cancer Therapy Efficacy
4.1. Radiotherapy
4.2. Chemotherapy Inefficacy and Chemoresistance
4.3. Immunotherapy
5. Therapeutic Strategies Targeting Hypoxia in Cancer
5.1. Hypoxia-Activated Prodrugs (HAPs)
5.1.1. Tirapazamine
5.1.2. Evofosfamide
5.1.3. CP-506
5.1.4. Tarloxotinib
5.2. Oxygenation Therapies
5.3. HIF Signaling Inhibitors
5.3.1. Inhibitors of HIF1 mRNA Expression
5.3.2. Inhibitors of HIF-1 Synthesis
5.3.3. Agents Reducing HIF-1 Stability
- Romidepsin
- Vorinostat
- Panobinostat
- Tanespimycin
- Lonafarnib
- Belinostat
- Chidamide
5.3.4. Inhibitors of HIF-1 Transcriptional Activity
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radicals |
| 13-cRA | 13-cis-retinoic acid |
| 5-FU | 5-fluorouracil |
| ABC | ATP-binding cassette |
| AMl | acute myeloid leukemia |
| ASCT | autologous stem cell transplantation |
| ATM | ataxia–telangiectasia-mutated kinase |
| ATMIN | ATM interactor |
| ATR | ataxia telangiectasia and Rad3-related kinase |
| ATRIP | ATR-interacting protein |
| BCRP | breast cancer resistance protein |
| Br-IPM | bromo-isophosphoramide mustard |
| CAFs | cancer-associated fibroblasts |
| CAIX | carbonic anhydrase IX |
| CHOP | cyclophosphamide, doxorubicin, vincristine, and prednisone |
| CR | complete response |
| CRi | incomplete count recovery |
| CRR | complete response rate |
| CSCC | cutaneous squamous-cell carcinoma |
| CSCs | cancer stem cells |
| CTCL | cutaneous T-cell lymphoma |
| DCR | disease control rate |
| DIPG | diffuse intrinsic pontine glioma |
| DLT | dose-limiting toxicity |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| dNTP | deoxyribonucleotide |
| DOR | duration of response |
| DOX | doxorubicin |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial–mesenchymal transition |
| EMT-TFs | EMT-related transcription factors |
| ENKTL | relapsed/refractory extranodal natural killer/T-cell lymphoma |
| EXO1 | nuclease, exonuclease 1 |
| FBP | fructose bisphosphatase |
| GEM | gemcitabine |
| H2O2 | hydrogen peroxide |
| H3K4 | fourth lysine residue on DNA packaging protein histone |
| HAPs | hypoxia-activated prodrugs |
| HBOT | hyperbaric oxygen therapy |
| HCC | hepatocellular carcinoma |
| HDAC | histone deacetylase |
| HIF | hypoxia-inducible factors |
| HK2 | hexokinase 2 |
| HLH | hemophagocytic lymphohistiocytosis |
| HNSCC | head and neck squamous cell carcinoma |
| HR | homologous recombination |
| HREs | hypoxia-response elements |
| ICI | immune checkpoint inhibitor |
| ITH | intratumoral heterogeneity |
| KDM | histone lysine demethylases |
| LOX | lysyl oxidase |
| MDSCs | myeloid-derived suppressor cells |
| MMP-9 | matrix metalloproteinase-9 |
| MRP1 | multidrug resistance related protein 1 |
| MTD | maximum tolerated dose |
| NHEJ | non-homologous end joining |
| NK | natural killer |
| NSCLC | non-small cell lung cancer |
| O2•− | oxygen superoxide |
| OER | Oxygen Enhancement Ratio |
| ORR | overall response rate |
| PARP-1 | poly (ADP-ribose) polymerase-1 |
| PDAC | pancreatic ductal adenocarcinoma |
| PFS | progression-free survival |
| P-gp | P-glycoprotein |
| PHDs | prolyl hydroxylases |
| PKM2 | pyruvate kinase M2 |
| PMB | primary mediastinal B-cell lymphoma |
| PR | partial response |
| PTCL | peripheral T-cell lymphoma |
| pVHL | von Hippel–Lindau tumor suppressor protein |
| R/R DLBCL | relapsed or refractory diffuse large B-cell lymphoma |
| R/R MCL | relapsed/refractory mantle cell lymphoma |
| R-GemOx | rituximab, gemcitabine, oxaliplatin |
| ROS | reactive oxygen species |
| RP2D | recommended phase II dose |
| RPA | replication protein A |
| SD | stable disease |
| SMO | smoothened |
| ssDNA | single-stranded DNA |
| TACE | trans-arterial chemoembolization |
| TAE | transarterial embolization |
| TAM | tumor-associated macrophage |
| TCA | tricarboxylic acid |
| TCR | T-cell receptor |
| TET | ten–eleven translocation |
| TGF-β | transforming growth factor β |
| TGI | tumor growth inhibition |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| T-PLL | T-cell prolymphocytic leukemia |
| VEGF | vascular endothelial growth factor |
| VGPR | very good partial responses |
| XPA | XP-A cells |
| XRCC4-LIGIV | DNA repair protein XRCC4 complex with ligase IV |
| ZEB1/2 | zinc finger E-box binding homeobox 1/2 |
References
- Bigos, K.J.; Quiles, C.G.; Lunj, S.; Smith, D.J.; Krause, M.; Troost, E.G.; West, C.M.; Hoskin, P.; Choudhury, A. Tumour Response to Hypoxia: Understanding the Hypoxic Tumour Microenvironment to Improve Treatment Outcome in Solid Tumours. Front. Oncol. 2024, 14, 1331355. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Hill, R.P. Hypoxia, DNA Repair and Genetic Instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Hypoxia in Cancer: Significance and Impact on Clinical Outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Godet, I.; Shin, Y.J.; Ju, J.A.; Ye, I.C.; Wang, G.; Gilkes, D.M. Fate-Mapping Post-Hypoxic Tumor Cells Reveals a ROS-Resistant Phenotype That Promotes Metastasis. Nat. Commun. 2019, 10, 4862. [Google Scholar] [CrossRef]
- Shi, R.; Liao, C.; Zhang, Q. Hypoxia-Driven Effects in Cancer: Characterization, Mechanisms, and Therapeutic Implications. Cells 2021, 10, 678. [Google Scholar] [CrossRef]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Barbeau, L.M.O. Harnessing Autophagy in Cancer. Doctoral Thesis, Maastricht University, Maastricht, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Rahane, D.; Dhingra, T.; Chalavady, G.; Datta, A.; Ghosh, B.; Rana, N.; Borah, A.; Saraf, S.; Bhattacharya, P. Hypoxia and Its Effect on the Cellular System. Cell Biochem. Funct. 2024, 42, e3940. [Google Scholar] [CrossRef]
- Chen, N.; Chen, X.; Huang, R.; Zeng, H.; Gong, J.; Meng, W.; Lu, Y.; Zhao, F.; Wang, L.; Zhou, Q. BCL-XL Is a Target Gene Regulated by Hypoxia-Inducible Factor-1α. J. Biol. Chem. 2009, 284, 10004–10012. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Rocha, H.L.; Godet, I.; Kurtoglu, F.; Metzcar, J.; Konstantinopoulos, K.; Bhoyar, S.; Gilkes, D.M.; Macklin, P. A Persistent Invasive Phenotype in Post-Hypoxic Tumor Cells Is Revealed by Fate Mapping and Computational Modeling. iScience 2021, 24, 102935. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Solnes, L.; Vallabhajosula, B. A Broad Overview of Positron Emission Tomography Radiopharmaceuticals and Clinical Applications: What Is New? Semin. Nucl. Med. 2011, 41, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Wozny, A.-S.; Alphonse, G.; Cassard, A.; Malésys, C.; Louati, S.; Beuve, M.; Lalle, P.; Ardail, D.; Nakajima, T.; Rodriguez-Lafrasse, C. Impact of Hypoxia on the Double-Strand Break Repair after Photon and Carbon Ion Irradiation of Radioresistant HNSCC Cells. Sci. Rep. 2020, 10, 21357. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, S.E.; Glazer, P.M. Multifaceted Control of DNA Repair Pathways by the Hypoxic Tumor Microenvironment. DNA Repair 2015, 32, 180–189. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Qian, J.; Rankin, E.B. Hypoxia-Induced Phenotypes That Mediate Tumor Heterogeneity. Adv. Exp. Med. Biol. 2019, 1136, 43–55. [Google Scholar] [CrossRef]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral Heterogeneity in Cancer Progression and Response to Immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef]
- Watson, I.R.; Takahashi, K.; Futreal, P.A.; Chin, L. Emerging Patterns of Somatic Mutations in Cancer. Nat. Rev. Genet. 2013, 14, 703–718. [Google Scholar] [CrossRef]
- Ramón y Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical Implications of Intratumor Heterogeneity: Challenges and Opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Thompson, L.L.; Jeusset, L.M.-P.; Lepage, C.C.; McManus, K.J. Evolving Therapeutic Strategies to Exploit Chromosome Instability in Cancer. Cancers 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Chakraborty, D.; Pradhan, J.; Imran Khan, M.; Dandapat, J. Epigenomic Interplay in Tumor Heterogeneity: Potential of Epidrugs as Adjunct Therapy. Cytokine 2022, 157, 155967. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Peng, Y.; Gao, A.; Du, C.; Herman, J.G. Epigenetic Heterogeneity in Cancer. Biomark. Res. 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Meir, Z.; Mukamel, Z.; Chomsky, E.; Lifshitz, A.; Tanay, A. Single-Cell Analysis of Clonal Maintenance of Transcriptional and Epigenetic States in Cancer Cells. Nat. Genet. 2020, 52, 709–718. [Google Scholar] [CrossRef]
- Dietz, S.; Lifshitz, A.; Kazdal, D.; Harms, A.; Endris, V.; Winter, H.; Stenzinger, A.; Warth, A.; Sill, M.; Tanay, A.; et al. Global DNA Methylation Reflects Spatial Heterogeneity and Molecular Evolution of Lung Adenocarcinomas. Int. J. Cancer 2019, 144, 1061–1072. [Google Scholar] [CrossRef]
- Runa, F.; Hamalian, S.; Meade, K.; Shisgal, P.; Gray, P.C.; Kelber, J.A. Tumor Microenvironment Heterogeneity: Challenges and Opportunities. Curr. Mol. Biol. Rep. 2017, 3, 218–229. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, A.; Yuan, Y.; Zhu, B.; Long, H. Heterogeneity of the Tumor Immune Microenvironment and Its Clinical Relevance. Exp. Hematol. Onco.l 2022, 11, 24. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Cheng, L. Tumor Microenvironment Heterogeneity an Important Mediator of Prostate Cancer Progression and Therapeutic Resistance. NPJ Precis. Oncol. 2022, 6, 31. [Google Scholar] [CrossRef]
- Murphy, K.J.; Chambers, C.R.; Herrmann, D.; Timpson, P.; Pereira, B.A. Dynamic Stromal Alterations Influence Tumor-Stroma Crosstalk to Promote Pancreatic Cancer and Treatment Resistance. Cancers 2021, 13, 3481. [Google Scholar] [CrossRef]
- Wahl, G.M.; Spike, B.T. Cell State Plasticity, Stem Cells, EMT, and the Generation of Intra-Tumoral Heterogeneity. NPJ Breast Cancer 2017, 3, 14. [Google Scholar] [CrossRef]
- Danhier, P.; Bański, P.; Payen, V.L.; Grasso, D.; Ippolito, L.; Sonveaux, P.; Porporato, P.E. Cancer Metabolism in Space and Time: Beyond the Warburg Effect. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, V.; Antoine, M.; Dom, G.; Detours, V.; Maenhaut, C.; Dumont, J.E. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Strickaert, A.; Saiselet, M.; Dom, G.; De Deken, X.; Dumont, J.E.; Feron, O.; Sonveaux, P.; Maenhaut, C. Cancer Heterogeneity Is Not Compatible with One Unique Cancer Cell Metabolic Map. Oncogene 2017, 36, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; DeBerardinis, R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Pandkar, M.R.; Dhamdhere, S.G.; Shukla, S. Oxygen Gradient and Tumor Heterogeneity: The Chronicle of a Toxic Relationship. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1876, 188553. [Google Scholar] [CrossRef]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef]
