Leucine Supplementation Prevents the Development of Skeletal Muscle Dysfunction in a Rat Model of HFpEF
Abstract
1. Introduction
2. Materials and Methods
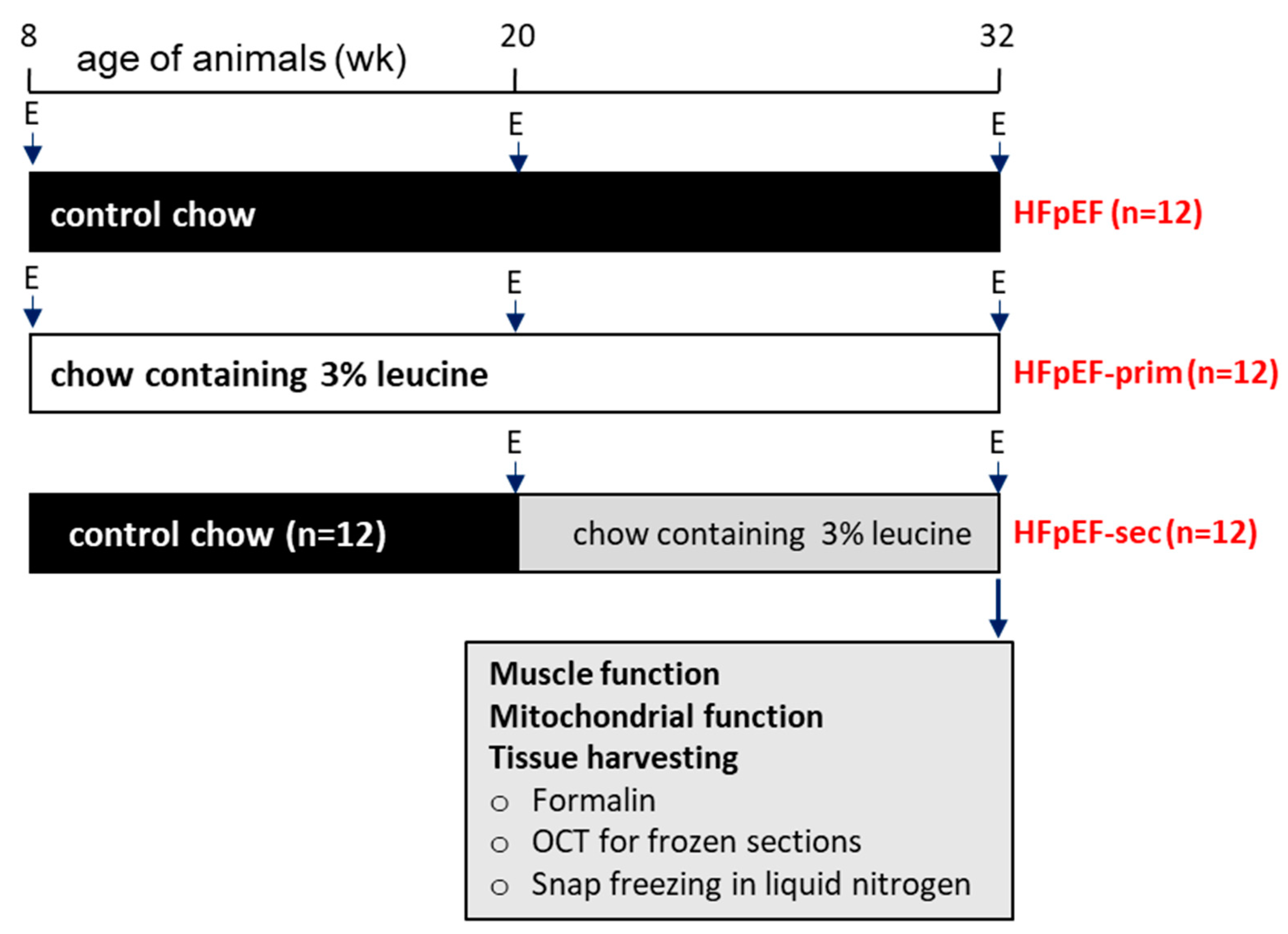
2.1. Study Design
2.2. Echocardiography
2.3. Skeletal Muscle Function
2.4. Assessment of Mitochondrial Function
2.5. Western Blot Analyses
2.6. Citrate Synthase Activity Measurement
2.7. Statistical Analyses
3. Results
3.1. Animal Characteristics
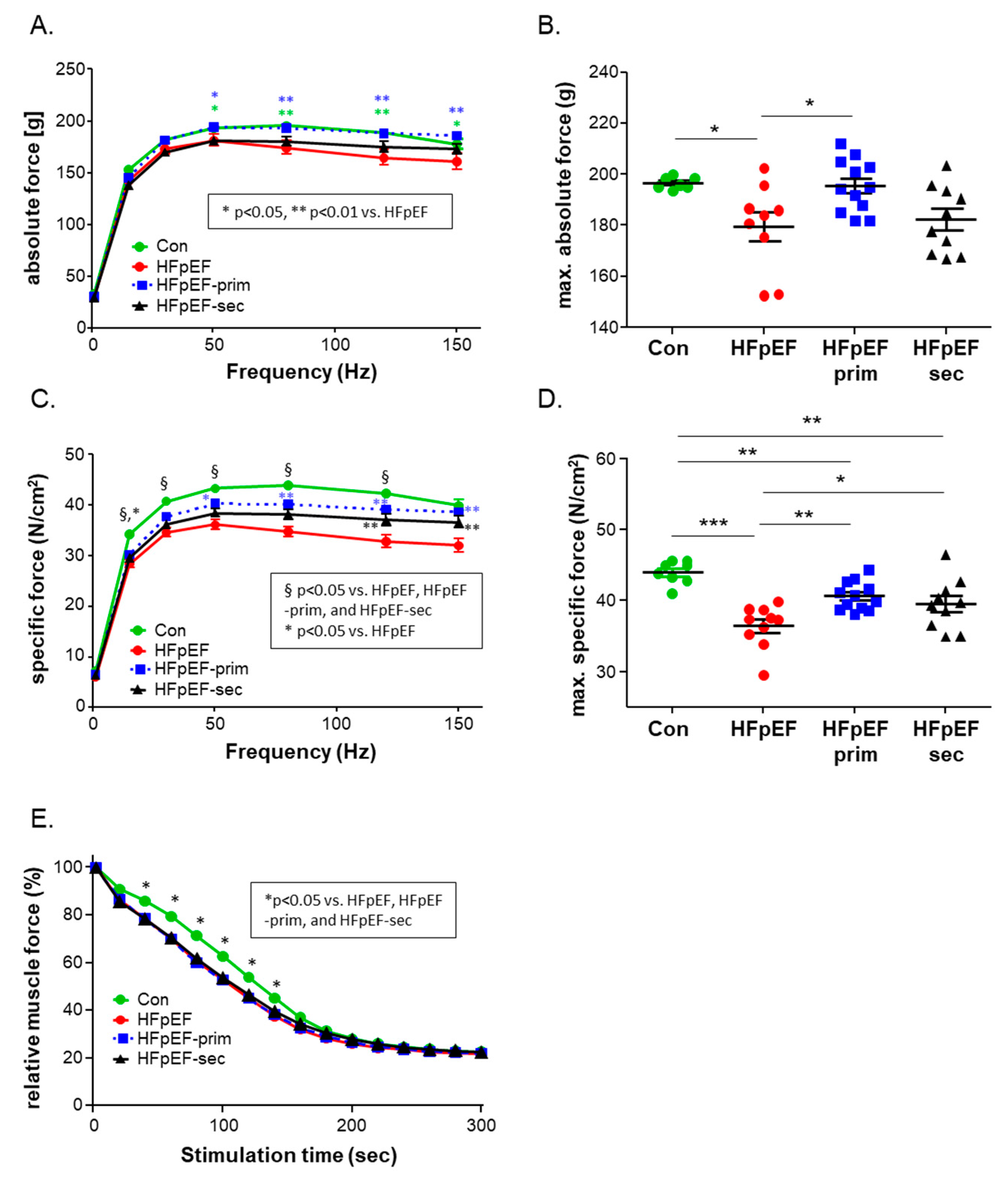
3.2. Impact of Leucine on Muscle Trophicity and Function
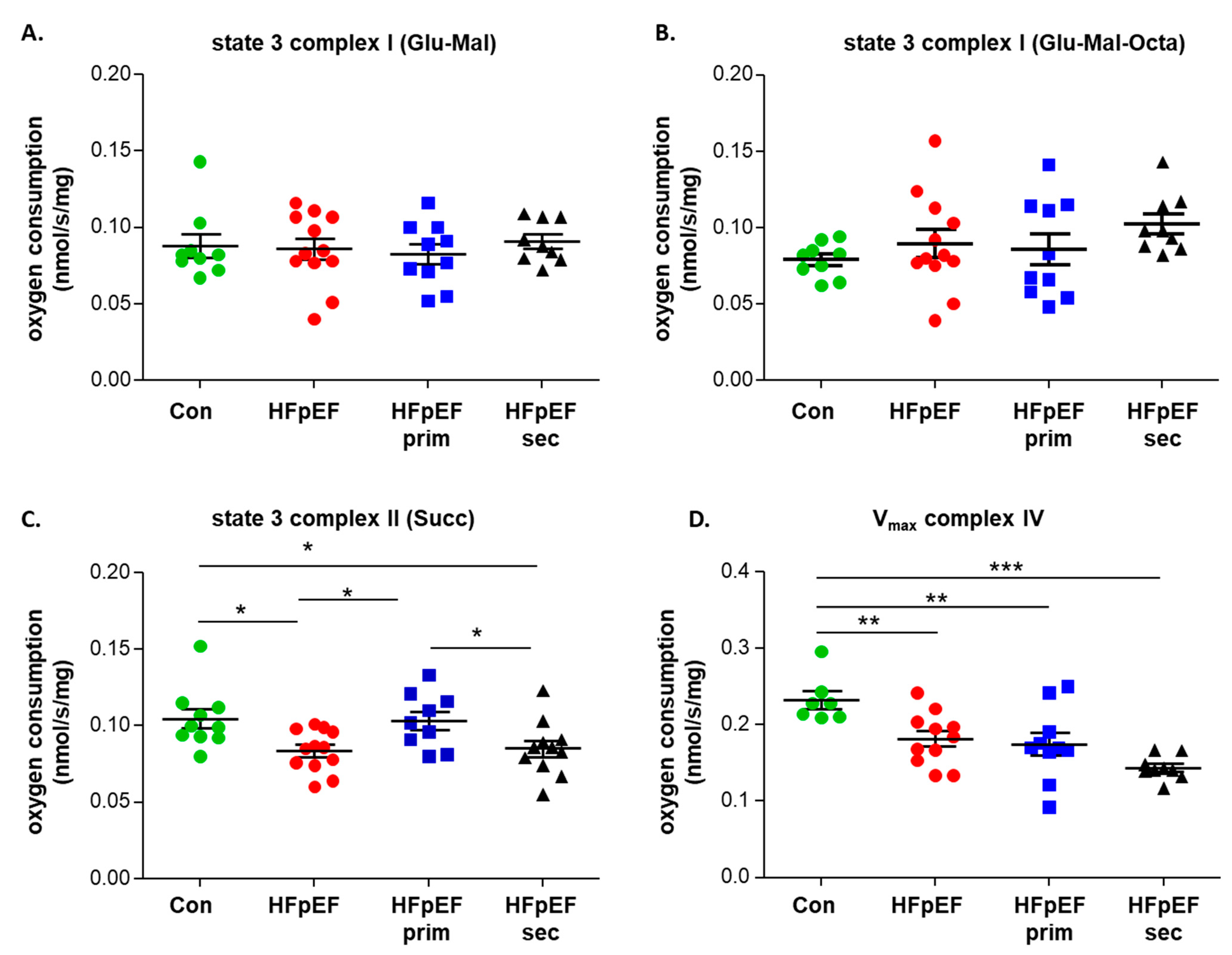
3.3. Mitochondrial Function
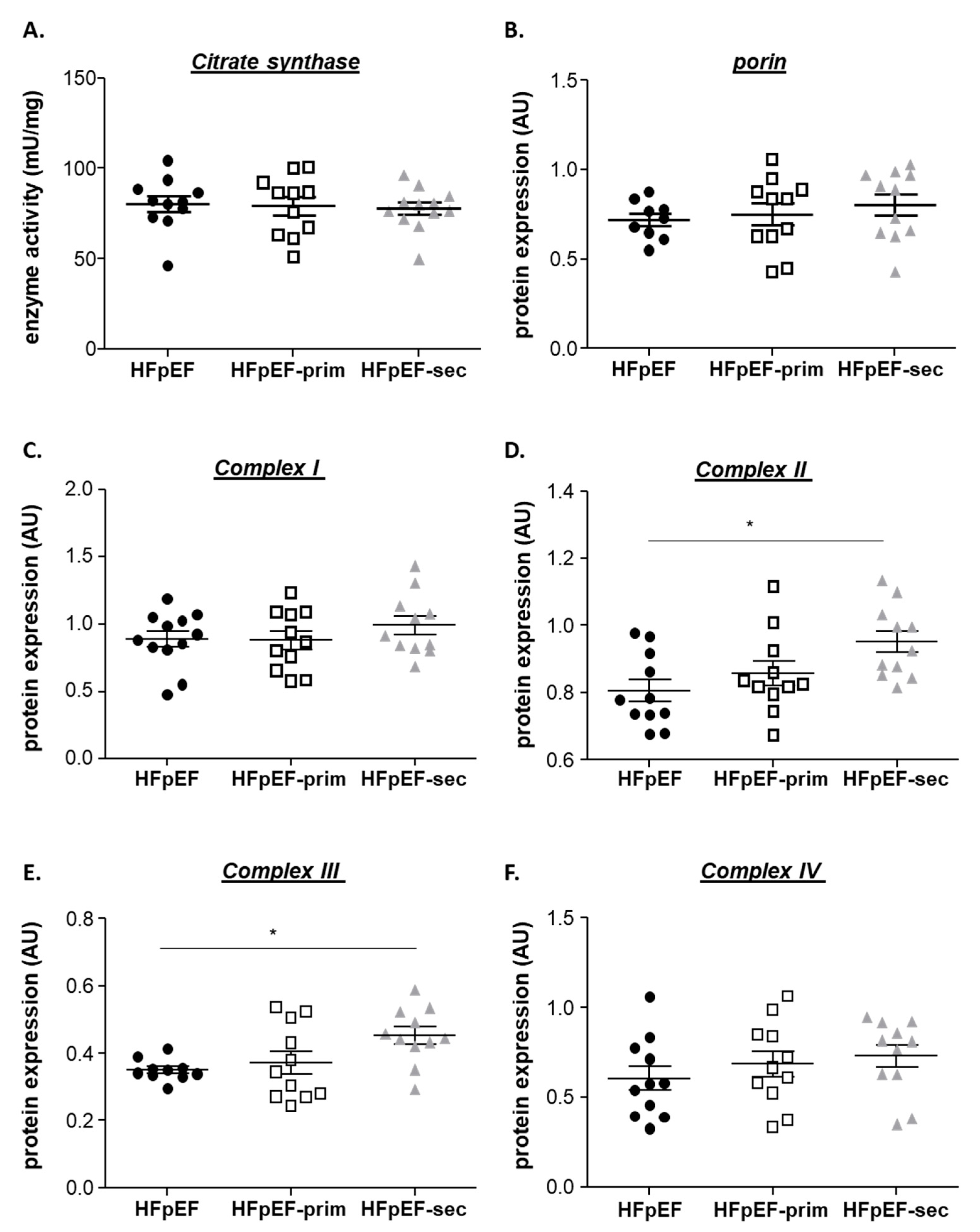
3.4. Proteins Related to Mitochondrial Function
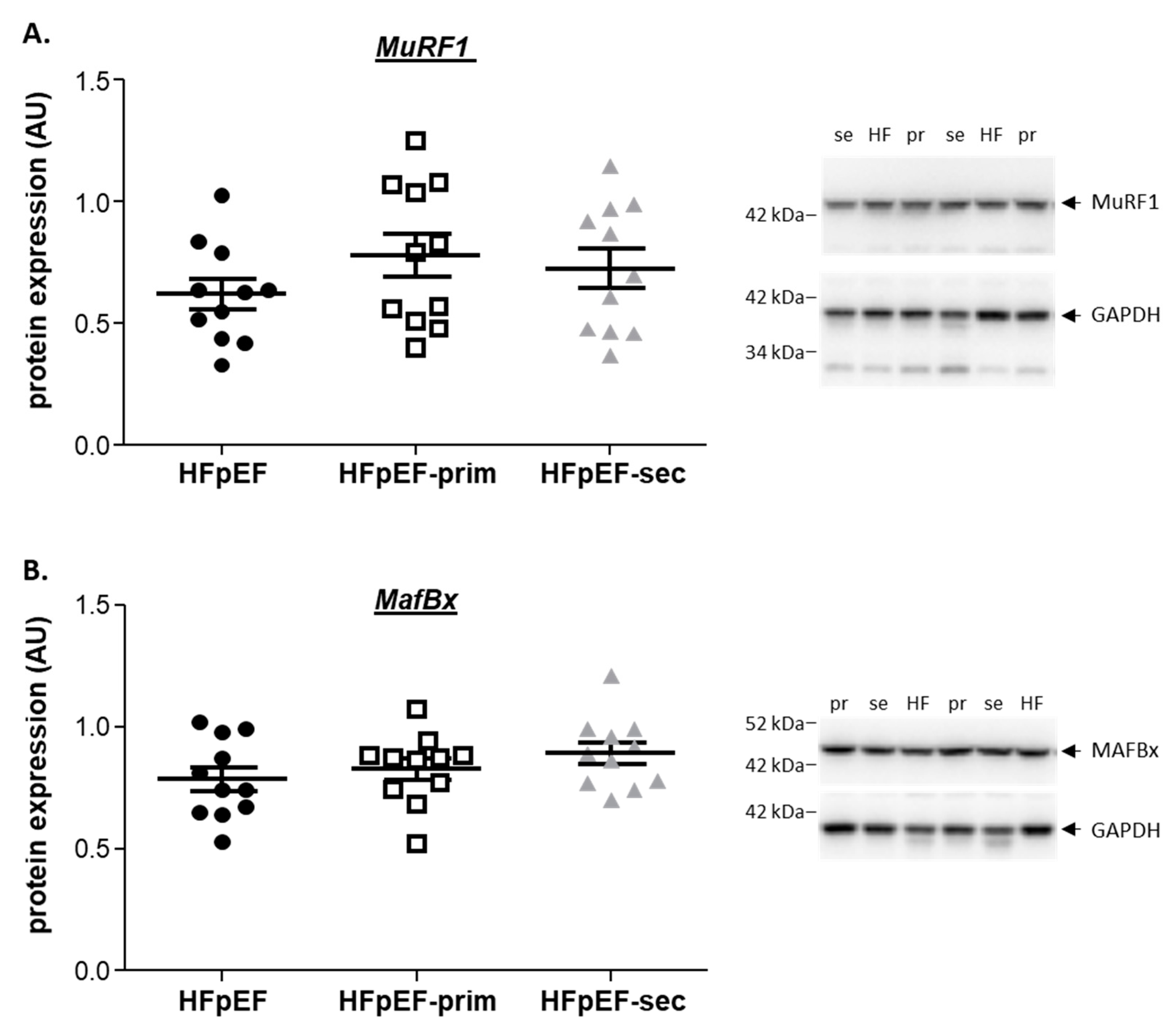
3.5. Proteins Related to Muscle Atrophy
4. Discussion
- prevents the development of muscle dysfunction (soleus absolute and specific muscle force) without modulation of muscle fatigability;
- improves mitochondrial function (mainly complex II induced respiration), without modulating mitochondrial content and the protein expression of respiratory chain complexes;
- increases the expression of mitofilin, mi-CK and reduces the protein expression of UCP3.
4.1. Leucine Supplementation and Skeletal Muscle Function
4.2. Leucine Supplementation and Mitochondrial Function
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection-Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Shen, J.L.; Xie, X.J. Insight into the Pro-inflammatory and Profibrotic Role of Macrophage in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Pharmacol. 2020, 76, 276–285. [Google Scholar] [CrossRef]
- Akiyama, E.; Sugiyama, S.; Matsuzawa, Y.; Konishi, M.; Suzuki, H.; Nozaki, T.; Ohba, K.; Matsubara, J.; Maeda, H.; Horibata, Y.; et al. Incremental Prognostic Significance of Peripheral Endothelial Dysfunction in Patients with Heart Failure with Normal Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2012, 60, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, L.; Rouault, P.; Duplaa, C.; Couffinhal, T.; Renault, M.A. Endothelial dysfunction in heart failure with preserved ejection fraction: What are the experimental proofs? Front. Physiol. 2022, 13, 906272. [Google Scholar] [CrossRef]
- Anderson, M.; Parrott, C.F.; Mark, J.H.; Peter, H.B.; Brubaker, P.H.; Ye, F.; Upadhya, B. Skeletal muscle abnormalities in heart failure with preserved ejection fraction. Heart Fail. Rev. 2023, 28, 157–168. [Google Scholar]
