LINC00665: An Emerging Biomarker for Cancer Diagnostics and Therapeutics
Abstract
:1. Introduction
2. Aberrant Expression of LINC00665 in Human Tumors
3. Association of LINC00665 with Clinicopathological Characteristics
4. The Prognostic and Diagnostic Value of LINC00665
5. Signaling Pathways Associated with LINC00665 in Cancer
5.1. Wnt/β-Catenin Signaling Pathway
5.2. TGF-β Signaling Pathway
5.3. NF-κB Signaling Pathway
5.4. PI3K/AKT Signaling Pathway
5.5. MAPK Signaling Pathway
6. The ceRNA Network Centered on LINC00665
7. The Encoded Micro-Peptide of LINC00665 and the Protein-Coding Genes It Directly Targets
8. The Relationship between LINC00665 and the Efficacy of Anticancer Drugs
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cong, Z.; Diao, Y.; Xu, Y.; Li, X.; Jiang, Z.; Shao, C.; Ji, S.; Shen, Y.; De, W.; Qiang, Y. Long non-coding RNA linc00665 promotes lung adenocarcinoma progression and functions as ceRNA to regulate AKR1B10-ERK signaling by sponging miR-98. Cell Death Dis. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, X.; Yu, L.; Liu, B.; Ma, J.; Yang, W. LINC00665 Facilitates the Malignant Processes of Osteosarcoma by Increasing the RAP1B Expression via Sponging miR-708 and miR-142-5p. Anal. Cell. Pathol. 2021, 2021, 5525711. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.Y.; Lin, P.; Pang, Y.Y.; Chen, G.; He, Y.; Dang, Y.W.; Yang, H. Expression of the Long Intergenic Non-Protein Coding RNA 665 (LINC00665) Gene and the Cell Cycle in Hepatocellular Carcinoma Using the Cancer Genome Atlas, the Gene Expression Omnibus, and Quantitative Real-Time Polymerase Chain Reaction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2786–2808. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Wang, Y.; Zhang, S.; Wu, R.; Lu, J.; et al. Micropeptide CIP2A-BP encoded by LINC00665 inhibits triple-negative breast cancer progression. EMBO J. 2020, 39, e102190. [Google Scholar] [CrossRef]
- Liu, X.Y.; Lu, X.Y.; Zhen, F.X.; Jin, S.D.; Yu, T.F.; Zhu, Q.; Wang, W.; Xu, K.; Yao, J.Q.; Guo, R.H. LINC00665 Induces Acquired Resistance to Gefitinib through Recruiting EZH2 and Activating PI3K/AKT Pathway in NSCLC. Mol. Ther. Nucleic Acids 2019, 16, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Feng, W.; Zhuang, Y.; Liu, J.; Feng, Z.; Xu, T.; Wang, W.; Zhu, Y.; Wang, Z. Long non-coding RNA linc00665 inhibits CDKN1C expression by binding to EZH2 and affects cisplatin sensitivity of NSCLC cells. Mol. Ther. Nucleic Acids 2021, 23, 1053–1065. [Google Scholar] [CrossRef]
- Lu, M.; Qin, X.; Zhou, Y.; Li, G.; Liu, Z.; Geng, X.; Yue, H. Long non-coding RNA LINC00665 promotes gemcitabine resistance of Cholangiocarcinoma cells via regulating EMT and stemness properties through miR-424-5p/BCL9L axis. Cell Death Dis. 2021, 12, 72. [Google Scholar] [CrossRef]
- Dai, H.; Sheng, X.; Sha, R.; Peng, J.; Yang, F.; Zhou, L.; Lin, Y.; Xu, Y.; Zhang, S.; Yin, W.; et al. Linc00665 Can Predict the Response to Cisplatin-Paclitaxel Neoadjuvant Chemotherapy for Breast Cancer Patients. Front. Oncol. 2021, 11, 604319. [Google Scholar] [CrossRef]
- Ji, W.; Diao, Y.L.; Qiu, Y.R.; Ge, J.; Cao, X.C.; Yu, Y. LINC00665 promotes breast cancer progression through regulation of the miR-379-5p/LIN28B axis. Cell Death Dis. 2020, 11, 16. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zou, L.; Zhu, T. Long non-coding RNA LINC00665 promotes metastasis of breast cancer cells by triggering EMT. Eur Rev. Med. Pharm. Sci 2020, 24, 3097–3104. [Google Scholar]
