Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines
Abstract
:1. Introduction
2. Pathomechanism of Changes in Skeletal Muscle in Sarcopenia and Sarcopenic Obesity
3. Obesity
3.1. Adipokines
3.2. Myosteatosis
3.3. Bone Marrow Adipose Tissue
4. Physical Exercise as a Method of Preventing Sarcopenia
5. A Role for Myokines in Sarcopenia: Cross-Talk between Muscle and Adipose Tissue
5.1. IGF-1
5.2. Myostatin
5.3. Irisin
5.4. Meteorin-like Factor (metrnl)
5.5. Brain-Derived Neurotrophic Factor (BDNF)
5.6. Fibroblast Growth Factor 21 (FGF21)
5.7. β-Aminoisobutyric Acid (BAIBA)
5.8. Apelin
5.9. Decorin
5.10. Il-6
5.11. IL-7
5.12. IL-15
5.13. Other Myokines
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Duda, K.; Majerczak, J.; Nieckarz, Z.; Heymsfield, S.B.; Zoladz, J.A. Human body composition and muscle mass. In Muscle and Exercise Physiology; Zoladz, J.A., Ed.; Academic Press: London, UK, 2019; pp. 3–26. [Google Scholar]
- Lee, R.C.; Wang, Z.M.; Heymsfield, S.B. Skeletal muscle mass and aging: Regional and whole-body measurement methods. Can. J. Appl. Physiol. 2001, 26, 102–122. [Google Scholar] [CrossRef]
- Degens, H. Human ageing: Impact on muscle force and power. In Muscle and Exercise Physiology; Zoladz, J.A., Ed.; Academic Press: London, UK, 2019; pp. 423–432. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-H.; Choi, K.M. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [Green Version]
- Poggiogalle, E.; Mendes, I.; Ong, B.; Prado, C.M.; Mocciaro, G.; Mazidi, M.; Lubrano, C.; Lenzi, A.; Donini, L.M.; Siervo, M. Sarcopenic obesity and insulin resistance: Application of novel body composition models. Nutrition 2020, 75–76, 110765. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, L.Y.; Wu, L.; Ma, H.; Li, X.M.; Li, Q.; Aleteng, Q.; Hu, Y.; He, W.Y.; Gao, J.; et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin. Nutr. 2021, 40, 571–580. [Google Scholar] [CrossRef]
- Kang, D.O.; Park, S.Y.; Choi, B.G.; Na, J.O.; Choi, C.U.; Kim, E.J.; Rha, S.W.; Park, C.G.; Hong, S.J.; Seo, H.S. Prognostic Impact of Low Skeletal Muscle Mass on Major Adverse Cardiovascular Events in Coronary Artery Disease: A Propensity Score-Matched Analysis of a Single Center All-Comer Cohort. J. Clin. Med. 2019, 8, 712. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, J.; Geerlings, M.A.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef]
- Matkovic, V.; Jelic, T.; Wardlaw, G.M.; Ilich, J.Z.; Goel, P.K.; Wright, J.K.; Andon, M.B.; Smith, K.T.; Heaney, R.P. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J. Clin. Investig. 1994, 93, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef] [Green Version]
- Zamboni, M.; Gattazzo, S.; Rossi, A.P. Myosteatosis: A relevant, yet poorly explored element of sarcopenia. Eur. Geriatr. Med. 2019, 10, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Landi, F.; Picca, A.; Calvani, R.; Marzetti, E. Anorexia of Aging: Assessment and Management. Clin. Geriatr. Med. 2017, 33, 315–323. [Google Scholar] [CrossRef]
- Schoufour, J.D.; Tieland, M.; Barazzoni, R.; Ben Allouch, S.; van der Bie, J.; Boirie, Y.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinkova, E.; Visser, B.; et al. The Relevance of Diet, Physical Activity, Exercise, and Persuasive Technology in the Prevention and Treatment of Sarcopenic Obesity in Older Adults. Front. Nutr. 2021, 8, 661449. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Gros, J.; Manceau, M.; Thome, V.; Marcelle, C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 2005, 435, 954–958. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res. Rev. 2014, 15, 51–60. [Google Scholar] [CrossRef]
- Gruberg, L.; Weissman, N.J.; Waksman, R.; Fuchs, S.; Deible, R.; Pinnow, E.E.; Ahmed, L.M.; Kent, K.M.; Pichard, A.D.; Suddath, W.O.; et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: The obesity paradox? J. Am. Coll. Cardiol. 2002, 39, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat Weight Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Bahat, G.; Kilic, C.; Ozkok, S.; Ozturk, S.; Karan, M.A. Associations of sarcopenic obesity versus sarcopenia alone with functionality. Clin. Nutr. 2021, 40, 2851–2859. [Google Scholar] [CrossRef]
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J. Aging Res. 2018, 2018, 6147426. [Google Scholar] [CrossRef] [Green Version]
- Alalwan, T.A. Phenotypes of Sarcopenic Obesity: Exploring the Effects on Peri-Muscular Fat, the Obesity Paradox, Hormone-Related Responses and the Clinical Implications. Geriatrics 2020, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro, M. Leptin Resistance and Lipolysis of White Adipose Tissue: An Implication to Ectopic Fat Disposition and Its Consequences. J. Atheroscler. Thromb. 2017, 24, 1088–1089. [Google Scholar] [CrossRef] [Green Version]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. 2010, 34, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Gustafsson, T.; Ulfhake, B. Sarcopenia: What Is the Origin of This Aging-Induced Disorder? Front. Genet. 2021, 12, 688526. [Google Scholar] [CrossRef]
- Wickramasinghe, K.; Mathers, J.C.; Wopereis, S.; Marsman, D.S.; Griffiths, J.C. From lifespan to healthspan: The role of nutrition in healthy ageing. J. Nutr. Sci. 2020, 9, e33. [Google Scholar] [CrossRef]
- Murphy, C.H.; Roche, H. Nutrition and physical activity countermeasures for sarcopenia: Time to get personal? Nutr. Bull. 2018, 43, 374–387. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Oh, J.; Tufts, H. The role of the neuromuscular junction in sarcopenia. In Sarcopenia; Sakuma, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 59–80. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [Green Version]
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [Green Version]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musaro, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: Recent research advances. Pflug. Arch. 2017, 469, 573–591. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Garcia-Prat, L.; Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell. Biol. 2021. [Google Scholar] [CrossRef]
- Metcalf, D. The Autonomous Behaviour of Normal Thymus Grafts. Aust. J. Exp. Biol. Med. Sci. 1963, 41, SUPPL437–SUPPL447. [Google Scholar] [CrossRef]
- Metcalf, D. Restricted Growth Capacity of Multiple Spleen Grafts. Transplantation 1964, 2, 387–392. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Callus, B.A.; Verhagen, A.M.; Vaux, D.L. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006, 273, 4264–4276. [Google Scholar] [CrossRef]
- Vassilev, A.; Kaneko, K.J.; Shu, H.; Zhao, Y.; DePamphilis, M.L. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001, 15, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Pobbati, A.V.; Chan, S.W.; Lee, I.; Song, H.; Hong, W. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure 2012, 20, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Guan, K.L. The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian Hippo pathway. Semin. Cell. Dev. Biol. 2012, 23, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, M.; Berman, K.S.; Cobb, M.H. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem. 1998, 273, 28625–28632. [Google Scholar] [CrossRef] [Green Version]
- Boggiano, J.C.; Vanderzalm, P.J.; Fehon, R.G. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell. 2011, 21, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hutchison, M.; Cobb, M.H. Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J. Biol. Chem. 1999, 274, 28803–28807. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef] [Green Version]
- Praskova, M.; Xia, F.; Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 2008, 18, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Sudol, M.; Bork, P.; Einbond, A.; Kastury, K.; Druck, T.; Negrini, M.; Huebner, K.; Lehman, D. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J. Biol. Chem. 1995, 270, 14733–14741. [Google Scholar] [CrossRef] [Green Version]
