To Detach, Migrate, Adhere, and Metastasize: CD97/ADGRE5 in Cancer
Abstract
1. Introduction: CD97—A Retrospect
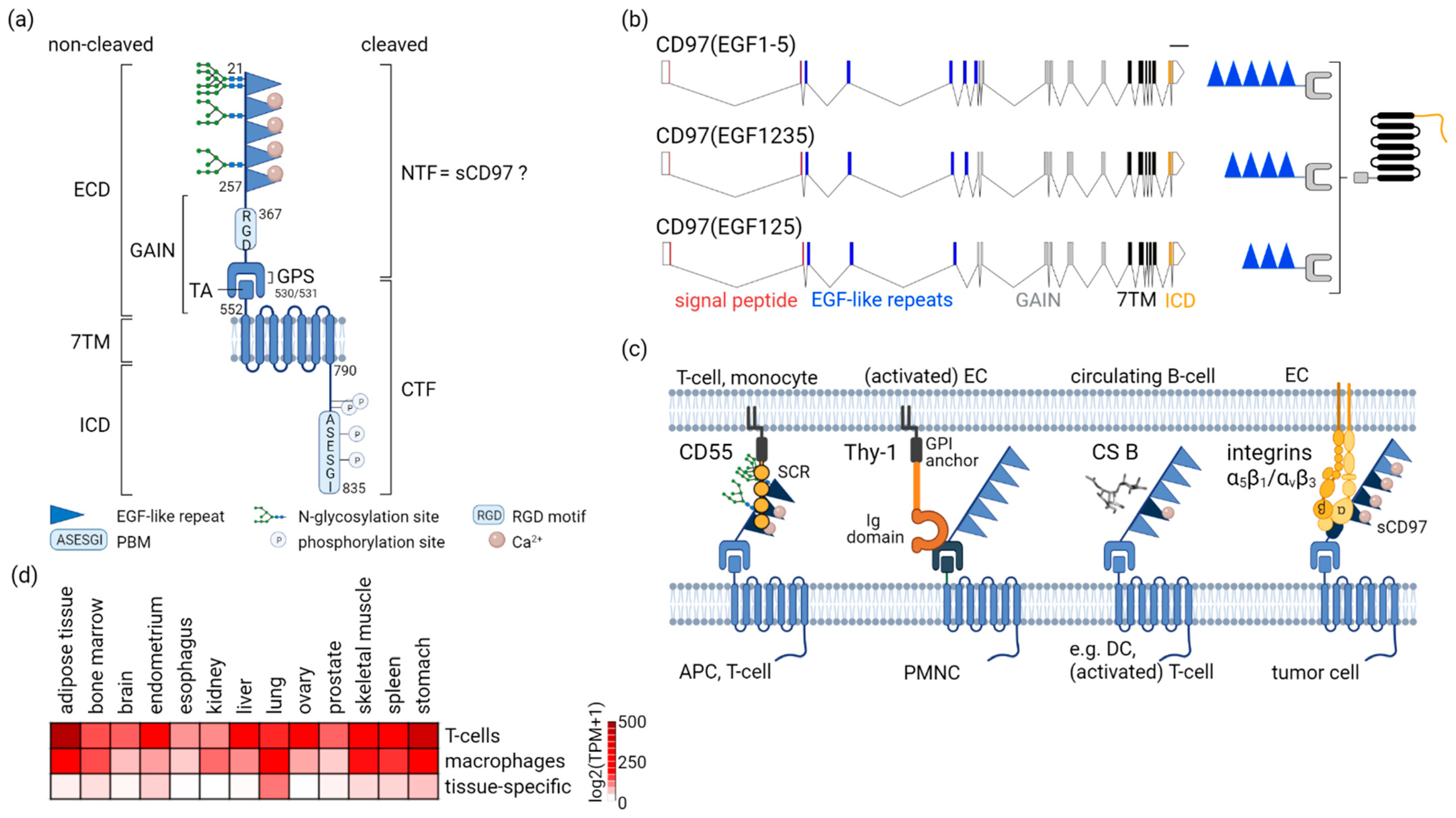
2. Overview of CD97/ADGRE5
2.1. CD97-A Prototypic aGPCR by Structure
2.2. Multifaceted CD97 Interactions
2.3. Not Everywhere: ADGRE5 in Normal Tissues
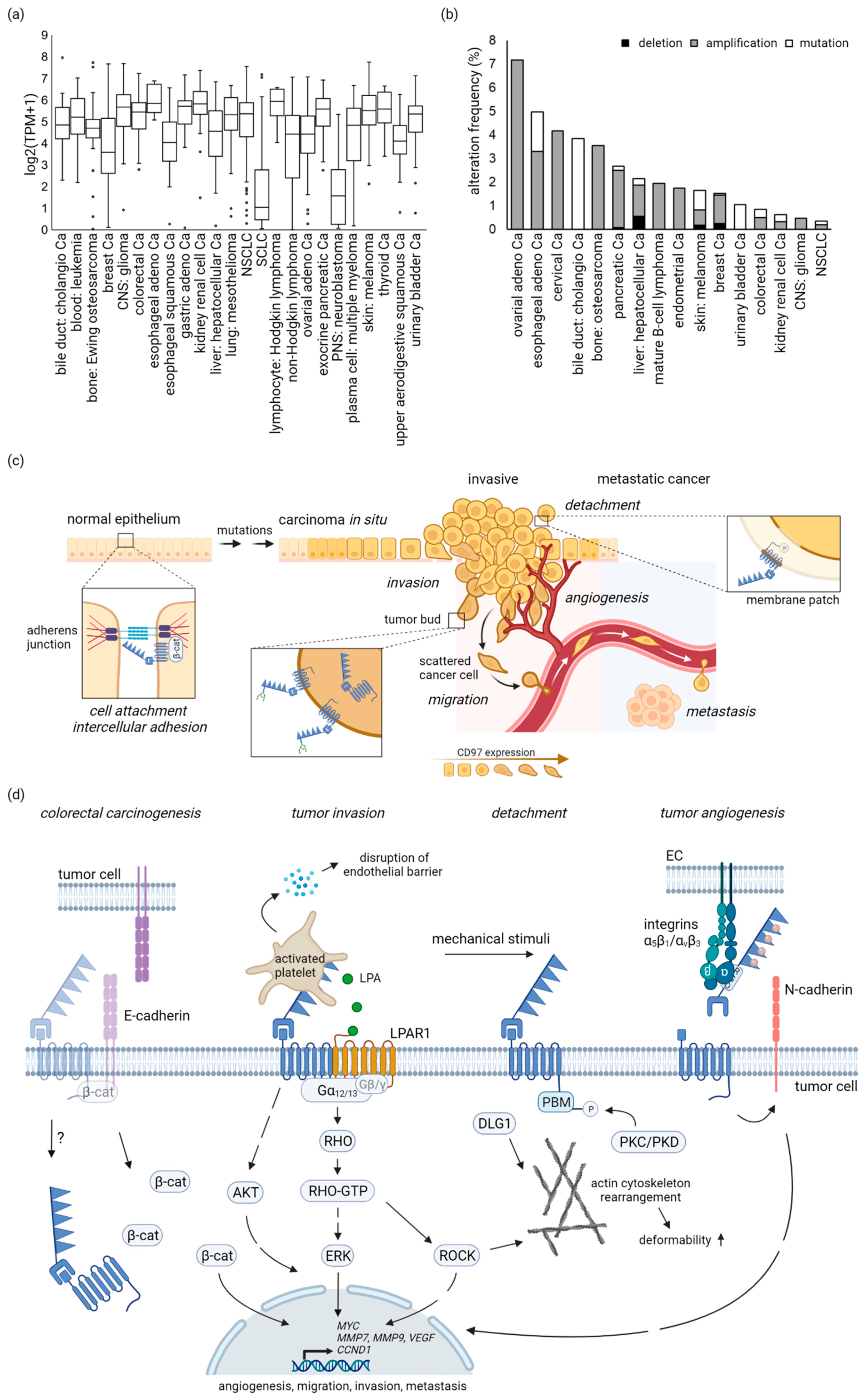
3. CD97 in Tumors
3.1. Most Tumor-Derived Cell Lines Are ADGRE5-Positive
3.2. No Enrichment of Cancer-Related ADGRE5 Mutations
3.3. Dysregulation of miRNAs and Epigenetic Changes Targeting ADGRE5 in Tumors
3.4. Present in Body Fluids of Tumor Patients: Soluble CD97 (sCD97)
4. CD97 in Specific Tumor Entities
4.1. CD97 in Carcinomas
4.2. CD97 in Primary Brain Tumors
4.3. CD97 in Myosarcomas
4.4. CD97 in Leukemias
5. CD97-Regulated Signaling Cascades and Functions in Tumors
6. Summary and Future Challenges
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| aGPCR | adhesion G protein-coupled receptor |
| CD97Ko | CD97 knockout |
| CNA | copy number alteration |
| CTF | C-terminal fragment |
| ECD | extracellular domain |
| ECM | extracellular matrix |
| GBM | glioblastoma multiforme |
| GAIN | GPCR autoproteolysis-inducing |
| GPS | GPCR proteolytic site |
| ICD | intracellular domain |
| miRNA, miR | micro RNA |
| NTF | N-terminal fragment |
| PBM | PDZ-binding motif |
| 7TM | seven-transmembrane |
| sCD97 | soluble CD97 |
| TA | tethered agonist |
| TCGA | The Cancer Genome Atlas |
References
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Eichler, W.; Aust, G.; Hamann, D. Characterization of the early activation-dependent antigen on lymphocytes defined by the monoclonal antibody BL-Ac(F2). Scand. J. Immunol. 1994, 39, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hamann, J.; Eichler, W.; Hamann, D.; Kerstens, H.M.; Poddighe, P.J.; Hoovers, J.M.; Hartmann, E.; Strauss, M.; van Lier, R.A. Expression cloning and chromosomal mapping of the leukocyte activation antigen CD97, a new seven-span transmembrane molecule of the secretion receptor superfamily with an unusual extracellular domain. J. Immunol. 1995, 155, 1942–1950. [Google Scholar]
- Pickl, W.F.; Majdic, O.; Mai, I.; Gadd, S.; Knapp, W. Overview of CD97. In Leucocyte Typing V; Schlossman, S.F., Boumsell, L., Gilks, W., Halan, J.M., Morimoto, C., Ritz, J., Shaw, S., Silverstein, R., Springer, T., Tedder, T.F., et al., Eds.; Oxford University Press: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1995; pp. 1151–1153. [Google Scholar]
- Carver, E.A.; Hamann, J.; Olsen, A.S.; Stubbs, L. Physical mapping of EMR1 and CD97 in human chromosome 19 and assignment of CD97 to mouse chromosome 8 suggest an ancient genomic duplication. Mamm. Genome 1999, 10, 1039–1040. [Google Scholar] [PubMed]
- Lin, H.H.; Stacey, M.; Hamann, J.; Gordon, S.; McKnight, A.J. Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics 2000, 67, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.; Lin, H.H.; Hilyard, K.L.; Gordon, S.; McKnight, A.J. Human epidermal growth factor (EGF) module-containing mucin-like hormone receptor 3 is a new member of the EGF-TM7 family that recognizes a ligand on human macrophages and activated neutrophils. J. Biol. Chem. 2001, 276, 18863–18870. [Google Scholar] [CrossRef]