- Lv, M.; Mao, Q.; Li, J.; Qiao, J.; Chen, X.; Luo, S. Knockdown of LINC00665 inhibits proliferation and invasion of breast cancer via competitive binding of miR-3619-5p and inhibition of catenin beta 1. Cell. Mol. Biol. Lett. 2020, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Sun, B.; Yang, B.; Lu, S. LINC00665 Stimulates Breast Cancer Progression via Regulating miR-551b-5p. Cancer Manag. Res. 2021, 13, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, Z.; Huang, W.; Yang, Y.; Wang, F.; Huang, H. LncRNA LINC00665 Promotes Prostate Cancer Progression via miR-1224-5p/SND1 Axis. OncoTargets Ther. 2020, 13, 2527–2535. [Google Scholar] [CrossRef] [Green Version]
- Xue, P.; Yan, M.; Wang, K.; Gu, J.; Zhong, B.; Tu, C. Up-Regulation of LINC00665 Facilitates the Malignant Progression of Prostate Cancer by Epigenetically Silencing KLF2 Through EZH2 and LSD1. Front. Oncol. 2021, 11, 639060. [Google Scholar] [CrossRef]
- Eke, I.; Bylicky, M.A.; Sandfort, V.; Chopra, S.; Martello, S.; Graves, E.E.; Coleman, C.N.; Aryankalayil, M.J. The lncRNAs LINC00261 and LINC00665 are upregulated in long-term prostate cancer adaptation after radiotherapy. Mol. Ther. Nucleic Acids 2021, 24, 175–187. [Google Scholar] [CrossRef]
- Xu, D.; Song, Q.; Liu, Y.; Chen, W.; Lu, L.; Xu, M.; Fang, X.; Zhao, W.; Zhou, H. LINC00665 promotes Ovarian Cancer progression through regulating the miRNA-34a-5p/E2F3 axis. J. Cancer 2021, 12, 1755–1763. [Google Scholar] [CrossRef]
- Meng, C.; Zhou, J.Q.; Liao, Y.S. Autophagy-related long non-coding RNA signature for ovarian cancer. J. Int. Med. Res. 2020, 48, 300060520970761. [Google Scholar] [CrossRef]
- Wu, M.; Shang, X.; Sun, Y.; Wu, J.; Liu, G. Integrated analysis of lymphocyte infiltration-associated lncRNA for ovarian cancer via TCGA, GTEx and GEO datasets. PeerJ 2020, 8, e8961. [Google Scholar] [CrossRef]
- Xia, L.; Chen, Y.X.; L, J.B. LINC00665 promotes HeLa cell proliferation, migration, invasion and epithelial-mesenchymal transition by activating the WNT-CTNNB1/β-catenin signaling pathway. Sheng Li Xue Bao 2021, 73, 233–243. [Google Scholar]
- Cai, Y.; Hao, M.; Chang, Y.; Liu, Y. LINC00665 enhances tumorigenicity of endometrial carcinoma by interacting with high mobility group AT-hook 1. Cancer Cell Int. 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bai, Q.; Chen, H.; Su, K.; Gao, C. LINC00665 induces gastric cancer progression through activating Wnt signaling pathway. J. Cell. Biochem. 2020, 121, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xiao, Z.; Wang, Y. Long non-coding RNA LINC00665 gastric cancer tumorigenesis by regulation miR-149-3p/RNF2 axis. OncoTargets Ther. 2019, 12, 6981–6990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, J. LINC00665 promotes cell proliferation, invasion, and metastasis by activating the TGF-beta pathway in gastric cancer. Pathol. Res. Pract. 2021, 224, 153492. [Google Scholar] [CrossRef]
- Yue, C.; Yu, C.; Peng, R.; Wang, J.; Li, G.; Xu, L. LINC00665/miR-379-5p/GRP78 regulates cisplatin sensitivity in gastric cancer by modulating endoplasmic reticulum stress. Cytotechnology 2021, 73, 413–422. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, J.; Huan, L.; Liu, Y.; Qiao, Y.; Wang, Z.; Chen, Z.; Huang, S.; Zhao, Y.; He, X. Inflammation-Induced Long Intergenic Noncoding RNA (LINC00665) Increases Malignancy Through Activating the Double-Stranded RNA-Activated Protein Kinase/Nuclear Factor Kappa B Pathway in Hepatocellular Carcinoma. Hepatology 2020, 72, 1666–1681. [Google Scholar] [CrossRef]
