Vitamin D in Cutaneous T-Cell Lymphoma
Abstract
1. Sunlight, Vitamin D, and Cutaneous T-Cell Lymphoma
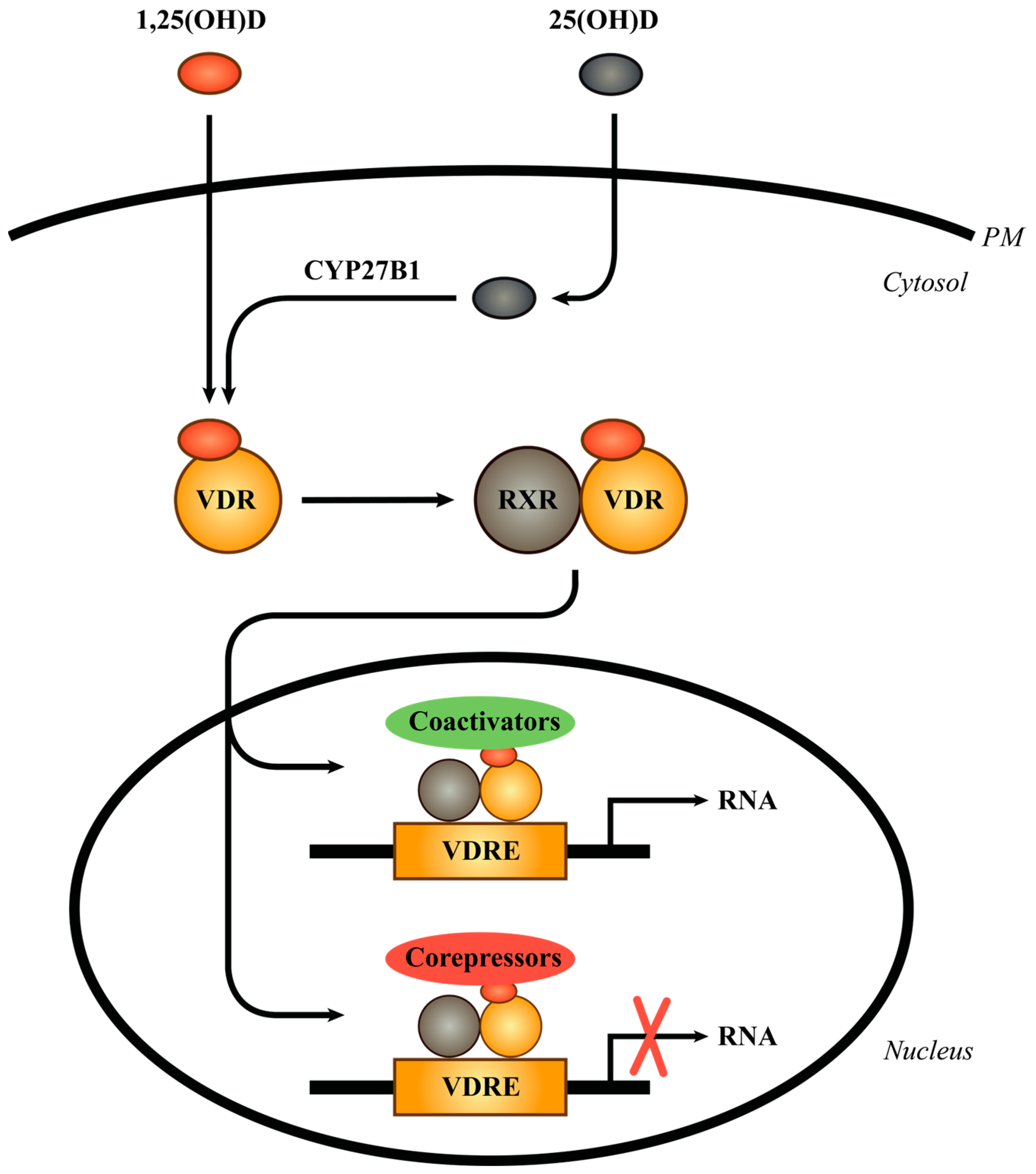
2. The Vitamin D Receptor
3. Effect of 1,25(OH)2D on Cells in the Skin
4. Overview of Cutaneous T-Cell Lymphoma
5. Vitamin D in CTCL
6. Vitamin D Serum Levels in CTCL
7. Vitamin D in the Pathogenesis of CTCL
8. Effects of Vitamin D Treatment in CTCL
9. Phototherapy and Sunlight Modulate Skin Inflammation in Vitamin D Independent Ways
10. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Holick, M.F.; Tian, X.Q.; Allen, M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci. USA 1995, 92, 3124–3126. [Google Scholar] [CrossRef]
- Holick, M.F.; MacLaughlin, J.A.; Doppelt, S.H. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science 1981, 211, 590–593. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Haddad, J.G.; Matsuoka, L.Y.; Hollis, B.W.; Hu, Y.Z.; Wortsman, J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Investig. 1993, 91, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Gospodarska, E.; Ghosh Dastidar, R.; Carlberg, C. Intervention Approaches in Studying the Response to Vitamin D3 Supplementation. Nutrients 2023, 15, 3382. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Palmer, M.T.; Lee, Y.K.; Maynard, C.L.; Oliver, J.R.; Bikle, D.D.; Jetten, A.M.; Weaver, C.T. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J. Biol. Chem. 2011, 286, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabrysova, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1alpha,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.W.; Bayraktar, U.D.; Kim, T.K.; St John, L.S.; Popat, U.; Khalili, J.; Molldrem, J.J.; Wieder, E.D.; Komanduri, K.V. Vitamin D receptor upregulation in alloreactive human T cells. Hum. Immunol. 2012, 73, 693–698. [Google Scholar] [CrossRef]
- Müller, K.; Odum, N.; Bendtzen, K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol. Lett. 1993, 35, 177–182. [Google Scholar] [CrossRef]
- Lemire, J.M.; Ince, A.; Takashima, M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 1992, 12, 143–148. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S.; Hou, T.Z.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D.M. Availability of 25-hydroxyvitamin D3 to APCs controls the balance between regulatory and inflammatory T cell responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; von Essen, M.R.; Levring, T.B.; Schjerling, P.; Woetmann, A.; Odum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D-binding protein controls T cell responses to vitamin D. BMC Immunol. 2014, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; Geisler, C. Macrophages Control the Bioavailability of Vitamin D and Vitamin D-Regulated T Cell Responses. Front. Immunol. 2021, 12, 722806. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Nemanic, M.K.; Gee, E.; Elias, P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Investig. 1986, 78, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Rode, A.K.O.; Kongsbak, M.; Hansen, M.M.; Lopez, D.V.; Levring, T.B.; Woetmann, A.; Odum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D Counteracts Mycobacterium tuberculosis-Induced Cathelicidin Downregulation in Dendritic Cells and Allows Th1 Differentiation and IFNgamma Secretion. Front. Immunol. 2017, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Talpur, R.; Cox, K.M.; Hu, M.; Geddes, E.R.; Parker, M.K.; Yang, B.Y.; Armstrong, P.A.; Liu, P.; Duvic, M. Vitamin D deficiency in mycosis fungoides and Sézary syndrome patients is similar to other cancer patients. Clin. Lymphoma Myeloma Leuk. 2014, 14, 518–524. [Google Scholar] [CrossRef]
- van Leeuwen, M.T.; Turner, J.J.; Falster, M.O.; Meagher, N.S.; Joske, D.J.; Grulich, A.E.; Giles, G.G.; Vajdic, C.M. Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. Int. J. Cancer 2013, 133, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.L.; Lish, K.M.; Yalowitz, C.B.; Soter, N.A. Ultraviolet-B phototherapy for early-stage cutaneous T-cell lymphoma. Arch. Dermatol. 1992, 128, 931–933. [Google Scholar] [CrossRef]