- McKnight, A.J.; Gordon, S. EGF-TM7: A novel subfamily of seven-transmembran-region leukocyte cell-surface molecules. Immunol. Today 1996, 17, 283–287. [Google Scholar] [CrossRef]
- Kwakkenbos, M.J.; Kop, E.N.; Stacey, M.; Matmati, M.; Gordon, S.; Lin, H.H.; Hamann, J. The human EGF-TM7 family: A postgenomic view. Immunogenetics 2004, 55, 655–666. [Google Scholar]
- Fredriksson, R.; Lagerstrom, M.C.; Lundin, L.G.; Schioth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Schioth, H.B.; Fredriksson, R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 2005, 142, 94–101. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2021/22: G protein-coupled receptors. Br. J. Pharmacol. 2021, 178 (Suppl. 1), S27–S156. [Google Scholar] [CrossRef]
- Hamann, J.; Aust, G.; Arac, D.; Engel, F.B.; Formstone, C.; Frederiksson, R.; Hall, R.A.; Harty, B.L.; Kirchhoff, C.; Knapp, B.; et al. International Union of Basic and Clinical Pharmacology. XCI. Adhesion G protein-coupled receptors. Pharmacol. Rev. 2015, 67, 338–367. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.X.; Haino, M.; Roth, M.J.; Maguire, J.E.; Jensen, P.N.; Yarme, A.; Stetler-Stevenson, M.A.; Siebenlist, U.; Kelly, K. CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J. Immunol. 1996, 157, 5438–5447. [Google Scholar] [PubMed]
- Wobus, M.; Vogel, B.; Schmücking, E.; Hamann, J.; Aust, G. N-glycosylation of CD97 within the EGF domains is crucial for epitope accessibility in normal and malignant cells as well as CD55 ligand binding. Int. J. Cancer 2004, 112, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Aust, G.; Wandel, E.; Boltze, C.; Sittig, D.; Schütz, A.; Horn, L.C.; Wobus, M. Diversity of CD97 in smooth muscle cells (SMCs). Cell Tissue Res. 2006, 323, 1–9. [Google Scholar]
- Zyryanova, T.; Schneider, R.; Adams, V.; Sittig, D.; Kerner, C.; Gebhardt, C.; Ruffert, H.; Glasmacher, S.; Hepp, P.; Punkt, K.; et al. Skeletal muscle expression of the adhesion-GPCR CD97: CD97 deletion induces an abnormal structure of the sarcoplasmatic reticulum but does not impair skeletal muscle function. PLoS ONE 2014, 9, e100513. [Google Scholar] [CrossRef]
- Wang, T.; Ward, Y.; Tian, L.; Lake, R.; Guedez, L.; Stetler-Stevenson, W.G.; Kelly, K. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counter receptors on endothelial cells. Blood 2004, 105, 2836–2844. [Google Scholar] [CrossRef]
- Tjong, W.Y.; Lin, H.H. The role of the RGD motif in CD97/ADGRE5-and EMR2/ADGRE2-modulated tumor angiogenesis. Biochem. Biophys. Res. Commun. 2019, 520, 243–249. [Google Scholar] [CrossRef]
- Tjong, W.Y.; Lin, H.H. The RGD motif is involved in CD97/ADGRE5-promoted cell adhesion and viability of HT1080 cells. Sci. Rep. 2019, 9, 1517. [Google Scholar] [CrossRef]