- Espino-Gonzalez, E.; Tickle, P.G.; Benson, A.P.; Kissane, R.W.P.; Askew, G.N.; Egginton, S.; Bowen, T.S. Abnormal skeletal muscle blood flow, contractile mechanics and fibre morphology in a rat model of obese-HFpEF. J. Physiol. 2021, 599, 981–1001. [Google Scholar] [CrossRef]
- Adams, V.; Schauer, A.; Augstein, A.; Kirchhoff, V.; Draskowski, R.; Jannasch, A.; Goto, K.; Lyall, G.; Männel, A.; Barthel, P.; et al. Targeting MuRF1 by small molecules in a HFpEF rat model improves myocardial diastolic function and skeletal muscle contractility. J. Cachexia Sarcopenia Muscle 2022, 13, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Winzer, E.B.; Schauer, A.; Langner, E.; Augstein, A.; Goto, K.; Männel, A.; Barthel, P.; Jannasch, A.; Labeit, S.; Mangner, N.; et al. Empagliflozin Preserves Skeletal Muscle Function in a HFpEF Rat Model. Int. J. Mol. Sci. 2022, 23, 10989. [Google Scholar] [CrossRef] [PubMed]
- Bekfani, T.; Bekhite, E.M.; Derlien, S.; Nisser, J.; Westermann, M.; Nietzsche, S.; Hamadanchi, A.; Fröb, E.; Westphal, J.; Haase, D.; et al. Skeletal Muscle Function, Structure, and Metabolism in Patients with Heart Failure with Reduced Ejection Fraction and Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, e007198. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Nicklas, B.; Kraus, W.E.; Lyles, M.F.; Eggebeen, J.; Morgan, T.M.; Haykowsky, M.J. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1364–H1370. [Google Scholar] [CrossRef]
- Tucker, W.J.; Haykowsky, M.J.; Seo, Y.; Stehling, E.; Forman, D.E. Impaired Exercise Tolerance in Heart Failure: Role of Skeletal Muscle Morphology and Function. Curr. Heart Fail. Rep. 2018, 15, 323–331. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Kouba, E.J.; Brubaker, P.H.; Nicklas, B.J.; Eggebeen, J.; Kitzman, D.W. Skeletal Muscle Composition and Its Relation to Exercise Intolerance in Older Patients with Heart Failure and Preserved Ejection Fraction. Am. J. Cardiol. 2014, 113, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Sharma, K.; Yanek, L.R.; Vaidya, D.; Schär, M.; Markl, M.; Subramanya, V.; Soleimani, S.; Ouyang, P.; Michos, E.D.; et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Schauer, A.; Augstein, A.; Methawasin, M.; Granzier, H.; Halle, M.; Craenenbroeck, E.M.V.; Rolim, N.; Gielen, S.; Pieske, B.; et al. Muscular changes in animal models of heart failure with preserved ejection fraction: What comes closest to the patient? ESC Heart Fail. 2021, 8, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.J.A.; Bharadwaj, M.S.; Van Horn, C.; Nicklas, B.J.; Lyles, M.F.; Eggebeen, J.; Haykowsky, M.J.; Brubaker, P.H.; Kitzman, D.W. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients with Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016, 4, 636–645. [Google Scholar] [CrossRef]