- Sudol, M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 1994, 9, 2145–2152. [Google Scholar]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007, 17, 2054–2060. [Google Scholar] [CrossRef] [Green Version]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef] [Green Version]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Richter, J.D.; Sonenberg, N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 2005, 433, 477–480. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [Green Version]
- Philp, A.; Hamilton, D.L.; Baar, K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J. Appl. Physiol. 2011, 110, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Watt, K.I.; Judson, R.; Medlow, P.; Reid, K.; Kurth, T.B.; Burniston, J.G.; Ratkevicius, A.; De Bari, C.; Wackerhage, H. Yap is a novel regulator of C2C12 myogenesis. Biochem. Biophys. Res. Commun. 2010, 393, 619–624. [Google Scholar] [CrossRef]
- Sudol, M.; Chen, H.I.; Bougeret, C.; Einbond, A.; Bork, P. Characterization of a novel protein-binding module–the WW domain. FEBS Lett. 1995, 369, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Carson, J.A.; Schwartz, R.J.; Booth, F.W. SRF and TEF-1 control of chicken skeletal alpha-actin gene during slow-muscle hypertrophy. Am. J. Physiol. 1996, 270, C1624–C1633. [Google Scholar] [CrossRef]
- Watt, K.I.; Turner, B.J.; Hagg, A.; Zhang, X.; Davey, J.R.; Qian, H.; Beyer, C.; Winbanks, C.E.; Harvey, K.F.; Gregorevic, P. The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat. Commun. 2015, 6, 6048. [Google Scholar] [CrossRef]
- Judson, R.N.; Gray, S.R.; Walker, C.; Carroll, A.M.; Itzstein, C.; Lionikas, A.; Zammit, P.S.; De Bari, C.; Wackerhage, H. Constitutive expression of Yes-associated protein (Yap) in adult skeletal muscle fibres induces muscle atrophy and myopathy. PLoS ONE 2013, 8, e59622. [Google Scholar] [CrossRef] [Green Version]
- Hulmi, J.J.; Oliveira, B.M.; Silvennoinen, M.; Hoogaars, W.M.; Ma, H.; Pierre, P.; Pasternack, A.; Kainulainen, H.; Ritvos, O. Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E41–E50. [Google Scholar] [CrossRef]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell. Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [Green Version]
- Judson, R.N.; Tremblay, A.M.; Knopp, P.; White, R.B.; Urcia, R.; De Bari, C.; Zammit, P.S.; Camargo, F.D.; Wackerhage, H. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J. Cell Sci. 2012, 125, 6009–6019. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.M.; Missiaglia, E.; Galli, G.G.; Hettmer, S.; Urcia, R.; Carrara, M.; Judson, R.N.; Thway, K.; Nadal, G.; Selfe, J.L.; et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell 2014, 26, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Dui, W.; Liu, D.; Xing, Y.; Yuan, Z.; Ji, G. MST1, a key player, in enhancing fast skeletal muscle atrophy. BMC Biol. 2013, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; De Mello, V.; Mohamed, A.; Ortuste Quiroga, H.P.; Garcia-Munoz, A.; Al Bloshi, A.; Tremblay, A.M.; von Kriegsheim, A.; Collie-Duguid, E.; Vargesson, N.; et al. Common and Distinctive Functions of the Hippo Effectors Taz and Yap in Skeletal Muscle Stem Cell Function. Stem Cells 2017, 35, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- You, B.; Yan, G.; Zhang, Z.; Yan, L.; Li, J.; Ge, Q.; Jin, J.P.; Sun, J. Phosphorylation of cardiac troponin I by mammalian sterile 20-like kinase 1. Biochem. J. 2009, 418, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Odashima, M.; Usui, S.; Takagi, H.; Hong, C.; Liu, J.; Yokota, M.; Sadoshima, J. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ. Res. 2007, 100, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.; Yung, R. Immune senescence, epigenetics and autoimmunity. Clin. Immunol. 2018, 196, 59–63. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Barberi, L.; Scicchitano, B.M.; De Rossi, M.; Bigot, A.; Duguez, S.; Wielgosik, A.; Stewart, C.; McPhee, J.; Conte, M.; Narici, M.; et al. Age-dependent alteration in muscle regeneration: The critical role of tissue niche. Biogerontology 2013, 14, 273–292. [Google Scholar] [CrossRef] [Green Version]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting—the role of the ubiquitin–proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.H.; Frost, R.A.; Vary, T.C. Regulation of muscle protein synthesis during sepsis and inflammation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E453–E459. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.A.; Lang, C.H. Protein kinase B/Akt: A nexus of growth factor and cytokine signaling in determining muscle mass. J. Appl. Physiol. 2007, 103, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity Alters the Muscle Protein Synthetic Response to Nutrition and Exercise. Front. Nutr. 2019, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Atherton, P.J.; Selby, A.; Rankin, D.; Williams, J.; Smith, K.; Hiscock, N.; Rennie, M.J. Muscle protein synthetic responses to exercise: Effects of age, volume, and intensity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Meex, R.C.; Blaak, E.E.; van Loon, L.J. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 2019, 20, 1205–1217. [Google Scholar] [CrossRef] [Green Version]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Saini, J.; McPhee, J.S.; Al-Dabbagh, S.; Stewart, C.E.; Al-Shanti, N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res. Rev. 2016, 27, 67–76. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Vasson, M.P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 2016, 26, 22–36. [Google Scholar] [CrossRef]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef] [Green Version]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.S.; Petrocelli, J.J.; Nelson, D.B.; Lindsay, C.C.; Gardner, J.E.; Morrow, V.R.; Keefe, A.C.; Huffaker, T.B.; et al. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E85–E98. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Afzali, A.M.; Muntefering, T.; Wiendl, H.; Meuth, S.G.; Ruck, T. Skeletal muscle cells actively shape (auto)immune responses. Autoimmun. Rev. 2018, 17, 518–529. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, bnaa016. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2018, 23, 570. [Google Scholar] [CrossRef] [Green Version]
- Strzepa, A.; Lobo, F.M.; Majewska-Szczepanik, M.; Szczepanik, M. Antibiotics and autoimmune and allergy diseases: Causative factor or treatment? Int. Immunopharmacol. 2018, 65, 328–341. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and their microbes: The unexpected friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2009, 28, 623–667. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Janssen, A.W.; Kersten, S. The role of the gut microbiota in metabolic health. FASEB J. 2015, 29, 3111–3123. [Google Scholar] [CrossRef] [Green Version]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Fanelli, F.; Calvani, R.; Mule, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Conway, J.; Duggal, N.A. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef]
- Buigues, C.; Fernandez-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martinez, R.; Martinez-Martinez, M.; Verdejo, Y.; Mascaros, M.C.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hanninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Huang, Y.; Yu, X. A Narrative Review of Gut-Muscle Axis and Sarcopenia: The Potential Role of Gut Microbiota. Int. J. Gen. Med. 2021, 14, 1263–1273. [Google Scholar] [CrossRef]
- Lee, M.-C.; Hsu, Y.-J.; Ho, H.-H.; Hsieh, S.-H.; Kuo, Y.-W.; Sung, H.-C.; Huang, C.-C. Lactobacillus salivarius Subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Hsu, Y.J.; Chuang, H.L.; Hsieh, P.S.; Ho, H.H.; Chen, W.L.; Chiu, Y.S.; Huang, C.C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef] [Green Version]