- Liebscher, I.; Schon, J.; Petersen, S.C.; Fischer, L.; Auerbach, N.; Demberg, L.M.; Mogha, A.; Coster, M.; Simon, K.U.; Rothemund, S.; et al. A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep. 2014, 9, 2018–2026. [Google Scholar] [CrossRef]
- Okajima, D.; Kudo, G.; Yokota, H. Brain-specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. J. Recept. Signal Transduct. 2010, 30, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ward, Y.; Lake, R.; Yin, J.J.; Heger, C.D.; Raffeld, M.; Goldsmith, P.K.; Merino, M.; Kelly, K. LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res. 2011, 71, 7301–7311. [Google Scholar] [CrossRef] [PubMed]
- Paavola, K.J.; Stephenson, J.R.; Ritter, S.L.; Alter, S.P.; Hall, R.A. The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J. Biol. Chem. 2011, 286, 28914–28921. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, D.; Sittig, D.; Hoffmann, F.; Rothemund, S.; Warmt, E.; Quaas, M.; Sturmer, J.; Seiler, L.; Liebscher, I.; Hoang, N.A.; et al. Mechano-dependent phosphorylation of the PDZ-binding motif of CD97/ADGRE5 modulates cellular detachment. Cell Rep. 2018, 24, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Beliu, G.; Altrichter, S.; Guixà-González, R.; Hemberger, M.; Brauer, I.; Dahse, A.-K.; Scholz, N.; Wieduwild, R.; Kuhlemann, A.; Batebi, H.; et al. Tethered agonist exposure in intact adhesion/class B2 GPCRs through intrinsic structural flexibility of the GAIN domain. Mol. Cell 2021, 81, 905–921.e5. [Google Scholar] [CrossRef]
- Dunn, H.A.; Ferguson, S.S. PDZ protein regulation of G protein-coupled receptor trafficking and signaling pathways. Mol. Pharmacol. 2015, 88, 624–639. [Google Scholar] [CrossRef]
- Hamann, J.; Vogel, B.; van Schijndel, G.M.; van Lier, R.A. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 1996, 184, 1185–1189. [Google Scholar] [CrossRef]
- Wandel, E.; Saalbach, A.; Sittig, D.; Gebhardt, C.; Aust, G. Thy-1 (CD90) is an interaction partner for CD97 on activated endothelial cells. J. Immunol. 2012, 188, 1442–1450. [Google Scholar] [CrossRef]
- Kwakkenbos, M.J.; Pouwels, W.; Matmati, M.; Stacey, M.; Lin, H.H.; Gordon, S.; van Lier, R.A.; Hamann, J. Expression of the largest CD97 and EMR2 isoforms on leukocytes facilitates a specific interaction with chondroitin sulfate on B cells. J. Leukoc. Biol. 2005, 77, 112–119. [Google Scholar] [CrossRef]
- Durrant, L.G.; Chapman, M.A.; Buckley, D.J.; Spendlove, I.; Robins, R.A.; Armitage, N.C. Enhanced expression of the complement regulatory protein CD55 predicts a poor prognosis in colorectal cancer patients. Cancer Immunol. Immunother. 2003, 52, 638–642. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Peng, S.; Chen, Z.; Gimm, O.; Finke, R.; Hoang-Vu, C. The expression of CD97EGF and its ligand CD55 on marginal epithelium is related to higher stage and depth of tumor invasion of gastric carcinomas. Oncol. Rep. 2005, 14, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Wojno, K.J.; Day, L.L.; Pienta, K.J. Analysis of membrane-bound complement regulatory proteins in prostate cancer. Urology 2005, 66, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Han, S.L.; Xu, C.; Wu, X.L.; Li, J.L.; Liu, Z.; Zeng, Q.Q. The impact of expressions of CD97 and its ligand CD55 at the invasion front on prognosis of rectal adenocarcinoma. Int. J. Colorectal Dis. 2010, 25, 695–702. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, H.; Jiao, Y.; Zheng, J. Expression and prognostic value of CD97 and its ligand CD55 in pancreatic cancer. Oncol. Lett. 2015, 9, 793–797. [Google Scholar] [CrossRef]
- He, Y.; Wang, W.; Xu, L.; Li, L.; Liu, J.; Feng, M.; Bu, H. Immunohistochemical expression and prognostic significance of CD97 and its ligand DAF in human cervical squamous cell carcinoma. Int. J. Gynecol. Pathol. 2015, 34, 473–479. [Google Scholar] [CrossRef]
- Meng, Z.-W.; Liu, M.-C.; Hong, H.-J.; Du, Q.; Chen, Y.-L. Expression and prognostic value of soluble CD97 and its ligand CD55 in intrahepatic cholangiocarcinoma. Tumour Biol. 2017, 39, 1010428317694319. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, X.; Tang, J.; Zhang, W.; Zhangyuan, G.; Ji, J.; Deng, L.; Lu, S.; Zhuo, H.; Sun, B. CD97 promotes tumor aggressiveness through the traditional G protein-coupled receptor-mediated signaling in hepatocellular carcinoma. Hepatology 2018, 68, 1865–1878. [Google Scholar] [CrossRef]
- Hamann, J.; Hsiao, C.C.; Lee, C.S.; Ravichandran, K.S.; Lin, H.H. Adhesion GPCRs as modulators of immune cell function. Handb. Exp. Pharmacol. 2016, 234, 329–350. [Google Scholar]
- Cerny, O.; Godlee, C.; Tocci, R.; Cross, N.E.; Shi, H.; Williamson, J.C.; Alix, E.; Lehner, P.J.; Holden, D.W. CD97 stabilises the immunological synapse between dendritic cells and T cells and is targeted for degradation by the Salmonella effector SteD. PLoS Pathog. 2021, 17, e1009771. [Google Scholar] [CrossRef]
- Liu, D.; Wu, J.; Zhu, H.; Zhu, X.; Jin, Y.; Yu, Y.; Zhang, X. Treatment of microvascular invasion in hepatocellular carcinoma with drug-loaded nanocomposite platform under synergistic effect of magnetic field/near-infrared light. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 110, 712–724. [Google Scholar] [CrossRef]
- Karpus, O.N.; Veninga, H.; Hoek, R.M.; Flierman, D.; van Buul, J.D.; Vandenakker, C.C.; vanBavel, E.; Medof, M.E.; van Lier, R.A.; Reedquist, K.A.; et al. Shear stress-dependent downregulation of the adhesion-G protein-coupled receptor CD97 on circulating leukocytes upon contact with its ligand CD55. J. Immunol. 2013, 190, 3740–3748. [Google Scholar] [CrossRef]
- Liu, D.; Duan, L.; Rodda, L.B.; Lu, E.; Xu, Y.; An, J.; Qiu, L.; Liu, F.; Looney, M.R.; Yang, Z.; et al. CD97 promotes spleen dendritic cell homeostasis through the mechanosensing of red blood cells. Science 2022, 375, eabi5965. [Google Scholar] [CrossRef] [PubMed]