- Odutola, M.K.; van Leeuwen, M.T.; Bruinsma, F.; Turner, J.; Hertzberg, M.; Seymour, J.F.; Prince, H.M.; Trotman, J.; Verner, E.; Roncolato, F.; et al. A Population-Based Family Case-Control Study of Sun Exposure and Follicular Lymphoma Risk. Cancer Epidemiol. Biomark. Prev. 2024, 33, 106–116. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, T.; Liu, H.; Li, W.; Liu, X.; Qiu, L.; Li, L.; Zhou, S.; Qian, Z.; Dong, S.; et al. Risk factors for POD24 in patients with previously untreated follicular lymphoma: A systematic review and meta-analysis. Ann. Hematol. 2022, 101, 2383–2392. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, H.; Zhou, Y.; Shi, Y. Meta-analysis of the prognostic and clinical value of serum 25-hydroxyvitamin D levels in previously untreated lymphoma. Future Oncol. 2021, 17, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Tung-Hahn, E.; Mogilevskiy, V.; Black, E.; Morgan, M.; Tung, R. Pediatric primary cutaneous anaplastic large-cell lymphoma with associated hypovitaminosis D. Arch. Dermatol. Res. 2023, 316, 50. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Bain, D.L.; Heneghan, A.F.; Connaghan-Jones, K.D.; Miura, M.T. Nuclear receptor structure: Implications for function. Annu. Rev. Physiol. 2007, 69, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B.; Bishop, K.A. Regulation of target gene expression by the vitamin D receptor—An update on mechanisms. Rev. Endocr. Metab. Disord. 2012, 13, 45–55. [Google Scholar] [CrossRef]
- Carlberg, C. Genomic signaling of vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An Overview of Gene Regulation, Ranging from Metabolism to Genomic Effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Zouboulis, C.C.; Vogt, T.; Holick, M.F. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev. Endocr. Metab. Disord. 2016, 17, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Walworth, N.C.; Holick, M.F. Effect of 1 alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J. Investig. Dermatol. 1986, 86, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Uchida, Y.; Moradian, S.; Crumrine, D.; Elias, P.M.; Bikle, D.D. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J. Investig. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef]
- Tu, C.L.; Crumrine, D.A.; Man, M.Q.; Chang, W.; Elalieh, H.; You, M.; Elias, P.M.; Bikle, D.D. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J. Investig. Dermatol. 2012, 132, 2350–2359. [Google Scholar] [CrossRef]
- Oda, Y.; Hu, L.; Nguyen, T.; Fong, C.; Zhang, J.; Guo, P.; Bikle, D.D. Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair. J. Investig. Dermatol. 2018, 138, 2423–2431. [Google Scholar] [CrossRef]
- Zinser, G.M.; Sundberg, J.P.; Welsh, J. Vitamin D3 receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 2002, 23, 2103–2109. [Google Scholar] [CrossRef]
- Ellison, T.I.; Smith, M.K.; Gilliam, A.C.; MacDonald, P.N. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J. Investig. Dermatol. 2008, 128, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Peelen, E.; Knippenberg, S.; Muris, A.H.; Thewissen, M.; Smolders, J.; Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. Effects of vitamin D on the peripheral adaptive immune system: A review. Autoimmun. Rev. 2011, 10, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin D receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Al-Jaberi, F.A.H.; Crone, C.G.; Lindenstrøm, T.; Arildsen, N.S.; Lindeløv, E.S.; Aagaard, L.; Gravesen, E.; Mortensen, R.; Andersen, A.B.; Olgaard, K.; et al. Reduced vitamin D-induced cathelicidin production and killing of Mycobacterium tuberculosis in macrophages from a patient with a non-functional vitamin D receptor: A case report. Front. Immunol. 2022, 13, 1038960. [Google Scholar] [CrossRef]
- Feentved Ødum, S.L.; Kongsbak-Wismann, M. Vitamin D and SARS-CoV-2. Basic Clin. Pharmacol. Toxicol. 2023, 133, 6–15. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, C.Q. PD-1/PD-L1 Interaction Maintains Allogeneic Immune Tolerance Induced by Administration of Ultraviolet B-Irradiated Immature Dendritic Cells. J. Immunol. Res. 2016, 2016, 2419621. [Google Scholar] [CrossRef]