- Magagnin, M.G.; Koritzinsky, M.; Wouters, B.G. Patterns of Tumor Oxygenation and Their Influence on the Cellular Hypoxic Response and Hypoxia-Directed Therapies. Drug Resist. Updates 2006, 9, 185–197. [Google Scholar] [CrossRef]
- Michiels, C.; Tellier, C.; Feron, O. Cycling Hypoxia: A Key Feature of the Tumor Microenvironment. Biochim. Biophys. Acta (BBA) Rev. Cancer 2016, 1866, 76–86. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Venkatesh, G.H. Hypoxic Stress Perturb DNA Repair Mechanisms Leading to Genetic Instability. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ed.; Springer Nature: Singapore, 2022; pp. 859–874. [Google Scholar] [CrossRef]
- de Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.R.; Tennant, D.A. Hypoxia and Metabolic Adaptation of Cancer Cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed]
- Abd, G.M.; Laird, M.C.; Ku, J.C.; Li, Y. Hypoxia-Induced Cancer Cell Reprogramming: A Review on How Cancer Stem Cells Arise. Front. Oncol. 2023, 13, 1227884. [Google Scholar] [CrossRef] [PubMed]
- Lehuédé, C.; Dupuy, F.; Rabinovitch, R.; Jones, R.G.; Siegel, P.M. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016, 76, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Špaková, I.; Rabajdová, M.; Mičková, H.; Graier, W.F.; Mareková, M. Effect of Hypoxia Factors Gene Silencing on ROS Production and Metabolic Status of A375 Malignant Melanoma Cells. Sci. Rep. 2021, 11, 10325. [Google Scholar] [CrossRef]
- Terry, S.; Engelsen, A.S.T.; Buart, S.; Elsayed, W.S.; Venkatesh, G.H.; Chouaib, S. Hypoxia-Driven Intratumor Heterogeneity and Immune Evasion. Cancer Lett. 2020, 492, 1–10. [Google Scholar] [CrossRef]
- Simon, M.C.; Keith, B. The Role of Oxygen Availability in Embryonic Development and Stem Cell Function. Nat. Rev. Mol. Cell. Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Okoye, I.; Esmaily, M.; Ghasemi Chaleshtari, M.; Masjedi, A.; Azizi, G.; Irandoust, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F. Hypoxia Inducible Factors in the Tumor Microenvironment as Therapeutic Targets of Cancer Stem Cells. Life Sci. 2019, 237, 116952. [Google Scholar] [CrossRef]
- Wang, P.; Gong, S.; Liao, B.; Pan, J.; Wang, J.; Zou, D.; Zhao, L.; Xiong, S.; Deng, Y.; Yan, Q.; et al. HIF1α/HIF2α Induces Glioma Cell Dedifferentiation into Cancer Stem Cells through Sox2 under Hypoxic Conditions. J. Cancer 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Z.; Zhu, Y.; Chen, J.; Li, W. Role of Hypoxia Inducible Factor-1 in Cancer Stem Cells (Review). Mol. Med. Rep. 2020, 23, 17. [Google Scholar] [CrossRef]
- Onishi, H.; Nakamura, K.; Yanai, K.; Nagai, S.; Nakayama, K.; Oyama, Y.; Fujimura, A.; Ozono, K.; Yamasaki, A. Cancer Therapy That Targets the Hedgehog Signaling Pathway Considering the Cancer Microenvironment (Review). Oncol. Rep. 2022, 47, 93. [Google Scholar] [CrossRef]
- Kanwal, R.; Esposito, J.E.; Jawed, B.; Zakir, S.K.; Pulcini, R.; Martinotti, R.; Botteghi, M.; Gaudio, F.; Martinotti, S.; Toniato, E. Exploring the Role of Epithelial–Mesenchymal Transcriptional Factors Involved in Hematological Malignancy and Solid Tumors: A Systematic Review. Cancers 2025, 17, 529. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-Cadherin in Cancer Metastasis, Its Emerging Role in Haematological Malignancies and Potential as a Therapeutic Target in Cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, G.; Li, X.; Zhang, Y.; Jiang, Y.; Shen, J.; Liu, J.; Wang, Q.; Zhu, J.; Feng, X.; et al. Hypoxia Induces Epithelial-Mesenchymal Transition via Activation of SNAI1 by Hypoxia-Inducible Factor-1α in Hepatocellular Carcinoma. BMC Cancer 2013, 13, 108. [Google Scholar] [CrossRef]
- Zheng, H.; Kang, Y. Multilayer Control of the EMT Master Regulators. Oncogene 2014, 33, 1755–1763. [Google Scholar] [CrossRef]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef]
- Shen, Z.; Yu, N.; Zhang, Y.; Jia, M.; Sun, Y.; Li, Y.; Zhao, L. The Potential Roles of HIF-1α in Epithelial-Mesenchymal Transition and Ferroptosis in Tumor Cells. Cell Signal. 2024, 122, 111345. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhang, Y.; Zhu, D.; Zhang, L.; Li, Y.; Zhu, Y.; Li, D.; Zhou, J. HIF-2α Promotes Epithelial-Mesenchymal Transition through Regulating Twist2 Binding to the Promoter of E-Cadherin in Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2016, 35, 26. [Google Scholar] [CrossRef]
- Belisario, D.C.; Kopecka, J.; Pasino, M.; Akman, M.; De Smaele, E.; Donadelli, M.; Riganti, C. Hypoxia Dictates Metabolic Rewiring of Tumors: Implications for Chemoresistance. Cells 2020, 9, 2598. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, S.; Wang, J.; Long, H.; Fang, L.; Wang, G.; Li, Z.; Que, T.; Liu, Y.; Li, Y.; et al. Hypoxia-Induced PLOD2 Promotes Proliferation, Migration and Invasion via PI3K/Akt Signaling in Glioma. Oncotarget 2017, 8, 41947–41962. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, L.; Zhang, G.; Shan, A.; Ye, C.; Liang, B.; Sun, J.; Liao, X.; Zhu, C.; Chen, Y.; et al. MicroRNAs Target the Wnt/Β catenin Signaling Pathway to Regulate Epithelial mesenchymal Transition in Cancer (Review). Oncol. Rep. 2020, 44, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, X.; Chen, W.; Ma, T.; Hu, Q.; Liang, C.; Xie, S.; Chen, C.; Hu, L.; Xu, S.; et al. Wnt/β-Catenin Signaling Enhances Hypoxia-Induced Epithelial–Mesenchymal Transition in Hepatocellular Carcinoma via Crosstalk with Hif-1α Signaling. Carcinogenesis 2013, 34, 962–973. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Maggiolini, M.; Musti, A.M. Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 2018, 19, 2011. [Google Scholar] [CrossRef]
- Guo, M.; Niu, Y.; Xie, M.; Liu, X.; Li, X. Notch Signaling, Hypoxia, and Cancer. Front. Oncol. 2023, 13, 1078768. [Google Scholar] [CrossRef]
- Bao, B.; Azmi, A.S.; Ali, S.; Ahmad, A.; Li, Y.; Banerjee, S.; Kong, D.; Sarkar, F.H. The Biological Kinship of Hypoxia with CSC and EMT and Their Relationship with Deregulated Expression of MiRNAs and Tumor Aggressiveness. Biochim. Biophys. Acta (BBA) Rev. Cancer 2012, 1826, 272–296. [Google Scholar] [CrossRef]
- Fluegen, G.; Avivar-Valderas, A.; Wang, Y.; Padgen, M.R.; Williams, J.K.; Nobre, A.R.; Calvo, V.; Cheung, J.F.; Bravo-Cordero, J.J.; Entenberg, D.; et al. Phenotypic Heterogeneity of Disseminated Tumour Cells Is Preset by Primary Tumour Hypoxic Microenvironments. Nat. Cell Biol. 2017, 19, 120–132. [Google Scholar] [CrossRef]
- Pribluda, A.; de la Cruz, C.C.; Jackson, E.L. Intratumoral Heterogeneity: From Diversity Comes Resistance. Clin. Cancer Res. 2015, 21, 2916–2923. [Google Scholar] [CrossRef]
- Januškevičienė, I.; Petrikaitė, V. Heterogeneity of Breast Cancer: The Importance of Interaction between Different Tumor Cell Populations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef]
- Kiwerska, K.; Szyfter, K. DNA Repair in Cancer Initiation, Progression, and Therapy—A Double-Edged Sword. J. Appl. Genet. 2019, 60, 329–334. [Google Scholar] [CrossRef]
- Zhou, J.; Albert Zhou, X.; Zhang, N.; Wang, J. Evolving Insights: How DNA Repair Pathways Impact Cancer Evolution. Cancer Biol. Med. 2020, 17, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.F.; Ud Din, S.; Shah, A.A. Analysis of Machine Learning Techniques for Detection Framework for DNA Repair Genes to Help Diagnose Cancer: A Systematic Literature Review. In Proceedings of the 2021 International Conference on Innovative Computing (ICIC), Lahore, Pakistan, 9–10 November 2021; pp. 1–10. [Google Scholar] [CrossRef]
- Hassan Venkatesh, G.; Bravo, P.; Shaaban Moustafa Elsayed, W.; Amirtharaj, F.; Wojtas, B.; Abou Khouzam, R.; Hussein Nawafleh, H.; Mallya, S.; Satyamoorthy, K.; Dessen, P.; et al. Hypoxia Increases Mutational Load of Breast Cancer Cells through Frameshift Mutations. Oncoimmunology 2020, 9, 1750750. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.R.; Glazer, P.M. Impact of Hypoxia on DNA Repair and Genome Integrity. Mutagenesis 2020, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, S.E.; Hegan, D.C.; Sulkowski, P.L.; Glazer, P.M. Suppression of Homology-Dependent DNA Double-Strand Break Repair Induces PARP Inhibitor Sensitivity in VHL-Deficient Human Renal Cell Carcinoma. Oncotarget 2018, 9, 4647–4660. [Google Scholar] [CrossRef]
- Tang, M.; Bolderson, E.; O’Byrne, K.J.; Richard, D.J. Tumor Hypoxia Drives Genomic Instability. Front. Cell Dev. Biol. 2021, 9, 626229. [Google Scholar] [CrossRef]
- Begg, K.; Tavassoli, M. Inside the Hypoxic Tumour: Reprogramming of the DDR and Radioresistance. Cell Death Discov. 2020, 6, 77. [Google Scholar] [CrossRef]
- Weterings, E.; Chen, D.J. The Endless Tale of Non-Homologous End-Joining. Cell Res. 2008, 18, 114–124. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A Key Player in DNA Damage Response and a Promising Target for Cancer Therapy. Biomed. Pharmacother. 2024, 175, 116663. [Google Scholar] [CrossRef]
- Olcina, M.M.; Grand, R.J.; Hammond, E.M. ATM Activation in Hypoxia-Causes and Consequences. Mol. Cell. Oncol. 2014, 1, e29903. [Google Scholar] [CrossRef]
- Paull, T.T. Mechanisms of ATM Activation. Annu. Rev. Biochem. 2015, 84, 711–738. [Google Scholar] [CrossRef]
- Bencokova, Z.; Kaufmann, M.R.; Pires, I.M.; Lecane, P.S.; Giaccia, A.J.; Hammond, E.M. ATM Activation and Signaling under Hypoxic Conditions. Mol. Cell. Biol. 2009, 29, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Fallone, F.; Britton, S.; Nieto, L.; Salles, B.; Muller, C. ATR Controls Cellular Adaptation to Hypoxia through Positive Regulation of Hypoxia-Inducible Factor 1 (HIF-1) Expression. Oncogene 2013, 32, 4387–4396. [Google Scholar] [CrossRef] [PubMed]
