Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis
Abstract
1. Introduction
2. Cellular and Molecular Aspects of Folliculogenesis
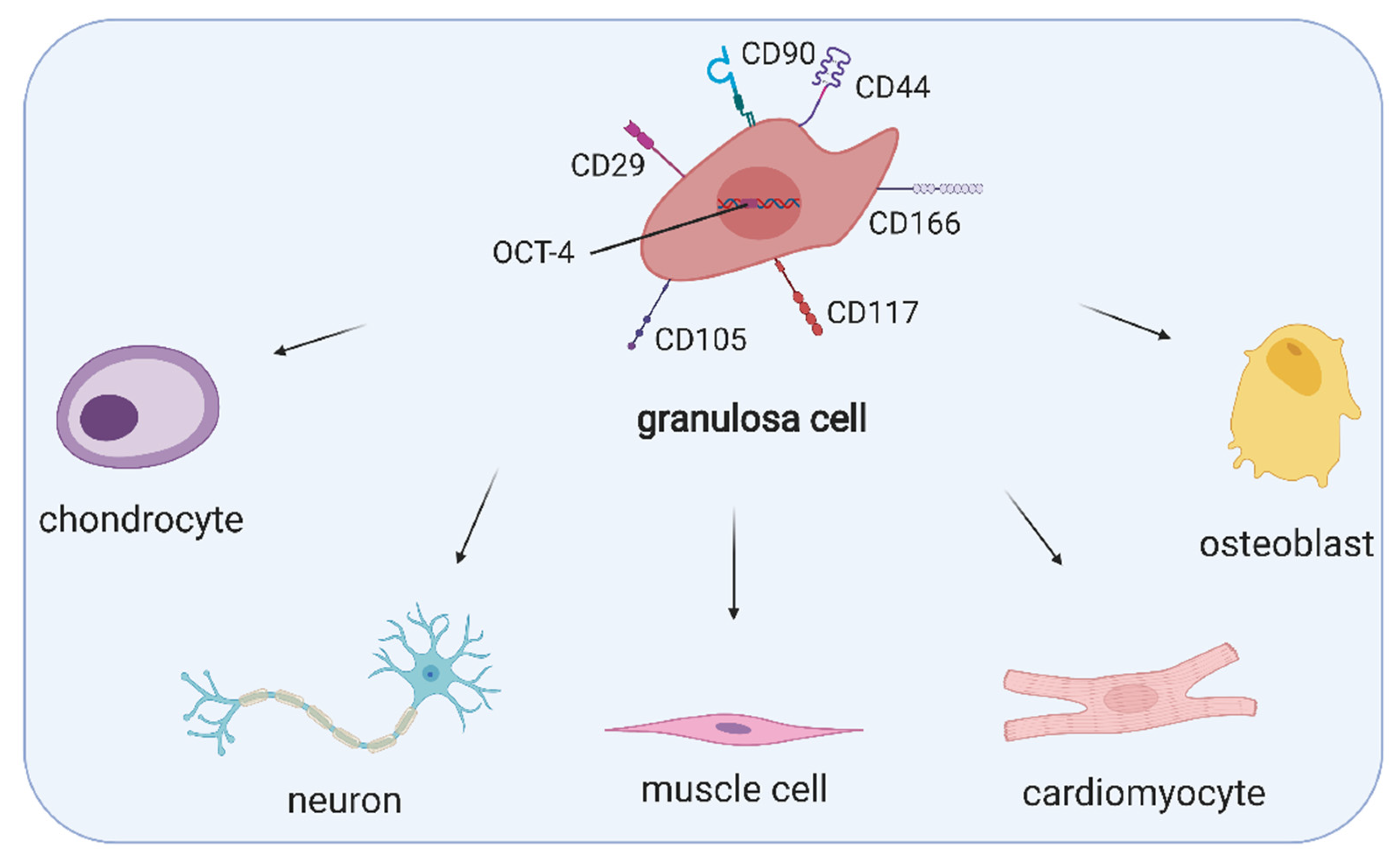
3. Ovarian Cell Stem-Like Plasticity
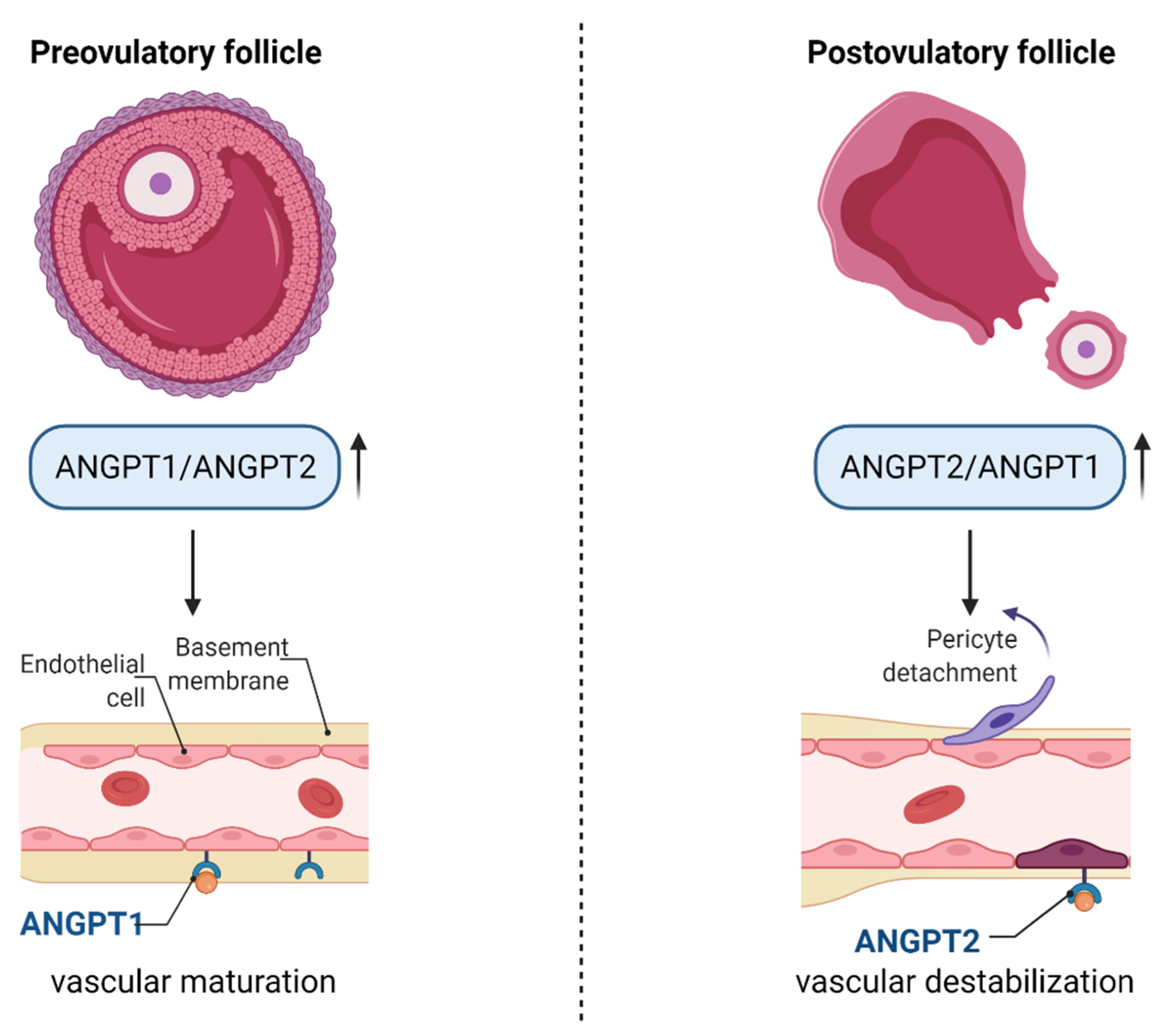
4. Regulation of Angiogenesis in Ovarian Follicles
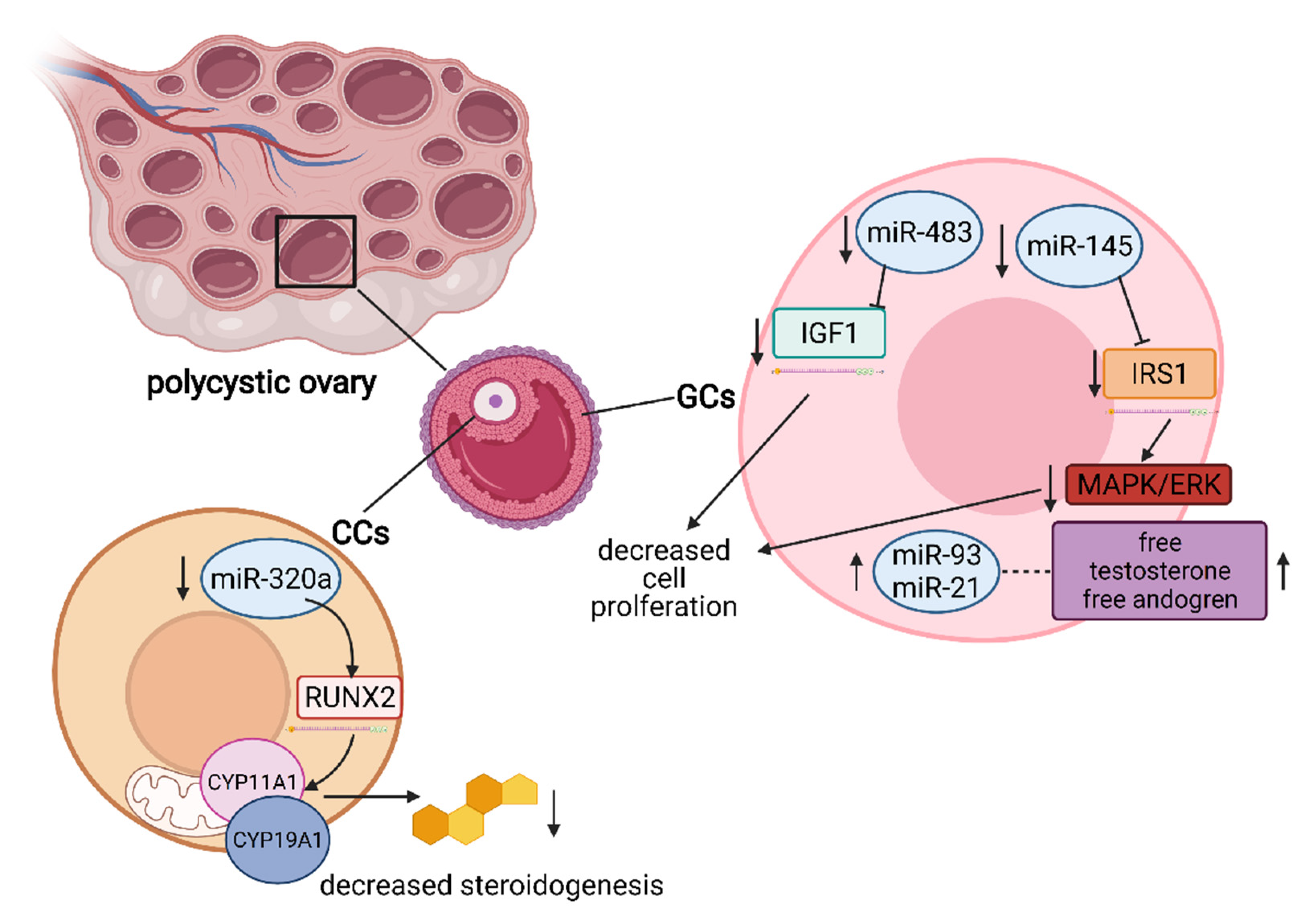
5. The Role of miRNA in the Regulation of Granulosa and Cumulus Cells’ Function
6. Ovarian Follicular Cells’ Molecular “Cross-Talk” and Interaction
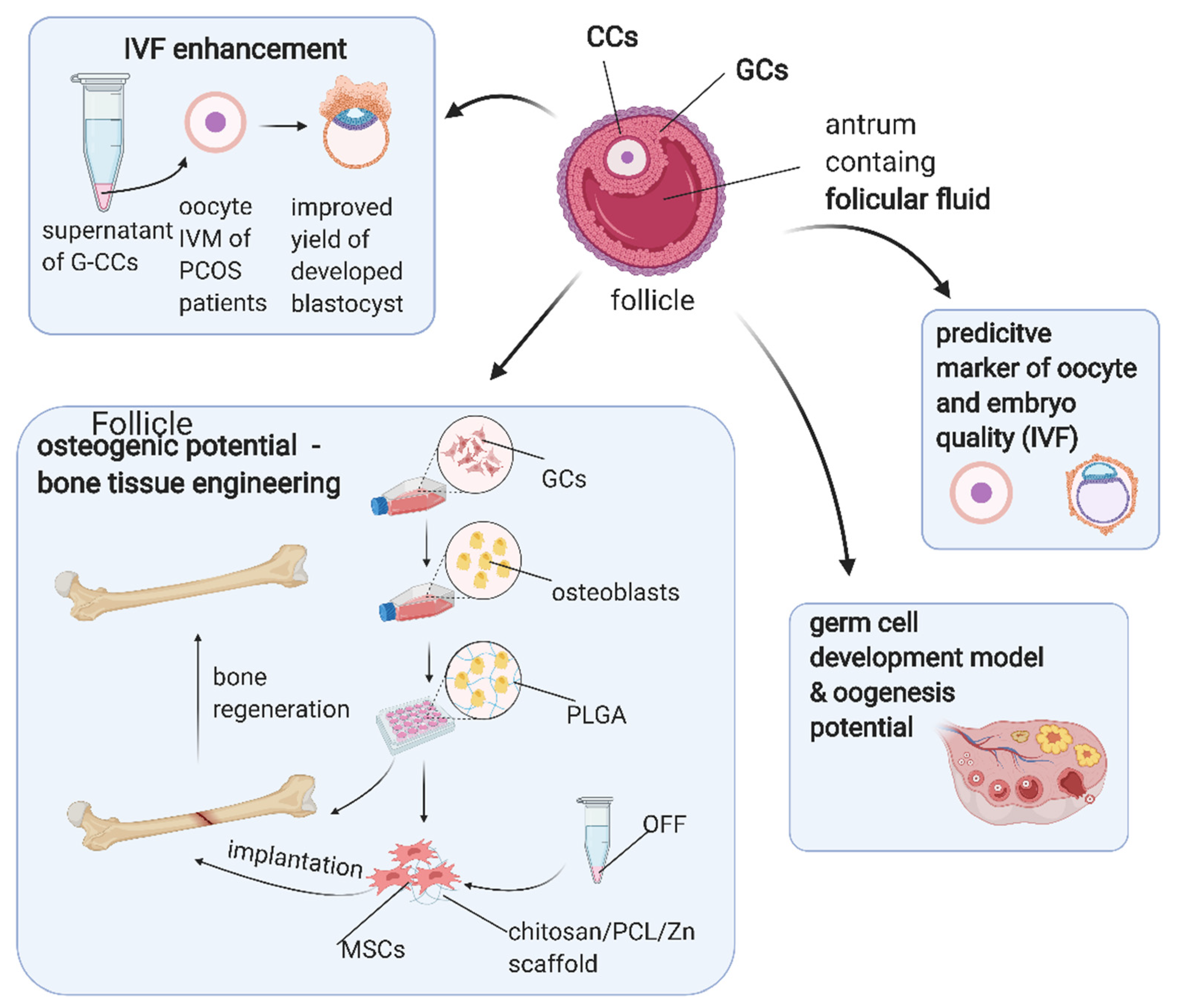
7. Application of Ovarian Follicular Stem Cells in Translational Medicine
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ndefo, U.A.; Eaton, A.; Green, M.R. Polycystic ovary syndrome: A review of treatment options with a focus on pharmacological approaches. P T 2013, 38, 336–355. [Google Scholar]
- Zhang, C. The Roles of Different Stem Cells in Premature Ovarian Failure. Curr. Stem Cell Res. Ther. 2019, 15, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rybska, M.; Knap, S.; Jankowski, M.; Borowiec, B.; Jeseta, M.; Bukowska, D.; Antosik, P.; Nowicki, M.; Zabel, M.; Kempisty, B.; et al. Pathogenesis and pathophysiology of ovarian follicular cysts in mammals. Med. J. Cell Biol. 2018, 6, 120–124. [Google Scholar] [CrossRef]
- Zhang, T.; Lee, W.Y.W.; Rui, Y.F.; Cheng, T.Y.; Jiang, X.H.; Li, G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Celikkan, F.T.; Cinar, O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy 2017, 19, 1351–1382. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C.; De Geyter, M.; Martin, I.; Holzgreve, W.; Scherberich, A.; Zhang, H. The Multipotency of Luteinizing Granulosa Cells Collected from Mature Ovarian Follicles. Stem Cells 2009, 27, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Cote, R.J.; Suster, S.; Weiss, L.M. Modern Surgical Pathology; Saunders: Philadelphia, PA, USA, 2009; ISBN 9781416039662. [Google Scholar]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef]
- McGee, E.A.; Hsueh, A.J.W. Initial and Cyclic Recruitment of Ovarian Follicles*. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef]
- Da Silva-Buttkus, P.; Jayasooriya, G.S.; Mora, J.M.; Mobberley, M.; Ryder, T.A.; Baithun, M.; Stark, J.; Franks, S.; Hardy, K. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J. Cell Sci. 2008, 121, 3890–3900. [Google Scholar] [CrossRef]
- Shah, J.S.; Sabouni, R.; Cayton Vaught, K.C.; Owen, C.M.; Albertini, D.F.; Segars, J.H. Biomechanics and mechanical signaling in the ovary: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 1135–1148. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C. Cells with stem cell characteristics in somatic compartments of the ovary. Biomed Res. Int. 2013, 2013, 310859. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.K.; Kerr, J.B.; Britt, K.; Liew, S.H.; Simpson, E.R.; Rosairo, D.; Drummond, A. Ovarian physiology: Follicle development, oocyte and hormone relationships. Anim. Reprod. 2009, 6, 16–19. [Google Scholar]
- Nguyen, T.; Lee, S.; Hatzirodos, N.; Hummitzsch, K.; Sullivan, T.R.; Rodgers, R.J.; Irving-Rodgers, H.F. Spatial differences within the membrana granulosa in the expression of focimatrix and steroidogenic capacity. Mol. Cell. Endocrinol. 2012, 363, 62–73. [Google Scholar] [CrossRef]
- Kranc, W.; Chachula, A.; Wojtanowicz-Markiewicz, K.; Ciesiólka, S.; Ociepa, E.; Bukowska, D.; Borys, S.; Piotrowska, H.; Bryja, A.; Antosik, P.; et al. The Insight into Developmental Capacity of Mammalian Cocs and Cumulus-Granulosa Cells-Recent Studies and Perspectives. Austin J. Invit. Fertilzation 2015, 2, 1023. [Google Scholar]
- Fraser, H.M.; Wulff, C. Angiogenesis in the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 88. [Google Scholar] [CrossRef] [PubMed]
- Rybska, M.; Knap, S.; Jankowski, M.; Jeseta, M.; Bukowska, D.; Antosik, P.; Nowicki, M.; Zabel, M.; Kempisty, B.; Jaśkowski, J.M. Characteristic of factors influencing the proper course of folliculogenesis in mammals. Med. J. Cell Biol. 2018, 6, 33–38. [Google Scholar] [CrossRef]