- Kazmierczak-Siedlecka, K.; Folwarski, M.; Skonieczna-Zydecka, K.; Ruszkowski, J.; Makarewicz, W. The use of Lactobacillus plantarum 299v (DSM 9843) in cancer patients receiving home enteral nutrition - study protocol for a randomized, double-blind, and placebo-controlled trial. Nutr. J. 2020, 19, 98. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rue, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Liao, X.; Wu, M.; Hao, Y.; Deng, H. Exploring the Preventive Effect and Mechanism of Senile Sarcopenia Based on "Gut-Muscle Axis". Front. Bioeng. Biotechnol. 2020, 8, 590869. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota-mitochondria inter-talk: Consequence for microbiota-host interaction. Pathog. Dis. 2016, 74, ftv096. [Google Scholar] [CrossRef] [Green Version]
- Houghton, M.J.; Kerimi, A.; Mouly, V.; Tumova, S.; Williamson, G. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J. 2019, 33, 1887–1898. [Google Scholar] [CrossRef] [Green Version]
- Mottawea, W.; Chiang, C.K.; Muhlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Aon, M.A.; Bhatt, N.; Cortassa, S.C. Mitochondrial and cellular mechanisms for managing lipid excess. Front. Physiol. 2014, 5, 282. [Google Scholar] [CrossRef] [Green Version]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.-S. Molecular mechanisms of exercise providing therapeutic rationale to counter sarcopenia. In Sarcopenia; Elsevier: Amsterdam, The Netherlands, 2021; pp. 159–169. [Google Scholar]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013, 17, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Lanthier, N.; Leclercq, I.A. Adipose tissues as endocrine target organs. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 545–558. [Google Scholar] [CrossRef]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef]
- Dempersmier, J.; Sul, H.S. Shades of brown: A model for thermogenic fat. Front. Endocrinol. 2015, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Kredel, L.I.; Siegmund, B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front. Immunol. 2014, 5, 462. [Google Scholar] [CrossRef] [Green Version]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and Metabolic Implications of Regional Differences among Fat Depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef] [Green Version]
- Roman, S.; Agil, A.; Peran, M.; Alvaro-Galue, E.; Ruiz-Ojeda, F.J.; Fernandez-Vazquez, G.; Marchal, J.A. Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Transl. Res. 2015, 165, 464–479. [Google Scholar] [CrossRef]
- Nam, S.Y. Obesity-Related Digestive Diseases and Their Pathophysiology. Gut Liver 2017, 11, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Galliano, D.; Bellver, J. Female obesity: Short- and long-term consequences on the offspring. Gynecol. Endocrinol. 2013, 29, 626–631. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Watt, K.I.; Henstridge, D.C.; Ziemann, M.; Sim, C.B.; Montgomery, M.K.; Samocha-Bonet, D.; Parker, B.L.; Dodd, G.T.; Bond, S.T.; Salmi, T.M.; et al. Yap regulates skeletal muscle fatty acid oxidation and adiposity in metabolic disease. Nat. Commun. 2021, 12, 2887. [Google Scholar] [CrossRef]
- Sun, S.; Ji, Y.; Kersten, S.; Qi, L. Mechanisms of inflammatory responses in obese adipose tissue. Annu. Rev. Nutr. 2012, 32, 261–286. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Flavell, R.A. Innate sensors of pathogen and stress: Linking inflammation to obesity. J. Allergy Clin. Immunol. 2013, 132, 287–294. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Goody, D.; Pfeifer, A. MicroRNAs in brown and beige fat. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2019, 1864, 29–36. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, M.; Oh, K.J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and their role in intestinal inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Bucci, L.; Yani, S.L.; Fabbri, C.; Bijlsma, A.Y.; Maier, A.B.; Meskers, C.G.; Narici, M.V.; Jones, D.A.; McPhee, J.S.; Seppet, E.; et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 2013, 14, 261–272. [Google Scholar] [CrossRef]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [Green Version]
- Adams, V.; Mangner, N.; Gasch, A.; Krohne, C.; Gielen, S.; Hirner, S.; Thierse, H.-J.; Witt, C.C.; Linke, A.; Schuler, G. Induction of MuRF1 is essential for TNF-α-induced loss of muscle function in mice. J. Mol. Biol. 2008, 384, 48–59. [Google Scholar] [CrossRef]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef]
- Burgos-Ramos, E.; Canelles, S.; Rodriguez, A.; Frago, L.M.; Gomez-Ambrosi, J.; Chowen, J.A.; Fruhbeck, G.; Argente, J.; Barrios, V. The increase in fiber size in male rat gastrocnemius after chronic central leptin infusion is related to activation of insulin signaling. Mol. Cell. Endocrinol. 2018, 470, 48–59. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Herberg, S.; Arounleut, P.; He, H.-Z.; Shiver, A.; Qi, R.-Q.; Zhou, L.; Isales, C.M.; Mi, Q.-S. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem. Biophys. Res. Commun. 2010, 400, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Tazawa, R.; Uchida, K.; Fujimaki, H.; Miyagi, M.; Inoue, G.; Sekiguchi, H.; Murata, K.; Takata, K.; Kawakubo, A.; Takaso, M. Elevated leptin levels induce inflammation through IL-6 in skeletal muscle of aged female rats. BMC Musculoskelet. Disord. 2019, 20, 199. [Google Scholar] [CrossRef] [Green Version]
- Vella, C.A.; Cushman, M.; Van Hollebeke, R.B.; Allison, M.A. Associations of Abdominal Muscle Area and Radiodensity with Adiponectin and Leptin: The Multiethnic Study of Atherosclerosis. Obesity 2018, 26, 1234–1241. [Google Scholar] [CrossRef]
- Lana, A.; Valdés-Bécares, A.; Buño, A.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. 2017, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Y.; Chen, W.L. Examining the Association Between Serum Leptin and Sarcopenic Obesity. J. Inflamm. Res. 2021, 14, 3481–3487. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Gamberi, T.; Modesti, A.; Magherini, F.; D’Souza, D.M.; Hawke, T.; Fiaschi, T. Activation of autophagy by globular adiponectin is required for muscle differentiation. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 694–702. [Google Scholar] [CrossRef]
- Gamberi, T.; Magherini, F.; Fiaschi, T. Adiponectin in myopathies. Int. J. Mol. Sci. 2019, 20, 1544. [Google Scholar] [CrossRef] [Green Version]
- Fiaschi, T.; Tedesco, F.S.; Giannoni, E.; Diaz-Manera, J.; Parri, M.; Cossu, G.; Chiarugi, P. Globular adiponectin as a complete mesoangioblast regulator: Role in proliferation, survival, motility, and skeletal muscle differentiation. Mol. Biol. Cell 2010, 21, 848–859. [Google Scholar] [CrossRef] [Green Version]
- Fiaschi, T. Mechanisms of Adiponectin Action; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2019. [Google Scholar]
- Fiaschi, T.; Giannoni, E.; Taddei, M.L.; Chiarugi, P. Globular adiponectin activates motility and regenerative traits of muscle satellite cells. PLoS ONE 2012, 7, e34782. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sweeney, G. Adiponectin action in skeletal muscle. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 33–41. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, J.; Hu, Z.; Walsh, K.; Wang, X.H. Evidence for adipose-muscle cross talk: Opposing regulation of muscle proteolysis by adiponectin and Fatty acids. Endocrinology 2007, 148, 5696–5705. [Google Scholar] [CrossRef] [Green Version]
- Can, B.; Kara, O.; Kizilarslanoglu, M.C.; Arik, G.; Aycicek, G.S.; Sumer, F.; Civelek, R.; Demirtas, C.; Ulger, Z. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin. Exp. Res. 2017, 29, 745–752. [Google Scholar] [CrossRef]
