Abstract
Even though cancers have been widely studied and real advances in therapeutic care have been made in the last few decades, relapses are still frequently observed, often due to therapeutic resistance. Cancer Stem Cells (CSCs) are, in part, responsible for this resistance. They are able to survive harsh conditions such as hypoxia or nutrient deprivation. Autophagy and Extracellular Vesicles (EVs) secretion are cellular processes that help CSC survival. Autophagy is a recycling process and EVs secretion is essential for cell-to-cell communication. Their roles in stemness maintenance have been well described. A common pathway involved in these processes is vesicular trafficking, and subsequently, regulation by Rab GTPases. In this review, we analyze the role played by Rab GTPases in stemness status, either directly or through their regulation of autophagy and EVs secretion.
1. Introduction
Oncology has been a widespread field of research in the last few decades. Despite many advances, there are still some problems to solve. Indeed, in some cases, relapses are frequently observed, linked to resistance to therapeutic treatments. This is, in part, due to a particular cancer cell population called Cancer Stem Cells (CSCs). These represent a small part of the tumor mass: 0.5–5% growing up to 10% in some cases. First discovered in leukemia [1,2], their existence has been rapidly demonstrated in solid tumors such as breast cancer [3] and brain cancer [4] and nowadays, in almost all cancers. CSCs share with physiological stem cells self-renewal, unlimited proliferation rate and multipotent capacities. The first characterization of CSC relied on their ability to regrow a tumor in vivo [1]. However, even today, CSCs remain difficult to characterize and isolate. Based on the same rationales as for other cellular subpopulations, CSCs are thought to be identified by the expression of specific markers. Indeed, membranal proteins such as CD133, CD44, LGR5 or intracellular actors—mainly transcriptional factors, i.e., BMI1, Oct4, Nanog, sox family—were described to be enhanced in stemness [5,6]. Nevertheless, unlike for other cellular subsets, it is not possible to establish a solid link between CSCs and unequivocal stemness markers. This explains why scientists also consider functional properties to define this peculiar population. Therefore, clonogenic faculty, chemotherapeutic resistance, metastatic propension [7] as well as quiescent stage and drug efflux are also commonly evaluated to identify CSCs [8]. However, these properties do not really define if these cancer cells are really cancer stem cells, progenitors or cancer stem-like aggressive cells. It is important to be aware that in many studies, including those cited in this review, the tumorigenicity requirement, which should be unavoidable, is not always verified. Readers, by referring to the cited publications, will be able to make up their own minds of stemness. Recently, the concept regarding CSCs has been evolving and no longer considers CSCs as a static entity, but rather as a continuum, constantly sprouting and adapting to changes in the microenvironment [9]. Thus, instead of having a unique CSC clone, tumors are composed of several CSC microstates, reflecting the high heterogeneity of the tumor. It appears that there is a dynamic reversibility between non-stem cell and stem cell states, which makes CSCs even more complicated to understand and target. CSCs are indeed highly regulated, including through the microenvironment. For example, it has been shown in breast and prostate cancer cell lines that IL-6 secretion may tip the balance in favor of a “stem-like cell” phenotype [10].
It is now well known that various processes are involved in the maintenance and status of CSCs. Among them, we decided to focus here on two particular processes, playing a key role in physiological as much as in pathological mechanisms: autophagy and Extracellular Vesicles (EVs) secretion. Three major types of autophagy have been described: micro-autophagy, chaperone-mediated autophagy and macro-autophagy. The last one, which we will be focusing on in this review, is commonly known as “autophagy”. It is a highly conserved degradation and recycling mechanism of cellular components, complementary to the proteasome. The autophagic process has an important role in the maintenance of cellular homeostasis and any dysfunction can easily lead to several pathologies, including cancer [11].
The second cellular process this review is focusing on is the secretion of Extracellular Vesicles (EVs). Among them are apoptotic bodies, microvesicles and exosomes. The last ones are nanovesicles secreted by a wide variety of cellular types, including tumor cells. They support tumor aggressiveness through the transfer of their content, thus changing the phenotype and/or behavior of the recipient cells. As an example, we showed in a previous study [12] that transfer of surface receptor TrkB (Tropomyosin receptor kinase B) by the secreted EVs of glioblastoma cells led to a restored aggressive phenotype of the non-aggressive shChi3L1 cell line. Furthermore, as EVs can easily be detected in many body fluids [13,14,15,16], some studies are pointing their advantages out as diagnosis and prognosis markers by performing a simple liquid biopsy. Indeed, in many cancer types, a difference in EV content between healthy persons and patients with cancer has been observed [17,18,19,20].
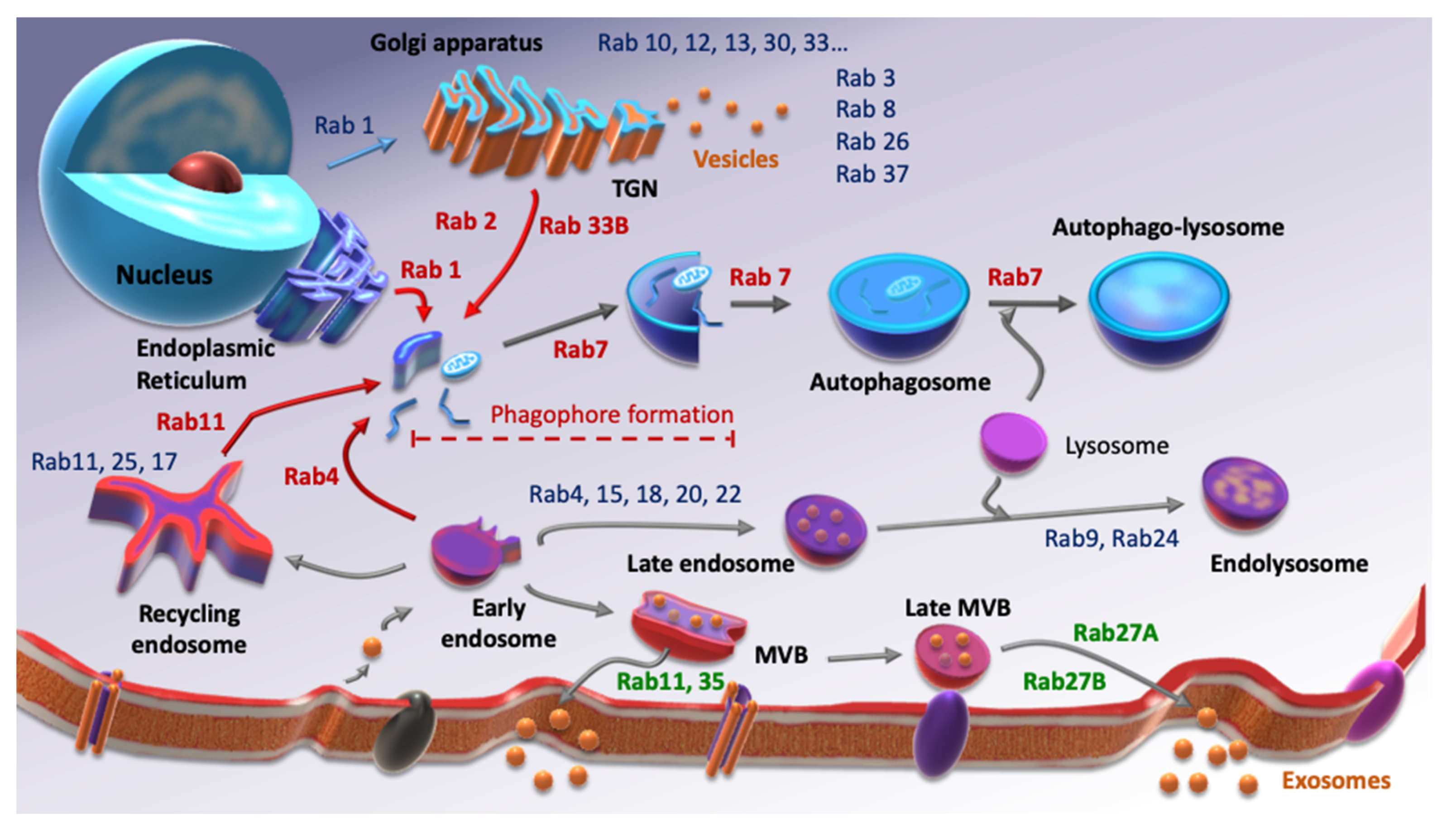
Autophagy and EVs secretion have clearly common points such as the involvement of the lysosome or their activation under stress conditions. Moreover, those two processes include vesicular trafficking, which means that Rab small G protein family is required for each of them. Rab GTPases are small G proteins belonging to the Ras superfamily. As with their counterparts, they balance between an active state, GTP-binding, and an inactive state, GDP-binding following GTP hydrolysis. To ensure this balance, they need the intervention of two factors. Indeed, the switch between GDP and GTP is performed by Guanine-nucleotide Exchanged Factors (GEFs), while GTP hydrolysis is amplified by GTPases’ Activated Proteins (GAPs). Over 70 Rab GTPases are described, each of them being able to interact with different effectors. Therefore, they are considered as markers of cellular compartments, since at least one of them is specific to each compartment (Figure 1). There is a considerable amount of evidence showing the involvement of Rab GTPases in cancer progression [21,22,23,24] but very little concerning their role in cancer stemness.