- Scandalis, L.; Kitzman, D.W.; Nicklas, B.J.; Lyles, M.; Brubaker, P.; Nelson, M.B.; Gordon, M.; Stone, J.; Bergstrom, J.; Neufer, P.D.; et al. Skeletal Muscle Mitochondrial Respiration and Exercise Intolerance in Patients with Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2023, 8, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Silva, D.; Wüst, R.C.I.; Conceicao, G.; Goncalves-Rodrigues, P.; Goncalves, N.; Goncalves, A.; Kuster, D.W.D.; Leite-Moreira, A.F.; van der Velden, J.; de Sousa Beleza, J.M.; et al. Disturbed cardiac mitochondrial and cytosolic calcium handling in a metabolic risk-related rat model of heart failure with preserved ejection fraction. Acta Physiol. 2019, 2019, e13378. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Schär, M.; Panjrath, G.S.; Zhang, Y.; Sharma, K.; Bottomley, P.A.; Golozar, A.; Steinberg, A.; Gerstenblith, G.; Russell, S.D.; et al. Fatigability, Exercise Intolerance, and Abnormal Skeletal Muscle Energetics in Heart Failure. Circ. Heart Fail. 2017, 10, e004129. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef]
- Jonathan, B.; Robertson, H.T. Cardiopulmonary exercise testing for heart failure: Pathophysiology and predictive markers. Heart 2023, 109, 256–263. [Google Scholar]
- Zhou, H.H.; Liao, Y.; Peng, Z.; Liu, F.; Wang, Q.; Yang, W. Association of muscle wasting with mortality risk among adults: A systematic review and meta-analysis of prospective studies. J. Cachexia Sarcopenia Muscle Clin. Rep. 2023, 14, 1596–1612. [Google Scholar] [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J.; et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients with Heart Failure with Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2021, 325, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, V.; Sharma, K.; Keteyian, S.J.; Alcain, C.F.; Desvigne-Nickens, P.; Fleg, J.L.; Florea, V.G.; Franklin, B.A.; Guglin, M.; Halle, M.; et al. Supervised Exercise Training for Chronic Heart Failure with Preserved Ejection Fraction: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2023, 147, e699–e715. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Parashar, A.; Kumbhani, D.; Agarwal, S.; Garg, J.; Kitzman, D.; Levine, B.; Drazner, M.; Berry, J.D. Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction: A Meta-analysis of Randomized Control Trials. Circ. Heart Fail. 2015, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [PubMed]