- Baker, J.F.; Newman, A.B.; Kanaya, A.; Leonard, M.B.; Zemel, B.; Miljkovic, I.; Long, J.; Weber, D.; Harris, T.B. The Adiponectin Paradox in the Elderly: Associations With Body Composition, Physical Functioning, and Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.E.; Lira, F.S.; Silva, B.S.A.; Freire, A.; Ramos, E.M.C.; Gobbo, L.A. Influence of skeletal muscle mass and fat mass on the metabolic and inflammatory profile in sarcopenic and non-sarcopenic overfat elderly. Aging Clin. Exp. Res. 2019, 31, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Van Hollebeke, R.B.; Cushman, M.; Schlueter, E.F.; Allison, M.A. Abdominal muscle density is inversely related to adiposity inflammatory mediators. Med. Sci. Sports Exerc. 2018, 50, 1495. [Google Scholar] [CrossRef]
- Sheng, C.H.; Du, Z.W.; Song, Y.; Wu, X.D.; Zhang, Y.C.; Wu, M.; Wang, Q.; Zhang, G.Z. Human resistin inhibits myogenic differentiation and induces insulin resistance in myocytes. Biomed. Res. Int. 2013, 2013, 804632. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, M.F.; Wallace, G.R.; Davis, E.T.; Murphy, D.P.; Nicholson, T.; Bennett, A.J.; Tsintzas, K.; Jones, S.W. Obese subcutaneous adipose tissue impairs human myogenesis, particularly in old skeletal muscle, via resistin-mediated activation of NFkappaB. Sci. Rep. 2018, 8, 15360. [Google Scholar] [CrossRef]
- Waters, D.L. Intermuscular Adipose Tissue: A Brief Review of Etiology, Association With Physical Function and Weight Loss in Older Adults. Ann. Geriatr. Med. Res. 2019, 23, 3–8. [Google Scholar] [CrossRef]
- Konopka, A.R.; Wolff, C.A.; Suer, M.K.; Harber, M.P. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R461–R468. [Google Scholar] [CrossRef]
- Rivas, D.A.; McDonald, D.J.; Rice, N.P.; Haran, P.H.; Dolnikowski, G.G.; Fielding, R.A. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2016, 310, R561–R569. [Google Scholar] [CrossRef] [Green Version]
- Gueugneau, M.; Coudy-Gandilhon, C.; Theron, L.; Meunier, B.; Barboiron, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Picard, B.; et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 566–576. [Google Scholar] [CrossRef]
- Mastrocola, R.; Collino, M.; Nigro, D.; Chiazza, F.; D’Antona, G.; Aragno, M.; Minetto, M.A. Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle. PLoS ONE 2015, 10, e0119587. [Google Scholar] [CrossRef] [Green Version]
- Moratal, C.; Raffort, J.; Arrighi, N.; Rekima, S.; Schaub, S.; Dechesne, C.; Chinetti, G.; Dani, C. IL-1β-and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci. Rep. 2018, 8, 17005. [Google Scholar]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef]
- Collao, N.; Farup, J.; De Lisio, M. Role of Metabolic Stress and Exercise in Regulating Fibro/Adipogenic Progenitors. Front. Cell Dev. Biol. 2020, 8, 9. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Y.; Yu, X. The Unique Metabolic Characteristics of Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Krings, A.; Rahman, S.; Huang, S.; Lu, Y.; Czernik, P.; Lecka-Czernik, B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 2012, 50, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Sulston, R.J.; Cawthorn, W.P. Bone marrow adipose tissue as an endocrine organ: Close to the bone? Horm. Mol. Biol. Clin. Investig. 2016, 28, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef] [Green Version]
- Yakabe, M.; Hosoi, T.; Akishita, M.; Ogawa, S. Updated concept of sarcopenia based on muscle-bone relationship. J. Bone Miner. Metab. 2020, 38, 7–13. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef]
- Musumeci, G. Sarcopenia and exercise “The State of the Art”. J. Funct. Morphol. Kinesiol. 2017, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef]
- Beckwee, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef]
- Lazarus, N.R.; Harridge, S.D.R. Declining performance of master athletes: Silhouettes of the trajectory of healthy human ageing? J. Physiol. 2017, 595, 2941–2948. [Google Scholar] [CrossRef] [Green Version]
- Johnston, A.P.; De Lisio, M.; Parise, G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl. Physiol. Nutr. Metab. 2008, 33, 191–199. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef] [Green Version]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.-W.; No, M.-H.; Min, D.-H.; Kang, J.-H.; Kwak, H.-B. Aging-induced Sarcopenia and Exercise. Off. J. Korean Acad. Kinesiol. 2017, 19, 43–59. [Google Scholar]
- Csapo, R.; Alegre, L.M. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand. J. Med. Sci. Sports 2016, 26, 995–1006. [Google Scholar] [CrossRef]
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Liao, C.D.; Tsauo, J.Y.; Lin, L.F.; Huang, S.W.; Ku, J.W.; Chou, L.C.; Liou, T.H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef]
- Gadelha, A.B.; Paiva, F.M.; Gauche, R.; de Oliveira, R.J.; Lima, R.M. Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 168–173. [Google Scholar] [CrossRef]
- Forbes, S.C.; Little, J.P.; Candow, D.G. Exercise and nutritional interventions for improving aging muscle health. Endocrine 2012, 42, 29–38. [Google Scholar] [CrossRef]
- Erlich, A.T.; Tryon, L.D.; Crilly, M.J.; Memme, J.M.; Moosavi, Z.S.M.; Oliveira, A.N.; Beyfuss, K.; Hood, D.A. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr. Med. Res. 2016, 5, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E92–E101. [Google Scholar] [CrossRef] [Green Version]
- Ko, I.G.; Jeong, J.W.; Kim, Y.H.; Jee, Y.S.; Kim, S.E.; Kim, S.H.; Jin, J.J.; Kim, C.J.; Chung, K.J. Aerobic exercise affects myostatin expression in aged rat skeletal muscles: A possibility of antiaging effects of aerobic exercise related with pelvic floor muscle and urethral rhabdosphincter. Int. Neurourol. J. 2014, 18, 77–85. [Google Scholar] [CrossRef]
- Bouaziz, W.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health benefits of endurance training alone or combined with diet for obese patients over 60: A review. Int. J. Clin. Pract. 2015, 69, 1032–1049. [Google Scholar] [CrossRef]
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 30–53. [Google Scholar] [CrossRef]
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Porcari, J.P.; McLean, K.P.; Foster, C.; Kernozek, T.; Crenshaw, B.; Swenson, C. Effects of electrical muscle stimulation on body composition, muscle strength, and physical appearance. J. Strength Cond. Res. 2002, 16, 165–172. [Google Scholar]
- Musumeci, G. The use of vibration as physical exercise and therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, P. Physical activity, health benefits, and mortality risk. Int. Sch. Res. Not. Cardiol. 2012, 2012, 718789. [Google Scholar] [CrossRef] [Green Version]
- Febbraio, M.A. Exercise metabolism in 2016: Health benefits of exercise-more than meets the eye! Nat. Rev. Endocrinol. 2017, 13, 72–74. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Who would have thought—Myokines two decades on. Nat. Rev. Endocrinol. 2020, 16, 619–620. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Li, C.; Zhong, R.; Zhao, Y.; Zhang, Q.; Tong, W.; Zhu, D.; Zhang, Y. Muscle-secreted granulocyte colony-stimulating factor functions as metabolic niche factor ameliorating loss of muscle stem cells in aged mice. EMBO J. 2019, 38, e102154. [Google Scholar] [CrossRef]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Scharf, G.; Heineke, J. Finding Good Biomarkers for Sarcopenia; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Han, L.; Wu, S.; Hu, P. The functions of sarcopenia related myokines. Transl. Med. Aging 2018, 2, 38–41. [Google Scholar] [CrossRef]
- Aryana, I.G.P.S.; Hapsari, A.A.A.R.; Kuswardhani, R.A.T. Myokine regulation as marker of sarcopenia in elderly. Mol. Cell. Biomed. Sci. 2018, 2, 38–47. [Google Scholar] [CrossRef]
- Castro, J.; Vale, R. Insulin-like growth factor i (igf-1) in older adults: A review. MOJ Gerontol. Geriatr. 2017, 1, 175–176. [Google Scholar]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Bando, H.; Zhang, C.; Takada, Y.; Yamasaki, R.; Saito, S. Impaired secretion of growth hormone-releasing hormone, growth hormone and IGF-I in elderly men. Acta Endocrinol. 1991, 124, 31–36. [Google Scholar] [CrossRef]