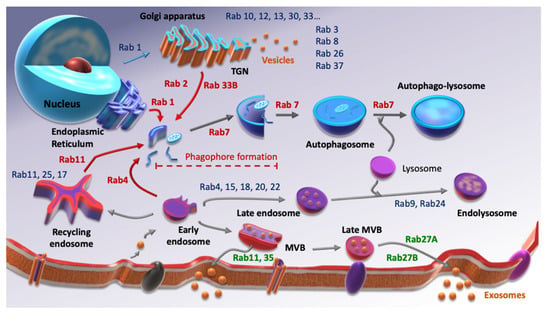
Figure 1.
Screening of some Rab GTPases involved in autophagy (red), EVs secretion (green) and other Rab proteins involved in other cellular processes (blue). Inspired from [25,26].
In this review, we focused on the role played by autophagy and EVs secretion in CSCs status and how Rab GTPases are acting in the triumvirate. Indeed, there are very few recent studies showing a direct role of this family in stemness, which will be explained.
2. Rab Family, Autophagy and CSCs
Autophagy is a mechanism that consists of the cytoplasmic formation of a double membrane vesicle, containing long-lived or damaged components such as proteins, lipids, organelles. This vesicle, called the autophagosome, will ultimately fuse with a lysosome, hence leading to the degradation of its content into elementary entities (amino acids, fatty acids, sugars, etc.). This is a way of providing nutrients and energy to the cell [27,28]. The formation of the autophagosome is a multistep process, in which several protein complexes are necessary for phagophore initiation (ULK1/2 complex), nucleation (Beclin-1 complex) and elongation (ATG5/12/16 and MAP-LC3 proteins) (for a review, see [11]). Whereas a family of almost 30 ATG (autophagy-related gene) proteins is involved, the main one is Microtubule-associated protein 1A/1B-light chain 3 (MAP-LC3) which allows closure of the vesicle. This protein is also used as a marker of the autophagic flux (fusion between autophagosome and lysosome).
As autophagy involves vesicular trafficking, it is not surprising to find Rab GTPases as essential actors. Among the Rab GTPases family, there are mainly 10 Rabs involved in the autophagic process [26]. Most of them act at the autophagosome level. One of the first Rab-GTPase described in autophagy regulation was Rab33B in 2008, a Golgi-associated Rab protein [29] via its interaction with the ATG16L protein. This protein is one of the components of a protein complex involved in autophagosome elongation. Additionally, Rab7 might be one of the most described Rab-GTPase protein in autophagic pathway regulation. Indeed, this particular protein plays an important role in lysosome biosynthesis and function [30]. This is why autophagosome fusion with the lysosome has been, in part, attributed to Rab7, particularly to Rab7a [31]. Moreover, Rab1 seems to participate in phagophore formation by regulating Atg9 localization [32,33], whereas Rab32 is required for autophagosome formation [34,35] and Rab5 for its closure [36]. In addition, Rab24 has been shown to be co-localized with MAP-LC3 [37]. Furthermore, Rab2 has recently been shown to be involved in the regulation of autophagosome and autolysosome formation in different mammalian cell lines [38]. A 2019 study on colon cancer cell lines demonstrated that the inhibition of Rab5/7, both playing a role in the endolysosomal pathway, could decrease the CSCs compartment [39]. Authors used mefloquine (MQ), an antimalarian drug, as an inhibitor of Rab5/7. They highlighted an impairment of mitophagy, the specific recycling of mitochondria via the autophagic pathway, and lysosomal activity inhibition by suppressing LAMP1 and LAMP2 expressions (lysosomal-associated membrane protein) both in vitro and in vivo using mouse PDX models. The combination of MQ with conventional colon cancer drugs led to a drastic decrease (down to 9.4%) in CD44v9+/CD133+ CSC population after MQ treatment. This effect was even stronger with the combined treatments and has also been shown in PDX models by an IHC staining of CD44v9+.
Autophagy has a critical role in embryonic development and is a key actor of cellular homeostasis, which is why its deregulation can easily lead to various diseases, including cancer [40]. Additionally, growing evidence is demonstrating the essential role of autophagy in physiological stemness in many cell types [41,42,43,44,45] as well as for CSCs maintenance and function. Nevertheless, the role played by autophagy in cancer is still not completely understood, as there is evidence of a pro- and anti-tumor autophagy, presuming a duality of the autophagic role in cancer [46,47]. Indeed, autophagy has been described as a cytoprotective mechanism against many cellular stresses that could be considered as an onco-suppressor, but it also protects cancer cells from their hypoxic environment, nutrient deprivation and treatments [48,49]. This is why we decided to investigate, on the one hand, autophagy as a “pro-CSCs” survival mechanism and, on the other hand, autophagy as an “anti-CSCs” mechanism, as there are few studies supporting the idea that autophagy can lead to CSCs differentiation.
The autophagic flux in the maintenance of CSCs stemness and function has been widely explored, in particular in Breast Cancer Stem Cells (BCSCs) [50,51,52,53]. This has particularly been demonstrated through studies on various autophagy proteins such as Beclin1 [54], Atg4A [55], implicated in MAP-LC3 maturation, or using drugs such as chloroquine or salinomycin to inhibit the fusion between the autophagosome and lysosome, i.e., autophagic flux [56,57]. Evidence of the implication of autophagy in CSCs survival for many other cancer types has also been given [58,59,60,61]. This is notably true for bladder cancer in which autophagy has been demonstrated as essential for stemness maintenance of bladder CSCs and for tumorigenic properties such as invasion and metastasis [62,63]. Autophagy-promoted metastasis is also known in gastric cancer [64]. Indeed, the Atg4A protein is shown to be responsible for inducing the epithelial–mesenchymal transition as well as stemness properties in various gastric cancer cell lines and in vivo using mice models. As previously mentioned, CSCs are key actors in therapeutic resistance, which is why numerous studies on different types of cancer have proven the beneficial effects of autophagy inhibition on therapy sensitization [50,65,66,67,68,69,70,71,72]. It has notably been demonstrated in non-small cell lung carcinoma (NSCLC) [67]. An enhanced CD133+ cell population after chemotherapeutic treatment using cisplatin has been shown both in vitro in the A549 cell line and ex vivo in clinical NSCLC samples. A higher autophagy level in those CD133+ cancer stem cells has been highlighted, which led the authors to try to inhibit this particular process in order to target the stem cells population. By using chloroquine, a loss in CD133+ cells and a decrease in sphere forming abilities in A549 cells were observed. Furthermore, a combined treatment using cisplatin and chloroquine showed a synergistic effect, inducing a decrease in tumor growth in engrafted NOD/SCID mice. Similar observations had already been made a few years earlier in ovarian cancer stem cells (OCSCs) [70]. A high level of autophagy has been shown is OCSCs. Furthermore, blocking the autophagic flux either by using chloroquine or knocking down ATG5 expression reduced the stemness capacities both in vitro and in vivo. Additionally, autophagy impairment increased chemotherapy sensitivity by significantly impacting cell viability in vitro as well as in vivo and by drastically reducing tumor weight and volume.