- Manic, G.; Obrist, F.; Sistigu, A.; Vitale, I. Trial Watch: Targeting ATM–CHK2 and ATR–CHK1 Pathways for Anticancer Therapy. Mol. Cell. Oncol. 2015, 2, e1012976. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.L.; Bindra, R.S.; Glazer, P.M. Hypoxia-Induced Phosphorylation of Chk2 in an Ataxia Telangiectasia Mutated–Dependent Manner. Cancer Res. 2005, 65, 10734–10741. [Google Scholar] [CrossRef]
- Basu, S.; Majumder, S.; Bhowal, A.; Ghosh, A.; Naskar, S.; Nandy, S.; Mukherjee, S.; Sinha, R.K.; Basu, K.; Karmakar, D.; et al. A Study of Molecular Signals Deregulating Mismatch Repair Genes in Prostate Cancer Compared to Benign Prostatic Hyperplasia. PLoS ONE 2015, 10, e0125560. [Google Scholar] [CrossRef]
- Bindra, R.S.; Glazer, P.M. Repression of RAD51 Gene Expression by E2F4/P130 Complexes in Hypoxia. Oncogene 2007, 26, 2048–2057. [Google Scholar] [CrossRef]
- Chan, N.; Koritzinsky, M.; Zhao, H.; Bindra, R.; Glazer, P.M.; Powell, S.; Belmaaza, A.; Wouters, B.; Bristow, R.G. Chronic Hypoxia Decreases Synthesis of Homologous Recombination Proteins to Offset Chemoresistance and Radioresistance. Cancer Res. 2008, 68, 605–614. [Google Scholar] [CrossRef]
- Valeri, N.; Gasparini, P.; Fabbri, M.; Braconi, C.; Veronese, A.; Lovat, F.; Adair, B.; Vannini, I.; Fanini, F.; Bottoni, A.; et al. Modulation of Mismatch Repair and Genomic Stability by MiR-155. Proc. Natl. Acad. Sci. USA 2010, 107, 6982–6987. [Google Scholar] [CrossRef]
- Crosby, M.E.; Kulshreshtha, R.; Ivan, M.; Glazer, P.M. MicroRNA Regulation of DNA Repair Gene Expression in Hypoxic Stress. Cancer Res. 2009, 69, 1221–1229. [Google Scholar] [CrossRef]
- Lu, Y.; Chu, A.; Turker, M.S.; Glazer, P.M. Hypoxia-Induced Epigenetic Regulation and Silencing of the BRCA1 Promoter. Mol. Cell. Biol. 2011, 31, 3339–3350. [Google Scholar] [CrossRef]
- Sulkowski, P.L.; Corso, C.D.; Robinson, N.D.; Scanlon, S.E.; Purshouse, K.R.; Bai, H.; Liu, Y.; Sundaram, R.K.; Hegan, D.C.; Fons, N.R.; et al. 2-Hydroxyglutarate Produced by Neomorphic IDH Mutations Suppresses Homologous Recombination and Induces PARP Inhibitor Sensitivity. Sci. Transl. Med. 2017, 9, eaal2463. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Jendrossek, V.; Matschke, J. Oncometabolites and the Response to Radiotherapy. Radiat. Oncol. 2020, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, F.; Ousset, M.; Biard, D.; Fallone, F.; Dauvillier, S.; Frit, P.; Salles, B.; Muller, C. A DNA-Dependent Stress Response Involving DNA-PK Occurs in Hypoxic Cells and Contributes to Cellular Adaptation to Hypoxia. J. Cell Sci. 2011, 124, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.G.W.; Walter, J.C.; Loparo, J.J. Two-Stage Synapsis of DNA Ends during Non-Homologous End Joining. Mol. Cell 2016, 61, 850–858. [Google Scholar] [CrossRef]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X.; et al. Molecular Landmarks of Tumor Hypoxia across Cancer Types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Ehteshamipour, S.; Mohebbi, S.; Rahimi, E.; Shabani Sadr, N.K.; Mohamad Soltani, B.; Behmanesh, M. HIF1α Contribution to the NHEJ DNA Repair Pathway Through Decreased Expression of XRCC4. Jentashapir J. Cell. Mol. Biol. 2024, 15, 142410. [Google Scholar] [CrossRef]
- Gomel, R.; Xiang, C.; Finniss, S.; Lee, H.K.; Lu, W.; Okhrimenko, H.; Brodie, C. The Localization of Protein Kinase C δ in Different Subcellular Sites Affects Its Proapoptotic and Antiapoptotic Functions and the Activation of Distinct Downstream Signaling Pathways. Mol. Cancer Res. 2007, 5, 627–639. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Zuo, X.; Basourakos, S.; Zhang, J.; Zhao, J.; Han, Y.; Lin, Y.; Wang, Y.; Jiang, Y.; et al. Β-catenin Nuclear Translocation Induced by HIF-1α Overexpression Leads to the Radioresistance of Prostate Cancer. Int. J. Oncol. 2018, 52, 1827–1840. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q. VHL and Hypoxia Signaling: Beyond HIF in Cancer. Biomedicines 2018, 6, 35. [Google Scholar] [CrossRef]
- Artemov, A.V.; Zhigalova, N.; Zhenilo, S.; Mazur, A.M.; Prokhortchouk, E.B. VHL Inactivation without Hypoxia Is Sufficient to Achieve Genome Hypermethylation. Sci. Rep. 2018, 8, 10667. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, S.E.; Lok, J.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H. Metabolic Markers in Relation to Hypoxia; Staining Patterns and Colocalization of Pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer 2011, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Mukherjee, C. Molecular Regulation of Hypoxia through the Lenses of Noncoding RNAs and Epitranscriptome. Wiley Interdiscip. Rev. RNA 2023, 14, e1750. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Wong, P.-F.; Ramasamy, T.S. Hypoxia-Regulated MicroRNAs: The Molecular Drivers of Tumor Progression. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 351–376. [Google Scholar] [CrossRef]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, H.; Li, Y.; Liu, Y.; Zou, J.; Zhu, Y. Clinicopathological and Prognostic Value of Hypoxia-Inducible Factor-1α in Breast Cancer: A Meta-Analysis Including 5177 Patients. Clin. Transl. Oncol. 2020, 22, 1892–1906. [Google Scholar] [CrossRef]
- Khojastehnezhad, M.A.; Seyedi, S.M.R.; Raoufi, F.; Asoodeh, A. Association of Hypoxia-Inducible Factor 1 Expressions with Prognosis Role as a Survival Prognostic Biomarker in the Patients with Osteosarcoma: A Meta-Analysis. Expert Rev. Mol. Diagn. 2022, 22, 1099–1106. [Google Scholar] [CrossRef]
- Li, Z.; Wei, R.; Yao, S.; Meng, F.; Kong, L. HIF-1A as a Prognostic Biomarker Related to Invasion, Migration and Immunosuppression of Cervical Cancer. Heliyon 2024, 10, e24664. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Y.; Lu, Y.; Liu, M.; Li, M.; Li, J.; Wu, L. Hypoxia-Inducible Factor 1 Alpha Promotes Cancer Stem Cells-like Properties in Human Ovarian Cancer Cells by Upregulating SIRT1 Expression. Sci. Rep. 2017, 7, 10592. [Google Scholar] [CrossRef]
- Chamie, K.; Klöpfer, P.; Bevan, P.; Störkel, S.; Said, J.; Fall, B.; Belldegrun, A.S.; Pantuck, A.J. Carbonic Anhydrase-IX Score Is a Novel Biomarker That Predicts Recurrence and Survival for High-Risk, Nonmetastatic Renal Cell Carcinoma: Data from the Phase III ARISER Clinical Trial. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 204.e25–204.e33. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Yaromina, A.; Houben, R.; Niemans, R.; Lambin, P.; Dubois, L.J. Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 2016, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sugisaka, J.; Shiraishi, Y.; Watanabe, Y.; Daga, H.; Azuma, K.; Nishino, K.; Mori, M.; Ota, T.; Saito, H.; et al. Serum VEGF-A as a Biomarker for the Addition of Bevacizumab to Chemo-Immunotherapy in Metastatic NSCLC. Nat. Commun. 2025, 16, 2825. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Liu, S.; Hardeman, A.; Rajagopal, P.S.; Mueller, J.; Khramtsova, G.; Sanni, A.; Ajani, M.; Clayton, W.; Hurley, I.W.; et al. The VEGF-Hypoxia Signature Is Upregulated in Basal-like Breast Tumors from Women of African Ancestry and Associated with Poor Outcomes in Breast Cancer. Clin. Cancer Res. 2024, 30, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, J.A.; Calkins-Adams, D.P.; Rinker, L.H.; Ballenger, C.A.; Weissler, M.C.; Fowler, W.C.; Novotny, D.B.; Varia, M.A. Hypoxia and Vascular Endothelial Growth Factor Expression in Human Squamous Cell Carcinomas Using Pimonidazole as a Hypoxia Marker. Cancer Res. 1998, 58, 3765–3768. [Google Scholar]
- Du, J.; Gu, J.; Deng, J.; Kong, L.; Guo, Y.; Jin, C.; Bao, Y.; Fu, D.; Li, J. The Expression and Survival Significance of Glucose Transporter-1 in Pancreatic Cancer: Meta-Analysis, Bioinformatics Analysis and Retrospective Study. Cancer Investig. 2021, 39, 741–755. [Google Scholar] [CrossRef]
- El-Benhawy, S.A.; Sakr, O.A.; Fahmy, E.I.; Ali, R.A.; Hussein, M.S.; Nassar, E.M.; Salem, S.M.; Abu-Samra, N.; Elzawawy, S. Assessment of Serum Hypoxia Biomarkers Pre- and Post-Radiotherapy in Patients with Brain Tumors. J. Mol. Neurosci. 2022, 72, 2303–2312. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Jiang, L.; Ren, X.; Cheng, B.; Xia, J. Prognostic Value of Glycolysis Markers in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Aging 2021, 13, 7284–7299. [Google Scholar] [CrossRef]
- Serganova, I.; Rizwan, A.; Ni, X.; Thakur, S.B.; Vider, J.; Russell, J.; Blasberg, R.; Koutcher, J.A. Metabolic Imaging: A Link between Lactate Dehydrogenase A, Lactate, and Tumor Phenotype. Clin. Cancer Res. 2011, 17, 6250–6261. [Google Scholar] [CrossRef]
- Radenkovic, S.; Milosevic, Z.; Konjevic, G.; Karadzic, K.; Rovcanin, B.; Buta, M.; Gopcevic, K.; Jurisic, V. Lactate Dehydrogenase, Catalase, and Superoxide Dismutase in Tumor Tissue of Breast Cancer Patients in Respect to Mammographic Findings. Cell Biochem. Biophys 2013, 66, 287–295. [Google Scholar] [CrossRef]
- Han, Y.-L.; Chen, L.; Qin, R.; Wang, G.-Q.; Lin, X.-H.; Dai, G.-H. Lysyl Oxidase and Hypoxia-Inducible Factor 1α: Biomarkers of Gastric Cancer. World J. Gastroenterol. 2019, 25, 1828–1839. [Google Scholar] [CrossRef]
- Schietke, R.; Warnecke, C.; Wacker, I.; Schödel, J.; Mole, D.R.; Campean, V.; Amann, K.; Goppelt-Struebe, M.; Behrens, J.; Eckardt, K.-U.; et al. The Lysyl Oxidases LOX and LOXL2 Are Necessary and Sufficient to Repress E-Cadherin in Hypoxia. J. Biol. Chem. 2010, 285, 6658–6669. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Li, W.; Jian, L.; Yin, S.; Yang, S.; Zhao, H.; Huang, W.; Zhang, Y.; Zhou, H. Identification of LOX as a Candidate Prognostic Biomarker in Glioblastoma Multiforme. Transl. Oncol. 2023, 36, 101739. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, X.; Ji, J.; Chen, L.; Cao, J.; Luo, J.; Zhang, S. High Expression Levels of MiR-21 and MiR-210 Predict Unfavorable Survival in Breast Cancer: A Systemic Review and Meta-Analysis. Int. J. Biol. Markers 2015, 30, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Peng, Q.; Zhu, J.; Shen, Y.; Lin, K.; Shen, Y.; Zhu, Y. Identification of MiR-210 and Combination Biomarkers as Useful Agents in Early Screening Non-Small Cell Lung Cancer. Gene 2020, 729, 144225. [Google Scholar] [CrossRef]
- Irlam-Jones, J.J.; Eustace, A.; Denley, H.; Choudhury, A.; Harris, A.L.; Hoskin, P.J.; West, C.M.L. Expression of MiR-210 in Relation to Other Measures of Hypoxia and Prediction of Benefit from Hypoxia Modification in Patients with Bladder Cancer. Br. J. Cancer 2016, 115, 571–578. [Google Scholar] [CrossRef]