- Naranjo, J.D.; Dziki, J.L.; Badylak, S.F. Regenerative Medicine Approaches for Age-Related Muscle Loss and Sarcopenia: A Mini-Review. Gerontology 2017, 63, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Sohmiya, M.; Oka, N.; Kato, Y. Effects of aging and sex on plasma insulin-like growth factor I (IGF-I) levels in normal adults. Acta Endocrinol. 1991, 124, 497–500. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, Y.; Gong, F.; Shan, G.; Yang, H.; Xu, K.; Zhang, D.; Cheng, X.; Zhang, Z.; Chen, S.; et al. Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS ONE 2017, 12, e0185561. [Google Scholar] [CrossRef]
- Haden, S.T.; Glowacki, J.; Hurwitz, S.; Rosen, C.; LeBoff, M.S. Effects of age on serum dehydroepiandrosterone sulfate, IGF-I, and IL-6 levels in women. Calcif. Tissue Int. 2000, 66, 414–418. [Google Scholar] [CrossRef]
- Moran, S.; Chen, Y.; Ruthie, A.; Nir, Y. Alterations in IGF-I affect elderly: Role of physical activity. Eur. Rev. Aging Phys. Act. 2007, 4, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Van den Beld, A.W.; Carlson, O.D.; Doyle, M.E.; Rizopoulos, D.; Ferrucci, L.; van der Lely, A.J.; Egan, J.M. IGFBP-2 and aging: A 20-year longitudinal study on IGFBP-2, IGF-I, BMI, insulin sensitivity and mortality in an aging population. Eur. J. Endocrinol. 2019, 180, 109–116. [Google Scholar] [CrossRef]
- Ferrari, U.; Schmidmaier, R.; Jung, T.; Reincke, M.; Martini, S.; Schoser, B.; Bidlingmaier, M.; Drey, M. IGF-I/IGFBP3/ALS Deficiency in Sarcopenia: Low GHBP Suggests GH Resistance in a Subgroup of Geriatric Patients. J. Clin. Endocrinol. Metab. 2021, 106, e1698–e1707. [Google Scholar] [CrossRef]
- Chew, J.; Tay, L.; Lim, J.; Leung, B.; Yeo, A.; Yew, S.; Ding, Y.; Lim, W. Serum myostatin and IGF-1 as gender-specific biomarkers of frailty and low muscle mass in community-dwelling older adults. J. Nutr. Health Aging 2019, 23, 979–986. [Google Scholar] [CrossRef]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age 2015, 37, 121. [Google Scholar] [CrossRef] [Green Version]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty Liver Index Associates with Relative Sarcopenia and GH/IGF-1 Status in Obese Subjects. PLoS ONE 2016, 11, e0145811. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Antunes, H.K.; Tufik, S.; de Mello, M.T. Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept. Mot. Ski. 2010, 110, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.L.; Wang, C.H.; Pan, C.Y.; Chen, F.C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.W.; Bressel, E.; Kim, D.Y. Effects of aquatic exercise on insulin-like growth factor-1, brain-derived neurotrophic factor, vascular endothelial growth factor, and cognitive function in elderly women. Exp. Gerontol. 2020, 132, 110842. [Google Scholar] [CrossRef]
- Arazi, H.; Babaei, P.; Moghimi, M.; Asadi, A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021, 21, 50. [Google Scholar] [CrossRef]
- Deuschle, M.; Blum, W.; Frystyk, J.; Ørskov, H.; Schweiger, U.; Weber, B.; Körner, A.; Gotthardt, U.; Schmider, J.; Standhardt, H. Endurance training and its effect upon the activity of the GH-IGFs system in the elderly. Int. J. Sports Med. 1998, 19, 250–254. [Google Scholar] [CrossRef]
- Singh, M.A.; Ding, W.; Manfredi, T.J.; Solares, G.S.; O’Neill, E.F.; Clements, K.M.; Ryan, N.D.; Kehayias, J.J.; Fielding, R.A.; Evans, W.J. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am. J. Physiol. 1999, 277, E135–E143. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef]
- Sharma, M.; Kambadur, R.; Matthews, K.G.; Somers, W.G.; Devlin, G.P.; Conaglen, J.V.; Fowke, P.J.; Bass, J.J. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell. Physiol. 1999, 180, 1–9. [Google Scholar] [CrossRef]
- Goodman, C.A.; McNally, R.M.; Hoffmann, F.M.; Hornberger, T.A. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol. Endocrinol. 2013, 27, 1946–1957. [Google Scholar] [CrossRef]
- Morissette, M.R.; Cook, S.A.; Buranasombati, C.; Rosenberg, M.A.; Rosenzweig, A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol. -Cell Physiol. 2009, 297, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Amirouche, A.; Durieux, A.C.; Banzet, S.; Koulmann, N.; Bonnefoy, R.; Mouret, C.; Bigard, X.; Peinnequin, A.; Freyssenet, D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 2009, 150, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Eilers, W.; Chambers, D.; Cleasby, M.; Foster, K. Local myostatin inhibition improves skeletal muscle glucose uptake in insulin-resistant high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E163–E174. [Google Scholar] [CrossRef]
- Takahashi, H.; Sato, K.; Yamaguchi, T.; Miyake, M.; Watanabe, H.; Nagasawa, Y.; Kitagawa, E.; Terada, S.; Urakawa, M.; Rose, M.T.; et al. Myostatin alters glucose transporter-4 (GLUT4) expression in bovine skeletal muscles and myoblasts isolated from double-muscled (DM) and normal-muscled (NM) Japanese shorthorn cattle. Domest. Anim. Endocrinol. 2014, 48, 62–68. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Cao, L.; Zhang, Y.; Xia, W.; Zhu, D. Myostatin regulates glucose metabolism via the AMP-activated protein kinase pathway in skeletal muscle cells. Int. J. Biochem. Cell. Biol. 2010, 42, 2072–2081. [Google Scholar] [CrossRef]
- Willis, S.A.; Sargeant, J.A.; Thackray, A.E.; Yates, T.; Stensel, D.J.; Aithal, G.P.; King, J.A. Effect of exercise intensity on circulating hepatokine concentrations in healthy men. Appl. Physiol. Nutr. Metab. 2019, 44, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef] [Green Version]
- Haines, M.S.; Dichtel, L.E.; Kimball, A.; Bollinger, B.; Gerweck, A.V.; Bredella, M.A.; Miller, K.K. OR26-03 Lower Serum Myostatin Levels Are Associated with Higher Insulin Sensitivity in Adults with Overweight/Obesity. J. Endocr. Soc. 2020, 4, OR26-03. [Google Scholar] [CrossRef]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef] [Green Version]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Amor, M.; Itariu, B.K.; Moreno-Viedma, V.; Keindl, M.; Jurets, A.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Serum Myostatin is Upregulated in Obesity and Correlates with Insulin Resistance in Humans. Exp. Clin. Endocrinol. Diabetes 2019, 127, 550–556. [Google Scholar] [CrossRef]
- Lin, J.; Arnold, H.B.; Della-Fera, M.A.; Azain, M.J.; Hartzell, D.L.; Baile, C.A. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem. Biophys. Res. Commun. 2002, 291, 701–706. [Google Scholar] [CrossRef]
- Hjorth, M.; Pourteymour, S.; Gorgens, S.W.; Langleite, T.M.; Lee, S.; Holen, T.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A.; et al. Myostatin in relation to physical activity and dysglycaemia and its effect on energy metabolism in human skeletal muscle cells. Acta Physiol. 2016, 217, 45–60. [Google Scholar] [CrossRef]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.S.; Li, G.; Blumenthal, J.B.; Ortmeyer, H.K. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity 2013, 21, 1350–1356. [Google Scholar] [CrossRef]
- Peng, L.N.; Lee, W.J.; Liu, L.K.; Lin, M.H.; Chen, L.K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2018, 9, 635–642. [Google Scholar] [CrossRef]
- Yarasheski, K.; Bhasin, S.; Sinha-Hikim, I.; Pak-Loduca, J.; Gonzalez-Cadavid, N. Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. J. Nutr. Health Aging 2002, 6, 343–348. [Google Scholar]
- Leger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163–175B. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Rodriguez, A.; Becerril, S.; Ezquerro, S.; Mendez-Gimenez, L.; Fruhbeck, G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol. 2017, 219, 362–381. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxid. Med. Cell. Longev. 2020, 2020, 6946037. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernandez-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef] [Green Version]