Aside from its pro-tumoral effects through CSCs maintenance and function, since a few years ago, autophagy has also been attributed a role in CSCs differentiation and thus, their sensitization to chemotherapeutic treatment. Some even point out the beneficial role of curcumin in inducing autophagy-mediated differentiation [73,74]. In the peripheral nervous system, Li and colleagues [75], working on neuroblastoma cell lines, showed that calcium/calmodulin-dependent protein kinase II was responsible for autophagy activation through Beclin1 phosphorylation. Furthermore, there was an induction of Id—inhibitor differentiation—degradation through autophagy, leading to cell differentiation. This has also been highlighted in the central nervous system and particularly in glioma-initiating cells [74,75,76,77,78,79,80]. Furthermore, Ciechomska and co-workers [78] observed autophagy activation using an inhibitor of Histone Methyl-Transferase (HMT), BIX01294, on Glioblastoma Stem Cells (GSCs). Moreover, after this treatment, an increase in astrocytic (GFAP; Glial Fibrillary Acidic Protein) and neuronal (TUBB3; Tubulin Beta 3 class III) differentiation markers in GSCs was revealed. This autophagy-mediated differentiation has been highlighted in some other cancer types, including colon, liver and gastric cancers [81,82,83]. A 2019 study [84] demonstrated both in vitro and in vivo that metformin, a common drug used to treat type II diabetes, could suppress the self-renewal and tumorigenicity abilities of Osteosarcoma Stem Cells (OSCs). Furthermore, metformin was shown to induce a cell cycle arrest in OSCs cell lines, as well as Reactive Oxygen Species (ROS)-mediated apoptosis and autophagy.Treated OSCs were impaired in their capacity to form spheres and showed a significant decrease in stemness markers Oct4 and Sox2. Finally, treated OSCs transplanted in mice led to a significant decrease in both tumor weight and volume. Then, a 2020 study also found out a link between ROS-mediated autophagy and proliferation and stemness status by working on colorectal cancer cells [85]. They investigated the effects of silencing LETM1 (Leucine zipper-EF-hand-containing Transmembrane protein 1), which is overexpressed in CRC tissues compared to normal ones and of poor prognosis. They first found a decrease in colony forming and proliferation capacities, in addition to an accumulation of S-phase cells. The monitoring of autophagy in esi-LETM1 cells showed a significant increase in Beclin1 expression and MAP-LC3II/I ratio, meaning an enhanced autophagic process. Few years earlier, Sharif et al. [86] worked on PHosphoGlycerate DeHydrogenase (PHGDH), previously shown as required for CSCs maintenance in hypoxia-induced Breast Cancer Stem Cells (BCSCs) [87]. They observed a significant positive correlation between PHGDH and Oct4 expressions both in vitro in Embryonic Carcinoma Stem-Like Cells and in Cancer Stem-Like Cells from patients. Therefore, they decided to undertake a PHGDH knockdown (KD) and demonstrated that it decreased the stemness characteristics and promoted Embryonic Carcinoma Stem-Like Cells differentiation in multilineage. Moreover, by looking for different autophagy proteins’ expression, they revealed an activation of autophagic flux in cells KD for PHGDH.
To summarize, autophagy has been widely studied for a few decades, but its involvement in cancer and CSCs remains uncertain. Indeed, in some cases, it appears that inhibiting autophagy impairs CSCs compartment and increases therapy sensitization, whereas in some others, inducing autophagy seems to be a better strategy to promote CSCs differentiation. Taken together, those data suggest that, although cancer therapy research is likely focused on finding anti-cancerous molecules that could be used in any type of cancer, concerning autophagy-targeted treatments, it might be of better interest to consider a cancer-dependent treatment.
3. Rab Family, Extracellular Vesicles and CSCs
Extracellular vesicles (EVs) include apoptotic bodies, microvesicles and exosomes. The last ones’ secretion is initiated by invagination of the endosomal membrane, leading to Multivesicular Bodies (MVBs) formation containing Intraluminal Vesicles (ILVs). They are finally targeted to the plasma membrane, fuse with it and release the ILVs into the extracellular environment. These exosomes are secreted with a size comprising between 30 and 150 nm (for review see [88]). EVs reflect the physiological status of the secretory cell [89] through their varied content, which includes proteins such as surface receptors, nucleic acids, lipids, etc. They are of major importance for cell-to-cell and cell-to-microenvironment communications.
As the secretion of Extracellular Vesicles (EVs) involves the endosomal pathway, it is not surprising that Rab GTPases are essential to this process. There are mainly three Rab-GTPases described in EVs biogenesis and secretion [90]: Rab11, Rab35 and Rab27A. All three proteins are implicated in the docking and fusion of the MVBs. Rab11 activity has been shown to be calcium-dependent in the erythroleukemia cell line K562 in 2005 [91]. More recently, the promotion of exosome secretion by Rab35 has been observed in hepatocellular carcinoma [92], when it had been previously demonstrated few years earlier in Oli-neu cell line, i.e., oligodendroglial precursor [93]. Although, the most studied and commonly known is Rab27A/B, also involved in the docking and fusion of MVBs at the plasma membrane [94]. One of the first studies showing the role of Rab27A in EVs secretion was carried out on HeLa cells [95] and then, confirmed in breast cancer cells [96]. There are some studies on the expression of Rab27A in different cancer types and its prognostic relevance. A high expression appears to be of poor prognosis in pancreatic cancer [97], bladder cancer [98], hepatocellular carcinoma [99] and gliomas [100]. This is also the case concerning an overexpression of Rab27B in non-small cell lung carcinoma [101]. On the contrary, for colon and prostate cancer, it seems that a high expression of Rab27A or Rab27B is correlated with a better outcome [102,103].
EVs secretion is an essential mechanism for cell-to-cell communication and cell-to-microenvironment communication. This is notably true concerning CSCs, which are permanently communicating, modulating and adapting to their microenvironment. Even though stem cell-derived EVs are studied as therapeutic tools in cancer [104,105] and also in other kinds of diseases [106,107,108,109] or even as regenerative tools [110], little is known about the action mechanism of EVs on CSCs maintenance. EVs have been attributed an emerging role of transfer of nucleic acids, such as miRNAs [111,112,113,114] or lncRNAs [115,116] and particularly for their involvement in stemness status. Ren et al. [115] worked on colorectal cancer (CRC) and the potential transfer of long non-coding RNA (lncRNA) H19, known to be of poor prognosis in CRC [117]. They found that H19 is overexpressed in CRC patients and particularly in Carcinoma-Associated Fibroblasts (CAFs). Using siRNA targeting H19 in CRC cell lines SW480 and HCT116, a significant decrease in the number of spheres and sensitization to oxaliplatin treatment was observed. A higher level of H19 was exhibited in patients’ CAF exosomes compared to normal fibroblast exosomes, thus imputing the stemness capacities to a transfer of H19 via exosomes secreted by CAFs. In 2017, Figueroa and colleagues [113] investigated the role played by exosomes from Glioma-Associated human Mesenchymal Stem Cells (GA-hMSC) on Glioma Stem Cells (GSCs). They observed in vitro an increase in clonogenic capacities of GSCs and in vivo an increase in the GSCs’ tumorigenicity. Among GA-hMSC exosomes’ miRNAs, miR-1587 was found to be responsible for the proliferation and self-renewal abilities of GSCs by targeting the tumor suppressor NCOR1 (Nuclear hormone receptor Co-repressor 1). Furthermore, another team worked on GSCs secreted exosomes and their mode of action on non-GSCs in several glioblastoma cell lines [118]. Indeed, those two cellular populations coexist in the tumor mass and understanding the way they communicate is as challenging as it is interesting to find new treatments. A neurosphere formation assay revealed colonies of higher number and size after GSC-derived exosomes treatment. Enhanced expression of several stemness markers in non-GSCs treated with GSC-derived exosomes was also highlighted, meaning a stemness transfer in the recipient cells. This has been confirmed in a mouse model by injecting glioblastoma cells treated or not with GSC-derived exosomes. When treated, the cells promoted the formation of bigger and heavier tumors. Afterwards, Notch1 transfer via GSCs derived exosomes into non-GSCs cells has been demonstrated to be responsible for transferring stemness capacities and inducing dedifferentiation. Then, a 2020 study on group 4 medulloblastoma primary cultures showed that miR-135a- and -135b-containing EVs could regulate stemness [119]. Tumor tissues were enzymatically dissociated to form single cells and to be cultured into Bulk Tumor Cells (BTCs) and Brain Tumor Spheroid-forming Cells (BTSCs) supposed to be enriched in stem cells and progenitors. Authors then performed a miRNA profiling on the cells and secreted EVs and the expression of numerous miRNAs was found to be significantly increased in BTSCs and BTSCs-EVs compared to the BTCs. Among them were miR-135a and -135b, which led to an impairment of the stemness capacities in BTSCs when downregulated. There is growing evidence on the impact of EVs on CSCs stemness and aggressiveness in several cancer types such as esophageal carcinoma [120] or pancreatic cancer [121,122]. Yan et al. [123] demonstrated that EVs derived from Lewis Lung Carcinoma could reprogram mouse-induced pluripotent stem cells into CSCs in vitro, endowing them with sphere formation abilities. Moreover, those converted CSCs were given tumorigenic and metastatic capacities in a nude mice model.