- Kałuzna, S.; Bryl, R.; Chermuła, B.; Sibiak, R.; Stefańska, K.; Pieńkowski, W.; Kranc, W.; Jeseta, M.; Ventruba, P.; Zakova, J.; et al. Expression of genes involved in the inflammatory response in human granulosa cells in short-term in vitro culture. Med. J. Cell Biol. 2020, 8, 190–195. [Google Scholar] [CrossRef]
- Erickson, G.F.; Hofeditz, C.; Unger, M.; Allen, W.R.; Dulbecco, R. A monoclonal antibody to a mammary cell line recognizes two distinct subtypes of ovarian granulosa cells. Endocrinology 1985, 117, 1490–1499. [Google Scholar] [CrossRef]
- Plancha, C.E.; Sanfins, A.; Rodrigues, P.; Albertini, D. Cell polarity during folliculogenesis and oogenesis. Reprod. Biomed. Online 2005, 10, 478–484. [Google Scholar] [CrossRef]
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Mora, J.M.; Fenwick, M.A.; Castle, L.; Baithun, M.; Ryder, T.A.; Mobberley, M.; Carzaniga, R.; Franks, S.; Hardy, K. Characterization and significance of adhesion and junction-related proteins in mouse ovarian follicles. Biol. Reprod. 2012, 86, 1–14. [Google Scholar] [CrossRef]
- Vanderhyden, B.C.; Tonary, A.M. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by a factor(s) secreted by the oocyte. Biol. Reprod. 1995, 53, 1243–1250. [Google Scholar] [CrossRef]
- Kranc, W.; Budna, J.; Kahan, R.; Chachuła, A.; Bryja, A.; Ciesiółka, S.; Borys, S.; Antosik, M.P.; Bukowska, D.; Brussow, K.P.; et al. Molecular basis of growth, proliferation, and differentiation of mammalian follicular granulosa cells. J. Biol. Regul. Homeost. Agents 2017, 31, 1–8. [Google Scholar] [PubMed]
- Rodgers, R.J.; Lavranos, T.C.; Van Wezel, I.L.; Irving-Rodgers, H.F. Development of the ovarian follicular epithelium. Mol. Cell. Endocrinol. 1999, 151, 171–179. [Google Scholar] [CrossRef]
- Jagarlamudi, K.; Rajkovic, A. Oogenesis: Transcriptional regulators and mouse models. Mol. Cell. Endocrinol. 2012, 356, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-W.; Chen, M.-J.; Jan, P.-S.; Chen, H.-F.; Ho, H.-N. Differentiation of Human Embryonic Stem Cells Into Functional Ovarian Granulosa-like Cells. J. Clin. Endocrinol. Metab. 2013, 98, 3713–3723. [Google Scholar] [CrossRef] [PubMed]
- Bowen, N.J.; Walker, L.D.; Matyunina, L.V.; Logani, S.; Totten, K.A.; Benigno, B.B.; McDonald, J.F. Gene expression profiling supports the hypothesis that human ovarian surface epithelia are multipotent and capable of serving as ovarian cancer initiating cells. BMC Med. Genomics 2009, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Skutella, T.; Stimpfel, M.; Sinkovec, J. Ovarian surface epithelium in patients with severe ovarian infertility: A potential source of cells expressing markers of pluripotent/multipotent stem cells. J. Biomed. Biotechnol. 2011, 2011, 381928. [Google Scholar] [CrossRef]
- Bhartiya, D.; Patel, H. Ovarian stem cells—resolving controversies. J. Assist. Reprod. Genet. 2018, 35, 393–398. [Google Scholar] [CrossRef]
- Stefańska, K.; Sibiak, R.; Hutchings, G.; Dompe, C.; Moncrieff, L.; Janowicz, K.; Jeseta, M.; Kempisty, B.; Machatkova, M.; Mozdziak, P. Evidence for existence of molecular stemness markers in porcine ovarian follicular granulosa cells. Med. J. Cell Biol. 2019, 7, 183–188. [Google Scholar] [CrossRef]
- Moncrieff, L.; Mozdziak, P.; Jeseta, M.; Machatkova, M.; Kranc, W.; Kempisty, B. Ovarian follicular cells—Living in the shadow of stemness cellular competence. Med. J. Cell Biol. 2019, 7, 134–140. [Google Scholar] [CrossRef]
- Wagner, M.; Yoshihara, M.; Douagi, I.; Damdimopoulos, A.; Panula, S.; Petropoulos, S.; Lu, H.; Pettersson, K.; Palm, K.; Katayama, S.; et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 2020, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Kezele, P.; Skinner, M.K. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol. Cell. Endocrinol. 2002, 188, 65–73. [Google Scholar] [CrossRef]
- Kranc, W.; Brązert, M.; Celichowski, P.; Bryja, A.; Nawrocki, M.J.; Ożegowska, K.; Jankowski, M.; Jeseta, M.; Pawelczyk, L.; Bręborowicz, A.; et al. ‘Heart development and morphogenesis’ is a novel pathway for human ovarian granulosa cell differentiation during long-term in vitro cultivation-a microarray approach. Mol. Med. Rep. 2019, 19, 1705–1715. [Google Scholar] [CrossRef]