- Oguz, A.; Sahin, M.; Tuzun, D.; Kurutas, E.B.; Ulgen, C.; Bozkus, O.; Gul, K. Irisin is a predictor of sarcopenic obesity in type 2 diabetes mellitus: A cross-sectional study. Medicine 2021, 100, e26529. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenia--The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71. [Google Scholar] [CrossRef]
- Bluher, S.; Panagiotou, G.; Petroff, D.; Markert, J.; Wagner, A.; Klemm, T.; Filippaios, A.; Keller, A.; Mantzoros, C.S. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity 2014, 22, 1701–1708. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Daskalopoulou, S.S.; Cooke, A.B.; Gomez, Y.H.; Mutter, A.F.; Filippaios, A.; Mesfum, E.T.; Mantzoros, C.S. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur. J. Endocrinol. 2014, 171, 343–352. [Google Scholar] [CrossRef]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Senechal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef]
- Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Liu, J.; Cui, X.Y.; Yang, Y.Q.; Gao, W.; Sun, L.; Dong, Y.C.; Kou, X.J. Effects of high-intensity treadmill training on timeliness and plasticity expression of irisin in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2168–2173. [Google Scholar]
- Loffler, D.; Muller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum irisin levels are regulated by acute strenuous exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef] [Green Version]
- Mazur-Bialy, A.I.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto-Mikami, E.; Sato, K.; Kurihara, T.; Hasegawa, N.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE 2015, 10, e0120354. [Google Scholar] [CrossRef] [Green Version]
- Nygaard, H.; Slettalokken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Ronnestad, B.R.; Ellefsen, S. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS ONE 2015, 10, e0121367. [Google Scholar] [CrossRef]
- Palacios-Gonzalez, B.; Vadillo-Ortega, F.; Polo-Oteyza, E.; Sanchez, T.; Ancira-Moreno, M.; Romero-Hidalgo, S.; Meraz, N.; Antuna-Puente, B. Irisin levels before and after physical activity among school-age children with different BMI: A direct relation with leptin. Obesity 2015, 23, 729–732. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Rodriguez, A.; Gouveia, A.M.; Goncalves, I.O.; Becerril, S.; Ramirez, B.; Beleza, J.; Fruhbeck, G.; Ascensao, A.; Magalhaes, J. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci. 2016, 165, 100–108. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ferreira, E.; Carneiro-Júnior, M.; Natali, A.; Bressan, J. Effects of exercise on the circulating concentrations of irisin in healthy adult individuals: A review. Sci. Sports 2016, 31, 251–260. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism 2015, 64, 1042–1050. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Peterson, J.M.; Mart, R.; Bond, C.E. Effect of obesity and exercise on the expression of the novel myokines, Myonectin and Fibronectin type III domain containing 5. PeerJ 2014, 2, e605. [Google Scholar] [CrossRef] [Green Version]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pollanen, E.; Makela, K.A.; Kainulainen, H.; Hakkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdes, S.; Botas, P.; Ricart, W.; Fernandez-Real, J.M. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, I.D. Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflug. Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Dong, Y.; Chen, F.; Mitch, W.; Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.; Baniyas, M.; Adeghate, E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Alam, A.; San, C.Y.; Eguchi, S.; Chen, Q.; Lian, Q.; Ma, D. Molecular mechanisms of brain-derived neurotrophic factor in neuro-protection: Recent developments. Brain Res. 2017, 1665, 1–21. [Google Scholar] [CrossRef]
- Mohammadi, A.; Amooeian, V.G.; Rashidi, E. Dysfunction in Brain-Derived Neurotrophic Factor Signaling Pathway and Susceptibility to Schizophrenia, Parkinson’s and Alzheimer’s Diseases. Curr. Gene Ther. 2018, 18, 45–63. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Han, X.; Zhou, X.; Zhang, S.; Cai, X.; Zhang, L.; Li, Y.; Li, M.; Gong, S.; Ji, L. Brain derived neurotrophic factor in newly diagnosed diabetes and prediabetes. Mol. Cell. Endocrinol. 2016, 429, 106–113. [Google Scholar] [CrossRef]
- Li, B.; Lang, N.; Cheng, Z.F. Serum Levels of Brain-Derived Neurotrophic Factor Are Associated with Diabetes Risk, Complications, and Obesity: A Cohort Study from Chinese Patients with Type 2 Diabetes. Mol. Neurobiol. 2016, 53, 5492–5499. [Google Scholar] [CrossRef]
- Matthews, V.B.; Astrom, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerstrom, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef] [Green Version]
- Colombo, E.; Bedogni, F.; Lorenzetti, I.; Landsberger, N.; Previtali, S.C.; Farina, C. Autocrine and immune cell-derived BDNF in human skeletal muscle: Implications for myogenesis and tissue regeneration. J. Pathol. 2013, 231, 190–198. [Google Scholar] [CrossRef]
- Mousavi, K.; Jasmin, B.J. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 2006, 26, 5739–5749. [Google Scholar] [CrossRef] [Green Version]
- Delezie, J.; Weihrauch, M.; Maier, G.; Tejero, R.; Ham, D.J.; Gill, J.F.; Karrer-Cardel, B.; Ruegg, M.A.; Tabares, L.; Handschin, C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 16111–16120. [Google Scholar] [CrossRef] [Green Version]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 2008, 59, 119–132. [Google Scholar]
- Zoladz, J.A.; Pilc, A. The effect of physical activity on the brain derived neurotrophic factor: From animal to human studies. J. Physiol. Pharmacol. 2010, 61, 533–541. [Google Scholar]
- Zoladz, J.A.; Majerczak, J.; Zeligowska, E.; Mencel, J.; Jaskolski, A.; Jaskolska, A.; Marusiak, J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol. Pharmacol. 2014, 65, 441–448. [Google Scholar]
- Marusiak, J.; Zeligowska, E.; Mencel, J.; Kisiel-Sajewicz, K.; Majerczak, J.; Zoladz, J.A.; Jaskolski, A.; Jaskolska, A. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J. Rehabil. Med. 2015, 47, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Coelho, F.M.; Pereira, D.S.; Lustosa, L.P.; Silva, J.P.; Dias, J.M.; Dias, R.C.; Queiroz, B.Z.; Teixeira, A.L.; Teixeira, M.M.; Pereira, L.S. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012, 54, 415–420. [Google Scholar] [CrossRef]
- Miyazaki, S.; Iino, N.; Koda, R.; Narita, I.; Kaneko, Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr. Gerontol. Int. 2021, 21, 27–33. [Google Scholar] [CrossRef]
- Coelho, F.G.; Gobbi, S.; Andreatto, C.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduroz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef]
- Iglesias, P.; Selgas, R.; Romero, S.; Diez, J.J. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur. J. Endocrinol. 2012, 167, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, V.; Jambrina, C.; Casana, E.; Sacristan, V.; Munoz, S.; Darriba, S.; Rodo, J.; Mallol, C.; Garcia, M.; Leon, X.; et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 2018, 10, e8791. [Google Scholar] [CrossRef]
- Kim, C.S.; Joe, Y.; Choi, H.S.; Back, S.H.; Park, J.W.; Chung, H.T.; Roh, E.; Kim, M.S.; Ha, T.Y.; Yu, R. Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J. Inflamm. 2019, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Hou, L.; Xiong, Y.; Zhao, S. Fibroblast Growth Factor 21 (FGF21) Promotes Formation of Aerobic Myofibers via the FGF21-SIRT1-AMPK-PGC1alpha Pathway. J. Cell. Physiol. 2017, 232, 1893–1906. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hou, L.; Wu, W.; Zhao, S.; Xiong, Y. Fibroblast growth factor 21 suppresses adipogenesis in pig intramuscular fat cells. Int. J. Mol. Sci. 2016, 17, 11. [Google Scholar] [CrossRef] [Green Version]