- Shan, Y.; Li, P. Long Intergenic Non-Protein Coding RNA 665 Regulates Viability, Apoptosis, and Autophagy via the MiR-186-5p/MAP4K3 Axis in Hepatocellular Carcinoma. Yonsei Med. J. 2019, 60, 842–853. [Google Scholar] [CrossRef]
- Zhao, X.; Weng, W.; Long, Y.; Pan, W.; Li, Z.; Sun, F. LINC00665/miR-9-5p/ATF1 is a novel axis involved in the progression of colorectal cancer. Hum. Cell 2020, 33, 1142–1154. [Google Scholar] [CrossRef]
- Han, T.; Gao, M.; Wang, X.; Li, W.; Zhuo, J.; Qu, Z.; Chen, Y. LINC00665 activates Wnt/beta-catenin signaling pathway to facilitate tumor progression of colorectal cancer via upregulating CTNNB1. Exp. Mol. Pathol. 2021, 120, 104639. [Google Scholar] [CrossRef]
- Pádua, D.; Pinto, D.F.; Figueira, P.; Pereira, C.F.; Almeida, R.; Mesquita, P. HMGA1 Has Predictive Value in Response to Chemotherapy in Gastric Cancer. Curr. Oncol. 2021, 29, 56–67. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, S.; Xu, Z.; Zhang, G. Downregulation of LINC00665 confers decreased cell proliferation and invasion via the miR-138-5p/E2F3 signaling pathway in NSCLC. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 127, 110214. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Diao, Y.; Li, X.; Jiang, Z.; Xu, Y.; Zhou, H.; Qiang, Y.; Wu, H.; Shen, Y. Long non-coding RNA linc00665 interacts with YB-1 and promotes angiogenesis in lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2020, 527, 545–552. [Google Scholar] [CrossRef] [PubMed]
- W, W.; Zhao, X.L.; Liu, J.; Zhang, Z.F. Downregulation of LINC00665 suppresses the progression of lung adenocarcinoma via regulating miR-181c-5p/ZIC2 axis. Aging 2021, 13, 17499–17515. [Google Scholar]
- Wang, A.; Zhang, T.; Wei, W.; Wang, H.; Zhang, Z.; Yang, W.; Xia, W.; Mao, Q.; Xu, L.; Jiang, F.; et al. The Long Noncoding RNA LINC00665 Facilitates c-Myc Transcriptional Activity via the miR-195-5p MYCBP Axis to Promote Progression of Lung Adenocarcinoma. Front. Oncol. 2021, 11, 666551. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, L.; Nie, K.; Li, L.; Song, S.; Liu, F.; Li, P.; Cao, D.; Liu, Y. Identification of LINC00665-miR-let-7b-CCNA2 competing endogenous RNA network associated with prognosis of lung adenocarcinoma. Sci. Rep. 2021, 11, 4434. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Hao, M.; Zhu, R. LINC00665 functions as a competitive endogenous RNA to regulate AGTR1 expression by sponging miR34a5p in glioma. Oncol. Rep. 2021, 45, 1202–1212. [Google Scholar] [CrossRef]
- Chen, K.; Bai, L.; Ji, L.; Wu, L.; Li, G. Bioinformatics analysis of the key potential ceRNA biomarkers in human thymic epithelial tumors. Medicine 2021, 100, e26271. [Google Scholar] [CrossRef]
- Zhang, D.W.; Gu, G.Q.; Chen, X.Y.; Zha, G.C.; Yuan, Z.; Wu, Y. LINC00665 facilitates the progression of osteosarcoma via sponging miR-3619-5p. Eur Rev. Med. Pharm. Sci 2020, 24, 9852–9859. [Google Scholar]
- Wang, C.; Li, M.; Wang, S.; Jiang, Z.; Liu, Y. LINC00665 Promotes the Progression of Multiple Myeloma by Adsorbing miR-214-3p and Positively Regulating the Expression of PSMD10 and ASF1B. OncoTargets Ther. 2020, 13, 6511–6522. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Lin, F.; Xu, M.; Zhao, X. Long non-coding RNA LINC00665 promotes melanoma cell growth and migration via regulating the miR-224-5p/VMA21 axis. Exp. Dermatol. 2022, 31, 64–73. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Pang, S.; Li, X.; Wang, P.; Ma, R.; Ma, Y.; Song, C. LINC00665 promotes the progression of acute myeloid leukemia by regulating the miR-4458/DOCK1 pathway. Sci. Rep. 2021, 11, 5009. [Google Scholar] [CrossRef] [PubMed]