Some investigations described EVs as being responsible for inducing therapeutic resistance in CSCs by enhancing and maintaining stemness in cancer cells, mainly in colorectal cancer [111,124,125,126]. One of the most recent studies also investigated the CAF-secreted exosomes and their way of action on stemness and therapeutic resistance [111]. The transfer of miR-92a-3p from CAFs to cancer cells via exosomes allowed the promotion of resistance to the chemotherapeutic agent 5-FU (5-Fluorouracile), metastasis and epithelial to mesenchymal transition. An enhancement of colorectal cancer cells stemness was also demonstrated by performing plate colony formation and sphere formation assays. A previous study [126] had already shown the contribution of CAF-derived exosomes to the chemotherapy resistance of colon CSCs to 5-FU and oxaliplatin, thus leading to the idea of targeting CAFs before therapy to unravel the exosomes’ secretion. Another team recently demonstrated the potential of CAF-derived exosomes to participate in radiotherapy resistance by promoting the stemness abilities of colon cancer cells [127]. They succeeded in demonstrating a higher number of spheres and resistance to radiotherapy when cells are treated with those particular EVs. Furthermore, in vivo experiments showed a greater capacity of rapidly forming larger tumors after radiation when CRC cells are previously treated with CAF-derived exosomes. Furthermore, EVs secreted following environmental stresses such as chemotherapy [112] or hypoxia [128] are able to promote stemness in surrounding cancer cells. Indeed, Ramteke and colleagues worked on prostate cancer cell lines LNCaP and PC3 and investigated the role of exosomes secreted under normoxic (21% O2) or hypoxic (1% O2) conditions. It appeared that treating the cells with exosomes secreted under hypoxia led to a higher invasiveness, migration rate and an especially higher number of prostaspheres compared to the normoxic exosomes treatment condition.
Altogether, these data suggest that EVs are essential actors of CSCs maintenance. This is why targeting them could be a new therapeutic approach to prevent the transfer of stemness abilities and henceforth, resistance to treatment. A recent study [129] used engineered biological nanoparticles similar to EVs to treat hepatocellular cancer by targeting liver CSCs. It was also demonstrated a few years earlier in early-stage breast cancer cells that targeting EVs could be efficient [130]. One team also investigated a possible way to use EVs as therapeutic tools to reprogram CSCs and trigger their differentiation [104]. For this purpose, they used exosomes from osteogenic differentiation of human adipose-derived stem cells on CD133+ cells. They were able to observe the induction of an osteogenic differentiation in the recipient cells.
4. Rab GTPases and CSCs
Rab GTPases have been described in normal stemness in some studies [131,132]. For instance, Rab8a has been highlighted in intestinal stem cells [133,134], or Rab31 in neural progenitor cells [135]. There is not a lot of evidence concerning a direct link between Rab GTPases and CSCs status. However, the overexpression of Rab5b in breast cancer stem cells was highlighted indirectly in a 2018 study [136]. Indeed, authors showed that miR-130a-3p, involved in Rab5b regulation, is downregulated in such CSCs. Furthermore, some Rabs are at the crossroad with the role played by several Rab GTPases in EVs release, especially concerning Rab27A/B. A recent study realized by Peng and coworkers [137] showed that the specific targeting of Rab27B in order to interfere with exosomes secretion could eliminate acute myeloid leukemia (AML) stem cells. The miR-34c-5p has been shown to be downregulated in primary AML CD34+/CD38− cells. This is why authors investigated the effect of miR-34c-5p overexpression. They were able to highlight an elimination of AML stem cells via inducing senescence and especially by decreasing Rab27B expression and thus, exosome release. Cheng et al. [24] also investigated the role of Rab27B in the stemness of two colorectal cancer cell lines. Colorectal cancer stem cells from HT29 and HCT15 cells were isolated and grown in a serum-free spheroid cultivation system to be enriched in stem-like and progenitor cells. Those cultures were called sphere-derived CSCs (SDCSCs). Silencing Rab27B led to a decrease in sphere formation of SDCSCs and to an attenuation of the tumor growth and CD44+ population in CD44High CRC PDX. Then, miR-146a-5p was found to be increased in SDCSCs exosomes, which are allowed to be released via Rab27B. Authors demonstrated that downregulating miR-146a-5p was responsible for an impairment in the sphere formation and tumorigenic capacities first given by SDCSC-derived exosomes. Finally, a positive correlation between exosomal miR-146a-5p expression in patients’ sera and a stemness cellular profile was shown.
Concerning the importance of Rab27A in the stemness status of cancer cells, there was a 2016 study that focused on the role played by miR-134-3p in human ovarian cancer stem cells [138]. It appeared that this miRNA is able to bind to the 3′UTR (Untranslated Region) site of Rab27A mRNA, thus interfering with its expression. Ovarian Cancer Stem Cells (OCSCs) were treated either with a wild-type miR-134-3p or with a mutant, and CSCs markers’ expression was analyzed. Authors observed a significant decrease in the expression of those markers, with the wild-type condition corresponding to a loss of Rab27A. In a xenograft model, they were able to show an impairment of tumor growth when miR-134-3p is overexpressed. In 2018, Chano et al. [139] demonstrated the role played by Rab39A in cancer stemness. This isoform is localized at late endosomes and lysosomes [140] and regulates phagosomes acidification [141], whereas Rab39b is rather localized at the Golgi apparatus. The authors used shRNA targeting Rab39A in human osteosarcoma CSCs and showed an impairment of stemness capacities in vitro by performing a clonogenic assay. They observed a significant decrease in the size and number of colonies. In vivo, shRab39A impaired tumorigenesis in xenografted mice. Then, using RNA-seq analysis, they tried to identify a Rab39A downstream effector. It appeared that RXRB (Retinoid X Receptor Beta) could be a potential candidate. Indeed, in shRab39A cell lines, RXRB expression decreased and when authors induced an overexpression of RXRB gene, the ability of shRab39A CSCs to form spheres was restored. Finally, targeting RXRB expression by shRNA had the same effect on CSCs as shRab39A, showing the implication of the Rab39A–RXRB axis in the maintenance of CSCs stemness.
Another study showed that Rab6 could have a role in lung CSCs (LCSCs) sensitivity to cisplatin-based chemotherapy [142]. Rab6 is located at the Golgi apparatus and regulates the vesicular trafficking from the Golgi toward the endoplasmic reticulum (ER) [143]. Authors demonstrated that miR-5100 is responsible for LCSCs cisplatin resistance through the downregulation of Rab6 expression. It seems that miR-5100 is upregulated in LCSCs compared to non-CSCs and, when overexpressed, increases CSCs properties. By looking at miR-5100 target genes, they found that Rab6 was downregulated in LCSCs. Furthermore, when Rab6 expression is increased using pcDNA plasmids, the expression of the common stemness markers was decreased. Another team worked on Breast Cancer Stem Cells (BCSCs) and focused on the prolyl isomerase Pin1, as it has been shown to be increased in human BCSCs, playing a key role in their promotion and maintenance [144,145]. They identified Rab2A as a downstream effector of Pin1. Authors highlighted that Rab2A is overexpressed in human cancers and thus, increases the BCSCs-enriched population. Finally, the study showed that the role of Rab2A in BCSCs is mediated by the activation of the Erk1/2 pathway and the translocation of β-catenin to the nucleus [146]. Furthermore, as previously mentioned, there are some publications showing growing evidence of the role of Rab27A, but also of its counterpart Rab27B, in CSCs status. Indeed, studies in BCSCs and colon CSCs [147,148] attributed to Rab27A a role in stemness maintenance. A recent study focused on Rab37 small G protein in lung cancer. Contrary to the other Rab GTPases quoted above, this one is known for its tumor suppressor action [149,150]. Authors investigated its potential role in LCSCs and observed that Rab37 nullifies LCSCs stemness via inhibition of the Wnt signaling pathway [151]. Indeed, downregulation of Rab37 expression seemed to enhance the stem-like properties of lung cancer cells both in vitro and in vivo. To do so, on the one hand, they used shRNAs targeting Rab37 and performed three-dimensional sphere culture assays and observed an increase in the size and number of the spheres. Furthermore, RT-qPCR results showed a significant increase in the expression of several stemness markers, which was confirmed in a mouse xenograft model. On the other hand, they overexpressed Rab37 by either inducing a constitutively active form of the protein or using a dominant negative mutant. The sphere formation assay and RT-qPCR results were in favor of a significant loss of stemness properties. In fact, Rab37 is responsible for the exocytosis of SFRP1 (Secreted Frizzled-Related Protein 1), which inhibits the Wnt pathway in LCSCs. By performing analysis in a lung cancer patients’ cohort, they showed that a low expression of Rab37, and thus of SFRP1, is associated with poor prognosis and high Oct4 expression, a well-known stemness marker.