- Li, M.; Ma, X.; Li, M.; Zhang, B.; Huang, J.; Liu, L.; Wei, Y. Prognostic Role of MicroRNA-210 in Various Carcinomas: A Systematic Review and Meta-Analysis. Dis. Markers 2014, 2014, 106197. [Google Scholar] [CrossRef]
- Ci, X.; Chen, S.; Zhu, R.; Zarif, M.; Jain, R.; Guo, W.; Ramotar, M.; Gong, L.; Xu, W.; Singh, O.; et al. Oral Pimonidazole Unveils Clinicopathologic and Epigenetic Features of Hypoxic Tumour Aggressiveness in Localized Prostate Cancer. BMC Cancer 2024, 24, 744. [Google Scholar] [CrossRef]
- Ragnum, H.B.; Vlatkovic, L.; Lie, A.K.; Axcrona, K.; Julin, C.H.; Frikstad, K.M.; Hole, K.H.; Seierstad, T.; Lyng, H. The Tumour Hypoxia Marker Pimonidazole Reflects a Transcriptional Programme Associated with Aggressive Prostate Cancer. Br. J. Cancer 2015, 112, 382–390. [Google Scholar] [CrossRef]
- Swartz, J.E.; Smits, H.J.G.; Philippens, M.E.P.; de Bree, R.; Kaanders, J.H.A.M.; Willems, S.M. Correlation and Colocalization of HIF-1α and Pimonidazole Staining for Hypoxia in Laryngeal Squamous Cell Carcinomas: A Digital, Single-Cell-Based Analysis. Oral Oncol. 2022, 128, 105862. [Google Scholar] [CrossRef]
- Beckers, C.; Pruschy, M.; Vetrugno, I. Tumor Hypoxia and Radiotherapy: A Major Driver of Resistance Even for Novel Radiotherapy Modalities. Semin. Cancer Biol. 2024, 98, 19–30. [Google Scholar] [CrossRef]
- Minassian, L.M.; Cotechini, T.; Huitema, E.; Graham, C.H. Hypoxia-Induced Resistance to Chemotherapy in Cancer. Adv. Exp. Med. Biol. 2019, 1136, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.-S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. 2021, 27, e934116-1–e934116-7. [Google Scholar] [CrossRef]
- Marcu, L.G.; Toma Dasu, I.; Dasu, A. The Six Rs of Head and Neck Cancer Radiotherapy. In Contemporary Issues in Head and Neck Cancer Management; Marcu, L.G., Ed.; InTech: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Meijer, T.W.H.; Kaanders, J.H.A.M.; Span, P.N.; Bussink, J. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism to Improve Radiotherapy Efficacy. Clin. Cancer Res. 2012, 18, 5585–5594. [Google Scholar] [CrossRef]
- Rakotomalala, A.; Escande, A.; Furlan, A.; Meignan, S.; Lartigau, E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the “Six Rs” of Radiotherapy. Front. Endocrinol 2021, 12, 742215. [Google Scholar] [CrossRef]
- Ma, Y.; Hendershot, L.M. The Role of the Unfolded Protein Response in Tumour Development: Friend or Foe? Nat. Rev. Cancer 2004, 4, 966–977. [Google Scholar] [CrossRef]
- Tian, Y.; Lei, Y.; Wang, Y.; Lai, J.; Wang, J.; Xia, F. Mechanism of Multidrug Resistance to Chemotherapy Mediated by P-glycoprotein (Review). Int. J. Oncol. 2023, 63, 119. [Google Scholar] [CrossRef]
- Ozcan, G. The Hypoxia-Inducible Factor-1α in Stemness and Resistance to Chemotherapy in Gastric Cancer: Future Directions for Therapeutic Targeting. Front. Cell Dev. Biol. 2023, 11, 1082057. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, L.; Zhong, T. The Role of HIF-1 & alpha; in Chemo-/Radioresistant Tumors. Onco Targets Ther. 2018, 11, 3003–3011. [Google Scholar] [CrossRef]
- Zhao, Q.; Tan, B.-B.; Li, Y.; Fan, L.-Q.; Yang, P.-G.; Tian, Y. Enhancement of Drug Sensitivity by Knockdown of HIF-1α in Gastric Carcinoma Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 23, 129–136. [Google Scholar] [CrossRef]
- Rohwer, N.; Dame, C.; Haugstetter, A.; Wiedenmann, B.; Detjen, K.; Schmitt, C.A.; Cramer, T. Hypoxia-Inducible Factor 1α Determines Gastric Cancer Chemosensitivity via Modulation of P53 and NF-ΚB. PLoS ONE 2010, 5, e12038. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A Novel Mechanism of Chemoresistance in Cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, Y.; Fan, Z. The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef] [PubMed]
- Sowa, T.; Menju, T.; Chen-Yoshikawa, T.F.; Takahashi, K.; Nishikawa, S.; Nakanishi, T.; Shikuma, K.; Motoyama, H.; Hijiya, K.; Aoyama, A.; et al. Hypoxia-inducible Factor 1 Promotes Chemoresistance of Lung Cancer by Inducing Carbonic Anhydrase IX Expression. Cancer Med. 2017, 6, 288–297. [Google Scholar] [CrossRef]
- Sebestyén, A.; Kopper, L.; Dankó, T.; Tímár, J. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol. Oncol. Res. 2021, 27, 1609802. [Google Scholar] [CrossRef]
- Hielscher, A.; Gerecht, S. Hypoxia and Free Radicals: Role in Tumor Progression and the Use of Engineering-Based Platforms to Address These Relationships. Free. Radic. Biol. Med. 2015, 79, 281–291. [Google Scholar] [CrossRef]
- Doktorova, H.; Hrabeta, J.; Khalil, M.A.; Eckschlager, T. Hypoxia-Induced Chemoresistance in Cancer Cells: The Role of Not Only HIF-1. Biomed. Pap. 2015, 159, 166–177. [Google Scholar] [CrossRef]
- Burger, R.M.; Peisach, J.; Horwitz, S.B. Activated Bleomycin. A Transient Complex of Drug, Iron, and Oxygen That Degrades DNA. J. Biol. Chem. 1981, 256, 11636–11644. [Google Scholar] [CrossRef]
- Rouschop, K.M.; Dubois, L.J.; Keulers, T.G.; van den Beucken, T.; Lambin, P.; Bussink, J.; van der Kogel, A.J.; Koritzinsky, M.; Wouters, B.G. PERK/EIF2α Signaling Protects Therapy Resistant Hypoxic Cells through Induction of Glutathione Synthesis and Protection against ROS. Proc. Natl. Acad. Sci. USA 2013, 110, 4622–4627. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Panada, E.; Moiso, E.; Buondonno, I.; Provero, P.; Rubinstein, M.; Kopecka, J.; Riganti, C. PERK Induces Resistance to Cell Death Elicited by Endoplasmic Reticulum Stress and Chemotherapy. Mol. Cancer 2017, 16, 91. [Google Scholar] [CrossRef]
- Bi, M.; Naczki, C.; Koritzinsky, M.; Fels, D.; Blais, J.; Hu, N.; Harding, H.; Novoa, I.; Varia, M.; Raleigh, J.; et al. ER Stress-Regulated Translation Increases Tolerance to Extreme Hypoxia and Promotes Tumor Growth. EMBO J. 2005, 24, 3470–3481. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramirez, L.; Cao, H.; Nelson, D.; Hammond, E.; Lee, A.-H.; Yoshida, H.; Mori, K.; Glimcher, L.H.; Denko, N.C.; Giaccia, A.J.; et al. XBP1 Is Essential for Survival under Hypoxic Conditions and Is Required for Tumor Growth. Cancer Res. 2004, 64, 5943–5947. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.C.; Delgoffe, G.M.; Najjar, Y.G. Characteristics of the Tumor Microenvironment That Influence Immune Cell Functions: Hypoxia, Oxidative Stress, Metabolic Alterations. Cancers 2020, 12, 3802. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Buart, S.; Chouaib, S. Hypoxic Stress-Induced Tumor and Immune Plasticity, Suppression, and Impact on Tumor Heterogeneity. Front. Immunol. 2017, 8, 1625. [Google Scholar] [CrossRef]
- Phan, A.T.; Goldrath, A.W. Hypoxia-Inducible Factors Regulate T Cell Metabolism and Function. Mol. Immunol. 2015, 68, 527–535. [Google Scholar] [CrossRef]
- Tao, J.-H.; Barbi, J.; Pan, F. Hypoxia-Inducible Factors in T Lymphocyte Differentiation and Function. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C580–C589. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yao, H.; Li, C.; Fang, J.-Y.; Xu, J. Regulation of PD-L1: Emerging Routes for Targeting Tumor Immune Evasion. Front. Pharmacol. 2018, 9, 536. [Google Scholar] [CrossRef]
- Robainas, M.; Otano, R.; Bueno, S.; Ait-Oudhia, S. Understanding the Role of PD-L1/PD1 Pathway Blockade and Autophagy in Cancer Therapy. Onco Targets Ther. 2017, 10, 1803–1807. [Google Scholar] [CrossRef]
- Steingold, J.M.; Hatfield, S.M. Targeting Hypoxia-A2A Adenosinergic Immunosuppression of Antitumor T Cells During Cancer Immunotherapy. Front. Immunol. 2020, 11, 570041. [Google Scholar] [CrossRef]
- Graham, K.; Unger, E. Overcoming Tumor Hypoxia as a Barrier to Radiotherapy, Chemotherapy and Immunotherapy in Cancer Treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Editor’s Pick: Tumour-Associated Hypoxia: Can We Give Chimeric Antigen Receptor T Cells More Breathing Space? Eur. Med. J. 2020, 5, 30–37. [CrossRef]
- Schurich, A.; Magalhaes, I.; Mattsson, J. Metabolic Regulation of CAR T Cell Function by the Hypoxic Microenvironment in Solid Tumors. Immunotherapy 2019, 11, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving Immune–Vascular Crosstalk for Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Espinosa-Cotton, M.; Guo, H.; Monette, S.; Cheung, N.-K.V. Targeting Tumor Vasculature to Improve Antitumor Activity of T Cells Armed Ex Vivo with T Cell Engaging Bispecific Antibody. J. Immunother. Cancer 2023, 11, e006680. [Google Scholar] [CrossRef]
- Hendry, S.A.; Farnsworth, R.H.; Solomon, B.; Achen, M.G.; Stacker, S.A.; Fox, S.B. The Role of the Tumor Vasculature in the Host Immune Response: Implications for Therapeutic Strategies Targeting the Tumor Microenvironment. Front. Immunol. 2016, 7, 621. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.-F. Targeting Hypoxia: Hypoxia-Activated Prodrugs in Cancer Therapy. Front. Oncol. 2021, 11, 700407. [Google Scholar] [CrossRef]
- Baran, N.; Konopleva, M. Molecular Pathways: Hypoxia-Activated Prodrugs in Cancer Therapy. Clin. Cancer Res. 2017, 23, 2382–2390. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.-F. The Hypoxia-Activated Prodrug TH-302: Exploiting Hypoxia in Cancer Therapy. Front. Pharmacol. 2021, 12, 636892. [Google Scholar] [CrossRef]
- van der Wiel, A.M.A.; Jackson-Patel, V.; Niemans, R.; Yaromina, A.; Liu, E.; Marcus, D.; Mowday, A.M.; Lieuwes, N.G.; Biemans, R.; Lin, X.; et al. Selectively Targeting Tumor Hypoxia with the Hypoxia-Activated Prodrug CP-506. Mol. Cancer Ther. 2021, 20, 2372–2383. [Google Scholar] [CrossRef]
- Musleh Ud Din, S.; Streit, S.G.; Huynh, B.T.; Hana, C.; Abraham, A.-N.; Hussein, A. Therapeutic Targeting of Hypoxia-Inducible Factors in Cancer. Int. J. Mol. Sci. 2024, 25, 2060. [Google Scholar] [CrossRef]
- Abi-Jaoudeh, N.; Dayyani, F.; Chen, P.J.; Fernando, D.; Fidelman, N.; Javan, H.; Liang, P.-C.; Hwang, J.-I.; Imagawa, D.K. Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Peng, C.-M.; Hwang, J.-I.; Liang, P.-C.; Chen, P.-J.; Abi-Jaoudeh, N.; Giiang, L.-H.; Tyan, Y.-S. Phase I Dose-Escalation Study of Tirapazamine Chemoembolization for Unresectable Early- and Intermediate-Stage Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2022, 33, 926–933.e1. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.N.; Rao, S.; Roth, B.; Bryan, T.; Fernando, D.M.; Dayyani, F.; Imagawa, D.; Abi-Jaoudeh, N. Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma. Cancers 2023, 15, 2738. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Shi, Y.; Szymanska, B.; Carol, H.; Boehm, I.; Lu, H.; Konoplev, S.; Fang, W.; Zweidler-McKay, P.A.; Campana, D.; et al. Pronounced Hypoxia in Models of Murine and Human Leukemia: High Efficacy of Hypoxia-Activated Prodrug PR-104. PLoS ONE 2011, 6, e23108. [Google Scholar] [CrossRef]