- Abuduer, M.; A, E.Z.G. LINC00665 promotes the viability, migration and invasion of T cell acute lymphoblastic leukemia cells by targeting miR-101 via modulating PI3K/Akt pathway. Tissue Cell 2021, 71, 101579. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Zheng, J.; Liu, X.; Liu, Y.; Liu, L.; Ma, J.; He, Q.; Yang, C.; Wang, D.; Cai, H.; et al. lncRNA LINC00665 Stabilized by TAF15 Impeded the Malignant Biological Behaviors of Glioma Cells via STAU1-Mediated mRNA Degradation. Mol. Ther. Nucleic Acids 2020, 20, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, X.; Nie, X.; Guo, Q.; Liu, Q.; Qi, Y.; Liu, J.; Lin, B. Construction of novel mRNA-miRNA-lncRNA regulatory networks associated with prognosis of ovarian cancer. J. Cancer 2020, 11, 7057–7072. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, L.M.; Roberts, A.B. TGF-beta signaling: Positive and negative effects on tumorigenesis. Curr Opin Genet. Dev. 2002, 12, 22–29. [Google Scholar] [CrossRef]
- Xie, F.; Ling, L.; Dam, H.; Zhou, F.F.; Zhang, L. TGF-β signaling in cancer metastasis. Acta Biochim. Biophys. Sin. 2018, 50, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Ma, L.; Chen, T.; Yang, Y.; Ma, Y.; Ma, L. NF-kappaB 1-induced LINC00665 regulates inflammation and apoptosis of neurons caused by spinal cord injury by targeting miR-34a-5p. Neurol. Res. 2021, 43, 418–427. [Google Scholar] [CrossRef]
- Fresno, V.J.A.; Casado, E.; De, C.J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Iliasov, A.R.; Nizamov, T.R.; Naumenko, V.A.; Garanina, A.S.; Vodopyanov, S.S.; Nikitin, A.A.; Pershina, A.G.; Chernysheva, A.A.; Kan, Y.; Mogilnikov, P.S.; et al. Non-magnetic shell coating of magnetic nanoparticles as key factor of toxicity for cancer cells in a low frequency alternating magnetic field. Colloids Surf. B Biointerfaces 2021, 206, 111931. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int J. Mol. Sci 2022, 23, 3847. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.W.; Duan, L.L.; Hu, X.L.; Zhang, X.; Hu, J.; Huang, L.; He, R.Z.; Hu, Z.; Luo, W.H.; et al. AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget 2017, 8, 33694–33703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.L.; Shan, T.D.; Han, Y.; Kong, Y.; Li, Y.B.; Peng, X.G.; Shang, L.; Wang, P.G.; Li, L.P. Long intergenic noncoding RNA 00665 promotes proliferation and inhibits apoptosis in colorectal cancer by regulating miR-126-5p. Aging 2021, 13, 13571–13584. [Google Scholar] [CrossRef]
- Ding, J.H.; Ding, X.J.; Leng, Z.H. LPCAT1 promotes gefitinib resistance via upregulation of the EGFR/PI3K/AKT signaling pathway in lung adenocarcinoma. J. Cancer 2022, 13, 1837–1847. [Google Scholar] [CrossRef]
- Jia, Y.F.; Xie, J.W. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis 2015, 2, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Khongkow, P.; Gomes, A.R.; Gong, C.; Man, E.P.S.; Tsang, J.W.; Zhao, F.; Monteiro, L.J.; Coombes, R.C.; Medema, R.H.; Khoo, U.S.; et al. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene 2016, 35, 990–1002. [Google Scholar] [CrossRef] [Green Version]

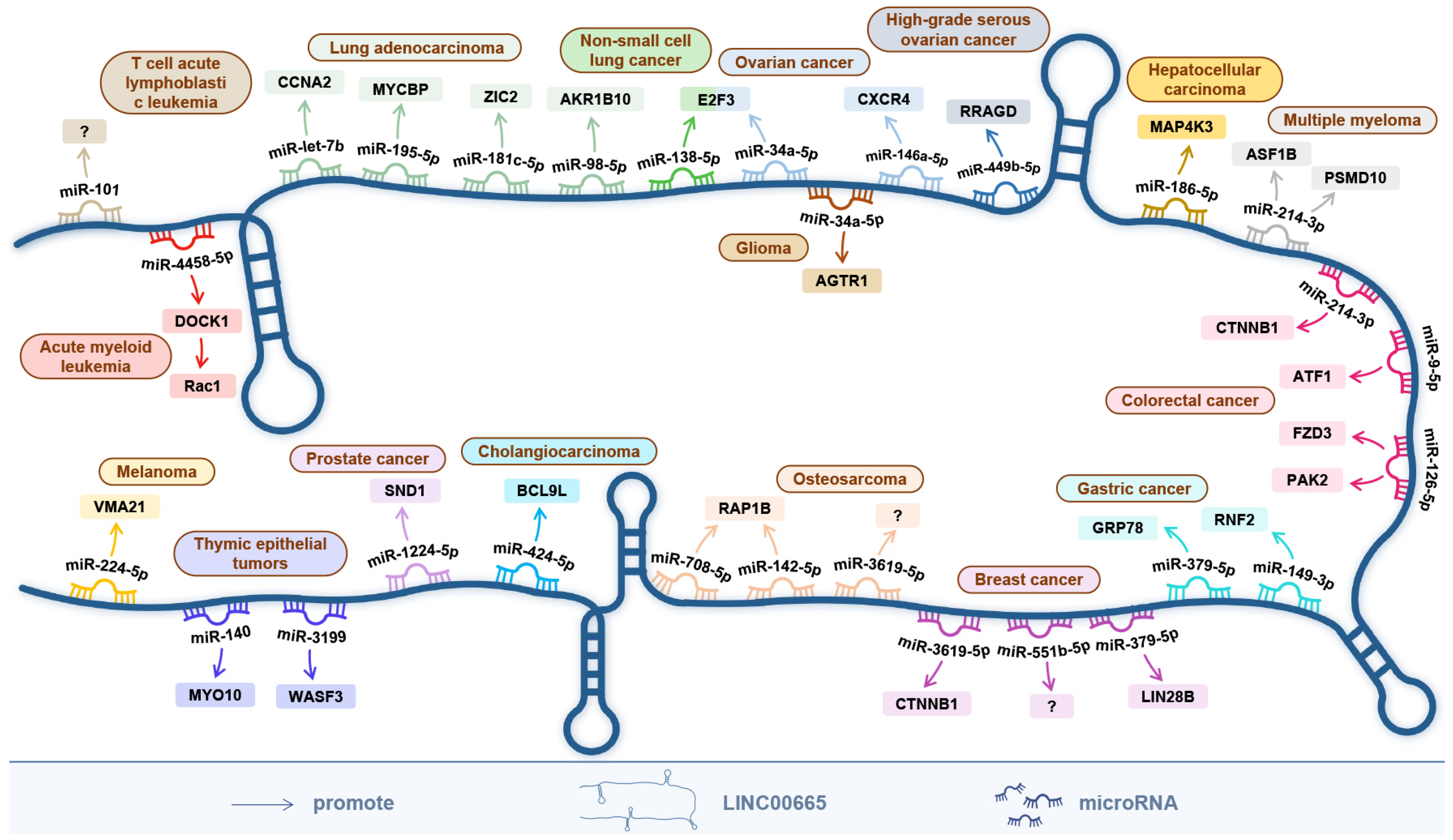
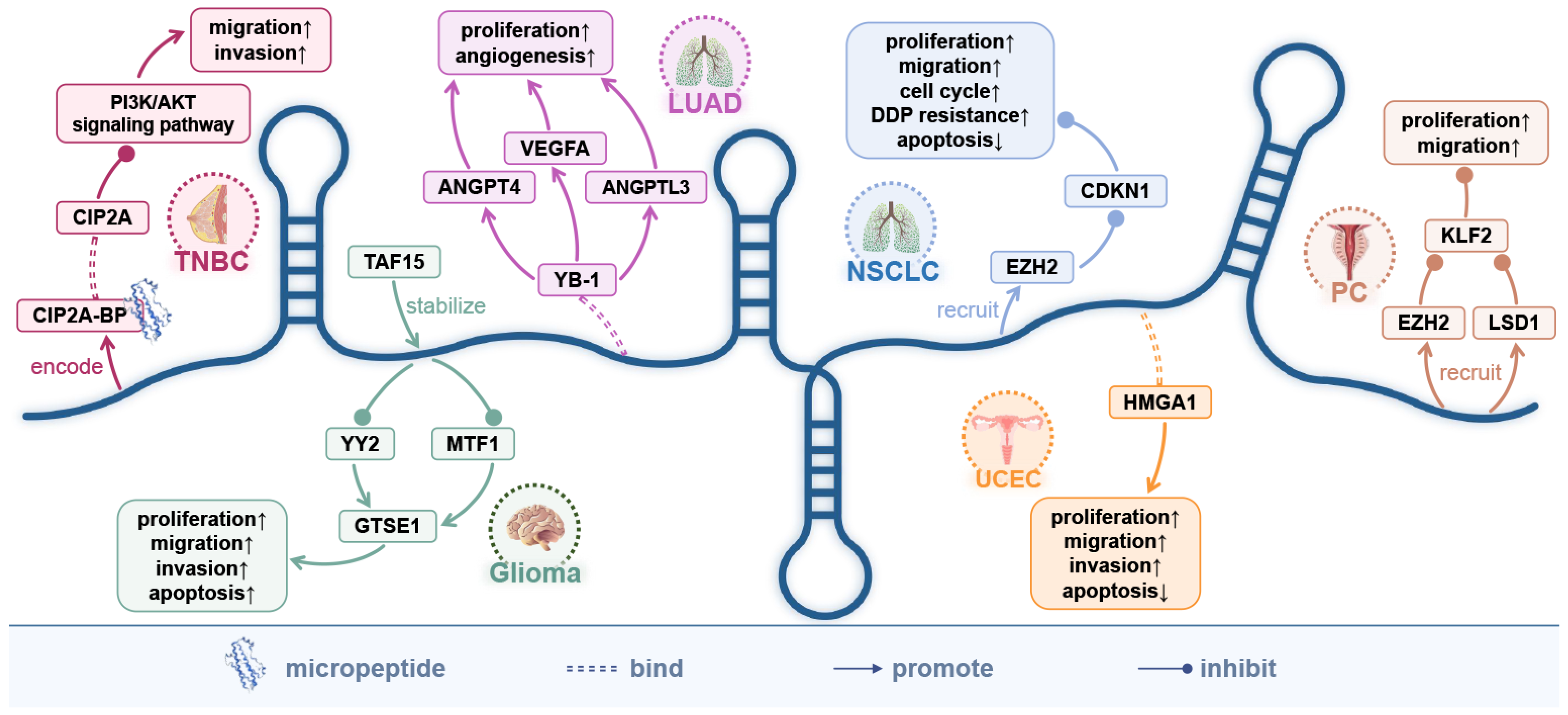


| Tumor Type | Assessed Cell Lines | Animals | Expression | Detection and Statistical Methods | Regulatory Mechanism | Effect In Vitro | Effect In Vivo | Ref. |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | BC (MCF-7, MDA-MB-231, BT474, BT549, MDA-MB-468, and T47D); Normal (MCF-10A) | 10 SCID mice (5-week-old, female) | Upregulation | qRT-PCR; Student’s t-test | LINC00665/miR-379-5p/LIN28B | proliferation↑ migration↑ invasion↑ EMT↑ | tumor growth↑ | [10] |