In addition, CSCs exhibiting high capacities to undertake Epithelial-to-Mesenchymal Transition (EMT) support metastasis, and subsequently, recurrence and poor prognosis in many cancers. This process consists of phenotypic modifications, notably the loss of E-cadherin toward N-cadherin expression. Various Rabs have been attributed a role in cancer cells migration and Epithelial-to-Mesenchymal Transition (EMT). Among them, Rab23 was found to be responsible for EMT in ovarian cancer and hepatocellular carcinoma [152,153]. Additionally, Rab11 has been described as a regulator of E-cadherin, thus promoting cell migration in colorectal cancer [154,155]. On the contrary, some Rab-GTPases negatively regulate EMT and their silencing has been proven to induce it. This is notably the case concerning the epigenetic silencing of Rab39A in cervical cancer [156] or the downregulation of Rab17 in non-small cell lung carcinoma [157].
All of these studies, in addition to what is known concerning the role played by those small G proteins in autophagy, extracellular vesicles secretion and/or cancer stem cells status (Figure 2), demonstrate the Rab GTPases’ potential to be new therapeutic targets to modulate cancer stemness and make the current treatments, especially chemotherapies, more efficient.
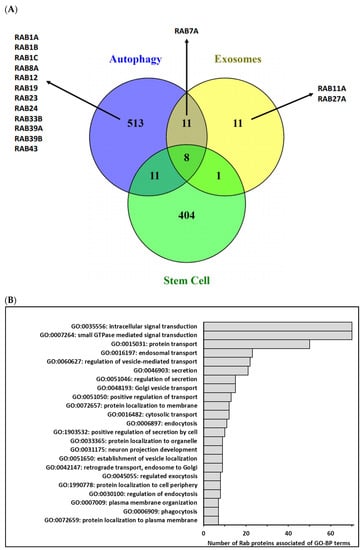
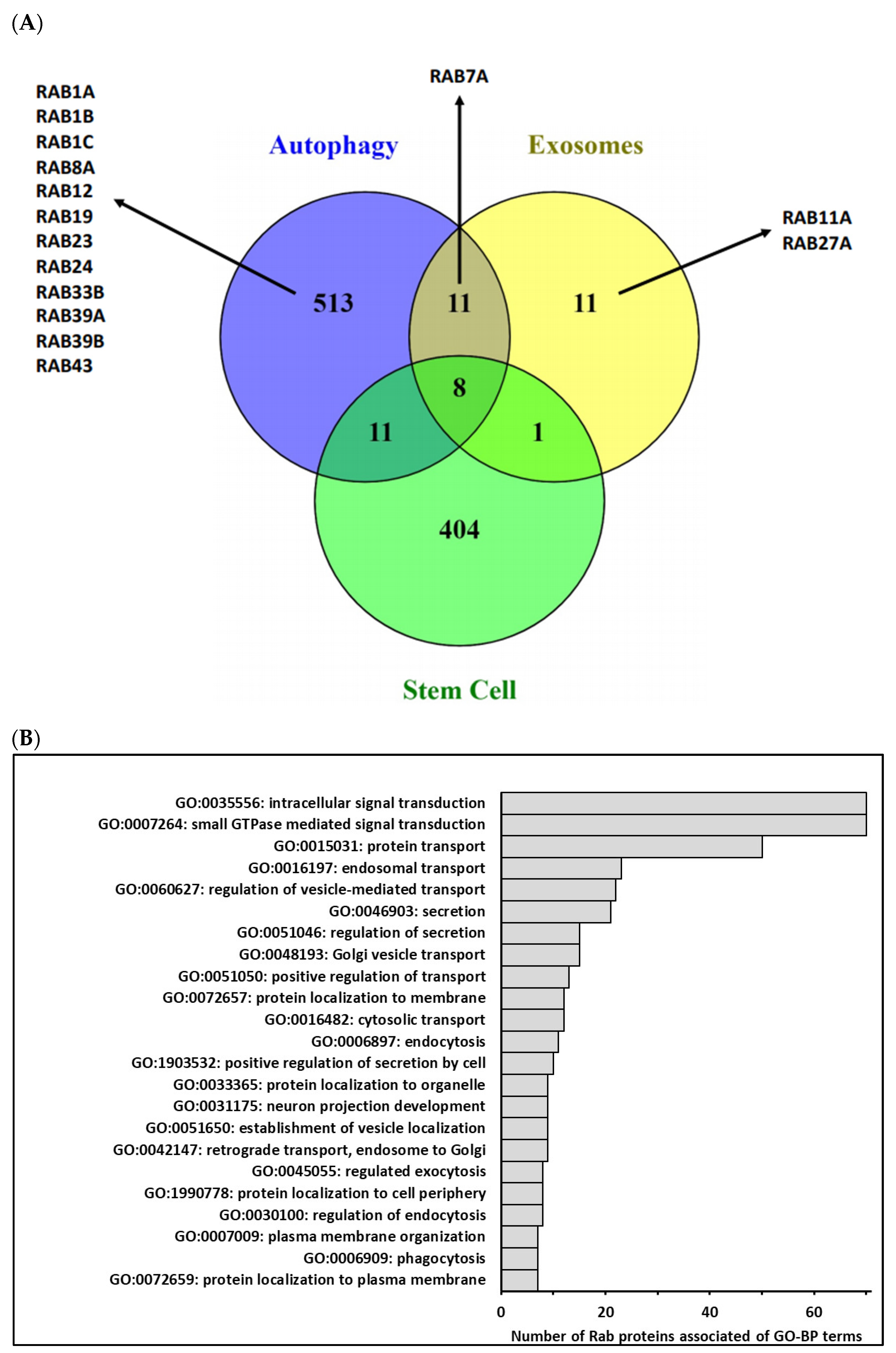
Figure 2.
(A)—Comparison of human genes associated with Gene Ontology terms related to autophagy, extracellular vesicles and/or exosomes biogenesis and stem cells biological process (http://geneontology.org/ and https://www.ebi.ac.uk/QuickGO/, accessed on 26 March 2021) using Venn diagram. In fact, 543 different genes were categorized into GO-BP related to autophagy (GO:0006914 and GO:0010506), 31 different genes were categorized into GO-BP related to extracellular vesicles/exosomes (GO:0140112, GO:0097734, GO:0099156, GO:1990182, GO:0071971 and GO:1903551) and 424 different genes were categorized into GO-BP related to stem cells (GO:0019827, GO:0017145, GO:0048863, GO:0072089 and GO:0048864). Few genes were commonly associated with three biological process, and among Rab identified in these processes, only Rab7A was common to 2 processes. (B)—Main terms of Gene Ontology-Biological Process (GO-BP) associated with Homo Sapiens Rab proteins. GO-BP terms associated with the 70 human Rab proteins were retrieved from the DAVID database (https://david.ncifcrf.gov/ accessed on 26 March 2021) by focusing on level 5 of the GO hierarchy. Among 81 enriched terms (p adjusted ≤ 0.05), 33 terms were associated with more than 10% of Rab. After removing redundant GO terms using REVIGO (http://revigo.irb.hr/ accessed on 26 March 2021) according to functional similarity, we selected a short list of 23 terms summarized in the above graphic, illustrating the functional diversity of the Rab protein family.