- Konopleva, M.; Thall, P.F.; Yi, C.A.; Borthakur, G.; Coveler, A.; Bueso-Ramos, C.; Benito, J.; Konoplev, S.; Gu, Y.; Ravandi, F.; et al. Phase I/II Study of the Hypoxia-Activated Prodrug PR104 in Refractory/Relapsed Acute Myeloid Leukemia and Acute Lymphoblastic Leukemia. Haematologica 2015, 100, 927–934. [Google Scholar] [CrossRef]
- Singleton, R.S.; Guise, C.P.; Ferry, D.M.; Pullen, S.M.; Dorie, M.J.; Brown, J.M.; Patterson, A.V.; Wilson, W.R. DNA Cross-Links in Human Tumor Cells Exposed to the Prodrug PR-104A: Relationships to Hypoxia, Bioreductive Metabolism, and Cytotoxicity. Cancer Res. 2009, 69, 3884–3891. [Google Scholar] [CrossRef]
- Yaromina, A.; Koi, L.; Schuitmaker, L.; van der Wiel, A.M.-M.A.; Dubois, L.J.; Krause, M.; Lambin, P. Overcoming Radioresistance with the Hypoxia-Activated Prodrug CP-506: A Pre-Clinical Study of Local Tumour Control Probability. Radiother. Oncol. 2023, 186, 109738. [Google Scholar] [CrossRef]
- Brenner, A.; Zuniga, R.; Sun, J.D.; Floyd, J.; Hart, C.P.; Kroll, S.; Fichtel, L.; Cavazos, D.; Caflisch, L.; Gruslova, A.; et al. Hypoxia-Activated Evofosfamide for Treatment of Recurrent Bevacizumab-Refractory Glioblastoma: A Phase I Surgical Study. Neuro. Oncol. 2018, 20, 1231–1239. [Google Scholar] [CrossRef]
- Brenner, A.J.; Floyd, J.; Fichtel, L.; Michalek, J.; Kanakia, K.P.; Huang, S.; Reardon, D.; Wen, P.Y.; Lee, E.Q. Phase 2 Trial of Hypoxia Activated Evofosfamide (TH302) for Treatment of Recurrent Bevacizumab-Refractory Glioblastoma. Sci. Rep. 2021, 11, 2306. [Google Scholar] [CrossRef]
- Sun, J.D.; Liu, Q.; Ahluwalia, D.; Ferraro, D.J.; Wang, Y.; Jung, D.; Matteucci, M.D.; Hart, C.P. Comparison of Hypoxia-Activated Prodrug Evofosfamide (TH-302) and Ifosfamide in Preclinical Non-Small Cell Lung Cancer Models. Cancer Biol. Ther. 2016, 17, 371–380. [Google Scholar] [CrossRef]
- Laubach, J.P.; Liu, C.-J.; Raje, N.S.; Yee, A.J.; Armand, P.; Schlossman, R.L.; Rosenblatt, J.; Hedlund, J.; Martin, M.; Reynolds, C.; et al. Phase I/II Study of Evofosfamide, A Hypoxia-Activated Prodrug with or without Bortezomib in Subjects with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. 2019, 25, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.D.; Liu, Q.; Ahluwalia, D.; Li, W.; Meng, F.; Wang, Y.; Bhupathi, D.; Ruprell, A.S.; Hart, C.P. Efficacy and Safety of the Hypoxia-Activated Prodrug TH-302 in Combination with Gemcitabine and Nab-Paclitaxel in Human Tumor Xenograft Models of Pancreatic Cancer. Cancer Biol. Ther. 2015, 16, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Badar, T.; Handisides, D.R.; Benito, J.M.; Richie, M.A.; Borthakur, G.; Jabbour, E.; Harutyunyan, K.; Konoplev, S.; Faderl, S.; Kroll, S.; et al. Phase I Study of Evofosfamide, an Investigational Hypoxia-activated Prodrug, in Patients with Advanced Leukemia. Am. J. Hematol. 2016, 91, 800–805. [Google Scholar] [CrossRef]
- McLean, L.S.; Morris, T.A.; Gramza, A.; Liu, S.; Khan, S.A.; Colevas, A.D.; Pearce, T.; Rischin, D. A Phase II Study of Tarloxotinib (a Hypoxia Activated Prodrug of a Pan-Erb Tyrosine Kinase Inhibitor) in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck or Skin. Investig. New Drugs 2022, 40, 782–788. [Google Scholar] [CrossRef]
- Estrada-Bernal, A.; Le, A.T.; Doak, A.E.; Tirunagaru, V.G.; Silva, S.; Bull, M.R.; Smaill, J.B.; Patterson, A.V.; Kim, C.; Liu, S.V.; et al. Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin. Cancer Res. 2021, 27, 1463–1475. [Google Scholar] [CrossRef]
- Xu, P.; Huang, J.-M.; Ren, Y.; Zha, X.; Deng, B.-F.; Wu, J.-H.; Lang, J.-Y. Regulation of Hypoxia-Induced MRNA Expressions of HIF-1alpha?And Osteopontin and in Vitro Radiosensitization by Tirapazamine in Human Nasopharyngeal Carcinoma HNE-1 and CNE-1 Cells. Chin. J. Cancer 2010, 29, 126–130. [Google Scholar] [CrossRef]
- Meng, F.; Evans, J.W.; Bhupathi, D.; Banica, M.; Lan, L.; Lorente, G.; Duan, J.-X.; Cai, X.; Mowday, A.M.; Guise, C.P.; et al. Molecular and Cellular Pharmacology of the Hypoxia-Activated Prodrug TH-302. Mol. Cancer Ther. 2012, 11, 740–751. [Google Scholar] [CrossRef]
- Spiegelberg, L.; Houben, R.; Niemans, R.; de Ruysscher, D.; Yaromina, A.; Theys, J.; Guise, C.P.; Smaill, J.B.; Patterson, A.V.; Lambin, P.; et al. Hypoxia-Activated Prodrugs and (Lack of) Clinical Progress: The Need for Hypoxia-Based Biomarker Patient Selection in Phase III Clinical Trials. Clin. Transl. Radiat. Oncol. 2019, 15, 62–69. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted Hypoxia Reduction Restores T Cell Infiltration and Sensitizes Prostate Cancer to Immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef]
- Jamieson, S.M.F.; Tsai, P.; Kondratyev, M.K.; Budhani, P.; Liu, A.; Senzer, N.N.; Chiorean, E.G.; Jalal, S.I.; Nemunaitis, J.J.; Kee, D.; et al. Evofosfamide for the Treatment of Human Papillomavirus-Negative Head and Neck Squamous Cell Carcinoma. JCI Insight 2018, 3, e122204. [Google Scholar] [CrossRef]
- Hegde, A.; Jayaprakash, P.; Couillault, C.A.; Piha-Paul, S.; Karp, D.; Rodon, J.; Pant, S.; Fu, S.; Dumbrava, E.E.; Yap, T.A.; et al. A Phase I Dose-Escalation Study to Evaluate the Safety and Tolerability of Evofosfamide in Combination with Ipilimumab in Advanced Solid Malignancies. Clin. Cancer Res. 2021, 27, 3050–3060. [Google Scholar] [CrossRef]
- Palmer, B.D.; Wilson, W.R.; Atwell, G.J.; Schultz, D.; Xu, X.Z.; Denny, W.A. Hypoxia-Selective Antitumor Agents. 9. Structure-Activity Relationships for Hypoxia-Selective Cytotoxicity among Analogs of 5-[N,N-Bis(2-Chloroethyl)Amino]-2,4-Dinitrobenzamide. J. Med. Chem. 1994, 37, 2175–2184. [Google Scholar] [CrossRef]
- Liu, S.V.; Villaruz, L.C.; Lee, V.H.F.; Zhu, V.W.; Baik, C.S.; Sacher, A.; McCoach, C.E.; Nguyen, D.; Li, J.Y.-C.; Pacheco, J.M.; et al. LBA61 First Analysis of RAIN-701: Study of Tarloxotinib in Patients with Non-Small Cell Lung Cancer (NSCLC) EGFR Exon 20 Insertion, HER2-Activating Mutations & Other Solid Tumours with NRG1/ERBB Gene Fusions. Ann. Oncol. 2020, 31, S1189. [Google Scholar] [CrossRef]
- Ciepła, J.; Smolarczyk, R. Tumor Hypoxia Unveiled: Insights into Microenvironment, Detection Tools and Emerging Therapies. Clin. Exp. Med. 2024, 24, 235. [Google Scholar] [CrossRef]
- Moen, I.; Stuhr, L.E.B. Hyperbaric Oxygen Therapy and Cancer—A Review. Target. Oncol. 2012, 7, 233–242. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Stirnemann, J.; Serratrice, J.; Mann, T.; Louge, P.; Christophe, C.; Samii, K.; Pignel, R.; Agoritsas, T.; Ansari, M.; Cannas, G.; et al. Protocol for a Multicentric, Double-Blind, Randomised Controlled Trial of Hyperbaric Oxygen Therapy (HBOT) versus Sham for Treating Vaso-Occlusive Crisis (VOC) in Sickle Cell Disease (SCD) in Patients Aged 8 Years or Older (HBOT-SCD Study). BMJ Open 2024, 14, e084825. [Google Scholar] [CrossRef]
- Stępień, K.; Ostrowski, R.P.; Matyja, E. Hyperbaric Oxygen as an Adjunctive Therapy in Treatment of Malignancies, Including Brain Tumours. Med. Oncol. 2016, 33, 101. [Google Scholar] [CrossRef]
- Canarslan Demir, K.; Avci, A.U.; Ozgok Kangal, M.K.; Ceylan, B.; Abayli, S.Y.; Ozler, I.; Yilmaz, K.B. Hyperbaric Oxygen Therapy for Managing Cancer Treatment Complications: A Safety Evaluation. Medicina 2025, 61, 385. [Google Scholar] [CrossRef]
- Granowitz, E.V.; Tonomura, N.; Benson, R.M.; Katz, D.M.; Band, V.; Makari-Judson, G.P.; Osborne, B.A. Hyperbaric Oxygen Inhibits Benign and Malignant Human Mammary Epithelial Cell Proliferation. Anticancer Res. 2005, 25, 3833–3842. [Google Scholar]
- Machado, V.; da Rocha, J.R.; Parra, R.; Feitosa, M.; Leite, C.; Minto, S.; Garcia, S.; Cunha, T.; Feres, O. Hyperbaric Oxygen Therapy Increases the Effect of 5-Fluorouracil Chemotherapy on Experimental Colorectal Cancer in Mice. Med. Gas. Res. 2023, 14, 121–126. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Tsuneyama, K.; Yen, M.-H.; Lee, J.-T.; Chen, J.-L.; Huang, S.-M. Hyperbaric Oxygen Suppressed Tumor Progression through the Improvement of Tumor Hypoxia and Induction of Tumor Apoptosis in A549-Cell-Transferred Lung Cancer. Sci. Rep. 2021, 11, 12033. [Google Scholar] [CrossRef]
- Fernández, E.; Morillo, V.; Salvador, M.; Santafé, A.; Beato, I.; Rodríguez, M.; Ferrer, C. Hyperbaric Oxygen and Radiation Therapy: A Review. Clin. Transl. Oncol. 2021, 23, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.-M.; Tsai, H.-P.; Tseng, T.-T.; Tseng, Y.-L.; Lieu, A.-S.; Kwan, A.-L.; Chang, A.Y.W. Hyperbaric Oxygen Therapy Adjuvant Chemotherapy and Radiotherapy through Inhibiting Stemness in Glioblastoma. Curr. Issues Mol. Biol. 2023, 45, 8309–8320. [Google Scholar] [CrossRef]
- Poff, A.M.; Ward, N.; Seyfried, T.N.; Arnold, P.; D’Agostino, D.P. Non-Toxic Metabolic Management of Metastatic Cancer in VM Mice: Novel Combination of Ketogenic Diet, Ketone Supplementation, and Hyperbaric Oxygen Therapy. PLoS ONE 2015, 10, e0127407. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, S.-Y.; Ho, P.-S.; Lin, C.-H.; Cheng, Y.-Y.; Wang, J.-K.; Sytwu, H.-K. Apoptosis of T-Leukemia and B-Myeloma Cancer Cells Induced by Hyperbaric Oxygen Increased Phosphorylation of P38 MAPK. Leuk. Res. 2007, 31, 805–815. [Google Scholar] [CrossRef]
- Liu, X.; Ye, N.; Xiao, C.; Wang, X.; Li, S.; Deng, Y.; Yang, X.; Li, Z.; Yang, X. Hyperbaric Oxygen Regulates Tumor Microenvironment and Boosts Commercialized Nanomedicine Delivery for Potent Eradication of Cancer Stem-like Cells. Nano Today 2021, 40, 101248. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, R.; Wang, C.; Ren, X.; Li, D.; Liu, Y.; Yu, Q. Key Genes Involved in the Beneficial Mechanism of Hyperbaric Oxygen for Glioblastoma and Predictive Indicators of Hyperbaric Oxygen Prolonging Survival in Glioblastoma Patients. Curr. Med. Sci. 2024, 44, 1036–1046. [Google Scholar] [CrossRef]