| BC (MCF-7, MDA-MB-231, ZR-75-30, and MDA-MB-415); Normal (MCF-10A) | - | Upregulation | TCGA RNA-seq and qRT-PCR; Independent sample t-test | - | proliferation↑ migration↑ invasion↑ EMT↑ | - | [11] | |
| BC (MCF-7 and MDA-MB-231); Normal (MCF-10A) | - | Upregulation | qRT-PCR and GEPIA; Student’s t-test and ANOVA | LINC00665/miR-3619-5p/CTNNB1 | proliferation↑ migration↑ invasion↑ cell cycle↑ apoptosis↓ | - | [12] | |
| - | - | Upregulation | TCGA RNA-seq and qRT-PCR; Student’s t-test and Chi-square test | - | cell cycle↑ DNA repair↑ | - | [9] | |
| BC (MCF-7, MDA-MB-231, and HCC-1937); Normal (MCF-10A) | 12 BALB/c nude mice (4-week-old, female) | Upregulation | TCGA RNA-seq and qRT-PCR; Student’s t-test, ANOVA, and Tukey post hoc tests | LINC00665/miR-551b-5p | proliferation↑ apoptosis↓ | tumor growth↑ | [13] | |
| Triple negative breast cancer | TNBC (BT549, Hs578T, and MDA-MB-231); Normal (MCF-10A and HEK293T) | 50 Nude mice (6–8-week-old, female) | Downregulation | qRT-PCR; two-tailed Student’s t-test | LINC00665/ CIP2A-BP/CIP2A | migration↓ invasion↓ | tumor growth↓ | [5] |
| Prostate cancer | PC (LNCaP, PC-3, DU-145, and 22RV1); Normal (RWPE-1) | 10 BALB/c nude mice (4-week-old, female) | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-1224-5p/SND1 | proliferation↑ migration↑ invasion↑ | tumor growth↑ | [14] |
| PC (PC-3, DU-145, 22RV1, and LNCaP) | 10 Nude mice (8-week-old, male) | Upregulation | qRT-PCR; Student’s t-test | LINC00665/ EZH2, LSD1/KLF2 | proliferation↑ migration↑ | tumor growth↑ | [15] | |
| PC (PC3) | - | Upregulation | TCGA RNA-seq and qRT-PCR; two-tailed Student’s t-test | - | DNA repair↑ radiosensitivity↓ | - | [16] | |
| Ovarian cancer | OC (A2780, OVCAR3, CAOV3, and SKOV3); Normal (IOSE80) | - | Upregulation | qRT-PCR; Student’s t-test and Pearson correlation test | LINC00665/miR-34a-5p/E2F3 | proliferation↑ migration↑ invasion↑ | - | [17] |
| - | - | Upregulation | TCGA RNA-seq and GTEx RNA-seq | LINC00665/ EDEM1, CAPNS1, SQSTM1, SERPINA1 | - | - | [18] | |
| - | - | Upregulation | GEO RNA-seq | LINC00665/miR-146a-5p/CXCR4 | - | - | [44] | |
| High-grade serous ovarian cancer | - | - | Upregulation | TCGA RNA-seq and GTEx RNA-seq | LINC00665/miR-449b-5p/RRAGD | - | - | [19] |
| Cervical cancer | CA (HeLa) | 10 BALB/c immuno-deficient mice (female) | Upregulation | RNA-seq and qRT-PCR; ANOVA | LINC00665/ CTNNB1/(Wnt/β-catenin signaling pathway) | proliferation↑ migration↑ invasion↑ EMT↑ | tumor growth↑ | [20] |
| Uterine corpus endometrial cancer | UCEC (RL-95-2, Ishikawa, HEC-1B, KLE, and HHUA) | 12 Mice (8-week-old, female) | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/ HMGA1 | proliferation↑ migration↑ invasion↑ apoptosis↓ | tumor growth↑ | [21] |
| Gastric cancer | GC (MKN28, BGC-823, SGC-7901, AGS, and HGC-27); Normal (GES-1) | 12 Nude mice (6-week-old, male) | Upregulation | qRT-PCR; two-tailed Student’s t-test and ANOVA | LINC00665/(Wnt/β-catenin signaling pathway) | proliferation↑ migration↑ invasion↑ apoptosis↓ | tumor growth↑ | [22] |
| GC (AGS, SGC-7901, HGC27, MGC-803, MKN-45, and BGC-823); Normal (GES-1) | - | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-149-3p/RNF2 | proliferation↑ migration↑ invasion↑ | - | [23] | |
| GC (AGS, MKN45, HGC27, MKN28, and SGC7901); Normal (GES) | BALB/c nude mice (6-week-old) | Upregulation | TCGA RNA-seq and qRT-PCR; Unpaired Student’s t-test and ANOVA | LINC00665/ TGF-β/Smad-2, α-SMA | EMT↑ migration↑ invasion↑ cell cycle↑ apoptosis↓ | tumor growth↑ | [24] | |