- Brevini, T.A.L.; Pennarossa, G.; Rahman, M.M.; Paffoni, A.; Antonini, S.; Ragni, G.; deEguileor, M.; Tettamanti, G.; Gandolfi, F. Morphological and Molecular Changes of Human Granulosa Cells Exposed to 5-Azacytidine and Addressed Toward Muscular Differentiation. Stem Cell Rev. Reports 2014, 10, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Lavranos, T.C.; Rodgers, H.F.; Young, F.M.; Vella, C.A. The physiology of the ovary: Maturation of ovarian granulosa cells and a novel role for antioxidants in the corpus luteum. J. Steroid Biochem. Mol. Biol. 1995, 53, 241–246. [Google Scholar] [CrossRef]
- Dzafic, E.; Stimpfel, M.; Novakovic, S.; Cerkovnik, P.; Virant-Klun, I. Expression of Mesenchymal Stem Cells-Related Genes and Plasticity of Aspirated Follicular Cells Obtained from Infertile Women. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chermuła, B.; Brazert, M.; Izycki, D.; Ciesiółka, S.; Kranc, W.; Celichowski, P.; Ozegowska, K.; Nawrocki, M.J.; Jankowski, M.; Jeseta, M.; et al. New Gene Markers of Angiogenesis and Blood Vessels Development in Porcine Ovarian Granulosa Cells during Short-Term Primary Culture in Vitro. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Bowdridge, E.C.; Vernon, M.W.; Flores, J.A.; Clemmer, M.J. In vitro progesterone production by luteinized human mural granulosa cells is modulated by activation of AMPK and cause of infertility. Reprod. Biol. Endocrinol. 2017, 15, 76. [Google Scholar] [CrossRef]
- Furukawa, K.; Fujiwara, H.; Sato, Y.; Zeng, B.X.; Fujii, H.; Yoshioka, S.; Nishi, E.; Nishio, T. Platelets are novel regulators of neovascularization and luteinization during human corpus luteum formation. Endocrinology 2007, 148, 3056–3064. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Grolli, S.; Ramoni, R.; Conti, V.; Quintavalla, F.; Grasselli, F. Platelets are involved in in vitro swine granulosa cell luteinization and angiogenesis. Anim. Reprod. Sci. 2018, 188, 51–56. [Google Scholar] [CrossRef]
- Dzafic, E.; Stimpfel, M.; Virant-Klun, I. Plasticity of granulosa cells: On the crossroad of stemness and transdifferentiation potential. J. Assist. Reprod. Genet. 2013, 30, 1255–1261. [Google Scholar] [CrossRef]
- Kinugawa, C.; Murakami, T.; Okamura, K.; Yajima, A. Telomerase activity in normal ovaries and premature ovarian failure. Tohoku J. Exp. Med. 2000, 190, 231–238. [Google Scholar] [CrossRef]
- Hoang, S.N.; Ho, C.N.Q.; Nguyen, T.T.P.; Doan, C.C.; Tran, D.H.; Le, L.T. Evaluation of stemness marker expression in bovine ovarian granulosa cells. Anim. Reprod. 2019, 16, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.É.S.; Gouveia, B.B.; Barberino, R.S.; Menezes, V.G.; Macedo, T.J.S.; Cavalcante, A.Y.P.; Monte, A.P.O.; Santos, J.M.S.; Matos, M.H.T. Resveratrol promotes in vitro activation of ovine primordial follicles by reducing DNA damage and enhancing granulosa cell proliferation via phosphatidylinositol 3-kinase pathway. Reprod. Domest. Anim. 2018, 53, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Fortune, J.E. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol. Reprod. Dev. 2007, 74, 1095–1104. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Detzel, C.; Skinner, M.K. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction 2006, 131, 1007–1015. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Navanukraw, C.; Johnson, M.L.; Vonnahme, K.A.; Ford, S.P.; Reynolds, L.P.; Redmer, D.A. Vascularity and expression of angiogenic factors in bovine dominant follicles of the first follicular wave. J. Anim. Sci. 2007, 85, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Woad, K.J.; Robinson, R.S. Luteal angiogenesis and its control. Theriogenology 2016, 86, 221–228. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Redmer, D.A. Growth and development of the corpus luteum. J. Reprod. Fertil. Suppl. 1999, 54, 181–191. [Google Scholar] [CrossRef][Green Version]
- Antczak, M.; Van Blerkom, J. The vascular character of ovarian follicular granulosa cells: Phenotypic and functional evidence for an endothelial-like cell population. Hum. Reprod. 2000, 15, 2306–2318. [Google Scholar] [CrossRef][Green Version]
- Merkwitz, C.; Ricken, A.M.; Lösche, A.; Sakurai, M.; Spanel-Borowski, K. Progenitor cells harvested from bovine follicles become endothelial cells. Differentiation 2010, 79, 203–210. [Google Scholar] [CrossRef]