- Barlow, J.P.; Karstoft, K.; Vigelso, A.; Gram, M.; Helge, J.W.; Dela, F.; Pappan, K.; O’Neil, D.; Dunn, W.; Solomon, T.P.J. Beta-aminoisobutyric acid is released by contracting human skeletal muscle and lowers insulin release from INS-1 832/3 cells by mediating mitochondrial energy metabolism. Metabol. Open 2020, 7, 100053. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Choi, K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia 2015, 58, 2096–2105. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 27. [Google Scholar] [CrossRef] [Green Version]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-aminoisobutyric acid as a novel regulator of carbohydrate and lipid metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.X.; Zhao, M.X.; Shu, X.D.; Xiong, X.Q.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci. Rep. 2016, 6, 21924. [Google Scholar] [CrossRef] [Green Version]
- Ginter, E.; Simko, V. Recent data on obesity research: Beta-aminoisobutyric acid. Bratisl. Lek. Listy 2014, 115, 492–493. [Google Scholar] [CrossRef]
- Hangelbroek, R.W.; Fazelzadeh, P.; Tieland, M.; Boekschoten, M.V.; Hooiveld, G.J.; van Duynhoven, J.P.; Timmons, J.A.; Verdijk, L.B.; de Groot, L.C.; van Loon, L.J. Expression of protocadherin gamma in skeletal muscle tissue is associated with age and muscle weakness. J. Cachexia Sarcopenia Muscle 2016, 7, 604–614. [Google Scholar] [CrossRef] [Green Version]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jahn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. Beta-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- Son, J.S.; Kim, H.J.; Son, Y.; Lee, H.; Chae, S.A.; Seong, J.K.; Song, W. Effects of exercise-induced apelin levels on skeletal muscle and their capillarization in type 2 diabetic rats. Muscle Nerve 2017, 56, 1155–1163. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, L.; Zhang, J.; Tang, C.S.; Qi, Y.F.; Zhang, J. Effect of Treadmill Running on Apelin and APJ Expression in Adipose Tissue and Skeletal Muscle in Rats Fed a High-fat Diet. Int. J. Sports Med. 2015, 36, 535–541. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Rai, R.; Ghosh, A.K.; Eren, M.; Mackie, A.R.; Levine, D.C.; Kim, S.Y.; Cedernaes, J.; Ramirez, V.; Procissi, D.; Smith, L.H.; et al. Downregulation of the Apelinergic Axis Accelerates Aging, whereas Its Systemic Restoration Improves the Mammalian Healthspan. Cell Rep. 2017, 21, 1471–1480. [Google Scholar] [CrossRef] [Green Version]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef]
- Vosadi, E.; Gholami, F.; Mortazavi, E. Effect of Eight Weeks of Exercise with Different Intensities on the Gene Expression of Decorin and Muscular TGF-β in the Male Adult Rats. Sci. J. Ilam Univ. Med. Sci. 2021, 29, 86–94. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Vuillermoz, B.; Wegrowski, Y.; Contet-Audonneau, J.L.; Danoux, L.; Pauly, G.; Maquart, F.X. Influence of aging on glycosaminoglycans and small leucine-rich proteoglycans production by skin fibroblasts. Mol. Cell. Biochem. 2005, 277, 63–72. [Google Scholar] [CrossRef]
- Svard, J.; Rost, T.H.; Sommervoll, C.E.N.; Haugen, C.; Gudbrandsen, O.A.; Mellgren, A.E.; Rodahl, E.; Ferno, J.; Dankel, S.N.; Sagen, J.V.; et al. Absence of the proteoglycan decorin reduces glucose tolerance in overfed male mice. Sci. Rep. 2019, 9, 4614. [Google Scholar] [CrossRef]
- El Shafey, N.; Guesnon, M.; Simon, F.; Deprez, E.; Cosette, J.; Stockholm, D.; Scherman, D.; Bigey, P.; Kichler, A. Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp. Cell. Res. 2016, 341, 187–195. [Google Scholar] [CrossRef]
- Bekki, M.; Hashida, R.; Kawaguchi, T.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Koya, S.; Hirota, K.; Matsuse, H.; Niizeki, T.; et al. The association between sarcopenia and decorin, an exercise-induced myokine, in patients with liver cirrhosis: A pilot study. JCSM Rapid Commun. 2018, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. IL-6 myokine signaling in skeletal muscle: A double-edged sword. FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. 2005, 98, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, L.; Berardinelli, M.G.; De Pasquale, L.; Nicoletti, C.; D’Amico, A.; Carvello, F.; Moneta, G.M.; Catizone, A.; Bertini, E.; De Benedetti, F.; et al. Functional and Morphological Improvement of Dystrophic Muscle by Interleukin 6 Receptor Blockade. EBioMedicine 2015, 2, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef]
- Alemán, H.; Esparza, J.; Ramirez, F.A.; Astiazaran, H.; Payette, H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing 2011, 40, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Tsujinaka, T.; Fujita, J.; Ebisui, C.; Yano, M.; Kominami, E.; Suzuki, K.; Tanaka, K.; Katsume, A.; Ohsugi, Y.; Shiozaki, H.; et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J. Clin. Investig. 1996, 97, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat. Cell. Biol. 2018, 20, 917–927. [Google Scholar] [CrossRef]
- Williams, A.; Wang, J.J.; Wang, L.; Sun, X.; Fischer, J.E.; Hasselgren, P.O. Sepsis in mice stimulates muscle proteolysis in the absence of IL-6. Am. J. Physiol. 1998, 275, R1983–R1991. [Google Scholar] [CrossRef]
- Ikeda, S.I.; Tamura, Y.; Kakehi, S.; Sanada, H.; Kawamori, R.; Watada, H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 473, 947–952. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef] [Green Version]
- Kristof, E.; Klusoczki, A.; Veress, R.; Shaw, A.; Combi, Z.S.; Varga, K.; Gyory, F.; Balajthy, Z.; Bai, P.; Bacso, Z.; et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp Cell Res 2019, 377, 47–55. [Google Scholar] [CrossRef]
- Tierney, M.T.; Aydogdu, T.; Sala, D.; Malecova, B.; Gatto, S.; Puri, P.L.; Latella, L.; Sacco, A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 2014, 20, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
- Begue, G.; Douillard, A.; Galbes, O.; Rossano, B.; Vernus, B.; Candau, R.; Py, G. Early activation of rat skeletal muscle IL-6/STAT1/STAT3 dependent gene expression in resistance exercise linked to hypertrophy. PLoS ONE 2013, 8, e57141. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.J.; Churchward-Venne, T.A.; Bellamy, L.; Parise, G.; Baker, S.K.; Phillips, S.M. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS ONE 2013, 8, e78636. [Google Scholar] [CrossRef] [Green Version]
- Toth, K.G.; McKay, B.R.; De Lisio, M.; Little, J.P.; Tarnopolsky, M.A.; Parise, G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS ONE 2011, 6, e17392. [Google Scholar] [CrossRef]
- Baeza-Raja, B.; Muñoz-Cánoves, P. p38 MAPK-induced nuclear factor-κB activity is required for skeletal muscle differentiation: Role of interleukin-6. Mol. Biol. Cell 2004, 15, 2013–2026. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Durstine, J.L.; Koh, H.J.; Carver, W.E.; Frizzell, N.; Carson, J.A. Acute myotube protein synthesis regulation by IL-6-related cytokines. Am. J. Physiol. Cell. Physiol. 2017, 313, C487–C500. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Wu, L.W.; Grivennikov, S.I.; de Jong, P.R.; Lian, I.; Yu, F.X.; Wang, K.; Ho, S.B.; Boland, B.S.; Chang, J.T.; et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015, 519, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.; Hellsten, Y.; Steensberg, A.; Pedersen, B.K. Differential regulation of IL-6 and TNF-alpha via calcineurin in human skeletal muscle cells. Cytokine 2006, 36, 141–147. [Google Scholar] [CrossRef]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases—Myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef]