- Sauzay, C.; Voutetakis, K.; Chatziioannou, A.; Chevet, E.; Avril, T. CD90/Thy-1, a cancer-associated cell surface signaling molecule. Front. Cell Dev. Biol. 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Ward, Y.; Lake, R.; Martin, P.L.; Killian, K.; Salerno, P.; Wang, T.; Meltzer, P.; Merino, M.; Cheng, S.Y.; Santoro, M.; et al. CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model. Oncogene 2013, 32, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Ward, Y.; Lake, R.; Faraji, F.; Sperger, J.; Martin, P.; Gilliard, C.; Ku, K.P.; Rodems, T.; Niles, D.; Tillman, H.; et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018, 23, 808–822. [Google Scholar] [CrossRef]
- Becker, S.; Wandel, E.; Wobus, M.; Schneider, R.; Amasheh, S.; Sittig, D.; Kerner, C.; Naumann, R.; Hamann, J.; Aust, G. Overexpression of CD97 in intestinal epithelial cells of transgenic mice attenuates colitis by strengthening adherens junctions. PLoS ONE 2010, 5, e8507. [Google Scholar] [CrossRef]
- Aust, G.; Kerner, C.; Gonsior, S.; Sittig, D.; Schneider, H.; Buske, P.; Scholz, M.; Dietrich, N.; Oldenburg, S.; Karpus, O.N.; et al. Mice overexpressing CD97 in intestinal epithelial cells provide a unique model for mammalian postnatal intestinal cylindrical growth. Mol. Biol. Cell. 2013, 24, 2256–2268. [Google Scholar] [CrossRef]
- Hilbig, D.; Dietrich, N.; Wandel, E.; Gonsior, S.; Sittig, D.; Hamann, J.; Aust, G. The interaction of CD97/ADGRE5 with beta-catenin in adherens junctions is lost during colorectal carcinogenesis. Front. Oncol. 2018, 8, 182. [Google Scholar] [CrossRef]
- Scholz, N.; Gehring, J.; Guan, C.; Ljaschenko, D.; Fischer, R.; Lakshmanan, V.; Kittel, R.J.; Langenhan, T. The Adhesion GPCR Latrophilin/CIRL shapes mechanosensation. Cell Rep. 2015, 11, 866–874. [Google Scholar] [CrossRef]
- Petersen, S.C.; Luo, R.; Liebscher, I.; Giera, S.; Jeong, S.J.; Mogha, A.; Ghidinelli, M.; Feltri, M.L.; Schoneberg, T.; Piao, X.; et al. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 2015, 85, 755–769. [Google Scholar] [CrossRef]
- Boyden, S.E.; Desai, A.; Cruse, G.; Young, M.L.; Bolan, H.C.; Scott, L.M.; Eisch, A.R.; Long, R.D.; Lee, C.C.; Satorius, C.L.; et al. Vibratory urticaria associated with a missense variant in ADGRE2. N. Engl. J. Med. 2016, 374, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wilde, C.; Fischer, L.; Lede, V.; Kirchberger, J.; Rothemund, S.; Schöneberg, T.; Liebscher, I. The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. FASEB J. 2016, 30, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.; Guan, C.; Nieberler, M.; Grotemeyer, A.; Maiellaro, I.; Gao, S.; Beck, S.; Pawlak, M.; Sauer, M.; Asan, E.; et al. Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons. eLife 2017, 6, e28360. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.G.; Seuwen, K.; Bridges, J.P. Adhesion GPCR function in pulmonary development and disease. Handb. Exp. Pharmacol. 2016, 234, 309–327. [Google Scholar] [PubMed]
- Wu, V.; Yeerna, H.; Nohata, N.; Chiou, J.; Harismendy, O.; Raimondi, F.; Inoue, A.; Russell, R.B.; Tamayo, P.; Gutkind, J.S. Illuminating the Onco-GPCRome: Novel G protein-coupled receptor-driven oncocrine networks and targets for cancer immunotherapy. J. Biol. Chem. 2019, 294, 11062–11086. [Google Scholar] [CrossRef]