- Berger, C.L.; Hanlon, D.; Kanada, D.; Dhodapkar, M.; Lombillo, V.; Wang, N.; Christensen, I.; Howe, G.; Crouch, J.; El-Fishawy, P.; et al. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood 2002, 99, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.D.; Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kabashima, K.; Miyachi, Y. The panoply of αβT cells in the skin. J. Dermatol. Sci. 2014, 76, 3–9. [Google Scholar] [CrossRef]
- Pham, J.P.; Wark, K.J.L.; Woods, J.; Frew, J.W. Resident cutaneous memory T cells: A clinical review of their role in chronic inflammatory dermatoses and potential as therapeutic targets. Br. J. Dermatol. 2023, 189, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; van Etten, E.; Gysemans, C.; Decallonne, B.; Kato, S.; Laureys, J.; Depovere, J.; Valckx, D.; Verstuyf, A.; Bouillon, R. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J. Bone Miner. Res. 2001, 16, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaberi, F.A.H.; Kongsbak-Wismann, M.; Aguayo-Orozco, A.; Krogh, N.; Buus, T.B.; Lopez, D.V.; Rode, A.K.O.; Gravesen, E.; Olgaard, K.; Brunak, S.; et al. Impaired Vitamin D Signaling in T Cells From a Family With Hereditary Vitamin D Resistant Rickets. Front. Immunol. 2021, 12, 684015. [Google Scholar] [CrossRef]
- Yu, S.; Bruce, D.; Froicu, M.; Weaver, V.; Cantorna, M.T. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc. Natl. Acad. Sci. USA 2008, 105, 20834–20839. [Google Scholar] [CrossRef]
- Yu, S.; Cantorna, M.T. The vitamin D receptor is required for iNKT cell development. Proc. Natl. Acad. Sci. USA 2008, 105, 5207–5212. [Google Scholar] [CrossRef]
- Kongsbak, M.; von Essen, M.R.; Boding, L.; Levring, T.B.; Schjerling, P.; Lauritsen, J.P.; Woetmann, A.; Odum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS ONE 2014, 9, e96695. [Google Scholar] [CrossRef]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Daryabor, G.; Gholijani, N.; Kahmini, F.R. A review of the critical role of vitamin D axis on the immune system. Exp. Mol. Pathol. 2023, 132–133, 104866. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Damas, N.D.; Weinert, B.T.; Pus, U.; Torres-Rusillo, S.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; et al. Vitamin D Inhibits IL-22 Production Through a Repressive Vitamin D Response Element in the il22 Promoter. Front. Immunol. 2021, 12, 715059. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Cua, D.J.; Boonstra, A.; Richards, D.F.; Crain, C.; Savelkoul, H.F.; de Waal-Malefyt, R.; Coffman, R.L.; Hawrylowicz, C.M.; O’Garra, A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002, 195, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Toews, G.B.; Bergstresser, P.R.; Streilein, J.W. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J. Immunol. 1980, 124, 445–453. [Google Scholar] [CrossRef] [PubMed]
- De Fabo, E.C.; Noonan, F.P. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 1983, 158, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Bergstresser, P.R.; Tigelaar, R.E.; Wood, P.J.; Streilein, J.W. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J. Exp. Med. 1983, 158, 781–794. [Google Scholar] [CrossRef]
- Robert, C.; Kupper, T.S. Inflammatory skin diseases, T cells, and immune surveillance. N. Engl. J. Med. 1999, 341, 1817–1828. [Google Scholar] [CrossRef]
- Schwarz, T. The dark and the sunny sides of UVR-induced immunosuppression: Photoimmunology revisited. J. Investig. Dermatol. 2010, 130, 49–54. [Google Scholar] [CrossRef]
- Kang, K.; Hammerberg, C.; Meunier, L.; Cooper, K.D. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J. Immunol. 1994, 153, 5256–5264. [Google Scholar] [CrossRef]