- Bender, H.R.; Campbell, G.E.; Aytoda, P.; Mathiesen, A.H.; Duffy, D.M. Thrombospondin 1 (THBS1) Promotes Follicular Angiogenesis, Luteinization, and Ovulation in Primates. Front. Endocrinol. 2019, 10, 727. [Google Scholar] [CrossRef]
- Garside, S.A.; Harlow, C.R.; Hillier, S.G.; Fraser, H.M.; Thomas, F.H. Thrombospondin-1 Inhibits Angiogenesis and Promotes Follicular Atresia in a Novel in Vitro Angiogenesis Assay. Endocrinology 2010, 151, 1280–1289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamanini, C.; De Ambrogi, M. Angiogenesis in Developing Follicle and Corpus Luteum. Reprod. Domest. Anim. 2004, 39, 206–216. [Google Scholar] [CrossRef]
- Grasselli, F.; Basini, G.; Bussolati, S.; Tamanini, C. Effects of VEGF and bFGF on proliferation and production of steroids and nitric oxide in porcine granulosa cells. Reprod. Domest. Anim. 2002, 37, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.S.; Nicklin, L.T.; Hammond, A.J.; Schams, D.; Hunter, M.G.; Mann, G.E. Fibroblast Growth Factor 2 Is More Dynamic than Vascular Endothelial Growth Factor A During the Follicle-Luteal Transition in the Cow1. Biol. Reprod. 2007, 77, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kamada, D.; Shirasuna, K.; Matsui, M.; Shimizu, T.; Kida, K.; Berisha, B.; Schams, D.; Miyamoto, A. Effect of local neutralization of basic fibroblast growth factor or vascular endothelial growth factor by a specific antibody on the development of the corpus luteum in the cow. Mol. Reprod. Dev. 2008, 75, 1449–1456. [Google Scholar] [CrossRef]
- Calvani, M.; Rapisarda, A.; Uranchimeg, B.; Shoemaker, R.H.; Melillo, G. Hypoxic induction of an HIF-1α-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 2006, 107, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Okuda, K. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J. Reprod. Dev. 2010, 56, 110–116. [Google Scholar] [CrossRef]
- Fraser, H.M.; Duncan, W.C. Vascular morphogenesis in the primate ovary. Angiogenesis 2005, 8, 101–116. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyamoto, A. Progesterone induces the expression of vascular endothelial growth factor (VEGF) 120 and Flk-1, its receptor, in bovine granulosa cells. Anim. Reprod. Sci. 2007, 102, 228–237. [Google Scholar] [CrossRef]
- Shimizu, T.; Jayawardana, B.C.; Nishimoto, H.; Kaneko, E.; Tetsuka, M.; Miyamoto, A. Hormonal regulation and differential expression of neuropilin (NRP)-1 and NRP-2 genes in bovine granulosa cells. Reproduction 2006, 131, 555–559. [Google Scholar] [CrossRef][Green Version]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–814. [Google Scholar] [CrossRef]
- Kuhnert, F.; Tam, B.Y.Y.; Sennino, B.; Gray, J.T.; Yuan, J.; Jocson, A.; Nayak, N.R.; Mulligan, R.C.; McDonald, D.M.; Kuo, C.J. Soluble receptor-mediated selective inhibition of VEGFR and PDGFRβ signaling during physiologic and tumor angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 10185–10190. [Google Scholar] [CrossRef] [PubMed]
- Sleer, L.S.; Taylor, C.C. Platelet-derived growth factors and receptors in the rat corpus luteum: Localization and identification of an effect on luteogenesis. Biol. Reprod. 2007, 76, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gurtan, A.M.; Sharp, P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Gebremedhn, S.; Hoelker, M.; Tholen, E.; Hailay, T.; Tesfaye, D. The role of micrornas in mammalian fertility: From gametogenesis to embryo implantation. Int. J. Mol. Sci. 2020, 21, 585. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.B.; Tesfaye, D.; Rings, F.; Hossien, M.; Hoelker, M.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Salilew-Wondim, D. MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation. J. Ovarian Res. 2017, 10, 37. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, Y.; Shen, C.; Zhang, L.; Wang, X. MicroRNA-375 regulates oocyte in vitro maturation by targeting ADAMTS1 and PGR in bovine cumulus cells. Biomed. Pharmacother. 2019, 118, 109350. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Jiang, H.; Gao, Y.; Xu, M.; Wang, J.; Liu, S.; Fu, Y.; Sun, X.; Xu, J.; et al. Regulatory Role of miRNA-375 in Expression of BMP15/GDF9 Receptors and its Effect on Proliferation and Apoptosis of Bovine Cumulus Cells. Cell. Physiol. Biochem. 2017, 41, 439–450. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Y.; Tang, X.; Gao, H.; Liu, N.; Gao, Y.; Hao, L.; Liu, S.; Jiang, Z. MiR-21-3p inhibits autophagy of bovine granulosa cells by targeting VEGFA via PI3K/AKT signaling. Reproduction 2019, 158, 441–452. [Google Scholar] [CrossRef]