- Esteves, L.; Caramelo, F.; Ribeiro, I.P.; Carreira, I.M.; de Melo, J.B. Probability distribution of copy number alterations along the genome: An algorithm to distinguish different tumour profiles. Sci. Rep. 2020, 10, 14868. [Google Scholar] [CrossRef]
- Sriram, K.; Moyung, K.; Corriden, R.; Carter, H.; Insel, P.A. GPCRs show widespread differential mRNA expression and frequent mutation and copy number variation in solid tumors. PLoS Biol. 2019, 17, e3000434. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Sweredoski, M.J.; Huss, D.; Lansford, R.; Hess, S.; Tirrell, D.A. Prometastatic GPCR CD97 is a direct target of tumor suppressor microRNA-126. ACS Chem. Biol. 2013, 9, 334–338. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D. MicroRNA-503-5p inhibits the CD97-mediated JAK2/STAT3 pathway in metastatic or paclitaxel-resistant ovarian cancer cells. Neoplasia 2019, 21, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Mojumdar, K.; Zhou, Z.; Jeong, K.J.; Mangala, L.S.; Yu, S.; Tsang, Y.H.; Rodriguez-Aguayo, C.; Lu, Y.; et al. A-to-I-edited miRNA-379-5p inhibits cancer cell proliferation through CD97-induced apoptosis. J. Clin. Investig. 2019, 129, 5343–5356. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, L.C.; Vasudevan, M.; Chattopadhyay, I.; Borthakar, B.B.; Rai, A.K.; Phukan, R.K.; Sharma, J.; Mahanta, J.; Kataki, A.C.; et al. Esophageal cancer epigenomics and integrome analysis of genome-wide methylation and expression in high risk northeast indian population. OMICS 2015, 19, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-Y.; Liao, Y.-P.; Wang, H.-C.; Chen, Y.-C.; Huang, R.-L.; Wang, Y.-C.; Yuan, C.-C.; Lai, H.-C. An epigenetic signature of adhesion molecules predicts poor prognosis of ovarian cancer patients. Oncotarget 2017, 8, 53432–53449. [Google Scholar] [CrossRef]
- Nieberler, M.; Kittel, R.J.; Petrenko, A.G.; Lin, H.H.; Langenhan, T. Control of adhesion GPCR function through proteolytic processing. Handb. Exp. Pharmacol. 2016, 234, 83–109. [Google Scholar]
- Steinert, M.; Wobus, M.; Boltze, C.; Schütz, A.; Wahlbuhl, M.; Hamann, J.; Aust, G. Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am. J. Pathol. 2002, 161, 1657–1667. [Google Scholar] [CrossRef]
- Vogl, U.M.; Öhler, L.; Rasic, M.; Frischer, J.M.; Modak, M.; Stöckl, J. Evaluation of prognostic immune signatures in patients with breast, colorectal and pancreatic cancer receiving chemotherapy. Anticancer Res. 2017, 37, 1947–1955. [Google Scholar] [CrossRef]
- Jinawath, N.; Vasoontara, C.; Jinawath, A.; Fang, X.; Zhao, K.; Yap, K.-L.; Guo, T.; Lee, C.S.; Wang, W.; Balgley, B.M.; et al. Oncoproteomic analysis reveals co-upregulation of RELA and STAT5 in carboplatin resistant ovarian carcinoma. PLoS ONE 2010, 5, e11198. [Google Scholar] [CrossRef]
- Aust, G.; Eichler, W.; Laue, S.; Lehmann, I.; Heldin, N.-E.; Lotz, O.; Scherbaum, W.A.; Dralle, H.; Hoang-Vu, C. CD97: A dedifferentiation marker in human thyroid carcinomas. Cancer Res. 1997, 57, 1798–1806. [Google Scholar]