| GC (SGC-7901, AGS, and HST2); Normal (GES) | - | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-379-5p/GRP78 | proliferation↑ apoptosis↓ | - | [25] | |
| Hepatocellular carcinoma | HCC (Huh-7 and HepG2) | - | Upregulation | TCGA RNA-seq; Student’s t-test, ANOVA, Dunnett’s multiple comparisons test, Wilcoxon tests, and nonparametric Mann–Whitney U-test | NF-κB/ LINC00665/ PKR/NF-κB loop | proliferation↑ | tumor growth↑ | [26] |
| - | - | Upregulation | TCGA RNA-seq, GEO RNA-seq and qRT-PCR; Independent sample t-tests | - | - | - | [3] | |
| HCC (Huh-7, HepG2, HCCLM6, MHCC-97H, and Hep3B); Normal (HL-7702) | 24 BALB/c nude mice (4–6-week-old, female) | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-186-5p/MAP4K3 | proliferation↑ apoptosis↓ autophagy↓ | tumor growth↑ | [27] | |
| Colorectal cancer | CRC (DLD-1, SW480, KM12, SW116, and SW620); Normal (NCM460) | - | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-9-5p/ATF1 | proliferation↑ migration↑ invasion↑ apoptosis↓ | - | [28] |
| CRC (SW620, LOVO, HCT-116, and SW480); Normal (NCM460) | Nude mice (Female) | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-214-3p, U2AF2/ CTNNB1/(Wnt/β-catenin signaling pathway) | proliferation↑ migration↑ invasion↑ apoptosis↓ | tumor growth↑ | [29] | |
| CRC (DLD1, RKO, HCT116, LOVO, SW480, and NCM460) | - | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-126-5p/PAK2, FZD3 | proliferation↑ invasion↑ apoptosis↓ | - | [30] | |
| Cholangiocarcinoma | CCA (SNU-1196, SNU-1079, SNU-308, SNU-245, SNU-478, and SNU-869) | 18 Nude mice | Upregulation | qRT-PCR and lncRNA microarray analysis; two-tailed Student’s t-test and ANOVA | LINC00665/miR-424-5p/BCL9L/(Wnt/β-catenin signaling pathway) | proliferation↑ drug resistance↑ EMT↑ stemness↑ apoptosis↓ | Drug resistance↑ | [8] |
| Non-small cell lung cancer | NSCLC (A549, H520, H1299, SPC-A1, and SK-MES-1) | 24 BALB/c nude mice | Upregulation | qRT-PCR and lncRNA microarray analysis; two-tailed Student’s t-test and ANOVA | LINC00665/miR-138-5p/E2F3 | proliferation↑ invasion↑ | tumor growth↑ | [31] |
| LUAD (PC9) | 24 Athymic BALB/c nude mice (5-week-old, male) | Upregulation | qRT-PCR; Student’s t-test | LINC00665/ EGFR/(PI3K/AKT signaling pathway) | proliferation↑ drug resistance↑ migration↑ cell cycle↑ apoptosis↓ | tumor growth↑ Drug resistance↑ | [6] | |
| NSCLC (PC9, SPC-A1, H1975, H1299, and A549); Normal (16HBE) | 24 BALB/c nude mice (4–5-week-old, male) | Upregulation | qRT-PCR; Student’s t-test, Wilcoxon test, and Chi-square test | LINC00665/ EZH2/ CDKN1C | proliferation↑ migration↑ cell cycle↑ drug resistance↑ apoptosis↓ | tumor growth↑ Drug resistance↑ | [7] | |
| Lung adenocarcinoma | NSCLC (A549, H1299, 16HBE, and D551) | 30 BALB/c athymic nude mice (4-week-old, female) | Upregulation | qRT-PCR; Student’s t-test, multiple Student’s t-test, and nonparametric Mann–Whitney U-test | LINC00665/ YB-1/ANGPT4, ANGPTL3, VEGFA | proliferation↑ angiogenesis↑ | angiogenesis↑ | [32] |
| NSCLC (A549, H1299, H1650, H520, SPC-A1, and SK-MES-1); Normal (16HBE) | 20 BALB/c athymic nude mice (4-week-old, female) | Upregulation | qRT-PCR; Student’s t-test and nonparametric Mann–Whitney U-test | SP1/ LINC00665/miR-98-5p/AKR1B10/(ERK signaling pathway) | proliferation↑ migration↑ invasion↑ EMT↑ | tumor growth↑ | [1] | |
| LUAD (SK-LU-1 and Calu-3) | 15 BALB/c nude mice (6–8-week-old, male) | Upregulation | qRT-PCR and GEPIA; two-tailed Student’s t-test, ANOVA and Tukey’s tests | LINC00665/miR-181c-5p/ZIC2 | proliferation↑ invasion↑ | tumor growth↑ | [33] | |