- Jansman, M.M.T.; Hosta-Rigau, L. Recent and Prominent Examples of Nano- and Microarchitectures as Hemoglobin-Based Oxygen Carriers. Adv. Colloid Interface Sci. 2018, 260, 65–84. [Google Scholar] [CrossRef]
- Kawasoe, Y.; Yokouchi, M.; Ueno, Y.; Iwaya, H.; Yoshida, H.; Komiya, S. Hyperbaric Oxygen as a Chemotherapy Adjuvant in the Treatment of Osteosarcoma. Oncol. Rep. 2009, 22, 1045–1050. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Wang, X.-Y.; Man, C.-F.; Gong, D.-D.; Fan, Y. Advances in Hyperbaric Oxygen to Promote Immunotherapy through Modulation of the Tumor Microenvironment. Front. Oncol. 2023, 13, 1200619. [Google Scholar] [CrossRef]
- Moen, I.; Øyan, A.M.; Kalland, K.-H.; Tronstad, K.J.; Akslen, L.A.; Chekenya, M.; Sakariassen, P.Ø.; Reed, R.K.; Stuhr, L.E.B. Hyperoxic Treatment Induces Mesenchymal-to-Epithelial Transition in a Rat Adenocarcinoma Model. PLoS ONE 2009, 4, e6381. [Google Scholar] [CrossRef]
- Alagoz, T.; Buller, R.E.; Anderson, B.; Terrell, K.L.; Squatrito, R.C.; Niemann, T.H.; Tatman, D.J.; Jebson, P. Evaluation of Hyperbaric Oxygen as a Chemosensitizer in the Treatment of Epithelial Ovarian Cancer in Xenografts in Mice. Cancer 1995, 75, 2313–2322. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Butler, G.J.; Beale, J.; Hamilton, R.W.; Lee, B.Y.; Lucas, P. Hyperbaric Oxygen and Malignancies: A Potential Role in Radiotherapy, Chemotherapy, Tumor Surgery and Phototherapy. Med. Sci. Monit. 2005, 11, RA279–RA289. [Google Scholar]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers 2022, 14, 6054. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Norris, R.E.; Shusterman, S.; Gore, L.; Muscal, J.A.; Macy, M.E.; Fox, E.; Berkowitz, N.; Buchbinder, A.; Bagatell, R. Phase 1 Evaluation of EZN-2208, a Polyethylene Glycol Conjugate of SN38, in Children Adolescents and Young Adults with Relapsed or Refractory Solid Tumors. Pediatr. Blood Cancer 2014, 61, 1792–1797. [Google Scholar] [CrossRef]
- Jeong, W.; Park, S.R.; Rapisarda, A.; Fer, N.; Kinders, R.J.; Chen, A.; Melillo, G.; Turkbey, B.; Steinberg, S.M.; Choyke, P.; et al. Weekly EZN-2208 (PEGylated SN-38) in Combination with Bevacizumab in Patients with Refractory Solid Tumors. Invest New Drugs 2014, 32, 340–346. [Google Scholar] [CrossRef]
- Kummar, S.; Raffeld, M.; Juwara, L.; Horneffer, Y.; Strassberger, A.; Allen, D.; Steinberg, S.M.; Rapisarda, A.; Spencer, S.D.; Figg, W.D.; et al. Multihistology, Target-Driven Pilot Trial of Oral Topotecan as an Inhibitor of Hypoxia-Inducible Factor-1α in Advanced Solid Tumors. Clin. Cancer Res. 2011, 17, 5123–5131. [Google Scholar] [CrossRef]
- Jeong, W.; Rapisarda, A.; Park, S.R.; Kinders, R.J.; Chen, A.; Melillo, G.; Turkbey, B.; Steinberg, S.M.; Choyke, P.; Doroshow, J.H.; et al. Pilot Trial of EZN-2968, an Antisense Oligonucleotide Inhibitor of Hypoxia-Inducible Factor-1 Alpha (HIF-1α), in Patients with Refractory Solid Tumors. Cancer Chemother. Pharmacol. 2014, 73, 343–348. [Google Scholar] [CrossRef]
- Ruan, J.; Zain, J.; Palmer, B.; Jovanovic, B.; Mi, X.; Swaroop, A.; Winter, J.N.; Gordon, L.I.; Karmali, R.; Moreira, J.; et al. Multicenter Phase 2 Study of Romidepsin plus Lenalidomide for Previously Untreated Peripheral T-Cell Lymphoma. Blood Adv. 2023, 7, 5771–5779. [Google Scholar] [CrossRef]
- Iyer, S.P.; Huen, A.; Ai, W.Z.; Jagadeesh, D.; Lechowicz, M.J.; Okada, C.; Feldman, T.A.; Ghione, P.; Alderuccio, J.P.; Champion, R.; et al. Safety and Efficacy of Tenalisib in Combination with Romidepsin in Patients with Relapsed/Refractory T-Cell Lymphoma: Results from a Phase I/II Open-Label Multicenter Study. Haematologica 2023, 109, 209–219. [Google Scholar] [CrossRef]
- Foley, N.; Riedell, P.A.; Bartlett, N.L.; Cashen, A.F.; Kahl, B.S.; Fehniger, T.A.; Fischer, A.; Moreno, C.; Liu, J.; Carson, K.R.; et al. A Phase I Study of Romidepsin in Combination with Gemcitabine, Oxaliplatin, and Dexamethasone in Patients with Relapsed or Refractory Aggressive Lymphomas Enriched for T-Cell Lymphomas. Clin. Lymphoma Myeloma Leuk. 2025, 25, 328–336. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Nirmal, A.J.; Rahman, J.; Xu, R.; Drill, E.; Galasso, N.; Ganesan, N.; Davey, T.; Hancock, H.; Perez, L.; et al. Duvelisib plus Romidepsin in Relapsed/Refractory T Cell Lymphomas: A Phase 1b/2a Trial. Nat. Med. 2024, 30, 2517–2527. [Google Scholar] [CrossRef]
- Pili, R.; Quinn, D.I.; Adra, N.; Logan, T.; Colligan, S.; Burney, H.N.; Hahn, N.M. A Phase I, Open Label, Dose Finding Study to Evaluate Safety, Pharmacodynamics and Efficacy of Pembrolizumab in Combination with Vorinostat in Patients with Advanced Prostate, Renal or Urothelial Carcinoma. Cancer Med. 2025, 14, TPS4581. [Google Scholar] [CrossRef]
- Godfrey, J.; Mei, M.; Chen, L.; Song, J.Y.; Bedell, V.; Budde, E.; Armenian, S.; Puverel, S.; Nikolaenko, L.; Chen, R.; et al. Results from a Phase I Trial of Pembrolizumab plus Vorinostat in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Haematologica 2023, 109, 533–542. [Google Scholar] [CrossRef]
- Stitzlein, L.M.; Baig, M.U.; Chandra, J.; McGovern, S.; Paulino, A.; Ketonen, L.M.; Khatua, S.; Zaky, W. Phase I Study of Vorinostat and Temsirolimus in Newly Diagnosed or Progressive Diffuse Intrinsic Pontine Glioma. Pediatr. Blood Cancer 2025, 72, e31619. [Google Scholar] [CrossRef]
- Arora, S.P.; Tenner, L.; Sarantopoulos, J.; Morris, J.; Liu, Q.; Mendez, J.A.; Curiel, T.; Michalek, J.; Mahalingam, D. Modulation of Autophagy: A Phase II Study of Vorinostat plus Hydroxychloroquine versus Regorafenib in Chemotherapy-Refractory Metastatic Colorectal Cancer (MCRC). Br. J. Cancer 2022, 127, 1153–1161. [Google Scholar] [CrossRef]
- Krug, L.M.; Kindler, H.L.; Calvert, H.; Manegold, C.; Tsao, A.S.; Fennell, D.; Öhman, R.; Plummer, R.; Eberhardt, W.E.E.; Fukuoka, K.; et al. Vorinostat in Patients with Advanced Malignant Pleural Mesothelioma Who Have Progressed on Previous Chemotherapy (VANTAGE-014): A Phase 3, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 447–456. [Google Scholar] [CrossRef]
- Jenner, M.W.; Pawlyn, C.; Davies, F.E.; Menzies, T.; Hockaday, A.; Olivier, C.; Jones, J.R.; Karunanithi, K.; Lindsay, J.; Kishore, B.; et al. The Addition of Vorinostat to Lenalidomide Maintenance for Patients with Newly Diagnosed Multiple Myeloma of All Ages: Results from ‘Myeloma XI’, a Multicentre, Open-label, Randomised, Phase III Trial. Br. J. Haematol. 2022, 201, 267–279. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Podoltsev, N.A.; Othus, M.; Pagel, J.M.; Radich, J.P.; Fang, M.; Rizzieri, D.A.; Marcucci, G.; Strickland, S.A.; Litzow, M.R.; et al. A Randomized Phase III Study of Standard versus High-Dose Cytarabine with or without Vorinostat for AML. Leukemia 2024, 38, 58–66. [Google Scholar] [CrossRef]
- Laubach, J.P.; Schjesvold, F.; Mariz, M.; Dimopoulos, M.A.; Lech-Maranda, E.; Spicka, I.; Hungria, V.T.M.; Shelekhova, T.; Abdo, A.; Jacobasch, L.; et al. Efficacy and Safety of Oral Panobinostat plus Subcutaneous Bortezomib and Oral Dexamethasone in Patients with Relapsed or Relapsed and Refractory Multiple Myeloma (PANORAMA 3): An Open-Label, Randomised, Phase 2 Study. Lancet Oncol. 2021, 22, 142–154. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Richardson, P.G.; Günther, A.; Sezer, O.; Siegel, D.; Bladé, J.; LeBlanc, R.; Sutherland, H.; Sopala, M.; Mishra, K.K.; et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J. Clin. Oncol. 2013, 31, 3696–3703. [Google Scholar] [CrossRef]
- Monje, M.; Cooney, T.; Glod, J.; Huang, J.; Peer, C.J.; Faury, D.; Baxter, P.; Kramer, K.; Lenzen, A.; Robison, N.J.; et al. Phase I Trial of Panobinostat in Children with Diffuse Intrinsic Pontine Glioma: A Report from the Pediatric Brain Tumor Consortium (PBTC-047). Neuro Oncol. 2023, 25, 2262–2272. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.M.; Yoon, S.-S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus Bortezomib and Dexamethasone versus Placebo plus Bortezomib and Dexamethasone in Patients with Relapsed or Relapsed and Refractory Multiple Myeloma: A Multicentre, Randomised, Double-Blind Phase 3 Trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- Richardson, P.G.; Schlossman, R.L.; Roy, A.N.; Panneerselvam, A.; Acharyya, S.; Sopala, M.; Lonial, S. Patient-reported Outcomes of Multiple Myeloma Patients Treated with Panobinostat after ≥2 Lines of Therapy Based on the International Phase 3, Randomized, Double-blind, Placebo-controlled PANORAMA-1 Trial. Br. J. Haematol. 2018, 181, 628–636. [Google Scholar] [CrossRef]
- Heath, E.I.; Hillman, D.W.; Vaishampayan, U.; Sheng, S.; Sarkar, F.; Harper, F.; Gaskins, M.; Pitot, H.C.; Tan, W.; Ivy, S.P.; et al. Carducci, M.A.; Erlichman, C.; Liu, G. A Phase II Trial of 17-Allylamino-17-Demethoxygeldanamycin in Patients with Hormone-Refractory Metastatic Prostate Cancer. Clin. Cancer Res. 2008, 14, 7940–7946. [Google Scholar] [CrossRef]
- Wahner Hendrickson, A.E.; Oberg, A.L.; Glaser, G.; Camoriano, J.K.; Peethambaram, P.P.; Colon-Otero, G.; Erlichman, C.; Ivy, S.P.; Kaufmann, S.H.; Karnitz, L.M.; et al. A Phase II Study of Gemcitabine in Combination with Tanespimycin in Advanced Epithelial Ovarian and Primary Peritoneal Carcinoma. Gynecol. Oncol. 2012, 124, 210–215. [Google Scholar] [CrossRef]
- Iyer, G.; Morris, M.J.; Rathkopf, D.; Slovin, S.F.; Steers, M.; Larson, S.M.; Schwartz, L.H.; Curley, T.; DeLaCruz, A.; Ye, Q.; et al. A Phase I Trial of Docetaxel and Pulse-Dose 17-Allylamino-17-Demethoxygeldanamycin in Adult Patients with Solid Tumors. Cancer Chemother. Pharmacol. 2012, 69, 1089–1097. [Google Scholar] [CrossRef]
- Walker, A.R.; Klisovic, R.; Johnston, J.S.; Jiang, Y.; Geyer, S.; Kefauver, C.; Binkley, P.; Byrd, J.C.; Grever, M.R.; Garzon, R.; et al. Pharmacokinetics and Dose Escalation of the Heat Shock Protein Inhibitor 17-Allyamino-17-Demethoxygeldanamycin in Combination with Bortezomib in Relapsed or Refractory Acute Myeloid Leukemia. Leuk. Lymphoma 2013, 54, 1996–2002. [Google Scholar] [CrossRef]
- Vaishampayan, U.N.; Burger, A.M.; Sausville, E.A.; Heilbrun, L.K.; Li, J.; Horiba, M.N.; Egorin, M.J.; Ivy, P.; Pacey, S.; LoRusso, P.M. Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of the Combination of Sorafenib and Tanespimycin. Clin. Cancer Res. 2010, 16, 3795–3804. [Google Scholar] [CrossRef]