- Cullen, T.; Thomas, A.W.; Webb, R.; Hughes, M.G. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: The effect of exercise intensity and volume. Appl. Physiol. Nutr. Metab. 2016, 41, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Mazzucchelli, R.; Durum, S.K. Interleukin-7 receptor expression: Intelligent design. Nat. Rev. Immunol. 2007, 7, 144–154. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. -Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; Bohn, S.K.; Raastad, T.; Paulsen, G.; Blomhoff, R.; Kadi, F. Differences in the inflammatory plasma cytokine response following two elite female soccer games separated by a 72-h recovery. Scand. J. Med. Sci. Sports 2010, 20, 740–747. [Google Scholar] [CrossRef]
- Lian, H. The Myokine Interleukin-7 May Influence Differentiation and Migration of Cultured Satellite Cells. Master’s Thesis, University of Oslo, Oslo, Norway, 2009. [Google Scholar]
- Ahn, N.; Kim, K. Effects of Aerobic and Resistance Exercise on Myokines in High Fat Diet-Induced Middle-Aged Obese Rats. Int. J. Environ. Res. Public Health 2020, 17, 2685. [Google Scholar] [CrossRef] [Green Version]
- Passtoors, W.M.; van den Akker, E.B.; Deelen, J.; Maier, A.B.; van der Breggen, R.; Jansen, R.; Trompet, S.; van Heemst, D.; Derhovanessian, E.; Pawelec, G.; et al. IL7R gene expression network associates with human healthy ageing. Immun. Ageing 2015, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17, e12750. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.R.; Hojman, P.; Erikstrup, C.; Fischer, C.P.; Plomgaard, P.; Mounier, R.; Mortensen, O.H.; Broholm, C.; Taudorf, S.; Krogh-Madsen, R. Association between interleukin-15 and obesity: Interleukin-15 as a potential regulator of fat mass. J. Clin. Endocrinol. Metab. 2008, 93, 4486–4493. [Google Scholar] [CrossRef] [Green Version]
- Carbo, N.; Lopez-Soriano, J.; Costelli, P.; Alvarez, B.; Busquets, S.; Baccino, F.M.; Quinn, L.S.; Lopez-Soriano, F.J.; Argiles, J.M. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: A potential role in body weight control. Biochim Biophys. Acta 2001, 1526, 17–24. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Stroud, A.M.; Argiles, J.M. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E191–E202. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.R.; Mounier, R.; Plomgaard, P.; Mortensen, O.H.; Penkowa, M.; Speerschneider, T.; Pilegaard, H.; Pedersen, B.K. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J. Physiol. 2007, 584, 305–312. [Google Scholar] [CrossRef]
- Crane, J.D.; MacNeil, L.G.; Lally, J.S.; Ford, R.J.; Bujak, A.L.; Brar, I.K.; Kemp, B.E.; Raha, S.; Steinberg, G.R.; Tarnopolsky, M.A. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015, 14, 625–634. [Google Scholar] [CrossRef]
- Tamura, Y.; Watanabe, K.; Kantani, T.; Hayashi, J.; Ishida, N.; Kaneki, M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: Is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr. J. 2011, 58, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chang, J.; Chen, W.; Zhao, L.; Qu, B.; Tang, C.; Qi, Y.; Zhang, J. Treadmill exercise promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor alpha expression in adipose tissue of high-fat diet rats. Endocrine 2013, 43, 579–585. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Pedersen, B.K. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839. [Google Scholar] [CrossRef]
- Argiles, J.M.; Lopez-Soriano, F.J.; Busquets, S. Therapeutic potential of interleukin-15: A myokine involved in muscle wasting and adiposity. Drug Discov. Today 2009, 14, 208–213. [Google Scholar] [CrossRef]
- Quinn, L.S. Interleukin-15: A muscle-derived cytokine regulating fat-to-lean body composition. J. Anim. Sci. 2008, 86, E75–E83. [Google Scholar] [CrossRef]
- Reyes, J.L.; Vannan, D.T.; Vo, T.; Gulamhusein, A.; Beck, P.L.; Reimer, R.A.; Eksteen, B. Neutralization of IL-15 abrogates experimental immune-mediated cholangitis in diet-induced obese mice. Sci. Rep. 2018, 8, 3127. [Google Scholar] [CrossRef]
- Pistilli, E.E.; Devaney, J.M.; Gordish-Dressman, H.; Bradbury, M.K.; Seip, R.L.; Thompson, P.D.; Angelopoulos, T.J.; Clarkson, P.M.; Moyna, N.M.; Pescatello, L.S.; et al. Interleukin-15 and interleukin-15R alpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine 2008, 43, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin--mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef]
- Kjobsted, R.; Hingst, J.; Fentz, J.; Foretz, M.; Sanz, M.; Pehmoller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [Green Version]
- Quinn, L.S.; Anderson, B.G.; Drivdahl, R.H.; Alvarez, B.; Argiles, J.M. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: Implications for treatment of muscle wasting disorders. Exp. Cell. Res. 2002, 280, 55–63. [Google Scholar] [CrossRef]
- Furmanczyk, P.S.; Quinn, L.S. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol. Int. 2003, 27, 845–851. [Google Scholar] [CrossRef]
- Busquets, S.; Figueras, M.T.; Meijsing, S.; Carbo, N.; Quinn, L.S.; Almendro, V.; Argiles, J.M.; Lopez-Soriano, F.J. Interleukin-15 decreases proteolysis in skeletal muscle: A direct effect. Int. J. Mol. Med. 2005, 16, 471–476. [Google Scholar] [CrossRef]
- Quinn, L.S.; Strait-Bodey, L.; Anderson, B.G.; Argiles, J.M.; Havel, P.J. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: Evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol. Int. 2005, 29, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Yang, M.-y.; Shi, Y.-x.; Xie, M.-m.; Zhu, M.; Zheng, X.-l.; Zhang, C.-k.; Ge, Z.-l.; Bian, X.-t.; Lv, J.-t. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun. Signal. 2018, 16, 42. [Google Scholar] [CrossRef] [Green Version]
- Kopinke, D.; Roberson, E.C.; Reiter, J.F. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell 2017, 170, 340–351.e12. [Google Scholar] [CrossRef] [Green Version]
- Lutz, C.T.; Quinn, L.S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: Altered cytokine levels as a common mechanism. Aging 2012, 4, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, K.; Yamaguchi, A. Sarcopenic obesity and endocrinal adaptation with age. Int. J. Endocrinol. 2013, 2013, 204164. [Google Scholar] [CrossRef] [Green Version]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Wolden-Hanson, T. Serum and muscle interleukin-15 levels decrease in aging mice: Correlation with declines in soluble interleukin-15 receptor alpha expression. Exp. Gerontol. 2010, 45, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Marzetti, E.; Carter, C.S.; Wohlgemuth, S.E.; Lees, H.A.; Giovannini, S.; Anderson, B.; Quinn, L.S.; Leeuwenburgh, C. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech. Ageing Dev. 2009, 130, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, A.; Silay, K.; Balik, A.R.; Avcioglu, G.; Aydin, A.S. The relationship between plasma interleukin-15 levels and sarcopenia in outpatient older people. Aging Clin. Exp. Res. 2018, 30, 783–790. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res. Rev. 2016, 28, 15–26. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araujo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Broholm, C.; Pedersen, B.K. Leukaemia inhibitory factor–an exercise-induced myokine. Exerc. Immunol. Rev. 2010, 16, 77–85. [Google Scholar]
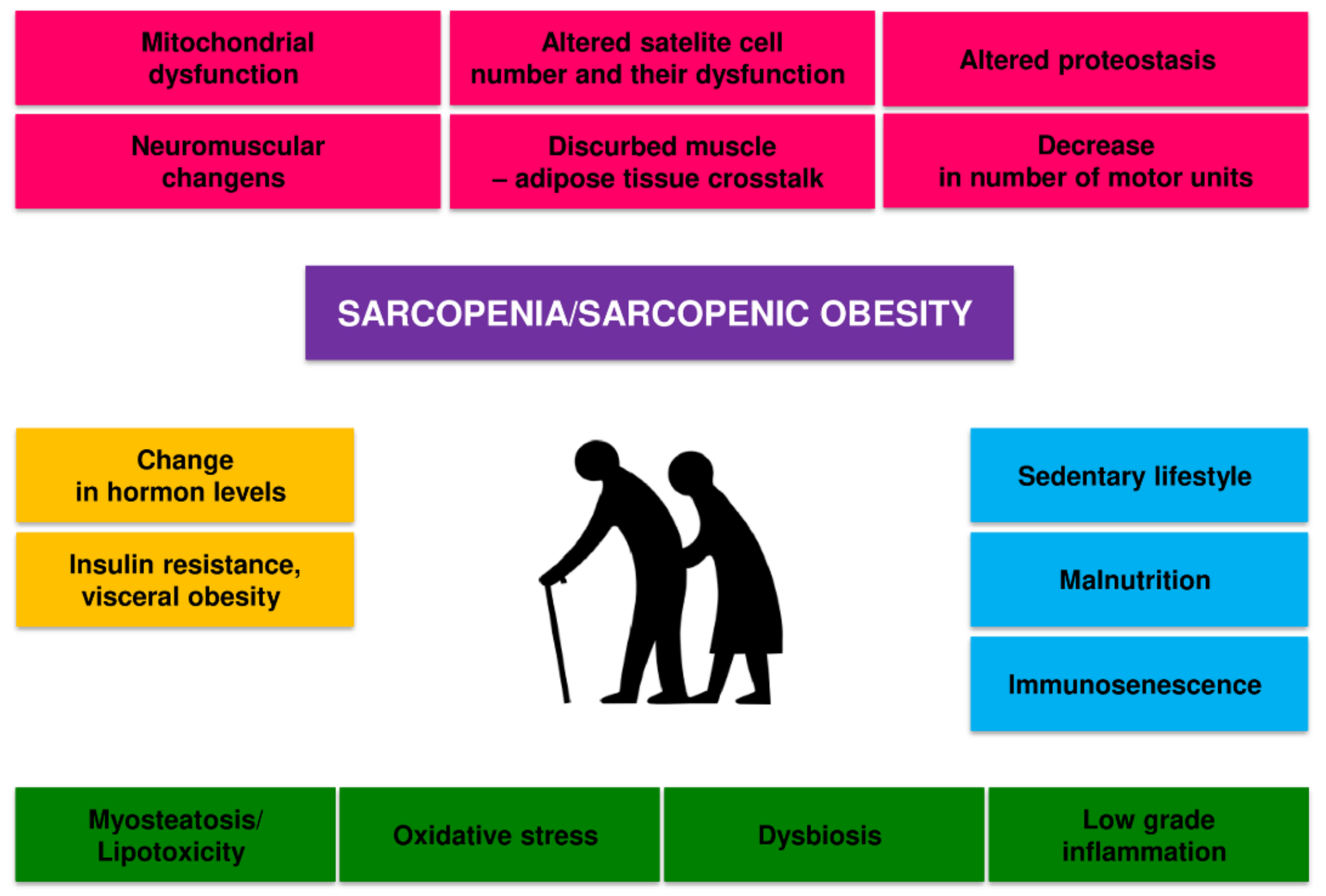
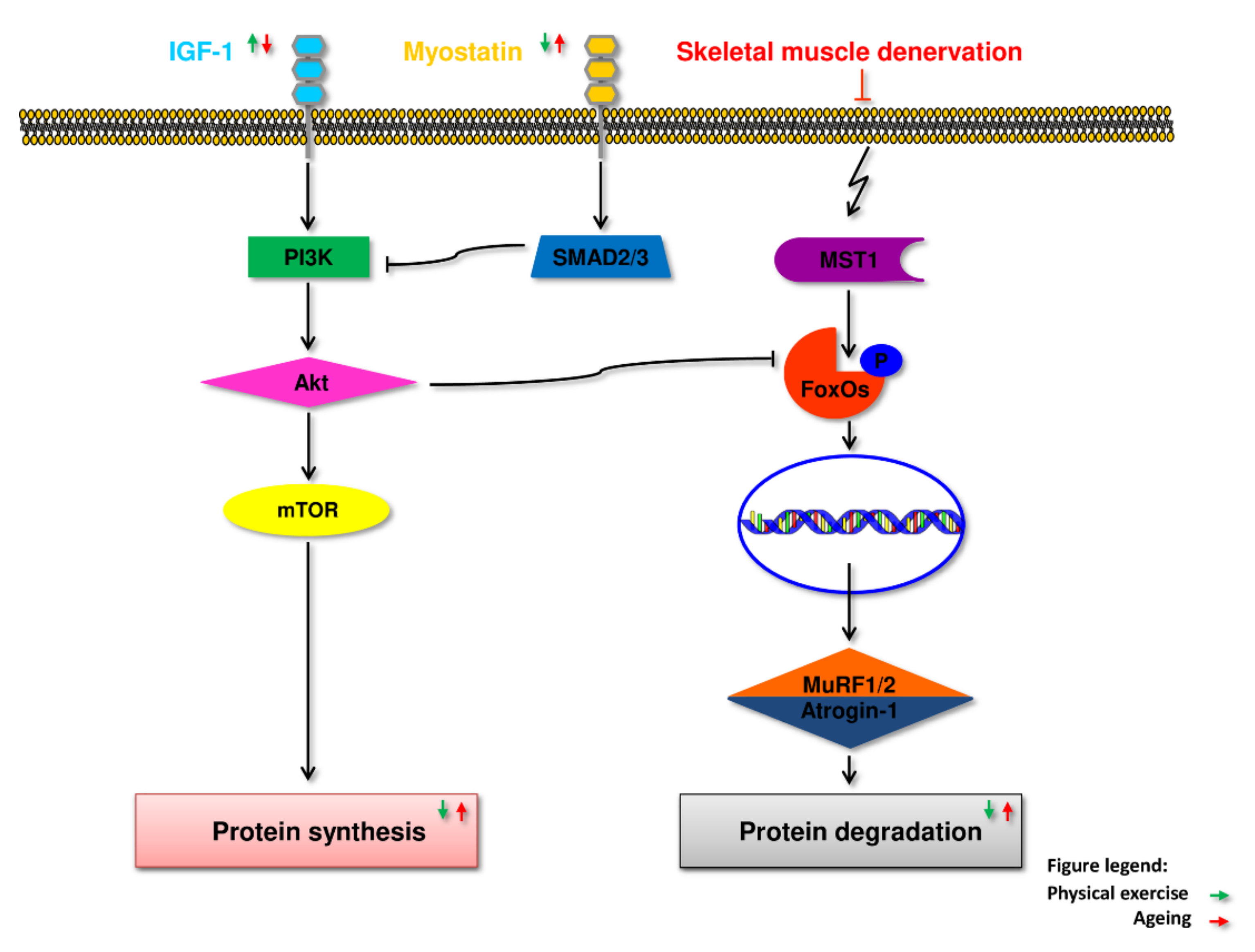
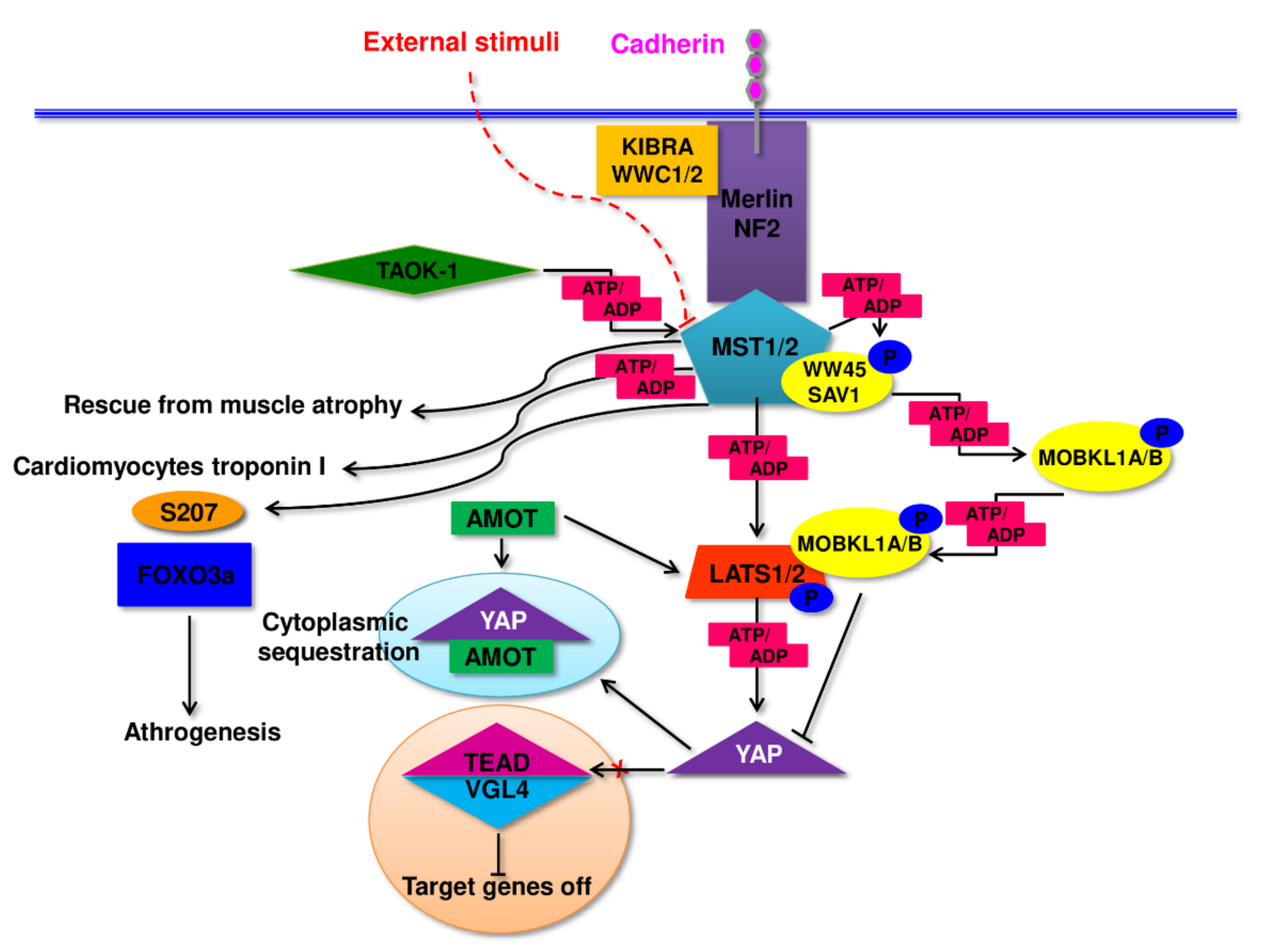

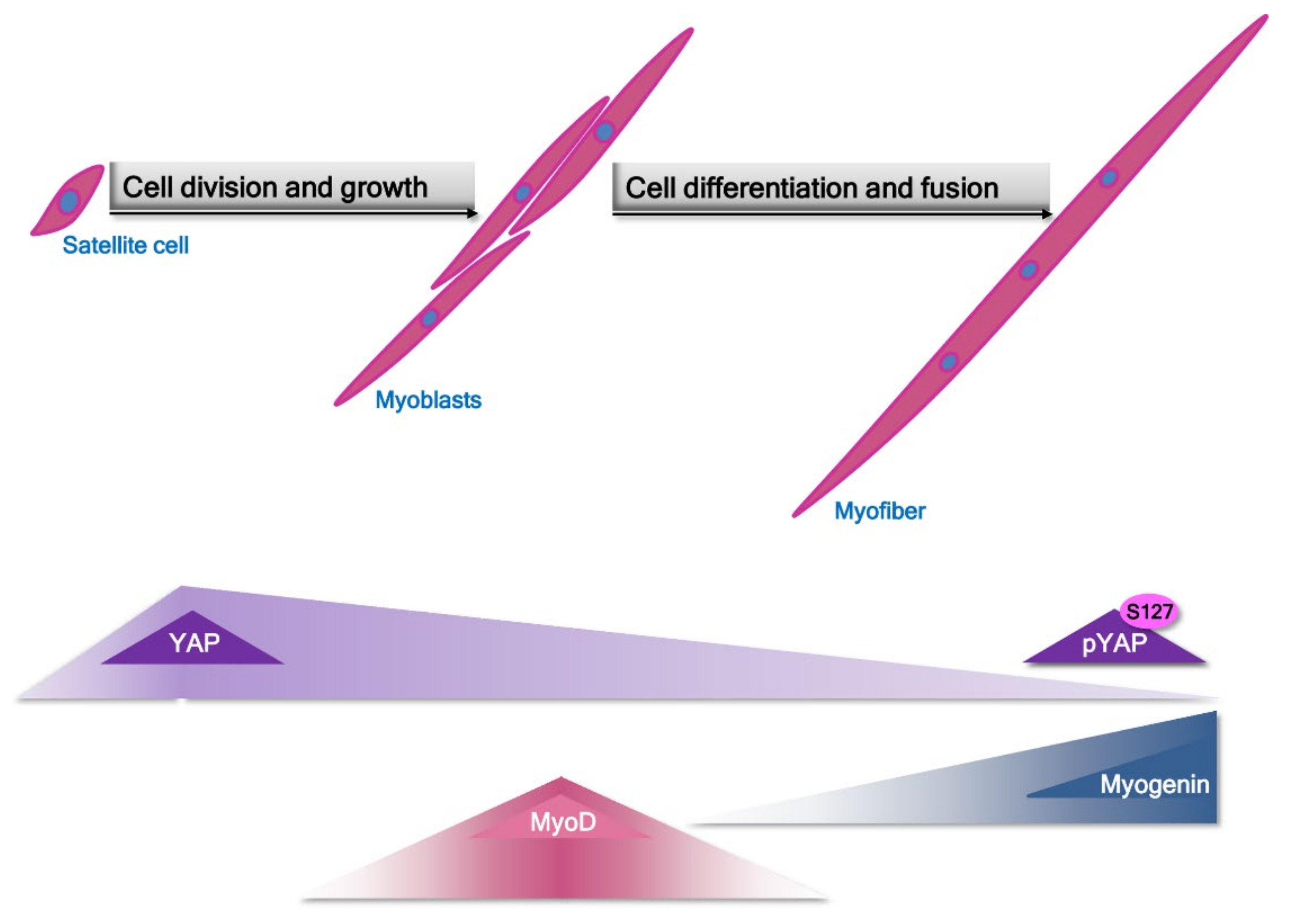
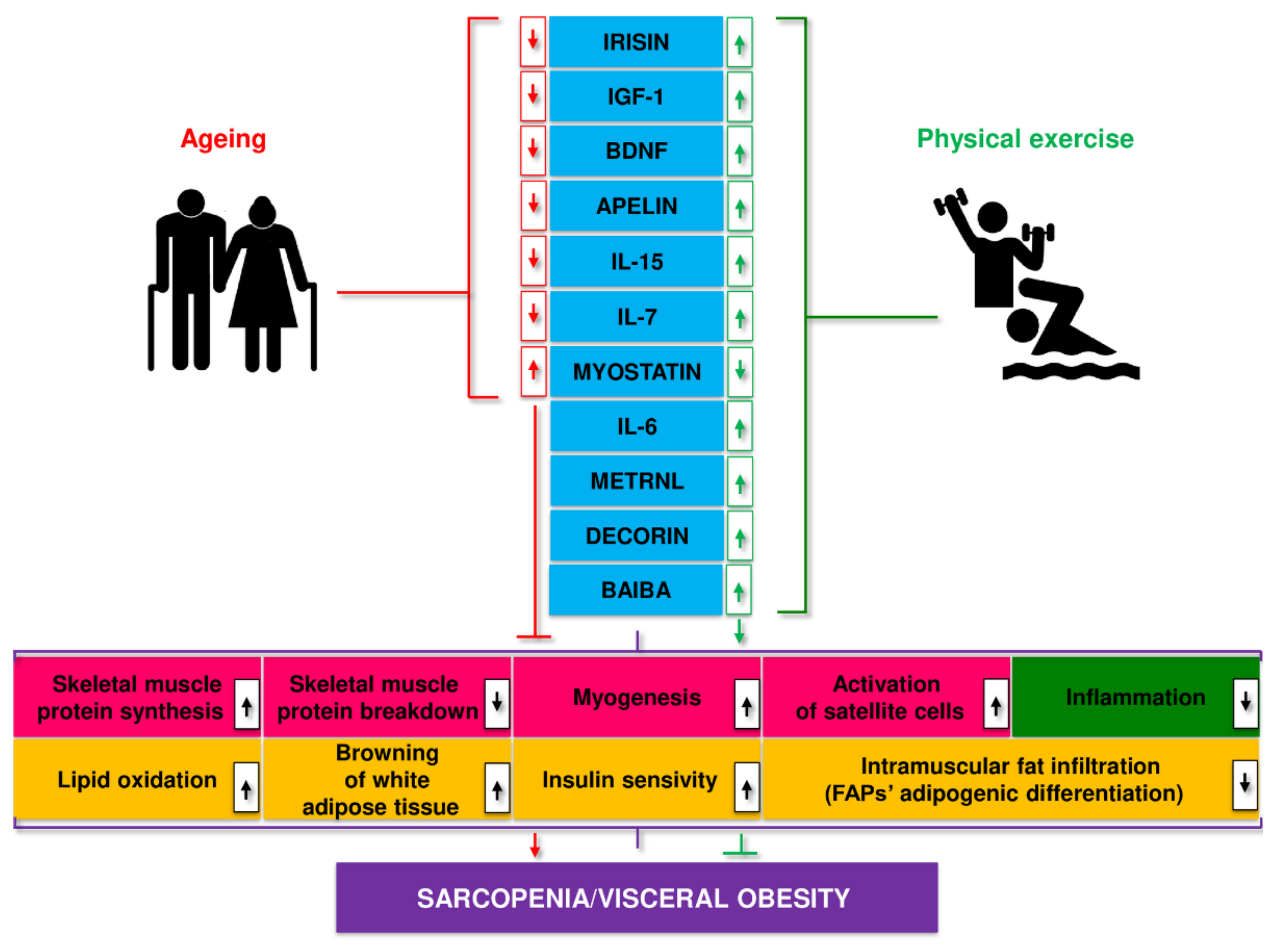
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. https://doi.org/10.3390/cells11010160
Bilski J, Pierzchalski P, Szczepanik M, Bonior J, Zoladz JA. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells. 2022; 11(1):160. https://doi.org/10.3390/cells11010160
Chicago/Turabian StyleBilski, Jan, Piotr Pierzchalski, Marian Szczepanik, Joanna Bonior, and Jerzy A. Zoladz. 2022. "Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines" Cells 11, no. 1: 160. https://doi.org/10.3390/cells11010160