| LUAD (HBE, A549, H1299, H1975, PC9, and SPC-A1) | 10 BALB/c nude mice (male) | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-195-5p/MYCBP | proliferation↑ invasion↑ cell cycle↑ apoptosis↓ | tumor growth↑ metastasis↑ | [34] | |
| - | - | Upregulation | GEO RNA-seq | LINC00665/miR-let-7b/CCNA2 | - | - | [35] | |
| Glioma | Glioma (U251 and U87); Normal (NHA) | BALB/c athymic nude mice(4-week-old) | Downregulation | qRT-PCR; Student’s t-test and ANOVA | TAF15/ LINC00665/ MTF1, YY2/ GTSE1 | proliferation↓ migration↓ invasion↓ apoptosis↑ | tumor growth↓ | [43] |
| Glioma (U87 MG, LN229, A172, U373, and U251); Normal (NHA) | 6 Specific pathogen-free mice (4-week-old) | Upregulation | TCGA RNA-seq and qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-34a-5p/AGTR1 | proliferation↑ migration↑ invasion↑ | tumor growth↑ | [36] | |
| Thymic epithelial tumors | - | - | Upregulation | TCGA RNA-seq | LINC00665/miR-140/MYO10 & LINC00665/miR-3199/WASF3 | - | - | [37] |
| Osteosarcoma | Osteosarcoma (143B, U2OS, MG-63, and Saos-2); Normal (hFOB1.19) | - | Upregulation | qRT-PCR; Student’s t-test, ANOVA, and Dunnett’s test | LINC00665/miR-3619 | proliferation↑ migration↑ invasion↑ | - | [38] |
| Osteosarcoma (MG-63, U2OS, 143B, and Saos-2); Normal (hFOB) | - | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-708-5p, miR-142-5p/RAP1B | proliferation↑ migration↑ invasion↑ | - | [2] | |
| Acute myelocytic leukemia | MM (MM.1S, U266, RPMI-8226, KM3, and H929); Normal (nPCs) | - | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-214-3p/PSMD10, ASF1B | proliferation↑ apoptosis↓ | - | [39] |
| Melanoma | Melanoma (A375, M21, A2058, and A-875) | BALB/c nude mice (6-week-old, male) | Upregulation | qRT-PCR; Student’s t-test and ANOVA | LINC00665/miR-224-5p/VMA21 | proliferation↑ migration↑ | - | [40] |
| Acute myelocytic leukemia | AML (KG1, U937, NB4, and HL60); Normal (HS-5) | - | Upregulation | qRT-PCR and GEPIA; Student’s t-test, ANOVA, and Dunnett’s test | LINC00665/miR-4458-5p/DOCK1/Rac1 | proliferation↑ migration↑ adhesion↑ apoptosis↓ | - | [41] |
| T cell acute lymphoblastic leukemia | T-ALL (MOLT-4 and CCRF-CEM); Normal (PBMC) | - | Upregulation | qRT-PCR and GEPIA; Student’s t-test, ANOVA, and Bonferroni post hoc test | LINC00665/miR-101 | viability↑ migration↑ adhesion↑ | - | [42] |
| System | Tumor Type | Sample Size | Expression | Prognostic/Diagnostic Value | Ref. |
|---|---|---|---|---|---|
| Reproductive system | Breast cancer | 60 patients | Upregulation | Positively associated with lymph node metastasis and TNM stage; prognostic factor of OS (p = 0.0209) and DFS (p = 0.0492) | [11] |
| 106 patients | Upregulation | Positively associated with tumor size, lymph node metastasis and TNM stage | [12] | ||
| 102 patients | Upregulation | Positively associated with lymph node metastasis; AUC = 0.785 | [9] | ||
| 36 patients | Upregulation | Positively associated with lymph node metastasis and TNM stage; prognostic factor of OS (p = 0.016) | [13] | ||
| Triple negative breast cancer | 217 patients | Downregulation | Prognostic factor of OS (MST: 29 versus 43 months, p = 0.02, HR = 2.64) | [5] | |
| Prostate cancer | 50 patients | Upregulation | Positively associated with lymph node metastasis and TNM stage; prognostic factor of OS (p < 0.05) | [15] | |
| - | Upregulation | Prognostic factor of OS (p = 0.022) | [16] | ||
| Ovarian cancer | 56 patients | Upregulation | Positively associated with tumor size, lymph node metastasis and FIGO stage; prognostic factor of OS (p = 0.0037, HR = 1.37) | [17] | |