- Ispoglou, T.; White, H.; Preston, T.; McElhone, S.; McKenna, J.; Hind, K. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65GÇô75 years. Eur. J. Clin. Nutr. 2016, 70, 182–188. [Google Scholar] [CrossRef] [PubMed]
- English, K.L.; Mettler, J.A.; Ellison, J.B.; Mamerow, M.M.; Arentson-Lantz, E.; Pattarini, J.M.; Ploutz-Snyder, R.; Sheffield-Moore, M.; Paddon-Jones, D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults12. Am. J. Clin. Nutr. 2016, 103, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Phillips, B.E.; Hill, I.; Greenhaff, P.; Lund, J.N.; Williams, J.; Rankin, D.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. Human skeletal muscle is refractory to the anabolic effects of leucine during the postprandial muscle-full period in older men. Clin. Sci. 2017, 131, 2643–2653. [Google Scholar] [CrossRef]
- Backx, E.M.P.; Horstman, A.M.H.; Marzuca-Nassr, G.N.; Van Kranenburg, J.; Smeets, J.S.; Fuchs, C.J.; Janssen, A.A.W.; De Groot, L.C.P.G.; Snijders, T.; Verdijk, L.B.; et al. Leucine Supplementation Does Not Attenuate Skeletal Muscle Loss during Leg Immobilization in Healthy, Young Men. Nutrients 2018, 10, 635. [Google Scholar] [CrossRef]
- Viana, L.R.; Chiocchetti, G.D.; Oroy, L.; Vieira, W.F.; Busanello, E.N.; Marques, A.C.; Salgado, C.D.; de Oliveira, A.L.; Vieira, A.S.; Suarez, P.S.; et al. Leucine-Rich Diet Improved Muscle Function in Cachectic Walker 256 Tumour-Bearing Wistar Rats. Cells 2021, 10, 3272. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-leucine dietary supplementation modulates muscle protein degradation and increases pro-inflammatory cytokines in tumour-bearing rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Ferian, A.; Alves, P.K.N.; Silva, W.J.; Bento, M.R.; Gasch, A.; Labeit, S.; Moriscot, A.S. Skeletal Muscle Anti-Atrophic Effects of Leucine Involve Myostatin Inhibition. DNA Cell Biol. 2020, 39, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.K.; Schauer, A.; Augstein, A.; Männel, A.; Barthel, P.; Joachim, D.; Friedrich, J.; Prieto, M.E.; Moriscot, A.S.; Linke, A.; et al. Leucine Supplementation Improves Diastolic Function in HFpEF by HDAC4 Inhibition. Cells 2023, 12, 2561. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Oliveira, A.; Viana, L.R.; Lopes-Aguiar, L.; Canevarolo, R.; Colombera, M.C.; Valentim, R.R.; Garcia-Fóssa, F.; de Sousa, L.M.; Castelucci, B.G.; et al. Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia. Cancers 2020, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Schauer, A.; Draskowski, R.; Jannasch, A.; Kirchhoff, V.; Goto, K.; Männel, A.; Barthel, P.; Augstein, A.; Winzer, E.; Tugtekin, M.; et al. ZSF1 rat as animal model for HFpEF: Development of reduced diastolic function and skeletal muscle dysfunction. ESC Heart Fail. 2020, 7, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Schauer, A.; Adams, V.; Augstein, A.; Jannasch, A.; Draskowski, R.; Kirchhoff, V.; Goto, K.; Mittag, J.; Galli, R.; Männel, A.; et al. Sacubitril/Valsartan Improves Diastolic Function but Not Skeletal Muscle Function in a Rat Model of HFpEF. Int. J. Mol. Sci. 2021, 22, 3570. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Rolim, N.P.L.; Fischer, T.; Baekkerud, F.H.; Medeiros, A.; Werner, S.; Bronstad, E.; Rognmo, O.; Mangner, N.; Linke, A.; et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur. J. Heart Fail. 2015, 17, 263–272. [Google Scholar] [CrossRef]
- Srere, P.A. Reactions on the cycle: Citrate synthase. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1969; Volume 13, pp. 3–5. [Google Scholar]
- Gallagher, H.; Hendrickse, P.W.; Pereira, M.G.; Bowen, T.S. Skeletal muscle atrophy, regeneration, and dysfunction in heart failure: Impact of exercise training. J. Sport Health Sci. 2023, 12, 557–567. [Google Scholar] [CrossRef]
- von Haehling, S.; Steinbeck, L.; Doehner, W.; Springer, J.; Anker, S.D. Muscle wasting in heart failure: An overview. Int. J. Biochem. Cell Biol. 2013, 45, 2257–2265. [Google Scholar] [CrossRef]
- Winzer, E.B.; Augstein, A.; Schauer, A.; Mueller, S.; Fischer-Schaepmann, T.; Goto, K.; Hommel, J.; Van Craenenbroeck, E.M.; Wisloff, U.; Pieske, B.; et al. Impact of Different Training Modalities on Molecular Alterations in Skeletal Muscle of Patients with Heart Failure with Preserved Ejection Fraction: A Substudy of the OptimEx Trial. Circ. Heart Fail. 2022, 15, e009124. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Arena, R.; Borlaug, B.A.; Carbone, S.; Canada, J.M.; Kirkman, D.L.; Garten, R.; Rodriguez-Miguelez, P.; Guazzi, M.; Lavie, C.J.; et al. Exercise Intolerance in Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2209–2225. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, N.; Haykowsky, M.; Forman, D.E.; Berry, J.; Pandey, A. Exercise Training for Prevention and Treatment of Heart Failure. Prog. Cardiovasc. Dis. 2017, 60, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Schulze, P.C. Exercise as a nonpharmacologic intervention in patients with heart failure. Phys. Sportsmed. 2011, 39, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.W.A.; Piedade, W.P.; Soares, L.C.; Souza, P.A.T.; Aguiar, A.F.; Vechetti-Junior, I.J.; Campos, D.H.S.; Fernandes, A.A.H.; Okoshi, K.; Carvalho, R.F.; et al. Aerobic Exercise Training Prevents Heart Failure-Induced Skeletal Muscle Atrophy by Anti-Catabolic, but Not Anabolic Actions. PLoS ONE 2014, 9, e110020. [Google Scholar] [CrossRef]
- Martinez-Arnau, F.M.; Fonfria-Vivas, R.; Cauli, O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef] [PubMed]
- Komar, B.; Schwingshackl, L.; Hoffmann, G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: A systematic review and meta-analysis. J. Nutr. Health Aging 2015, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.R.; Kim, H.J.; Lecker, S.H. Ubiquitin-protein ligases in muscle wasting. Int. J. Biochem. Cell Biol. 2005, 37, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.E.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 53. [Google Scholar] [CrossRef]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Baker, E.; Lee, K.M.; Persinger, A.M.; Hawkins, W.; Puppa, M. Effects of low-dose leucine supplementation on gastrocnemius muscle mitochondrial content and protein turnover in tumor-bearing mice. Appl. Physiol. Nutr. Metab. 2019, 44, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Arentson-Lantz, E.J.; Mikovic, J.; Bhattarai, N.; Fry, C.S.; Lamon, S.; Porter, C.; Paddon-Jones, D. Leucine augments specific skeletal muscle mitochondrial respiratory pathways during recovery following 7 days of physical inactivity in older adults. J. Appl. Physiol. 2021, 130, 1522–1533. [Google Scholar] [CrossRef]
- Kutsche, H.S.; Schreckenberg, R.; Schlüter, K.D. Uncoupling Proteins in Striated Muscle Tissue: Known Facts and Open Questions. Antioxid. Redox Signal 2022, 37, 324–335. [Google Scholar] [CrossRef]
- Gong, D.W.; He, Y.; Karas, M.; Reitman, M. Uncoupling Protein-3 Is a Mediator of Thermogenesis Regulated by Thyroid Hormone, ß3-Adrenergic Agonists, and Leptin. J. Biol. Chem. 1997, 272, 24129–24132. [Google Scholar] [CrossRef]