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Jaksic, A.; Noonan, F.P.; Finlay-Jones, J.J. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998, 187, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Vieyra-Garcia, P.A.; Wolf, P. From Early Immunomodulatory Triggers to Immunosuppressive Outcome: Therapeutic Implications of the Complex Interplay Between the Wavebands of Sunlight and the Skin. Front. Med. 2018, 5, 232. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Grigalavicius, M.; Juraleviciute, M.; Grant, W.B. Phototherapy and vitamin D. Clin. Dermatol. 2016, 34, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- El Bedewi, A.; El Anany, G.; El Mofty, M. Role of Synchrotron infra red microspectroscopy in studying epidermotropism of cutaneous T-cell lymphoma. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, A.; Surmanowicz, P.; Alhusayen, R.; Hull, P.; Litvinov, I.V.; Zhou, Y.; Gniadecki, R. Immunotherapy for Cutaneous T-Cell Lymphoma: Current Landscape and Future Developments. J. Cutan. Med. Surg. 2019, 23, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Assaf, C.; Waser, N.; Bagot, M.; He, M.; Li, T.; Dalal, M.; Gavini, F.; Trinchese, F.; Zomas, A.; Little, M.; et al. Contemporary Treatment Patterns and Response in Relapsed/Refractory Cutaneous T-Cell Lymphoma (CTCL) across Five European Countries. Cancers 2021, 14, 145. [Google Scholar] [CrossRef]
- Dai, J.; Duvic, M. Cutaneous T-Cell Lymphoma: Current and Emerging Therapies. Oncology 2023, 37, 55–62. [Google Scholar] [CrossRef]
- Weiner, D.M.; Durgin, J.S.; Wysocka, M.; Rook, A.H. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: Current and future approaches. J. Am. Acad. Dermatol. 2021, 84, 597–604. [Google Scholar] [CrossRef]
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J.; Kim, Y.H.; Stonesifer, C.; Tensen, C.P.; Geskin, L.J.; Quaglino, P.; Ramelyte, E. Cutaneous T cell lymphoma. Nat. Rev. Dis. Primers 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Ghazawi, F.M.; Alghazawi, N.; Le, M.; Netchiporouk, E.; Glassman, S.J.; Sasseville, D.; Litvinov, I.V. Environmental and Other Extrinsic Risk Factors Contributing to the Pathogenesis of Cutaneous T Cell Lymphoma (CTCL). Front. Oncol. 2019, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.R.; Adeuyan, O.; Schreidah, C.M.; Chen, C.; Trager, M.H.; Lapolla, B.A.; Fahmy, L.M.; Weng, C.; Geskin, L.J. Clusters, crop dusters, and myth busters: A scoping review of environmental exposures and cutaneous T-cell lymphoma. Ital. J. Dermatol. Venerol. 2023, 158, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Ghazawi, F.M.; Netchiporouk, E.; Rahme, E.; Tsang, M.; Moreau, L.; Glassman, S.; Provost, N.; Gilbert, M.; Jean, S.E.; Roshdy, O.; et al. Distribution and Clustering of Cutaneous T-Cell Lymphoma (CTCL) Cases in Canada During 1992 to 2010. J. Cutan. Med. Surg. 2018, 22, 154–165. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Netchiporouk, E.; Rahme, E.; Tsang, M.; Moreau, L.; Glassman, S.; Provost, N.; Gilbert, M.; Jean, S.E.; Pehr, K.; et al. Comprehensive analysis of cutaneous T-cell lymphoma (CTCL) incidence and mortality in Canada reveals changing trends and geographic clustering for this malignancy. Cancer 2017, 123, 3550–3567. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Tetzlaff, M.T.; Thibault, P.; Gangar, P.; Moreau, L.; Watters, A.K.; Netchiporouk, E.; Pehr, K.; Prieto, V.G.; Rahme, E.; et al. Gene expression analysis in Cutaneous T-Cell Lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. Oncoimmunology 2017, 6, e1306618. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Goh, G.; Walradt, T.; Hong, B.S.; Bunick, C.G.; Chen, K.; Bjornson, R.D.; Maman, Y.; Wang, T.; Tordoff, J.; et al. Genomic landscape of cutaneous T cell lymphoma. Nat. Genet. 2015, 47, 1011–1019. [Google Scholar] [CrossRef]
- da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat. Genet. 2015, 47, 1465–1470. [Google Scholar] [CrossRef]
- Buus, T.B.; Willerslev-Olsen, A.; Fredholm, S.; Blümel, E.; Nastasi, C.; Gluud, M.; Hu, T.; Lindahl, L.M.; Iversen, L.; Fogh, H.; et al. Single-cell heterogeneity in Sézary syndrome. Blood Adv. 2018, 2, 2115–2126. [Google Scholar] [CrossRef]
- Jiang, T.T.; Cao, S.; Kruglov, O.; Virmani, A.; Geskin, L.J.; Falo, L.D., Jr.; Akilov, O.E. Deciphering Tumor Cell Evolution in Cutaneous T-Cell Lymphomas: Distinct Differentiation Trajectories in Mycosis Fungoides and Sézary Syndrome. J. Investig. Dermatol. 2023. [Google Scholar] [CrossRef]