- Assou, S.; Al-Edani, T.; Haouzi, D.; Philippe, N.; Lecellier, C.H.; Piquemal, D.; Commes, T.; Aït-Ahmed, O.; Dechaud, H.; Hamamah, S. MicroRNAs: New candidates for the regulation of the human cumulus-oocyte complex. Hum. Reprod. 2013, 28, 3038–3049. [Google Scholar] [CrossRef]
- Andrei, D.; Nagy, R.A.; van Montfoort, A.; Tietge, U.; Terpstra, M.; Kok, K.; van den Berg, A.; Hoek, A.; Kluiver, J.; Donker, R. Differential miRNA Expression Profiles in Cumulus and Mural Granulosa Cells from Human Pre-ovulatory Follicles. MicroRNA 2018, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Lauková, M.; Ovcharenko, D.; Brenaut, P.; Mlynček, M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J. Cell. Physiol. 2010, 223, 49–56. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kisova, G.; Brenaut, P.; Ovcharenko, D.; Grossmann, R.; Mlyncek, M. Involvement of MicroRNA Mir15a in Control of Human Ovarian Granulosa Cell Proliferation, Apoptosis, Steroidogenesis, and Response to FSH. MicroRNA 2014, 3, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.X.; Zhang, X.; Lu, Y.; Li, L.; Cui, S. MiRNA-143 mediates the proliferative signaling pathway of FSH and regulates estradiol production. J. Endocrinol. 2017, 234, 1–14. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Peng, S.; Wu, L.; Lin, H.Y.; Wang, S.; Wang, H. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction 2012, 144, 234–244. [Google Scholar] [CrossRef]
- Nie, M.; Yu, S.; Peng, S.; Fang, Y.; Wang, H.; Yang, X. miR-23a and miR-27a promote human granulosa cell apoptosis by targeting SMAD51. Biol. Reprod. 2015, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; An, H.J.; Kim, K.A.; Ko, J.J.; Kim, J.H.; Kim, Y.R.; Ahn, E.H.; Rah, H.C.; Lee, W.S.; Kim, N.K. Single nucleotide polymorphisms at miR-146a/196a2 and their primary ovarian insufficiency-related target gene regulation in granulosa cells. PLoS ONE 2017, 12, e0183479. [Google Scholar] [CrossRef]
- Cho, S.H.; Ahn, E.H.; An, H.J.; Kim, J.H.; Ko, J.J.; Kim, Y.R.; Lee, W.S.; Kim, N.K. Association of mir-938G>A polymorphisms with primary ovarian insufficiency (POI)-related gene expression. Int. J. Mol. Sci. 2017, 18, 1255. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, X.; Fang, T.; Hou, Y.; Hu, Y. Hyaluronic acid promotes the expression of progesterone receptor membrane component 1 via epigenetic silencing of miR-139-5p in human and rat granulosa cells. Biol. Reprod. 2014, 91, 116. [Google Scholar] [CrossRef]
- Naji, M.; Aleyasin, A.; Nekoonam, S.; Arefian, E.; Mahdian, R.; Amidi, F. Differential Expression of miR-93 and miR-21 in Granulosa Cells and Follicular Fluid of Polycystic Ovary Syndrome Associating with Different Phenotypes. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, H.; Yan, C.Y.; Gao, X.F.; Ling, X.J. Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem. Biophys. Res. Commun. 2017, 482, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Song, Y.; Li, Y.; Zhao, D.; Ma, L.; Tan, L. miR-483 is down-regulated in polycystic ovarian syndrome and inhibits KGN cell proliferation via targeting insulin-like growth factor 1 (IGF1). Med. Sci. Monit. 2016, 22, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Ma, X.; Chen, B.; Huang, Y.; Liu, S.; Yang, H.; Zou, W. MicroRNA-145 Negatively Regulates Cell Proliferation Through Targeting IRS1 in Isolated Ovarian Granulosa Cells from Patients with Polycystic Ovary Syndrome. Reprod. Sci. 2017, 24, 902–910. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, R.; Eppig, J.J. Transcriptional activity of the mouse oocyte genome: Companion granulosa cells modulate transcription and chromatin remodeling. Dev. Biol. 2001, 229, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Brower, P.T.; Schultz, R.M. Intercellular communication between granulosa cells and mouse oocytes: Existence and possible nutritional role during oocyte growth. Dev. Biol. 1982, 90, 144–153. [Google Scholar] [CrossRef]