5. Conclusions
This quick update on the current knowledge concerning autophagy, EVs and Rab GTPases in CSCs maintenance identifies new possible therapeutic strategies, especially via targeting Rab GTPases. Indeed, even if the question of their real tumorigenicity is still debated, their intervention in key stages of the life of a cell justifies the development of therapies targeting them. Furthermore, this has been the topic of a quite recent review [158]. This goal could be achieved by targeting of Rabs’ regulatory proteins [159], particularly their GEFs. A team designed nine peptides that could target Rab GTPases through their different interactions [160], which was performed by Mitra and coworkers in 2017 using stapled peptides inhibitors of Rab25 [161] in various cell lines. This might also be achieved by the means of chemical agents such as Nexinhibs (Neutrophil-exocytosis inhibitors), which have been found to be inhibitors of the interaction between Rab27a and its effector JFC1, i.e., synaptotagmin-like protein1 [162]. Thus, even if these innovative therapies targeting Rab proteins are promising, further studies would have to be undertaken. Indeed, depending on cell type, tumoral stage/grade and even the cancer type, the targeted Rab might not be the same, leading to personalized patient care.
Author Contributions
Writing—original draft preparation, A.B.; writing—review and editing, G.B., C.A.; figure design, S.B.; Gene Ontology (GO) analysis, S.D.; supervision review and editing, B.B., M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by La Ligue contre le cancer, comité de la Haute-Vienne.
Acknowledgments
Authors would like to thank Camille Frugier, for her time and help with the English.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.-A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.-B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; He, Q.; Li, Z.; Zou, Q.; Xu, P.; Yu, H.; Ding, Y.; Zhu, W. Cancer stem cells in colorectal cancer and the association with chemotherapy resistance. Med. Oncol. 2021, 38, 43. [Google Scholar] [CrossRef]
- Malanchi, I.; Santamaria-Martínez, A.; Susanto, E.; Peng, H.; Lehr, H.-A.; Delaloye, J.-F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011, 481, 85–89. [Google Scholar] [CrossRef]
- Miyoshi, N.; Haraguchi, N.; Mizushima, T.; Ishii, H.; Yamamoto, H.; Mori, M. Targeting cancer stem cells in refractory cancer. Regen Ther. 2021, 17, 13–19. [Google Scholar] [CrossRef]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Hirsch, H.A.; Wang, G.; Struhl, K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc. Natl. Acad. Sci. USA 2011, 108, 1397–1402. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Pinet, S.; Bessette, B.; Vedrenne, N.; Lacroix, A.; Richard, L.; Jauberteau, M.-O.; Battu, S.; Lalloué, F. TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget 2016, 7, 50349–50364. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y.; Ohkubo, T.; Kawaji, H.; Machida, A.; Miyata, H.; Goda, S.; Roy, S.; Hayashizaki, Y.; Suzuki, H.; Yokota, T. Next-generation sequencing-based small RNA profiling of cerebrospinal fluid exosomes. Neurosci Lett. 2017, 636, 48–57. [Google Scholar] [CrossRef]
- Qin, W.; Tsukasaki, Y.; Dasgupta, S.; Mukhopadhyay, N.; Ikebe, M.; Sauter, E.R. Exosomes in Human Breast Milk Promote EMT. Clin. Cancer Res. 2016, 22, 4517–4524. [Google Scholar] [CrossRef]
- Machida, T.; Tomofuji, T.; Ekuni, D.; Maruyama, T.; Yoneda, T.; Kawabata, Y.; Mizuno, H.; Miyai, H.; Kunitomo, M.; Morita, M. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int. J. Mol. Sci. 2015, 16, 21294–21309. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kamada, H.; Kanasaki, S.; Maeda, Y.; Nagano, K.; Abe, Y.; Inoue, M.; Yoshioka, Y.; Tsutsumi, Y.; Katayama, S.; et al. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharmazie 2013, 68, 969–973. [Google Scholar]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; Lebleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Duijvesz, D.; Burnum-Johnson, K.E.; Gritsenko, M.A.; Hoogland, A.M.; Vredenbregt-van den Berg, M.S.; Willemsen, R.; Luider, T.; Paša-Tolić, L.; Jenster, G. Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS ONE 2013, 8, e82589. [Google Scholar] [CrossRef]
- Sugimachi, K.; Matsumura, T.; Hirata, H.; Uchi, R.; Ueda, M.; Ueo, H.; Shinden, Y.; Iguchi, T.; Eguchi, H.; Shirabe, K.; et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer 2015, 112, 532–538. [Google Scholar] [CrossRef]
- Geng, D.; Zhao, W.; Feng, Y.; Liu, J. Overexpression of Rab25 promotes hepatocellular carcinoma cell proliferation and invasion. Tumor Biol. 2016, 37, 7713–7718. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, S.-Y.; Park, C.-W.; Park, S.-H.; Yin, J.; Kim, J.; Park, J.-B.; Lee, J.-Y.; Kim, H.; Kim, S.-C. Rab3a promotes brain tumor initiation and progression. Mol. Biol. Rep. 2014, 41, 5903–5911. [Google Scholar] [CrossRef]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Théry, C. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef]
- Cheng, W.; Liao, T.; Lin, C.; Yuan, L.E.; Lan, H.; Lin, H.; Teng, H.; Chang, H.; Lin, C.; Yang, C.; et al. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int. J. Cancer 2019, 145, 2209–2224. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef]
- Szatmári, Z.; Sass, M. The autophagic roles of Rab small GTPases and their upstream regulators: A review. Autophagy 2014, 10, 1154–1166. [Google Scholar] [CrossRef]
- Jawhari, S.; Ratinaud, M.-H.; Verdier, M. Glioblastoma, hypoxia and autophagy: A survival-prone «ménage-à-trois». Cell Death Dis. 2016, 7, e2434. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fujita, N.; Kanno, E.; Yamamoto, A.; Yoshimori, T.; Fukuda, M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol. Biol. Cell. 2008, 19, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Langemeyer, L.; Fröhlich, F.; Ungermann, C. Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends Cell Biol. 2018, 28, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Stroupe, C. This Is the End: Regulation of Rab7 Nucleotide Binding in Endolysosomal Trafficking and Autophagy. Front. Cell Dev. Biol. 2018, 6, 129. [Google Scholar] [CrossRef]
- Zoppino, F.C.M.; Militello, R.D.; Slavin, I.; Alvarez, C.; Colombo, M.I. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic Cph Den. 2010, 11, 1246–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Menon, S.; Yamasaki, A.; Chou, H.-T.; Walz, T.; Jiang, Y.; Ferro-Novick, S. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc. Natl. Acad. Sci. USA 2013, 110, 9800–9805. [Google Scholar] [CrossRef]
- Hirota, Y.; Tanaka, Y. A small GTPase, human Rab32, is required for the formation of autophagic vacuoles under basal conditions. Cell Mol. Life Sci CMLS 2009, 66, 2913–2932. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Huang, X. Rab32 is important for autophagy and lipid storage in Drosophila. PLoS ONE 2012, 7, e32086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zou, S.; Chen, Y.; Lipatova, Z.; Sun, D.; Zhu, X.; Li, R.; Wu, Z.; You, W.; Cong, X.; et al. A Rab5 GTPase module is important for autophagosome closure. PLoS Genet. 2017, 13, e1007020. [Google Scholar] [CrossRef] [PubMed]