- Codella, R.; Alves, T.C.; Befroy, D.E.; Choi, C.S.; Luzi, L.; Rothman, D.L.; Kibbey, R.G.; Shulman, G.I. Overexpression of UCP3 decreases mitochondrial efficiency in mouse skeletal muscle in vivo. FEBS Lett. 2023, 597, 309–319. [Google Scholar] [CrossRef]
- Bessman, S.P.; Carpenter, C.L. The Creatine-Creatine Phosphate Energy Shuttle. Ann. Rev. Biochem. 1985, 54, 831–862. [Google Scholar] [CrossRef]
- Zoll, J.; Sanchez, H.; N’Guessan, B.; Ribera, F.; Lampert, E.; Bigard, X.; Serrurier, B.; Fortin, D.; Geny, B.; Veksler, V.; et al. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol. 2002, 543, 191–200. [Google Scholar] [CrossRef]
- Tateo, I.; Tohoru, I.; Yukie, M.; Fumio, H.; Kazuhiko, K.; Nobuo, T. A novel human gene that is preferentially transcribed in heart muscle. Gene 1994, 144, 301–306. [Google Scholar] [CrossRef]
- Li, H.; Ruan, Y.; Zhang, K.; Jian, F.; Hu, C.; Miao, L.; Gong, L.; Sun, L.; Zhang, X.; Chen, S.; et al. Mic60/Mitofilin determines MICOS assembly essential for mitochondrial dynamics and mtDNA nucleoid organization. Cell Death Differ. 2016, 23, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liang, S.C.; Diao, K.Y.; Wang, Q.; He, Y. Mitofilin Mitigates Myocardial Damage in Acute Myocardial Infarction by Regulating Pyroptosis of Cardiomyocytes. Front. Cardiovasc. Med. 2022, 9, 823591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dorn, G.W. PINK1-Phosphorylated Mitofusin 2 Is a Parkin Receptor for Culling Damaged Mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Tomczak, C.R.; Scott, J.M.; Paterson, D.I.; Kitzman, D.W. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J. Appl. Physiol. 2015, 119, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Garbade, J.; Heyne, E.; van den Berg, M.; Winzer, E.B.; Hommel, J.; Sandri, M.; Jozwiak-Nozdrzykowska, J.; Meyer, A.L.; Lehmann, S.; et al. Molecular Mechanisms of Diaphragm Myopathy in Humans with Severe Heart Failure. Circ. Res. 2021, 128, 706–719. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. Characteristics, Treatments, and Outcomes of Patients with Preserved Systolic Function Hospitalized for Heart Failure: A Report From the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef]
- Cheng, R.K.; Cox, M.; Neely, M.L.; Heidenreich, P.A.; Bhatt, D.L.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am. Heart J. 2014, 168, 721–730. [Google Scholar] [CrossRef]
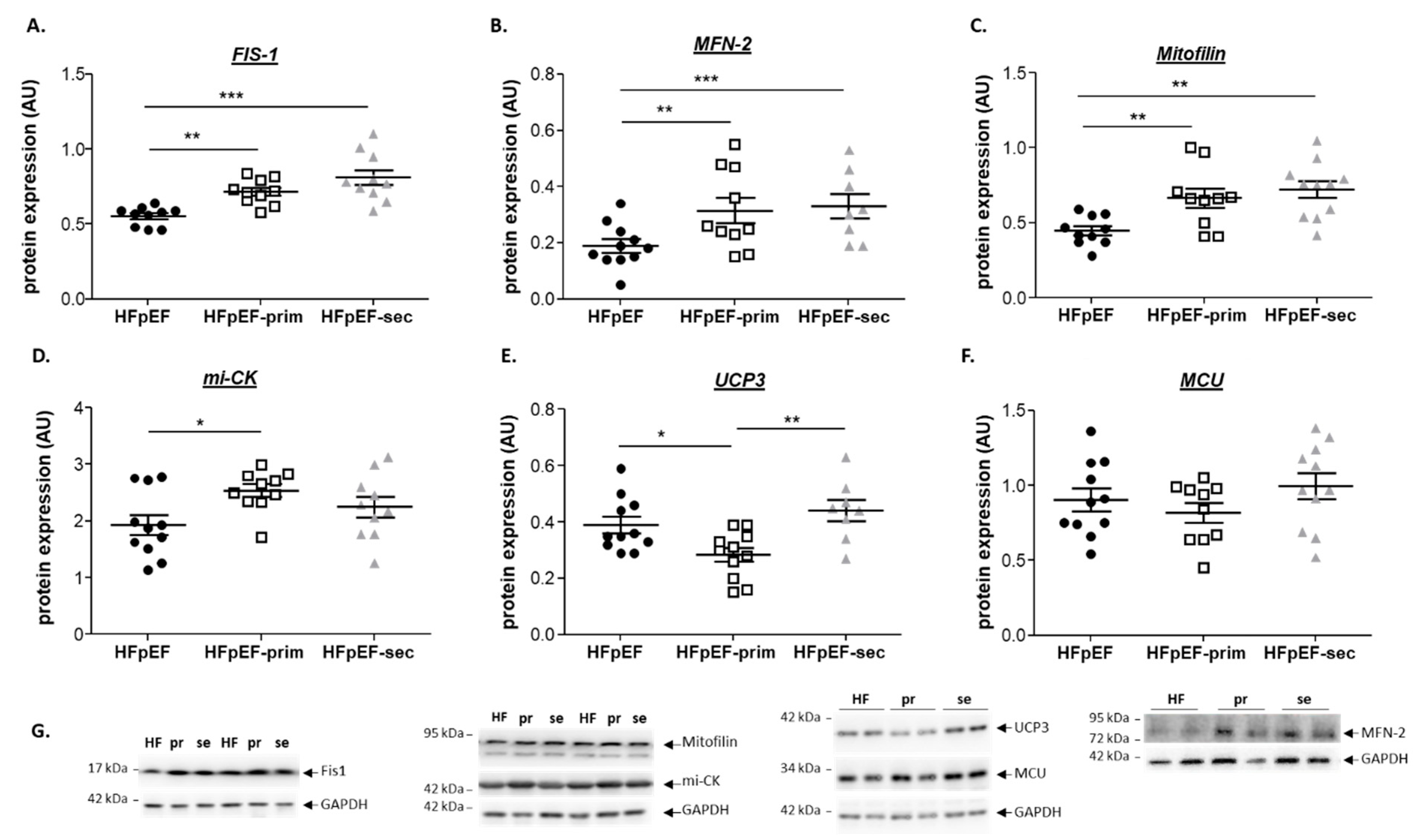
 no change vs. HfPEF.
no change vs. HfPEF.
 no change vs. HfPEF.