| - | Upregulation | Prognostic factor of OS (p = 5.006 × 10−3) | [18] | ||
| - | Upregulation | Prognostic factor of OS (p = 0.00051, HR = 1.43) | [44] | ||
| High-grade serous ovarian cancer | - | Upregulation | Negatively associated with the infiltration level of macrophages and dendritic cells | [19] | |
| Digestive system | Gastric cancer | 49 patients | Upregulation | Positively associated with poor differentiation and TNM stage | [23] |
| 116 patients | Upregulation | Positively associated with tumor depth, lymph node metastasis and TNM stage; prognostic factor of OS (p < 0.05, HR = 2.703); AUC = 0.828 | [24] | ||
| Hepatocellular carcinoma | 122 patients | Upregulation | Positively associated with lymph node metastasis and TNM stage; prognostic factor of OS (p < 0.05) | [26] | |
| 39 patients | Upregulation | Positively associated with poor differentiation, TNM stage and vascular invasion; prognostic factor of OS (MST: 46 versus 70 months, p = 0.027, HR = 1.477); AUC = 0.614, sensitivity = 0.55, specificity = 0.53 | [3] | ||
| 76 patients | Upregulation | Positively associated with tumor size and poor differentiation; prognostic factor of OS (p < 0.05) | [27] | ||
| Colorectal cancer | 46 patients | Upregulation | Positively associated with lymph node metastasis and poor differentiation | [28] | |
| Cholangiocarcinoma | 100 patients | Upregulation | Positively associated with lymph node metastasis and TNM stage; prognostic factor of OS (p = 0.0375, HR = 1.835) and RFS (p < 0.001, HR = 2.554) | [8] | |
| Respiratory system | Non-small cell lung cancer | 60 patients | Upregulation | Positively associated with tumor size, lymph node metastasis and TNM stage; prognostic factor of OS (p = 0.005) and PFS (p = 0.002) | [7] |
| Lung adenocarcinoma | 60 patients | Upregulation | Positively associated with tumor size, lymph node metastasis and TNM stage | [32] | |
| 80 patients | Upregulation | Positively associated with tumor size, lymph node metastasis and TNM stage; prognostic factor of OS (p = 0.0115) and RFS (p < 0.001) | [1] | ||
| 84 patients | Upregulation | Prognostic factor of OS (p = 0.035, HR = 1.44) | [33] | ||
| - | Upregulation | Prognostic factor of OS (p < 0.05) | [35] | ||
| Nervous system | Glioma | - | Downregulation | Negatively associated with pathological grade | [43] |
| 48 patients | Upregulation | Prognostic factor of OS (p = 0.0241) | [36] | ||
| Endocrine system | Thymic epithelial tumors | - | Upregulation | Prognostic factor of OS (p = 0.047) | [37] |
| Endocrine system | Osteosarcoma | 33 patients | Upregulation | Prognostic factor of OS (p < 0.05) | [38] |
| 42 patients | Upregulation | Prognostic factor of OS (p = 0.011) | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, C.; Xie, Z.; Shen, J.; Jia, Y.; Duan, S. LINC00665: An Emerging Biomarker for Cancer Diagnostics and Therapeutics. Cells 2022, 11, 1540. https://doi.org/10.3390/cells11091540
Zhong C, Xie Z, Shen J, Jia Y, Duan S. LINC00665: An Emerging Biomarker for Cancer Diagnostics and Therapeutics. Cells. 2022; 11(9):1540. https://doi.org/10.3390/cells11091540
Chicago/Turabian StyleZhong, Chenming, Zijun Xie, Jinze Shen, Yunhua Jia, and Shiwei Duan. 2022. "LINC00665: An Emerging Biomarker for Cancer Diagnostics and Therapeutics" Cells 11, no. 9: 1540. https://doi.org/10.3390/cells11091540
APA StyleZhong, C., Xie, Z., Shen, J., Jia, Y., & Duan, S. (2022). LINC00665: An Emerging Biomarker for Cancer Diagnostics and Therapeutics. Cells, 11(9), 1540. https://doi.org/10.3390/cells11091540