- Aust, G.; Steinert, M.; Schütz, A.; Wahlbuhl, M.; Hamann, J.; Wobus, M. CD97, but not its closely related EGF-TM7 family member EMR2, is expressed on gastric, pancreatic and esophageal carcinomas. Am. J. Clin. Pathol. 2002, 118, 699–707. [Google Scholar] [CrossRef]
- Wu, J.; Lei, L.; Wang, S.; Gu, D.; Zhang, J. Immunohistochemical expression and prognostic value of CD97 and its ligand CD55 in primary gallbladder carcinoma. J. Biomed. Biotechnol. 2012, 2012, 587672. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Vu, C.; Bull, K.; Schwarz, I.; Krause, G.; Schmutzler, C.; Aust, G.; Köhrle, J.; Dralle, H. Regulation of CD97 protein in thyroid carcinoma. J. Clin. Endocrinol. Metab. 1999, 84, 1104–1109. [Google Scholar] [CrossRef]
- Lugli, A.; Zlobec, I.; Berger, M.D.; Kirsch, R.; Nagtegaal, I.D. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2021, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Sittig, D.; Hanisch, I.; Wobus, M.; Wandel, E.; Loeffler, M.; Aust, G. Individual cell-based models of tumor–environment interactions. Multiple effects of CD97 on tumor invasion. Am. J. Pathol. 2006, 169, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L.A.; Knuechel, R.; Kirchner, T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 2001, 98, 10356–10361. [Google Scholar] [CrossRef] [PubMed]
- Hlubek, F.; Brabletz, T.; Budczies, J.; Pfeiffer, S.; Jung, A.; Kirchner, T. Heterogeneous expression of Wnt/beta-catenin target genes within colorectal cancer. Int. J. Cancer 2007, 121, 1941–1948. [Google Scholar] [CrossRef]
- Wobus, M.; Huber, O.; Hamann, J.; Aust, G. CD97 overexpression in tumor cells at the invasion front in colorectal cancer (CC) is independently regulated of the canonical Wnt pathway. Mol. Carcinog. 2006, 45, 881–886. [Google Scholar] [CrossRef]
- Safaee, M.; Clark, A.J.; Oh, M.C.; Ivan, M.E.; Bloch, O.; Kaur, G.; Sun, M.Z.; Kim, J.M.; Oh, T.; Berger, M.S.; et al. Overexpression of CD97 confers an invasive phenotype in glioblastoma cells and is associated with decreased survival of glioblastoma patients. PLoS ONE 2013, 8, e62765. [Google Scholar] [CrossRef]
- Mallawaaratchy, D.M.; Buckland, M.E.; McDonald, K.L.; Li, C.C.; Ly, L.; Sykes, E.K.; Christopherson, R.I.; Kaufman, K.L. Membrane proteome analysis of glioblastoma cell invasion. J. Neuropathol. Exp. Neurol. 2015, 74, 425–441. [Google Scholar] [CrossRef]
- Safaee, M.; Fakurnejad, S.; Bloch, O.; Clark, A.J.; Ivan, M.E.; Sun, M.Z.; Oh, T.; Phillips, J.J.; Parsa, A.T. Proportional upregulation of CD97 isoforms in glioblastoma and glioblastoma-derived brain tumor initiating cells. PLoS ONE 2015, 10, e0111532. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Lubelski, D.; Schonberg, D.L.; Wu, Q.; Hale, J.S.; Flavahan, W.A.; Mulkearns-Hubert, E.E.; Man, J.; Hjelmeland, A.B.; Yu, J.; et al. Phage display discovery of novel molecular targets in glioblastoma-initiating cells. Cell Death Differ. 2014, 21, 1325–1339. [Google Scholar] [CrossRef][Green Version]