- Rindler, K.; Bauer, W.M.; Jonak, C.; Wielscher, M.; Shaw, L.E.; Rojahn, T.B.; Thaler, F.M.; Porkert, S.; Simonitsch-Klupp, I.; Weninger, W.; et al. Single-Cell RNA Sequencing Reveals Tissue Compartment-Specific Plasticity of Mycosis Fungoides Tumor Cells. Front. Immunol. 2021, 12, 666935. [Google Scholar] [CrossRef]
- Gaydosik, A.M.; Tabib, T.; Geskin, L.J.; Bayan, C.A.; Conway, J.F.; Lafyatis, R.; Fuschiotti, P. Single-Cell Lymphocyte Heterogeneity in Advanced Cutaneous T-cell Lymphoma Skin Tumors. Clin. Cancer Res. 2019, 25, 4443–4454. [Google Scholar] [CrossRef] [PubMed]
- Hamrouni, A.; Fogh, H.; Zak, Z.; Ødum, N.; Gniadecki, R. Clonotypic Diversity of the T-cell Receptor Corroborates the Immature Precursor Origin of Cutaneous T-cell Lymphoma. Clin. Cancer Res. 2019, 25, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, N.; Voigt, A.P.; Liu, V.; Link, B.K.; Zhang, W.; Jabbari, A. Single-Cell Profiling of Cutaneous T-Cell Lymphoma Reveals Underlying Heterogeneity Associated with Disease Progression. Clin. Cancer Res. 2019, 25, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, C.B.; Desale, S.; Fernandez, S.J.; Shenoy, A.G. The impact of environmental ultraviolet exposure on the clinical course of mycosis fungoides. J. Am. Acad. Dermatol. 2019, 81, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Linser, K.; Harnack, K. Heliotherapy of mycosis fungoides. Arch. Klin. Exp. Dermatol. 1962, 215, 181–201. [Google Scholar] [CrossRef]
- Tippel, H.; Engst, R. Mycosis fungoides. Results of helioclimate therapy in high mountains (Davos, 1,560). Hautarzt 1986, 37, 450–453. [Google Scholar] [PubMed]
- Hodak, E.; Pavlovsky, L. Phototherapy of Mycosis Fungoides. Dermatol. Clin. 2015, 33, 697–702. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.M.; Olsen, J.; Johansen, P.; Kaerlev, L.; Guénel, P.; Arveux, P.; Wingren, G.; Hardell, L.; Ahrens, W.; Stang, A.; et al. Occupational sun exposure and mycosis fungoides: A European multicenter case-control study. J. Occup. Environ. Med. 2006, 48, 390–393. [Google Scholar] [CrossRef]
- Song, X.; Chang, S.; Seminario-Vidal, L.; de Mingo Pulido, A.; Tordesillas, L.; Song, X.; Reed, R.A.; Harkins, A.; Whiddon, S.; Nguyen, J.V.; et al. Genomic and Single-Cell Landscape Reveals Novel Drivers and Therapeutic Vulnerabilities of Transformed Cutaneous T-cell Lymphoma. Cancer Discov. 2022, 12, 1294–1313. [Google Scholar] [CrossRef]
- Jones, C.L.; Degasperi, A.; Grandi, V.; Amarante, T.D.; Mitchell, T.J.; Nik-Zainal, S.; Whittaker, S.J. Spectrum of mutational signatures in T-cell lymphoma reveals a key role for UV radiation in cutaneous T-cell lymphoma. Sci. Rep. 2021, 11, 3962. [Google Scholar] [CrossRef]
- Gniadecki, R.; O’Keefe, S.; Hennessey, D.; Iyer, A. Is Cutaneous T-Cell Lymphoma Caused by Ultraviolet Radiation? A Comparison of UV Mutational Signatures in Malignant Melanoma and Mycosis Fungoides. Cells 2023, 12, 1616. [Google Scholar] [CrossRef]
- Volden, G. A study of the photosensitive factor in relation to skin lesions of mycosis fungoides patients. Dermatologica 1980, 161, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.B.; Switchenko, J.; Ayers, A.; Kim, E.; Lechowicz, M.J. Risk of bacteremia in patients with cutaneous T-cell lymphoma (CTCL). Leuk. Lymphoma 2020, 61, 2652–2658. [Google Scholar] [CrossRef]
- Axelrod, P.I.; Lorber, B.; Vonderheid, E.C. Infections complicating mycosis fungoides and Sézary syndrome. JAMA 1992, 267, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Bonin, S.; Tothova, S.M.; Barbazza, R.; Brunetti, D.; Stanta, G.; Trevisan, G. Evidence of multiple infectious agents in mycosis fungoides lesions. Exp. Mol. Pathol. 2010, 89, 46–50. [Google Scholar] [CrossRef]
- Dobos, G.; Assaf, C. Transcriptomic changes during stage progression of mycosis fungoides: From translational analyses to their potential clinical implications. Br. J. Dermatol. 2022, 186, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Mirvish, E.D.; Pomerantz, R.G.; Geskin, L.J. Infectious agents in cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2011, 64, 423–431. [Google Scholar] [CrossRef]