- Schenk, E.; Hendrickson, A.E.W.; Northfelt, D.; Toft, D.O.; Ames, M.M.; Menefee, M.; Satele, D.; Qin, R.; Erlichman, C. Phase I Study of Tanespimycin in Combination with Bortezomib in Patients with Advanced Solid Malignancies. Investig. New Drugs 2013, 31, 1251–1256. [Google Scholar] [CrossRef]
- Richardson, P.G.; Badros, A.Z.; Jagannath, S.; Tarantolo, S.; Wolf, J.L.; Albitar, M.; Berman, D.; Messina, M.; Anderson, K.C. Tanespimycin with Bortezomib: Activity in Relapsed/Refractory Patients with Multiple Myeloma. Br. J. Haematol. 2010, 150, 428–437. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Liu, D.; Yuan, Y.; Liu, V.; Kang, S.; Groves, M.; Puduvalli, V.; Levin, V.; Conrad, C.; Colman, H.; et al. Phase 1/1b Study of Lonafarnib and Temozolomide in Patients with Recurrent or Temozolomide Refractory Glioblastoma. Cancer 2013, 119, 2747–2753. [Google Scholar] [CrossRef]
- Kerklaan, B.M.; Diéras, V.; Le Tourneau, C.; Mergui-Roelvink, M.; Huitema, A.D.R.; Rosing, H.; Beijnen, J.H.; Marreaud, S.; Govaerts, A.-S.; Piccart-Gebhart, M.J.; et al. Phase I Study of Lonafarnib (SCH66336) in Combination with Trastuzumab plus Paclitaxel in Her2/Neu Overexpressing Breast Cancer: EORTC Study 16023. Cancer Chemother. Pharmacol. 2013, 71, 53–62. [Google Scholar] [CrossRef]
- Wong, N.S.; Meadows, K.L.; Rosen, L.S.; Adjei, A.A.; Kaufmann, S.H.; Morse, M.A.; Petros, W.P.; Zhu, Y.; Statkevich, P.; Cutler, D.L.; et al. A Phase I Multicenter Study of Continuous Oral Administration of Lonafarnib (SCH 66336) and Intravenous Gemcitabine in Patients with Advanced Cancer. Cancer Investig. 2011, 29, 617–625. [Google Scholar] [CrossRef]
- Kim, E.S.; Kies, M.S.; Fossella, F.V.; Glisson, B.S.; Zaknoen, S.; Statkevich, P.; Munden, R.F.; Summey, C.; Pisters, K.M.W.; Papadimitrakopoulou, V.; et al. Phase II Study of the Farnesyltransferase Inhibitor Lonafarnib with Paclitaxel in Patients with Taxane-refractory/Resistant Nonsmall Cell Lung Carcinoma. Cancer 2005, 104, 561–569. [Google Scholar] [CrossRef]
- Hanrahan, E.O.; Kies, M.S.; Glisson, B.S.; Khuri, F.R.; Feng, L.; Tran, H.T.; Ginsberg, L.E.; Truong, M.T.; Hong, W.K.; Kim, E.S. A Phase II Study of Lonafarnib (SCH66336) in Patients with Chemorefractory, Advanced Squamous Cell Carcinoma of the Head and Neck. Am J Clin Oncol 2009, 32, 274–279. [Google Scholar] [CrossRef]
- Shafer, D.; Kagan, A.B.; Rudek, M.A.; Kmieciak, M.; Tombes, M.B.; Shrader, E.; Bandyopadhyay, D.; Hudson, D.; Sankala, H.; Weir, C.; et al. Phase 1 Study of Belinostat and Adavosertib in Patients with Relapsed or Refractory Myeloid Malignancies. Cancer Chemother. Pharmacol. 2023, 91, 281–290. [Google Scholar] [CrossRef]
- Luu, T.; Frankel, P.; Beumer, J.H.; Lim, D.; Cristea, M.; Appleman, L.J.; Lenz, H.J.; Gandara, D.R.; Kiesel, B.F.; Piekarz, R.L.; et al. Phase I Trial of Belinostat in Combination with 13-Cis-Retinoic Acid in Advanced Solid Tumor Malignancies: A California Cancer Consortium NCI/CTEP Sponsored Trial. Cancer Chemother. Pharmacol. 2019, 84, 1201–1208. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; Van Hoof, A.; Brown, P.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Thomas, C.M. Belinostat for the Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma. Journal of Oncology Pharm. Pract. 2017, 23, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Maher, K.R.; Shafer, D.; Schaar, D.; Bandyopadhyay, D.; Deng, X.; Wright, J.; Piekarz, R.; Rudek, M.A.; Harvey, R.D.; Grant, S. A Phase I Study of MLN4924 and Belinostat in Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancer Chemother. Pharmacol. 2025, 95, 24. [Google Scholar] [CrossRef]
- NIH. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Xu, K.; Ramesh, K.; Huang, V.; Gurbani, S.S.; Cordova, J.S.; Schreibmann, E.; Weinberg, B.D.; Sengupta, S.; Voloschin, A.D.; Holdhoff, M.; et al. Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma. Tomography 2022, 8, 688–700. [Google Scholar] [CrossRef]
- Herbaux, C.; Kornauth, C.; Poulain, S.; Chong, S.J.F.; Collins, M.C.; Valentin, R.; Hackett, L.; Tournilhac, O.; Lemonnier, F.; Dupuis, J.; et al. BH3 Profiling Identifies Ruxolitinib as a Promising Partner for Venetoclax to Treat T-Cell Prolymphocytic Leukemia. Blood 2021, 137, 3495–3506. [Google Scholar] [CrossRef]
- Zong, X.; Yang, Z.; Zhou, J.; Jin, Z.; Wu, D. Clinical Trial: Chidamide plus CHOP Improve the Survival of Newly Diagnosed Angioimmunoblastic T-Cell Lymphoma. Front. Immunol. 2024, 15. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, Y.; Zhou, H.; Lai, Y.; Cao, Y.; Li, Z.; Su, N.; Li, W.; Huang, H.; Liu, P.; et al. Chidamide, a Histone Deacetylase Inhibitor, Combined with R-GemOx in Relapsed/Refractory Diffuse Large B-Cell Lymphoma (TRUST): A Multicenter, Single-Arm, Phase 2 Trial. Cancer Med. 2025, 14. [Google Scholar] [CrossRef]
- Chen, G.; Xue, K.; Zhang, Q.; Xia, Z.; Jin, J.; Li, R.; Liu, Y.; Lv, F.; Hong, X.; Li, X.; et al. Chidamide plus R-GDP for Relapsed/Refractory Diffuse Large B-cell Lymphoma in Patients Ineligible for Autologous Transplantation: A Prospective, Single-arm, Phase II Study. Cancer Med. 2024, 13. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Su, L.; Liu, L.; Gao, Y.; Wang, Q.; Su, H.; Song, Y.; Zhang, H.; Shen, J.; et al. Efficacy of Chidamide Maintenance Therapy versus Autologous Stem Cell Transplantation versus Observation as a Post-Remission Choice in the Survival of Adult Patients with Peripheral T-Cell Lymphoma: Post Hoc Analysis of a Prospective, Multicenter, Phase 2 Study in China. Ann. Hematol. 2024, 103, 3061–3069. [Google Scholar] [CrossRef]
- Liang, J.; Wang, L.; Wang, X.; Cui, G.; Zhou, J.; Xing, T.; Du, K.; Xu, J.; Wang, L.; Liang, R.; et al. Chidamide plus Prednisone, Cyclophosphamide, and Thalidomide for Relapsed or Refractory Peripheral T-Cell Lymphoma: A Multicenter Phase II Trial. Chin. Med. J. 2024, 137, 1576–1582. [Google Scholar] [CrossRef]
- Gao, Y.; He, H.; Li, X.; Zhang, L.; Xu, W.; Feng, R.; Li, W.; Xiao, Y.; Liu, X.; Chen, Y.; et al. Sintilimab (Anti-PD-1 Antibody) plus Chidamide (Histone Deacetylase Inhibitor) in Relapsed or Refractory Extranodal Natural Killer T-Cell Lymphoma (SCENT): A Phase Ib/II Study. Signal Transduct. Target. Ther. 2024, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, J.; Wang, Z. The Efficacy and Safety of Chidamide in Combination with Etoposide and Glucocorticoids for the Treatment of Hemophagocytic Lymphohistiocytosis in Adult Patients: An Open-Label, Single-Center Study. Front. Immunol. 2024, 15, 1415597. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Wheler, J.J.; Naing, A.; Jackson, E.F.; Janku, F.; Hong, D.; Ng, C.S.; Tannir, N.M.; Lawhorn, K.N.; Huang, M.; et al. Targeting Hypoxia-Inducible Factor-1α (HIF-1α) in Combination with Antiangiogenic Therapy: A Phase I Trial of Bortezomib plus Bevacizumab. Oncotarget 2014, 5, 10280–10292. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Shim, J.; Kang, K.-W.; Yoon, S.E.; Hong, J.S.; Lim, S.N.; Yhim, H.-Y.; Kwon, J.H.; Lee, G.-W.; Yang, D.-H.; et al. Assessing the Efficacy of Bortezomib and Dexamethasone for Induction and Maintenance Therapy in Relapsed/Refractory Cutaneous T-Cell Lymphoma: A Phase II CISL1701/BIC Study. Cancer Res. Treat. 2025, 57, 267–279. [Google Scholar] [CrossRef]
- Zeng, T.; Jiang, T.; Yang, G.; Cheng, Z.; Lou, C.; Wei, W.; Tao, C.; Hu, S.; Wang, H.; Cui, X.; et al. Bortezomib in Previously Treated Phosphatase and Tension Homology-deficient Patients with Advanced Intrahepatic Cholangiocarcinoma: An Open-label, Prospective and Single-centre Phase II Trial. Clin. Transl. Med. 2024, 14, e1675. [Google Scholar] [CrossRef]
- Fischer, L.; Jiang, L.; Dürig, J.; Schmidt, C.; Stilgenbauer, S.; Bouabdallah, K.; Solal-Celigny, P.; Scholz, C.W.; Feugier, P.; de Wit, M.; et al. The Addition of Bortezomib to Rituximab, High-Dose Cytarabine and Dexamethasone in Relapsed or Refractory Mantle Cell Lymphoma—A Randomized, Open-Label Phase III Trial of the European Mantle Cell Lymphoma Network. Leukemia 2024, 38, 1307–1314. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Blonquist, T.; McMasters, M.; Hobbs, G.; McAfee, S.; Rosenblatt, J.; Amrein, P.; Connolly, C.; Ramos, A.; Logan, E.; et al. A Phase I Clinical Trial of Lenalidomide Combined with Bortezomib for Acute Myeloid Leukemia or Myelodysplastic Syndrome Relapsing after Allogeneic Stem Cell Transplantation. Leuk. Res. 2025, 153, 107693. [Google Scholar] [CrossRef]
- Gerber, D.E.; Boothman, D.A.; Fattah, F.J.; Dong, Y.; Zhu, H.; Skelton, R.A.; Priddy, L.L.; Vo, P.; Dowell, J.E.; Sarode, V.; et al. Phase 1 Study of Romidepsin plus Erlotinib in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2015, 90, 534–541. [Google Scholar] [CrossRef]
- Chiappella, A.; Dodero, A.; Evangelista, A.; Re, A.; Orsucci, L.; Usai, S.V.; Castellino, C.; Stefoni, V.; Pinto, A.; Zanni, M.; et al. Romidepsin-CHOEP Followed by High-Dose Chemotherapy and Stem-Cell Transplantation in Untreated Peripheral T-Cell Lymphoma: Results of the PTCL13 Phase Ib/II Study. Leukemia 2023, 37, 433–440. [Google Scholar] [CrossRef]
- Bachy, E.; Camus, V.; Thieblemont, C.; Sibon, D.; Casasnovas, R.-O.; Ysebaert, L.; Damaj, G.; Guidez, S.; Pica, G.M.; Kim, W.S.; et al. Romidepsin Plus CHOP Versus CHOP in Patients with Previously Untreated Peripheral T-Cell Lymphoma: Results of the Ro-CHOP Phase III Study (Conducted by LYSA). J. Clin. Oncol. 2022, 40, 242–251. [Google Scholar] [CrossRef]

| Biomarker | Biological Function | Detection Method | Clinical Relevance | Refs. |
|---|---|---|---|---|
| HIF-1α | A master regulator of cellular response to low oxygen; accumulates under hypoxia and binds hypoxia-response elements (HRE) on DNA to promote cell survival(targets include CA-IX, LOX, and GLUT-1). | Immunohistochemistry (IHC), Western blot (WB), qRT-PCR, ELISA, ChI | A meta-analysis involving 5177 patients demonstrated that high HIF-1α expression is associated with poorer survival and more aggressive breast cancer features, including overall survival (OS), disease-free survival (DFS), tumor stage (TNM), and estrogen receptor (ER) status; increased expression predicts poor prognosis in ovarian cancer and osteosarcoma. | [109,110,111,112] |