- Mirkowska, P.; Hofmann, A.; Sedek, L.; Slamova, L.; Mejstrikova, E.; Szczepanski, T.; Schmitz, M.; Cario, G.; Stanulla, M.; Schrappe, M.; et al. Leukemia surfaceome analysis reveals new disease-associated features. Blood 2013, 121, e149–e159. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kitamura, H.; Hijikata, A.; Tomizawa-Murasawa, M.; Tanaka, S.; Takagi, S.; Uchida, N.; Suzuki, N.; Sone, A.; Najima, Y.; et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci. Transl. Med. 2010, 2, 17ra9. [Google Scholar] [CrossRef] [PubMed]
- Coustan-Smith, E.; Song, G.; Clark, C.; Key, L.; Liu, P.; Mehrpooya, M.; Stow, P.; Su, X.; Shurtleff, S.; Pui, C.H.; et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2011, 117, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.H.; Roy, N.; Chakraborty, S.; Desrichard, A.; Chung, S.S.; Woolthuis, C.M.; Hu, W.; Berezniuk, I.; Garrett-Bakelman, F.E.; Hamann, J.; et al. CD97 is a critical regulator of acute myeloid leukemia stem cell function. J. Exp. Med. 2019, 216, 2362–2377. [Google Scholar] [CrossRef]
- Coustan-Smith, E.; Song, G.; Shurtleff, S.; Yeoh, A.E.-J.; Chng, W.J.; Chen, S.P.; Rubnitz, J.E.; Pui, C.-H.; Downing, J.R.; Campana, D. Universal monitoring of minimal residual disease in acute myeloid leukemia. JCI Insight 2018, 3, e98561. [Google Scholar] [CrossRef]
- Houtsma, R.; van der Meer, N.K.; Meijer, K.; Morsink, L.; Hogeling, S.M.; Woolthuis, C.; Ammatuna, E.; Nijk, M.; de Boer, B.; Huls, G.; et al. CombiFlow: Combinatorial AML-specific plasma membrane expression profiles allow longitudinal tracking of clones. Blood Adv. 2022, 6, 2129–2149. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Keysselt, K.; Chen, H.Y.; Sittig, D.; Hamann, J.; Lin, H.H.; Aust, G. The Adhesion GPCR CD97 inhibits apoptosis. Int. J. Biochem. Cell Biol. 2015, 65, 197–208. [Google Scholar] [CrossRef]
- Safaee, M.M.; Wang, E.J.; Jain, S.; Chen, J.-S.; Gill, S.; Zheng, A.C.; Garcia, J.H.; Beniwal, A.S.; Tran, Y.; Nguyen, A.T.; et al. CD97 is associated with mitogenic pathway activation, metabolic reprogramming, and immune microenvironment changes in glioblastoma. Sci. Rep. 2022, 12, 1464. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sethi, N.; Kang, Y. Unravelling the complexity of metastasis—Molecular understanding and targeted therapies. Nat. Rev. Cancer 2011, 11, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aust, G.; Zheng, L.; Quaas, M. To Detach, Migrate, Adhere, and Metastasize: CD97/ADGRE5 in Cancer. Cells 2022, 11, 1538. https://doi.org/10.3390/cells11091538
Aust G, Zheng L, Quaas M. To Detach, Migrate, Adhere, and Metastasize: CD97/ADGRE5 in Cancer. Cells. 2022; 11(9):1538. https://doi.org/10.3390/cells11091538
Chicago/Turabian StyleAust, Gabriela, Leyu Zheng, and Marianne Quaas. 2022. "To Detach, Migrate, Adhere, and Metastasize: CD97/ADGRE5 in Cancer" Cells 11, no. 9: 1538. https://doi.org/10.3390/cells11091538
APA StyleAust, G., Zheng, L., & Quaas, M. (2022). To Detach, Migrate, Adhere, and Metastasize: CD97/ADGRE5 in Cancer. Cells, 11(9), 1538. https://doi.org/10.3390/cells11091538