- Amsterdam, A.; Tajima, K.; Frajese, V.; Seger, R. Analysis of signal transduction stimulated by gonadotropins in granulosa cells. Mol. Cell. Endocrinol. 2003, 202, 77–80. [Google Scholar]
- Kocherova, I.; Bryl, R.; Crha, I.; Ventruba, P.; Zakova, J.; Ješeta, M. The extracellular reactive oxygen species levels in primary in vitro culture of human ovarian granulosa and cumulus cells. Med. J. Cell Biol. 2020, 8, 112–117. [Google Scholar] [CrossRef]
- Brazert, M.; Kranc, W.; Jopek, K.; Kempisty, B.; Pawelczyk, L. New markers of human cumulus oophorus cells cultured in vitro-transcriptomic profile. Med. J. Cell Biol. 2020, 8, 60–72. [Google Scholar] [CrossRef]
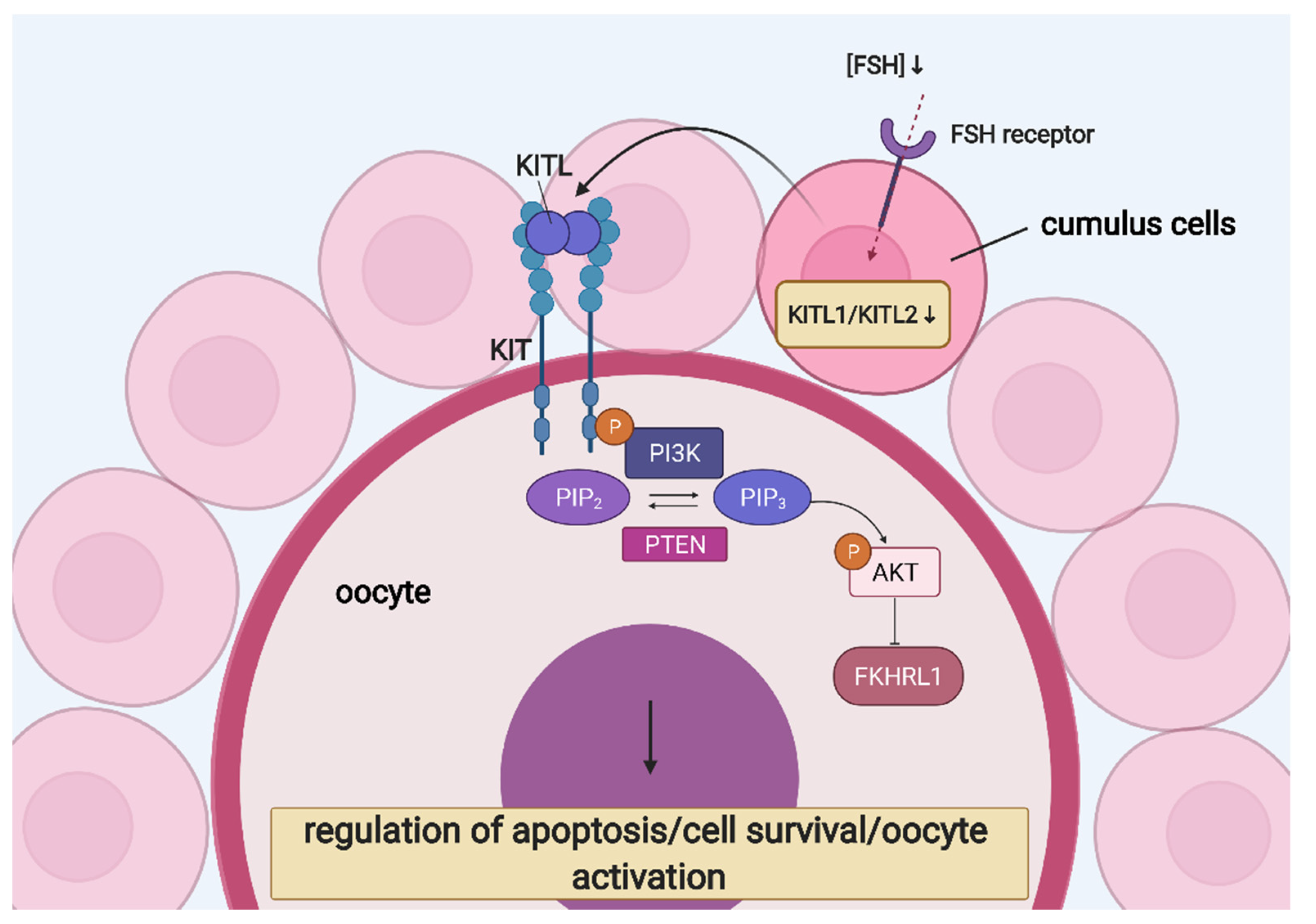
- Ismail, R.S.; Okawara, Y.; Fryer, J.N.; Vanderhyden, B.C. Hormonal regulation of the ligand for c-kit in the rat ovary and its effects on spontaneous oocyte meiotic maturation. Mol. Reprod. Dev. 1996, 43, 458–469. [Google Scholar] [CrossRef]
- Manova, K.; Huang, E.J.; Angeles, M.; De Leon, V.; Sanchez, S.; Pronovost, S.M.; Besmer, P.; Bachvarova, R.F. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev. Biol. 1993, 157, 85–99. [Google Scholar] [CrossRef]
- Laitinen, M.; Rutanen, E.M.; Ritvos, O. Expression of c-kit ligand messenger ribonucleic acids in human ovaries and regulation of their steady state levels by gonadotropins in cultured granulosa-luteal cells. Endocrinology 1995, 136, 4407–4414. [Google Scholar] [CrossRef]
- Parrott, J.A.; Skinner, M.K. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999, 140, 4262–4271. [Google Scholar] [CrossRef]
- Thomas, F.H.; Ethier, J.F.; Shimasaki, S.; Vanderhyden, B.C. Follicle-stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology 2005, 146, 941–949. [Google Scholar] [CrossRef]
- Huang, E.J.; Manova, K.; Packer, A.I.; Sanchez, S.; Bachvarova, R.F.; Besmer, P. The murine Steel Panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev. Biol. 1993, 157, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Takakura, N.; Kataoka, H.; Kunisada, T.; Okamura, H.; Nishikawa, S.I. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev. Biol. 1997, 184, 122–137. [Google Scholar] [CrossRef]
- Jin, X.; Han, C.S.; Zhang, X.S.; Yuan, J.X.; Hu, Z.Y.; Liu, Y.X. Signal transduction of stem cell factor in promoting early follicle development. Mol. Cell. Endocrinol. 2005, 229, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Hämäläinen, T.; Peng, S.L.; et al. Oocyte-specific deletion of pten causes premature activation of the primordial follicle pool. Science. 2008, 319, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Shen, L.; Ren, C.; Boman, K.; Lundin, E.; Ottander, U.; Lindgren, P.; Liu, Y.X.; Sun, Q.Y.; Liu, K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev. Biol. 2005, 281, 160–170. [Google Scholar] [CrossRef]
- Honda, A.; Hirose, M.; Inoue, K.; Hiura, H.; Miki, H.; Ogonuki, N.; Sugimoto, M.; Abe, K.; Kanatsu-Shinohara, M.; Kono, T.; et al. Large-scale production of growing oocytes in vitro from neonatal mouse ovaries. Int. J. Dev. Biol. 2009, 53, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Salustri, A.; Ulisse, S.; Yanagishita, M.; Hascall, V.C. Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-β. J. Biol. Chem. 1990, 265, 19517–19523. [Google Scholar] [CrossRef]
- Diaz, F.J.; O’Brien, M.J.; Wigglesworth, K.; Eppig, J.J. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev. Biol. 2006, 299, 91–104. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Ritter, L.J.; Schulz, S.J.; Amato, F.; Armstrong, D.T.; Gilchrist, R.B. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology 2005, 146, 2798–2806. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Eppig, J.J.; Wigglesworth, K.; Pendola, F.; Hirao, Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol. Reprod. 1997, 56, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Norman, R.J.; Armstrong, D.T.; Gilchrist, R.B. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol. Reprod. 2000, 63, 839–845. [Google Scholar] [CrossRef]