- Munafó, D.B.; Colombo, M.I. Induction of autophagy causes dramatic changes in the subcellular distribution of GFP-Rab24. Traffic 2002, 3, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jiang, X.; Tian, R.; Zhao, P.; Li, L.; Wang, X.; Chen, S.; Zhu, Y.; Mei, M.; Bao, S.; et al. RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells. Autophagy 2019, 15, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Koseki, J.; Takahashi, H.; Miyoshi, N.; Nishida, N.; Nishimura, J.; Hata, T.; Matsuda, C.; Mizushima, T.; Yamamoto, H.; et al. Disruption of Endolysosomal RAB5/7 Efficiently Eliminates Colorectal Cancer Stem Cells. Cancer Res. 2019, 79, 1426–1437. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Lyu, Z.-S.; Cao, X.-N.; Wen, Q.; Mo, X.-D.; Zhao, H.-Y.; Chen, Y.-H.; Wang, Y.; Chang, Y.-J.; Xu, L.-P.; Zhang, X.-H.; et al. Autophagy in endothelial cells regulates their haematopoiesis-supporting ability. EBioMedicine 2020, 53, 102677. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Yang, L.; Li, Y.; He, Y.; Liu, Z.; Zhang, Y.; Su, G. Bone marrow mesenchymal stem cells enhance autophagy and help protect cells under hypoxic and retinal detachment conditions. J. Cell Mol. Med. 2020, 24, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Tra, T.; Gong, L.; Kao, L.-P.; Li, X.-L.; Grandela, C.; Devenish, R.J.; Wolvetang, E.; Prescott, M. Autophagy in Human Embryonic Stem Cells. PLoS ONE 2011, 6, e27485. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerto, M.C.; Folkerts, H.; Wierenga, A.T.J.; Schepers, K.; Schuringa, J.J.; Coffer, P.J.; Vellenga, V. Autophagy Proteins ATG5 and ATG7 Are Essential for the Maintenance of Human CD34+ Hematopoietic Stem-Progenitor Cells: Autophagy in Human Hematopoietic Stem Cells. Stem Cells 2016, 34, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Ojha, R.; Bhattacharyya, S.; Singh, S.K. Autophagy in Cancer Stem Cells: A Potential Link Between Chemoresistance, Recurrence, and Metastasis. BioResearch Open Access 2015, 4, 97–108. [Google Scholar] [CrossRef] [PubMed]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, S.; Guo, X.; Sun, K.; Han, Z.; Li, R.; Zhao, Q.-D.; Deng, W.-J.; Xie, X.-Q.; Zhang, J.-W.; et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013, 339, 70–81. [Google Scholar] [CrossRef]
- Jawhari, S.; Bessette, B.; Hombourger, S.; Durand, K.; Lacroix, A.; Labrousse, F.; Jauberteau, M.-O.; Ratinaud, M.-H.; Verdier, M. Autophagy and TrkC/NT-3 signaling joined forces boost the hypoxic glioblastoma cell survival. Carcinogenesis 2017, 38, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Shen, S.; Zhang, Y.-J.; Xu, C.-F.; Cao, Z.-T.; Wen, L.-P.; Wang, J. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Han, Y.; Fan, S.; Qin, T.; Yang, J.; Sun, Y.; Lu, Y.; Mao, J.; Li, L. Role of autophagy in breast cancer and breast cancer stem cells. Int. J. Oncol. 2018, 52, 1057–1070. [Google Scholar] [CrossRef]
- Maycotte, P.; Jones, K.L.; Goodall, M.L.; Thorburn, J.; Thorburn, A. Autophagy Supports Breast Cancer Stem Cell Maintenance by Regulating IL6 Secretion. Mol. Cancer Res. 2015, 13, 651–658. [Google Scholar] [CrossRef]
- Cufí, S.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Martin-Castillo, B.; Vellon, L.; Menendez, J.A. Autophagy positively regulates the CD44+ CD24−/low breast cancer stem-like phenotype. Cell Cycle 2011, 10, 3871–3885. [Google Scholar] [CrossRef]
- Gong, C.; Bauvy, C.; Tonelli, G.; Yue, W.; Deloménie, C.; Nicolas, V.; Zhu, Y.; Domergue, V.; Marin-Esteban, V.; Tharinger, H.; et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 2013, 32, 2261–2272. [Google Scholar] [CrossRef]
- Wolf, J.; Dewi, D.L.; Fredebohm, J.; Müller-Decker, K.; Flechtenmacher, C.; Hoheisel, J.D.; Boettcher, M. A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype. Breast Cancer Res. 2013, 15, R109. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Blanco, E.; Kim, Y.-S.; Rodriguez, A.A.; Zhao, H.; Huang, T.H.-M.; Chen, C.-L.; Jin, G.; Landis, M.D.; Burey, L.A.; et al. Chloroquine Eliminates Cancer Stem Cells Through Deregulation of Jak2 and DNMT1: Chloroquine inhibits DNMT1 and Jak2 in CSC. Stem Cells 2014, 32, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Hamaï, A.; Tonelli, G.; Bauvy, C.; Nicolas, V.; Tharinger, H.; Codogno, P.; Mehrpour, M. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy 2013, 9, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, X.; Li, X.; Song, S.; Meng, Q.; Wang, L.; Lu, Y.; Xin, X.; Pu, H.; Gui, X.; et al. Long noncoding RNA HULC accelerates the growth of human liver cancer stem cells by upregulating CyclinD1 through miR675-PKM2 pathway via autophagy. Stem Cell Res. Ther. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bai, S.; Wang, R.; Xiong, S.; Li, Y.; Wang, X.; Chen, W.; Cheng, B. Cancer-associated fibroblasts endow stem-like qualities to liver cancer cells by modulating autophagy. Cancer Manag Res. 2019, 11, 5737–5744. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Martell, E.; Dai, C.; Kennedy, B.E.; Murphy, P.; Clements, D.R.; Kim, Y.; Lee, P.W.K.; Gujar, S.A. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy 2017, 13, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Hu, L.; Ge, R.; Yang, L.; Liu, K.; Li, Y.; Sun, Y.; Wang, K. Autophagy-deficiency in hepatic progenitor cells leads to the defects of stemness and enhances susceptibility to neoplastic transformation. Cancer Lett. 2016, 371, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Luo, Y.; Xu, J.; Liufu, H.; Tian, Z.; Huang, C.; Li, J.; Huang, C. A Feedback Loop Formed by ATG7/Autophagy, FOXO3a/miR-145 and PD-L1 Regulates Stem-Like Properties and Invasion in Human Bladder Cancer. Cancers 2019, 11, 349. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, G.; Hua, X.; Li, Y.; Yan, H.; Che, X.; Tian, Z.; Liufu, H.; Huang, C.; Li, J. CD44s is a crucial ATG7 downstream regulator for stem-like property, invasion, and lung metastasis of human bladder cancer (BC) cells. Oncogene 2019, 38, 3301–3315. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-W.; Ping, Y.-F.; Jiang, Y.-X.; Luo, X.; Zhang, X.; Bian, X.-W.; Yu, P.-W. ATG4A promotes tumor metastasis by inducing the epithelial-mesenchymal transition and stem-like properties in gastric cells. Oncotarget 2016, 7, 39279–39292. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, C.; Zeng, Y.; Chen, M.; Li, Y.; Xie, C.; Zhou, Y.; Zhong, Y.; Yu, H. Radiosensitization of Clioquinol Combined with Zinc in the Nasopharyngeal Cancer Stem-like Cells by Inhibiting Autophagy in vitro and in vivo. Int. J. Biol. Sci. 2020, 16, 777–789. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, P.; Zhang, Q.; Liu, Q.; Jiang, S.; Liu, L.; Guo, M.; Cheng, Q.; Zheng, J.; Yao, H. Peglated-H1/pHGFK1 nanoparticles enhance anti-tumor effects of sorafenib by inhibition of drug-induced autophagy and stemness in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 362. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Liu, G.; Tian, G. Autophagy inhibition of cancer stem cells promotes the efficacy of cisplatin against non-small cell lung carcinoma. Ther. Adv. Respir. Dis. 2019, 13, 175346661986609. [Google Scholar] [CrossRef] [PubMed]
- Rothe, K.; Porter, V.; Jiang, X. Current Outlook on Autophagy in Human Leukemia: Foe in Cancer Stem Cells and Drug Resistance, Friend in New Therapeutic Interventions. Int. J. Mol. Sci. 2019, 20, 461. [Google Scholar] [CrossRef]
- You, Y.; Bi, F.-F.; Jiang, Y.; Xu, Y.-T.; An, Y.-Y.; Li, D.; Yang, Q. BRCA1 affects the resistance and stemness of SKOV3-derived ovarian cancer stem cells by regulating autophagy. Cancer Med. 2019, 8, 656–668. [Google Scholar] [CrossRef]
- Pagotto, A.; Pilotto, G.; Mazzoldi, E.L.; Nicoletto, M.O.; Frezzini, S.; Pastò, A.; Amadori, A. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 2017, 8, e2943. [Google Scholar] [CrossRef]
- Yang, M.-C.; Wang, H.-C.; Hou, Y.-C.; Tung, H.-L.; Chiu, T.-J.; Shan, Y.-S. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol. Cancer 2015, 14, 179. [Google Scholar] [CrossRef]