| Carbonic anhydrase IX (CAIX) | Regulates pH in hypoxic tumor cells by converting CO2 to bicarbonate and protons, supporting survival in acidic conditions. | IHC, ELISA, PET imaging with CAIX-targeted radiotracers | A prospective analysis involving high-risk non-metastatic ccRCC patients confirmed CAIX expression as a statistically significant prognostic biomarker for DFS and OS. High plasma CAIX levels serve as an independent prognostic biomarker in patients with non-small cell lung cancer (NSCLC), particularly in early stages (I and II). | [113,114] |
| Vascular endothelial growth factor (VEGF) | Key regulator of angiogenesis, promoting blood vessel formation to supply oxygen and nutrients, and modulating the tumor immune microenvironment. | ELISA (serum/plasma), IHC, qRT-PCR | Serum VEGF-A helps identify NSCLC patients benefiting from bevacizumab; higher hypoxia-related VEGF scores in necrotic breast tumors, especially basal-like subtype VEGF levels are affected by various factors besides hypoxia, which results in a variable correlation with direct measures of tumor oxygenation like pimonidazole. | [115,116,117] |
| Glucose transporter 1 (GLUT-1) | Glucose transporter is upregulated by HIF-1α under hypoxia to increase glucose uptake for anaerobic metabolism | IHC, WB, qRT-PCR, ELISA | Serum levels rise after radiotherapy in glioma, indicating tumor metabolic adaptation; overexpression is linked to radioresistance in breast cancer. High GLUT-1 correlates with poor prognosis in head and neck, lung, and pancreatic cancers, serving as a potential prognostic and therapeutic biomarker. | [118,119,120] |
| Lactate dehydrogenase (LDH) | Critical metabolic enzyme involved in glucose and glutamine metabolism, tumor pH regulation, and TCA cycle activity. | ELISA, serum biochemical assays | Elevated salivary LDH is found in HNC and OPMD compared to controls; serum LDH is prognostic in several cancers, including colorectal and prostate cancers. Activity correlates with aggressive breast cancer features. | [121,122] |
| Lysyl oxidase (LOX) | Involved in ECM remodeling and hypoxia-induced repression of E-cadherin via HIF-1. | IHC, qRT-PCR (tissue and blood samples) | LOX and HIF-1α expression increase with lymph node metastasis and tumor invasion, correlating with gastric cancer progression and poor prognosis; additionally, LOX is linked to patient survival, glioma cell differentiation, tumor recurrence, and may serve as a prognostic biomarker and therapeutic target for glioblastoma. | [123,124,125] |
| miR-210 | Regulates cellular metabolism under hypoxia by shifting energy production from oxidative phosphorylation to glycolysis; suppresses mitochondrial enzymes and increases ROS to support survival. | qRT-PCR (from plasma, serum, or tissue), microarray, next-generation sequencing (NGS) | MicroRNA-210 may serve as a biomarker for NSCLC detection; its high expression predicts poor prognosis in breast cancer and reflects hypoxia in bladder cancer, correlating with other markers, while microarray analyses identify it as a key hypoxia-induced miRNA across breast, head and neck, lung, colon, and kidney cancer cell lines. | [126,127,128,129] |
| Pimonidazole (PIMO) | Exogenous hypoxia marker (2-nitroimidazole) binds covalently to cellular macromolecules when oxygen levels drop below 1.3%. | IHC after administration as hypoxia marker | Oral PIMO revealed aggressive clinico-pathological features in localized prostate cancer Imaging-guided biopsies from 52 patients (43 given pimonidazole) showed aggressive hypoxia-driven prostate cancer phenotype, validated in two cohorts. Digital image analysis shows distinct differences between HIF-1α and PIMO as hypoxia biomarkers, indicating coexistence of different hypoxia forms in laryngeal cancer. | [130,131,132] |
| Hypoxia-Activated Prodrug (HAP) | Mechanism of Action | Key Outcomes | References |
|---|---|---|---|
| Tirapazamine | reduced to cytotoxic radical species in a hypoxic environment | improves outcome in non-operative hepatocellular cancer combined with transarterial embolization | [175,176,177] |
| PR-104 | converted to PR-104A, activated under hypoxia via one-electron reduction by cytochrome P450 oxidoreductase, generating cytotoxic metabolites | decreased tumor burden and prolonged survival in preclinical models of ALL, T-ALL, and AML, associated with disease response in a Phase I/II clinical trial | [178,179,180] |
| CP-506 | undergoes metabolic activation in hypoxic conditions and targets negatively charged guanine bases, disrupting DNA replication and leading to the death of hypoxic tumor cells | improvement in patients with hypopharyngeal squamous cell carcinoma | [173,181] |
| Evofosfamide (TH-302) | releases a DNA-crosslinking bromo-isophosphoramide mustard (Br-IPM) under low-oxygen conditions | promising results in trials with pancreatic cancer, prostate cancer, and melanoma | [182,183,184,185,186,187] |
| Tarloxotinib | activated only in low-oxygen conditions, releasing tarloxotinib-TKI, which irreversibly inhibits the pan-HER family (EGFR, HER2, HER4) by binding to conserved cysteine residues, blocking ERK and AKT pathways to suppress cell proliferation and survival | may help avoid bone marrow suppression, a common issue with cytotoxic HAPs that could limit their effectiveness when paired with ICIs | [188,189] |
| Biological Aspect | Effect of HBO | Key Outcomes | References |
|---|---|---|---|
| Apoptosis | Apoptosis induction in tumor cells | Activation of pro-apoptotic MAPK and inhibition of anti-apoptotic ERK in hematopoietic cells; Induction of apoptosis in osteosarcoma cells. | [210,214] |
| Tumor Angiogenesis | May inhibit neovascularization, but remains controversial | Reduced tumor peripheral vessel diameter and density in breast cancer and glioma models; Improved angiogenesis without increasing tumor growth in lung cancer xenograft models; Downregulation of proangiogenic genes (VEGFα, VEGFβ, FGFM, PDGF, TGFα) in rat adenocarcinoma. | [206,215,216] |
| Metastatic Potential | Does not enhance metastasis, may reduce tumor invasiveness by promoting a MET-like phenotype | No metastasis induced by HBO was observed in vivo in mouse models of squamous cell carcinoma and head and neck cancer; HBO promotes MET in DMBA-induced breast tumors, leading to less aggressive phenotype. | [202,216] |
| Chemotherapeutic Efficacy | Increases tumor perfusion and cellular sensitivity, enhances drug delivery to hypoxic tumor regions | Increased vascularization in large tumors (e.g., epithelial ovarian cancer), improving chemotherapy response; Increased GBM sensitivity to temozolomide; Slowed tumor growth and increased chemo efficacy in rat mammary tumors. | [217,218] |
| Radiotherapy Efficacy | Therapeutic agent for late radiation injuries; radiosensitizer enhancing radiotherapy effect | Study on 320 cervical cancer patients treated with radiotherapy and HBO showed significant improvement in local control and survival in the HBO group; Shown to reduce late radiation-induced injuries in the head and neck region, bones, prostate, and bladder. | [164,207] |
| HIF-1 Inhibitor | Mechanism of Action | Key Outcomes | References |
|---|---|---|---|
| EZN-2208 | Decreasing HIF-1α mRNA expression | - Shown to be safe and well-tolerated in a dose of 12–30 mg/m2 in young adults with solid tumors; - Its addition to bevacizumab resulted in decreased levels of HIF-1α protein levels and prolonged disease stabilization. | [174,219,220,221,222] |
| Topotecan | Decreasing HIF-1α mRNA translation | Decreased HIF-1α expression in advanced solid tumors. | [223] |
| EZN-2968 | - Decreased HIF-1 levels in patients with refractory solid tumors; - Prolonged disease stability. | [174,219,220,224] | |
| Romidepsin (FK228) | Decreasing HIF-1α stabilization | - Safe and well-tolerable in dosage of 8–14 mg/m2; - Inconclusive efficacy results. | [174,219,220,225,226,227,228] |
| Vorinostat | - Good tolerability of 100–400 mg doses; - Approved in therapy for cutaneous T-cell lymphoma, but failed to prove its efficacy in other cancer therapies. | [174,219,220,229,230,231,232,233,234,235] | |
| Panobinostat (LBH589) | - A total of 20 mg panobinostat increased efficacy of multiple myeloma therapy. | [174,219,220,236,237,238,239,240] | |
| Tanespimycin (17-AAG) | - Safe and well-tolerated at dosage 150–500 mg/m2; - Unclear data concerning efficacy. | [174,219,220,241,242,243,244,245,246,247] | |
| Lonafarnib (SCH66336) | - Well-tolerated in a dose of 150–200 mg twice a day; - Some promising data in the literature; however, further investigation is required. | [174,219,248,249,250,251,252] | |
| Belinostat | - Effective in treatment of relapsed/refractory peripheral T-cell lymphoma; - Its addition was shown beneficial in relapsed/refractory acute leukemia, T-cell prolymphocytic leukemia, and glioblastoma. | [174,219,253,254,255,256,257,258,259,260]] | |
| Chidamide | - Dose 20–30 mg 2x/week is well-tolerable; - Showed benefits in therapy of lymphoma and hemophagocytic lymphohistiocytosis. | [174,219,261,262,263,264,265,266,267] | |
| Bortezomib | Decreasing HIF-1α transcriptional activity | - Safe and well-tolerated in a dose 1.3 mg/m2; - Effective in treatment of renal cell carcinoma, lymphoma, and intrahepatic cholangiocarcinoma. | [174,219,268,269,270,271,272] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunachowicz, D.; Tomecka, P.; Sędzik, M.; Kalinin, J.; Kuźnicki, J.; Rembiałkowska, N. Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies. Cells 2025, 14, 1057. https://doi.org/10.3390/cells14141057
Kunachowicz D, Tomecka P, Sędzik M, Kalinin J, Kuźnicki J, Rembiałkowska N. Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies. Cells. 2025; 14(14):1057. https://doi.org/10.3390/cells14141057
Chicago/Turabian StyleKunachowicz, Dominika, Paulina Tomecka, Mikołaj Sędzik, Jarosław Kalinin, Jacek Kuźnicki, and Nina Rembiałkowska. 2025. "Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies" Cells 14, no. 14: 1057. https://doi.org/10.3390/cells14141057
APA StyleKunachowicz, D., Tomecka, P., Sędzik, M., Kalinin, J., Kuźnicki, J., & Rembiałkowska, N. (2025). Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies. Cells, 14(14), 1057. https://doi.org/10.3390/cells14141057