- Hussein, T.S.; Froiland, D.A.; Amato, F.; Thompson, J.G.; Gilchrist, R.B. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J. Cell Sci. 2005, 118, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Sibiak, R.; Bryl, R.; Stefańska, K.; Chermuła, B.; Pieńkowski, W.; Jeseta, M.; Pawelczyk, L.; Mozdziak, P.; Spaczyński, R.Z.; Kempisty, B. Expression of the apoptosis regulatory gene family in the long-term in vitro cultured human cumulus cells. Med. J. Cell Biol. 2021, 9, 8–13. [Google Scholar] [CrossRef]
- Kocherova, I.; Stefańska, K.; Bryl, R.; Perek, J.; Pieńkowski, W.; Zakova, J.; Crha, I.; Ventruba, P.; Mozdziak, P.; Ješeta, M. Apoptosis-related genes expression in primary in vitro culture of human ovarian granulosa cells. Med. J. Cell Biol. 2020, 8, 176–182. [Google Scholar] [CrossRef]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, P.; Demayo, J.; Demayo, F.J.; Elvin, J.A.; Carino, C.; Prasad, S.V.; Skinner, S.S.; Dunbar, B.S.; Dube, J.L.; et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol. 2001, 15, 854–866. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Burns, K.H.; Viveiros, M.M.; Eppig, J.J. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science (80-. ). 2002, 296, 2178–2180. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the Genes for Oocyte-Derived Growth Factors GDF9 and BMP15 Are Associated with Both Increased Ovulation Rate and Sterility in Cambridge and Belclare Sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Sugiura, K.; Su, Y.Q.; Diaz, F.J.; Pangas, S.A.; Sharma, S.; Wigglesworth, K.; O’Brien, M.J.; Matzuk, M.M.; Shimasaki, S.; Eppig, J.J. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 2007, 134, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Mottershead, D.G.; Ritter, L.J.; Gilchrist, R.B. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol. Hum. Reprod. 2012, 18, 121–128. [Google Scholar] [CrossRef]
- Rybska, M.; Knap, S.; Stefańska, K.; Jankowski, M.; Chamier-Gliszczyńska, A.; Popis, M.; Jeseta, M.; Bukowska, D.; Antosik, P.; Kempisty, B.; et al. Transforming growth factor (TGF)—Is it a key protein in mammalian reproductive biology? Med. J. Cell Biol. 2018, 6, 127–130. [Google Scholar] [CrossRef]
- Joyce, I.M.; Clark, A.T.; Pendola, F.L.; Eppig, J.J. Comparison of recombinant growth differentiation factor-9 and oocyte regulation of KIT ligand messenger ribonucleic acid expression in mouse ovarian follicles. Biol. Reprod. 2000, 63, 1669–1675. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bryja, A.; Pieńkowski, W.; Stefańska, K.; Chermuła, B.; Bryl, R.; Wieczorkiewicz, M.; Kulus, J.; Wąsiatycz, G.; Bukowska, D.; Ratajczak, K.; et al. Analysis of TGFB1, CD105 and FSP1 expression in human granulosa cells during a 7-day primary in vitro culture. Med. J. Cell Biol. 2020, 8, 152–157. [Google Scholar] [CrossRef]
- Baena, V.; Terasaki, M. Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle. Sci. Rep. 2019, 9, 1262. [Google Scholar] [CrossRef]
- Macaulay, A.D.; Gilbert, I.; Caballero, J.; Barreto, R.; Fournier, E.; Tossou, P.; Sirard, M.A.; Clarke, H.J.; Khandjian, É.W.; Richard, F.J.; et al. The gametic synapse: RNA transfer to the bovine oocyte. Biol. Reprod. 2014, 91, 90. [Google Scholar] [CrossRef]
- Albertini, D.F.; Rider, V. Patterns of intercellular connectivity in the mammalian cumulus-oocyte complex. Microsc. Res. Tech. 1994, 27, 125–133. [Google Scholar] [CrossRef]
- Motta, P.M.; Correr, S.; Makabe, S.; Naguro, T. Oocyte Follicle Cells Association during Development of Human Ovarian Follicle. A Study by High Resolution Scanning and Transmission Electron Microscopy. Arch. Histol. Cytol. 1994, 57, 369–394. [Google Scholar] [CrossRef]
- Simon, A.M.; Goodenough, D.A. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998, 8, 477–483. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef]
- Pelland, A.M.D.; Corbett, H.E.; Baltz, J.M. Amino acid transport mechanisms in mouse oocytes during growth and meiotic maturation. Biol. Reprod. 2009, 81, 1041–1054. [Google Scholar] [CrossRef]