- Willerslev-Olsen, A.; Krejsgaard, T.; Lindahl, L.M.; Bonefeld, C.M.; Wasik, M.A.; Koralov, S.B.; Geisler, C.; Kilian, M.; Iversen, L.; Woetmann, A.; et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins 2013, 5, 1402–1421. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Gao, Y.; Liu, F.; Pan, H.; Tu, P.; Wang, Y. Characteristics of Staphylococcus aureus Colonization in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2024, 144, 188–191. [Google Scholar] [CrossRef]
- Guenova, E.; Ødum, N. Old Sins Cast Long Shadows: News on Staphylococcus aureus in Cutaneous T Cell Lymphoma. J. Investig. Dermatol. 2024, 144, 8–10. [Google Scholar] [CrossRef]
- Odum, N.; Lindahl, L.M.; Wod, M.; Krejsgaard, T.; Skytthe, A.; Woetmann, A.; Iversen, L.; Christensen, K. Investigating heredity in cutaneous T-cell lymphoma in a unique cohort of Danish twins. Blood Cancer J. 2017, 7, e517. [Google Scholar] [CrossRef]
- Gluud, M.; Pallesen, E.M.H.; Buus, T.B.; Gjerdrum, L.M.R.; Lindahl, L.M.; Kamstrup, M.R.; Bzorek, M.; Danielsen, M.; Bech, R.; Monteiro, M.N.; et al. Malignant T cells induce skin barrier defects through cytokine-mediated JAK/STAT signaling in cutaneous T-cell lymphoma. Blood 2023, 141, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Jackow, C.M.; Cather, J.C.; Hearne, V.; Asano, A.T.; Musser, J.M.; Duvic, M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood 1997, 89, 32–40. [Google Scholar] [CrossRef]
- Nguyen, V.; Huggins, R.H.; Lertsburapa, T.; Bauer, K.; Rademaker, A.; Gerami, P.; Guitart, J. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J. Am. Acad. Dermatol. 2008, 59, 949–952. [Google Scholar] [CrossRef]
- Kadin, M.E.; Hamilton, R.G.; Vonderheid, E.C. Evidence linking atopy and staphylococcal superantigens to the pathogenesis of lymphomatoid papulosis, a recurrent CD30+ cutaneous lymphoproliferative disorder. PLoS ONE 2020, 15, e0228751. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, C.K.; Willerslev-Olsen, A.; Namini, M.R.J.; Zeng, Z.; Yan, L.; Danielsen, M.; Gluud, M.; Pallesen, E.M.H.; Wojewoda, K.; Osmancevic, A.; et al. Staphylococcus aureus induce drug resistance in cancer T cells in Sézary Syndrome. Blood 2024. [Google Scholar] [CrossRef]
- Talpur, R.; Bassett, R.; Duvic, M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2008, 159, 105–112. [Google Scholar] [CrossRef]
- Lindahl, L.M.; Willerslev-Olsen, A.; Gjerdrum, L.M.R.; Nielsen, P.R.; Blümel, E.; Rittig, A.H.; Celis, P.; Herpers, B.; Becker, J.C.; Stausbøl-Grøn, B.; et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood 2019, 134, 1072–1083. [Google Scholar] [CrossRef]
- Lindahl, L.M.; Iversen, L.; Ødum, N.; Kilian, M. Staphylococcus aureus and Antibiotics in Cutaneous T-Cell Lymphoma. Dermatology 2022, 238, 551–553. [Google Scholar] [CrossRef]
- Emge, D.A.; Bassett, R.L.; Duvic, M.; Huen, A.O. Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen in erythrodermic cutaneous T-cell lymphoma (CTCL) patients. Arch. Dermatol. Res. 2020, 312, 283–288. [Google Scholar] [CrossRef]
- Lewis, D.J.; Holder, B.B.; Duvic, M. The “Duvic regimen” for erythrodermic flares secondary to Staphylococcus aureus in mycosis fungoides and Sézary syndrome. Int. J. Dermatol. 2018, 57, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, E.M.H.; Gluud, M.; Vadivel, C.K.; Buus, T.B.; de Rooij, B.; Zeng, Z.; Ahmad, S.; Willerslev-Olsen, A.; Röhrig, C.; Kamstrup, M.R.; et al. Endolysin Inhibits Skin Colonization by Patient-Derived Staphylococcus Aureus and Malignant T-Cell Activation in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2023, 143, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Kawaguchi, M.; Takahashi, N.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Skin barrier dysfunction and low antimicrobial peptide expression in cutaneous T-cell lymphoma. Clin. Cancer Res. 2014, 20, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Mitsui, H.; Witte, K.; Gellrich, S.; Gulati, N.; Humme, D.; Witte, E.; Gonsior, M.; Beyer, M.; Kadin, M.E.; et al. Deficient cutaneous antibacterial competence in cutaneous T-cell lymphomas: Role of Th2-mediated biased Th17 function. Clin. Cancer Res. 2014, 20, 5507–5516. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Miyagaki, T.; Kamijo, H.; Oka, T.; Shishido-Takahashi, N.; Suga, H.; Sugaya, M.; Sato, S. Decreased progranulin expression in Mycosis fungoides: A possible association with the high frequency of skin infections. Eur. J. Dermatol. 2018, 28, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Nakamura, M.; Kabashima-Kubo, R.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Defective epidermal innate immunity and resultant superficial dermatophytosis in adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2012, 18, 3772–3779. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Drake, M.T.; Maurer, M.J.; Link, B.K.; Habermann, T.M.; Ansell, S.M.; Micallef, I.N.; Kelly, J.L.; Macon, W.R.; Nowakowski, G.S.; Inwards, D.J.; et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 4191–4198. [Google Scholar] [CrossRef]
- Kelly, J.L.; Drake, M.T.; Fredericksen, Z.S.; Asmann, Y.W.; Liebow, M.; Shanafelt, T.D.; Feldman, A.L.; Ansell, S.M.; Macon, W.R.; Herr, M.M.; et al. Early life sun exposure, vitamin D-related gene variants, and risk of non-Hodgkin lymphoma. Cancer Causes Control 2012, 23, 1017–1029. [Google Scholar] [CrossRef]
- Kricker, A.; Armstrong, B.K.; Hughes, A.M.; Goumas, C.; Smedby, K.E.; Zheng, T.; Spinelli, J.J.; De Sanjosé, S.; Hartge, P.; Melbye, M.; et al. Personal sun exposure and risk of non Hodgkin lymphoma: A pooled analysis from the Interlymph Consortium. Int. J. Cancer 2008, 122, 144–154. [Google Scholar] [CrossRef]
- Yu, Z.; Wolf, P. How It Works: The Immunology Underlying Phototherapy. Dermatol. Clin. 2020, 38, 37–53. [Google Scholar] [CrossRef]
- Vieyra-Garcia, P.A.; Wolf, P. A deep dive into UV-based phototherapy: Mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol. Ther. 2021, 222, 107784. [Google Scholar] [CrossRef]
- Ma, Y.; Trump, D.L.; Johnson, C.S. Vitamin D in combination cancer treatment. J. Cancer 2010, 1, 101–107. [Google Scholar] [CrossRef]
- Rasheed, H.; Hegazy, R.A.; Gawdat, H.I.; Mehaney, D.A.; Kamel, M.M.; Fawzy, M.M.; Nooh, M.M.; Darwish, H.A. Serum Vitamin D and Vitamin D Receptor Gene Polymorphism in Mycosis Fungoides Patients: A Case Control Study. PLoS ONE 2016, 11, e0158014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Incel Uysal, P.; Alli, N.; Hayran, Y.; Candar, T. Mycosis Fungoides and Vitamin D Status: Analyses of Serum 25-Hydroxyvitamin D Levels and Single Nucleotide Polymorphisms in the Vitamin D Receptor Gene. Acta Dermatovenerol. Croat. 2018, 26, 8–14. [Google Scholar] [PubMed]
- Velissari, A.; Lakiotaki, E.; Nikolaou, V.; Argyropoulos, K.V.; Stratigos, A.; Daikos, G.; Konstantopoulos, K.; Siakantaris, M. Genetic polymorphisms in immunity related genes and the vitamin D receptor gene and risk of cutaneous T-cell lymphoma in Greek population. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e805–e807. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Belzberg, M.; Hughes, J.M.; Mahadevan, V.; Khanna, R.; Bakhshi, P.R.; Hong, M.S.; Williams, K.A.; Grossberg, A.L.; Kwatra, S.G.; et al. Comorbidities in Mycosis Fungoides and Racial Differences in Co-Existent Lymphomatoid Papulosis: A Cross-Sectional Study of 580 Patients in an Urban Tertiary Care Center. Medicines 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Mrotzek, C.; Felcht, M.; Sommer, A.; Schrader, A.; Klemke, C.D.; Herling, M.; Schlaak, M.; Fabri, M. Vitamin D controls apoptosis and proliferation of cutaneous T-cell lymphoma cells. Exp. Dermatol. 2015, 24, 798–800. [Google Scholar] [CrossRef]
- Kim, E.J.; Hess, S.; Richardson, S.K.; Newton, S.; Showe, L.C.; Benoit, B.M.; Ubriani, R.; Vittorio, C.C.; Junkins-Hopkins, J.M.; Wysocka, M.; et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J. Clin. Investig. 2005, 115, 798–812. [Google Scholar] [CrossRef]
- Ødum, N. Deregulated signalling and inflammation in cutaneous T-cell lymphoma. Br. J. Dermatol. 2020, 182, 16–17. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ikeda, S.; Abe, F.; Takahashi, Y.; Kitadate, A.; Takahashi, N.; Wakui, H.; Tagawa, H. Downregulation of miR-26 promotes invasion and metastasis via targeting interleukin-22 in cutaneous T-cell lymphoma. Cancer Sci. 2022, 113, 1208–1219. [Google Scholar] [CrossRef]