no change vs. HfPEF.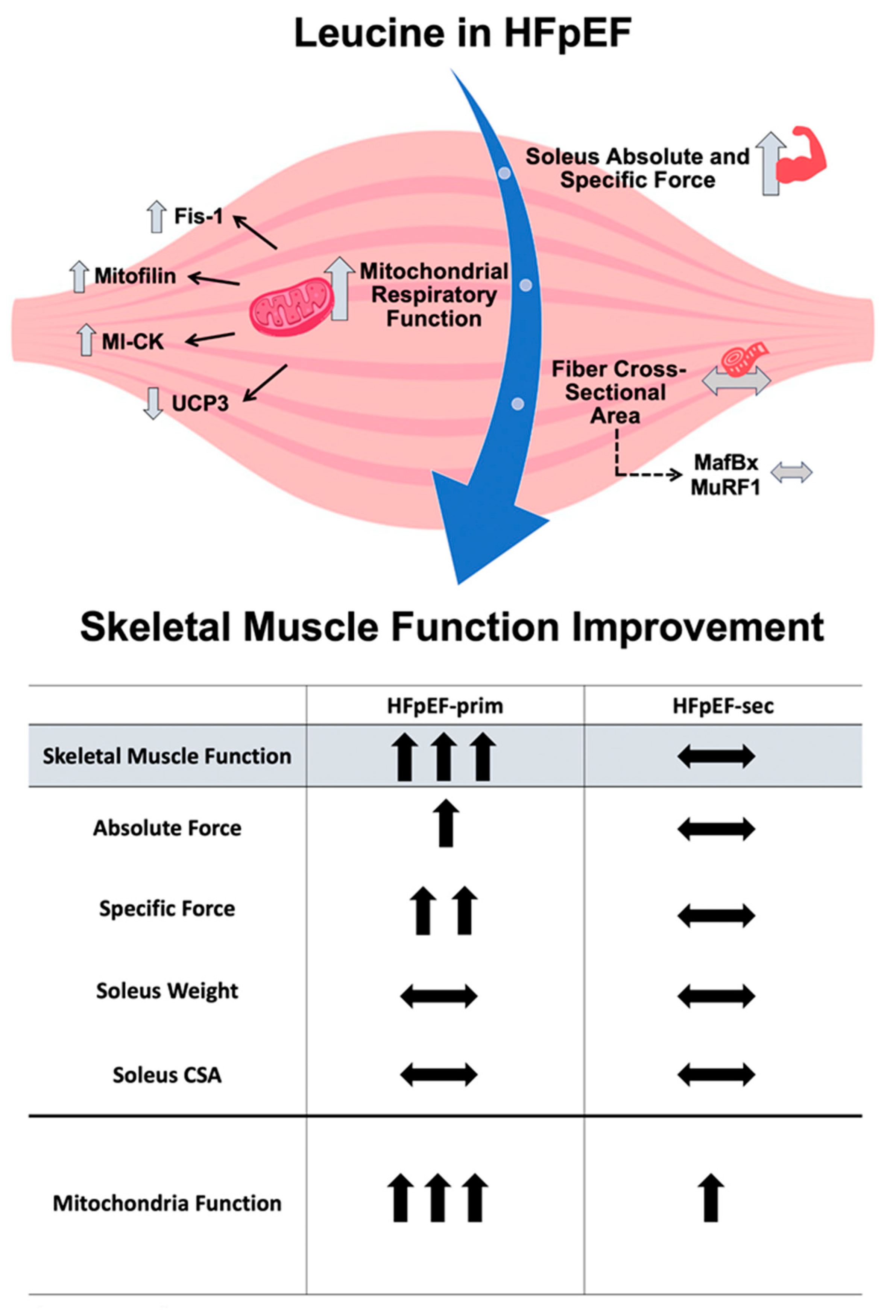
| Con | HFpEF | HFpEF-prim | HFpEF-sec | |
|---|---|---|---|---|
| Body weight (g) | 260 ± 3 | 512 ± 7 *** | 500 ± 7 *** | 486 ± 5 * |
| Tibia length (mm) | 35.3 ± 0.1 | 35.5 ± 0.1 | 35.5 ± 0.1 | 35.4 ± 0.2 |
| Heart weight/TL (mm/mg) | 26.5 ± 0.4 | 37.8 ± 0.7 *** | 37.3 ± 0.5 *** | 37.7 ± 1.0 ** |
| Lung weight (wet/dry) | 4.46 ± 0.02 | 4.14 ± 0.03 *** | 4.15 ± 0.02 *** | 4.20 ± 0.03 *** |
| Soleus weight/TL (mg/mm) | 4.00 ± 0.04 | 4.42 ± 0.11 ** | 4.27 ± 0.06 | 4.39 ± 0.10 * |
| Soleus CSA (µm2) | 3003 ± 162 | 3112 ± 118 | 2908 ± 150 | 2678 ± 79 |
| EDL weight/TL (mg/mm) | 3.78 ± 0.06 | 4.07 ± 0.04 ** | 4.07 ± 0.02 ** | 4.04 ± 0.06 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.K.N.; Schauer, A.; Augstein, A.; Prieto Jarabo, M.-E.; Männel, A.; Barthel, P.; Vahle, B.; Moriscot, A.S.; Linke, A.; Adams, V. Leucine Supplementation Prevents the Development of Skeletal Muscle Dysfunction in a Rat Model of HFpEF. Cells 2024, 13, 502. https://doi.org/10.3390/cells13060502
Alves PKN, Schauer A, Augstein A, Prieto Jarabo M-E, Männel A, Barthel P, Vahle B, Moriscot AS, Linke A, Adams V. Leucine Supplementation Prevents the Development of Skeletal Muscle Dysfunction in a Rat Model of HFpEF. Cells. 2024; 13(6):502. https://doi.org/10.3390/cells13060502
Chicago/Turabian StyleAlves, Paula Ketilly Nascimento, Antje Schauer, Antje Augstein, Maria-Elisa Prieto Jarabo, Anita Männel, Peggy Barthel, Beatrice Vahle, Anselmo S. Moriscot, Axel Linke, and Volker Adams. 2024. "Leucine Supplementation Prevents the Development of Skeletal Muscle Dysfunction in a Rat Model of HFpEF" Cells 13, no. 6: 502. https://doi.org/10.3390/cells13060502
APA StyleAlves, P. K. N., Schauer, A., Augstein, A., Prieto Jarabo, M.-E., Männel, A., Barthel, P., Vahle, B., Moriscot, A. S., Linke, A., & Adams, V. (2024). Leucine Supplementation Prevents the Development of Skeletal Muscle Dysfunction in a Rat Model of HFpEF. Cells, 13(6), 502. https://doi.org/10.3390/cells13060502