- Purcell, S.H.; Moley, K.H. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol. Metab. 2009, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Pocar, P.; Navarrete-Santos, A.; Wrenzycki, C.; Gandolfi, F.; Niemann, H.; Fischer, B. Glucose transporter expression is developmentally regulated in in vitro derived bovine preimplantation embryos. Mol. Reprod. Dev. 2001, 60, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.A.; Gosden, R.G.; Allison, V.; Spears, N. Effect of androgens on the development of mouse follicles growing in vitro. J. Reprod. Fertil. 1998, 113, 27–33. [Google Scholar] [CrossRef]
- Orisaka, M.; Jiang, J.Y.; Orisaka, S.; Kotsuji, F.; Tsang, B.K. Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology 2009, 150, 2740–2748. [Google Scholar] [CrossRef]
- Mattioli, M.; Gloria, A.; Turriani, M.; Berardinelli, P.; Russo, V.; Nardinocchi, D.; Curini, V.; Baratta, M.; Martignani, E.; Barboni, B. Osteo-regenerative potential of ovarian granulosa cells: An in vitro and in vivo study. Theriogenology 2012, 77, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, Y.; Jeganathan, K.; Sivanesan, S.; Koka, P.; Amritha, T.M.S.; Vimalraj, S.; Dhanasekaran, A. Assessment of human ovarian follicular fluid derived mesenchymal stem cells in chitosan/PCL/Zn scaffold for bone tissue regeneration. Life Sci. 2021, 264, 118502. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, L.; Ye, X.; Fu, H.; Sheng, X.; Wang, L.; Wang, H.; Heng, D.; Liu, L. Functional Oocytes Derived from Granulosa Cells. Cell Rep. 2019, 29, 4256–4267.e9. [Google Scholar] [CrossRef]
- Madkour, A.; Bouamoud, N.; Kaarouch, I.; Louanjli, N.; Saadani, B.; Assou, S.; Aboulmaouahib, S.; Sefrioui, O.; Amzazi, S.; Copin, H.; et al. Follicular fluid and supernatant from cultured cumulus-granulosa cells improve in vitro maturation in patients with polycystic ovarian syndrome. Fertil. Steril. 2018, 110, 710–719. [Google Scholar] [CrossRef]
- Atrabi, M.J.; Akbarinejad, V.; Khanbabaee, R.; Dalman, A.; Amorim, C.A.; Najar-Asl, M.; Valojerdi, M.R.; Fathi, R. Formation and activation induction of primordial follicles using granulosa and cumulus cells conditioned media. J. Cell. Physiol. 2019, 234, 10148–10156. [Google Scholar] [CrossRef]
- Taheri, M.; Saki, G.; Nikbakht, R.; Eftekhari, A.R. Bone morphogenetic protein 15 induces differentiation of mesenchymal stem cells derived from human follicular fluid to oocyte-like cell. Cell Biol. Int. 2021, 45, 127–139. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, T.H.; Lee, J.H.; Lee, W.J.; Jeon, R.H.; Jang, S.J.; Ock, S.A.; Lee, S.L.; Park, B.W.; Rho, G.J. Overexpression of Oct4 in porcine ovarian stem/stromal cells enhances differentiation of oocyte-like cells in vitro and ovarian follicular formation in vivo. J. Ovarian Res. 2016, 9, 24. [Google Scholar] [CrossRef]
- Kordus, R.J.; LaVoie, H.A. Granulosa cell biomarkers to predict pregnancy in ART: Pieces to solve the puzzle. Reproduction 2017, 153, R69–R83. [Google Scholar] [CrossRef] [PubMed]
- Luddi, A.; Gori, M.; Marrocco, C.; Capaldo, A.; Pavone, V.; Bianchi, L.; Boschi, L.; Morgante, G.; Piomboni, P.; de Leo, V. Matrix metalloproteinases and their inhibitors in human cumulus and granulosa cells as biomarkers for oocyte quality estimation. Fertil. Steril. 2018, 109, 930–939.e3. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. https://doi.org/10.3390/cells10061396
Dompe C, Kulus M, Stefańska K, Kranc W, Chermuła B, Bryl R, Pieńkowski W, Nawrocki MJ, Petitte JN, Stelmach B, et al. Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells. 2021; 10(6):1396. https://doi.org/10.3390/cells10061396
Chicago/Turabian StyleDompe, Claudia, Magdalena Kulus, Katarzyna Stefańska, Wiesława Kranc, Błażej Chermuła, Rut Bryl, Wojciech Pieńkowski, Mariusz J. Nawrocki, James N. Petitte, Bogusława Stelmach, and et al. 2021. "Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis" Cells 10, no. 6: 1396. https://doi.org/10.3390/cells10061396
APA StyleDompe, C., Kulus, M., Stefańska, K., Kranc, W., Chermuła, B., Bryl, R., Pieńkowski, W., Nawrocki, M. J., Petitte, J. N., Stelmach, B., Mozdziak, P., Jeseta, M., Pawelczyk, L., Jaśkowski, J. M., Piotrowska-Kempisty, H., Spaczyński, R. Z., Nowicki, M., & Kempisty, B. (2021). Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells, 10(6), 1396. https://doi.org/10.3390/cells10061396