- Wang, L.; DeMarco, S.S.; Chen, J.; Phillips, C.M.; Bridges, L.C. Retinoids Bias Integrin Expression and Function in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2015, 135, 2102–2108. [Google Scholar] [CrossRef]
- Krejsgaard, T.; Lindahl, L.M.; Mongan, N.P.; Wasik, M.A.; Litvinov, I.V.; Iversen, L.; Langhoff, E.; Woetmann, A.; Odum, N. Malignant inflammation in cutaneous T-cell lymphoma-a hostile takeover. Semin. Immunopathol. 2017, 39, 269–282. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Inducing endogenous antimicrobial peptides to battle infections. Proc. Natl. Acad. Sci. USA 2006, 103, 8913–8914. [Google Scholar] [CrossRef] [PubMed]
- Gunville, C.F.; Mourani, P.M.; Ginde, A.A. The role of vitamin D in prevention and treatment of infection. Inflamm. Allergy Drug Targets 2013, 12, 239–245. [Google Scholar] [CrossRef]
- Gaydosik, A.M.; Queen, D.S.; Trager, M.H.; Akilov, O.E.; Geskin, L.J.; Fuschiotti, P. Genome-wide transcriptome analysis of the STAT6-regulated genes in advanced-stage cutaneous T-cell lymphoma. Blood 2020, 136, 1748–1759. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Tang, R.; Ouyang, S.; Li, S.; Wu, J. Vitamin D inhibits palmitate-induced macrophage pro-inflammatory cytokine production by targeting the MAPK pathway. Immunol. Lett. 2018, 202, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, V.; Bouttier, M.; Boukhaled, G.; Salehi-Tabar, R.; Avramescu, R.G.; Memari, B.; Hasaj, B.; Lukacs, G.L.; Krawczyk, C.M.; White, J.H. Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J. Biol. Chem. 2017, 292, 20657–20668. [Google Scholar] [CrossRef] [PubMed]
- Lovato, P.; Norsgaard, H.; Tokura, Y.; Ropke, M.A. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J. Dermatol. Sci. 2016, 81, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Thien, R.; Baier, K.; Pietschmann, P.; Peterlik, M.; Willheim, M. Interactions of 1 alpha,25-dihydroxyvitamin D3 with IL-12 and IL-4 on cytokine expression of human T lymphocytes. J. Allergy Clin. Immunol. 2005, 116, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Bendix, M.; Greisen, S.; Dige, A.; Hvas, C.L.; Bak, N.; Jørgensen, S.P.; Dahlerup, J.F.; Deleuran, B.; Agnholt, J. Vitamin D increases programmed death receptor-1 expression in Crohn’s disease. Oncotarget 2017, 8, 24177–24186. [Google Scholar] [CrossRef]
- Koren, R.; Liberman, U.A.; Maron, L.; Novogrodsky, A.; Ravid, A. 1,25-Dihydroxyvitamin D3 acts directly on human lymphocytes and interferes with the cellular response to interleukin-2. Immunopharmacology 1989, 18, 187–194. [Google Scholar] [CrossRef]
- Scott-Mackie, P.; Hickish, T.; Mortimer, P.; Sloane, J.; Cunningham, D. Calcipotriol and regression in T-cell lymphoma of skin. Lancet 1993, 342, 172. [Google Scholar] [CrossRef]
- French, L.E.; Ramelet, A.A.; Saurat, J.H. Remission of cutaneous T-cell lymphoma with combined calcitriol and acitretin. Lancet 1994, 344, 686–687. [Google Scholar] [CrossRef]
- Bagot, M. Treatment of cutaneous T-cell lymphoma by retinoids and calcitriol. Lancet 1995, 346, 376–377. [Google Scholar] [PubMed]
- Thomsen, K. Cutaneous T-cell lymphoma and calcitriol and isotretinoin treatment. Lancet 1995, 345, 1583. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Vieyra-Garcia, P.; Benezeder, T.; Crouch, J.D.; Kim, I.R.; O’Malley, J.T.; Devlin, P.M.; Gehad, A.; Zhan, Q.; Gudjonsson, J.E.; et al. Phototherapy Restores Deficient Type I IFN Production and Enhances Antitumor Responses in Mycosis Fungoides. J. Investig. Dermatol. 2023, 144, 621–632. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ødum, A.-W.F.; Geisler, C. Vitamin D in Cutaneous T-Cell Lymphoma. Cells 2024, 13, 503. https://doi.org/10.3390/cells13060503
Ødum A-WF, Geisler C. Vitamin D in Cutaneous T-Cell Lymphoma. Cells. 2024; 13(6):503. https://doi.org/10.3390/cells13060503
Chicago/Turabian StyleØdum, August-Witte Feentved, and Carsten Geisler. 2024. "Vitamin D in Cutaneous T-Cell Lymphoma" Cells 13, no. 6: 503. https://doi.org/10.3390/cells13060503
APA StyleØdum, A.-W. F., & Geisler, C. (2024). Vitamin D in Cutaneous T-Cell Lymphoma. Cells, 13(6), 503. https://doi.org/10.3390/cells13060503