- Naik, P.P.; Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Das, C.K.; Mishra, R.; Patil, S.; Bhutia, S.K. Autophagy regulates cisplatin-induced stemness and chemoresistance via the upregulation of CD44, ABCB1 and ADAM17 in oral squamous cell carcinoma. Cell Prolif. 2018, 51, e12411. [Google Scholar] [CrossRef] [PubMed]
- Heebkaew, N.; Rujanapun, N.; Kunhorm, P.; Jaroonwitchawan, T.; Chaicharoenaudomrung, N.; Promjantuek, W.; Noisa, P. Curcumin Induces Neural Differentiation of Human Pluripotent Embryonal Carcinoma Cells through the Activation of Autophagy. BioMed. Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Long, L.; Zheng, B.; Ji, W.; Yang, N.; Zhang, Q.; Liang, Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012, 103, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.-Q.; Deng, R.; Li, D.-D.; Tang, J.; Chen, W.-D.; Chen, J.-H.; Ji, J.; Jiao, L.; Jiang, S.; et al. CaMKII-mediated Beclin 1 phosphorylation regulates autophagy that promotes degradation of Id and neuroblastoma cell differentiation. Nat. Commun. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Li, B.; Long, L.; Chen, L.; Huang, Q.; Liang, Z. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int. J. Cancer 2011, 129, 2720–2731. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Li, T.; Ma, H.; Yang, Y.; Zhang, C.; Hai, L.; Liu, P.; Yuan, F.; Li, J.; Yi, L.; et al. Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation. Cell Death Dis. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Ciechomska, I.A.; Przanowski, P.; Jackl, J.; Wojtas, B.; Kaminska, B. BIX01294, an inhibitor of histone methyltransferase, induces autophagy-dependent differentiation of glioma stem-like cells. Sci. Rep. 2016, 6, 38723. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner: Cannabidiol and GSCs differentiation. Int. J. Cancer 2015, 137, 1855–1869. [Google Scholar] [CrossRef]
- Zhuang, W.-Z.; Long, L.-M.; Ji, W.-J.; Liang, Z.-Q. Rapamycin induces differentiation of glioma stem/progenitor cells by activating autophagy. Chin. J. Cancer 2011, 30, 712–720. [Google Scholar] [CrossRef]
- Pouyafar, A.; Rezabakhsh, A.; Rahbarghazi, R.; Heydarabad, M.Z.; Shokrollahi, E.; Sokullu, E.; Khaksar, M.; Nourazarian, A.; Avci, C.B. Treatment of cancer stem cells from human colon adenocarcinoma cell line HT-29 with resveratrol and sulindac induced mesenchymal-endothelial transition rate. Cell Tissue Res. 2019, 376, 377–388. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Meng, X.; Wang, M.; Tan, J.; Jia, W.; Li, P.; Li, J.; Zhou, Q. ERas Enhances Resistance to Cisplatin-Induced Apoptosis by Suppressing Autophagy in Gastric Cancer Cell. Front. Cell Dev. Biol. 2020, 7, 375. [Google Scholar] [CrossRef]
- Zhang, H. CCND1 silencing suppresses liver cancer stem cell differentiation through inhibiting autophagy. Hum. Cell 2020, 33, 140–147. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, J.; Wang, Y.; Zhou, L.; Che, J.; Wang, F.; Peng, S.; Zhang, G.; Shang, P. Metformin Suppresses Self-Renewal Ability and Tumorigenicity of Osteosarcoma Stem Cells via Reactive Oxygen Species-Mediated Apoptosis and Autophagy. Oxid. Med. Cell Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Che, N.; Yang, Z.; Liu, X.; Li, M.; Feng, Y.; Zhang, C.; Li, C.; Cui, Y.; Xuan, Y. Suppression of LETM1 inhibits the proliferation and stemness of colorectal cancer cells through reactive oxygen species–induced autophagy. J. Cell Mol. Med. 2021, 25, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Martell, E.; Dai, C.; Ghassemi-Rad, M.S.; Lee, K.; Singh, S.K.; Weaver, I.C.G.; Gujar, S. Phosphoglycerate dehydrogenase inhibition induces p-mTOR-independent autophagy and promotes multilineage differentiation in embryonal carcinoma stem-like cells. Cell Death Dis. 2018, 9, 990. [Google Scholar] [CrossRef]
- Samanta, D.; Park, Y.; Andrabi, S.A.; Shelton, L.M.; Gilkes, D.M.; Semenza, G.L. PHGDH Expression Is Required for Mitochondrial Redox Homeostasis, Breast Cancer Stem Cell Maintenance, and Lung Metastasis. Cancer Res. 2016, 76, 4430–4442. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; van Balkom, B.W.M. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell Vesicles 2012, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 Promotes Docking and Fusion of Multivesicular Bodies in a Calcium-Dependent Manner: Ca2+-Dependent Multivesicular Body Fusion. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef]
- Yang, L.; Peng, X.; Li, Y.; Zhang, X.; Ma, Y.; Wu, C.; Fan, Q.; Wei, S.; Li, H.; Liu, J. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol. Cancer 2019, 18, 78. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Two Rabs for exosome release. Nat. Cell Biol. 2010, 12, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Campbell, E.C.; Lucocq, J.; Riches, A.; Powis, S.J. Monitoring the Rab27 associated exosome pathway using nanoparticle tracking analysis. Exp. Cell Res. 2013, 319, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ni, Q.; Wang, X.; Zhu, H.; Wang, Z.; Huang, J. High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med. Oncol. 2015, 32, 372. [Google Scholar] [CrossRef]
- Liu, J.; Gong, X.; Zhu, X.; Xue, D.; Liu, Y.; Wang, P. Rab27A overexpression promotes bladder cancer proliferation and chemoresistance through regulation of NF-κB signaling. Oncotarget 2017, 8, 75272–75283. [Google Scholar] [CrossRef]
- Dong, W.-W. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 1806. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Zhang, C.; Li, M.; Jiang, C.; Li, Y. Rab27a Was Identified as a Prognostic Biomaker by mRNA Profiling, Correlated with Malignant Progression and Subtype Preference in Gliomas. PLoS ONE 2014, 9, e89782. [Google Scholar] [CrossRef]
- Koh, H.M.; Song, D.H. Prognostic role of Rab27A and Rab27B expression in patients with non-small cell lung carcinoma: Prognostic role of Rab27A/B in NSCLC. Thorac. Cancer 2019, 10, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, X.; Ni, Y.; Hou, N.; Xu, L.; Zhan, F.; Zhu, H.; Xiong, L.; Chen, P. High Rab27A expression indicates favorable prognosis in CRC. Diagn Pathol. 2015, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Worst, T.S.; Meyer, Y.; Gottschalt, M.; Weis, C.-A.; Von Hardenberg, J.; Frank, C.; Steidler, A.; Michel, M.S.; Erben, P. RAB27A, RAB27B and VPS36 are downregulated in advanced prostate cancer and show functional relevance in prostate cancer cells. Int. J. Oncol. 2017, 50, 920–932. [Google Scholar] [CrossRef]
- Lee, K.S.; Choi, J.S.; Cho, Y.W. Reprogramming of cancer stem cells into non-tumorigenic cells using stem cell exosomes for cancer therapy. Biochem. Biophys. Res. Commun. 2019, 512, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gupta, D.; Shankar, S.; Srivastava, R.K. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget 2015, 6, 3280–3291. [Google Scholar] [CrossRef]
- Sun, X.; Meng, H.; Wan, W.; Xie, M.; Wen, C. Application potential of stem/progenitor cell-derived extracellular vesicles in renal diseases. Stem Cell Res. Ther. 2019, 10, 8. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Baulch, J.E.; Acharya, M.M.; Allen, B.D.; Ru, N.; Chmielewski, N.N.; Martirosian, V.; Giedzinski, E.; Syage, A.; Park, A.L.; Benke, A.L.; et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc. Natl Acad. Sci. USA 2016, 113, 4836–4841. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Oki, K.; Ochiya, T. Potential Application of Extracellular Vesicles of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Alzheimer’s Disease Therapeutics. In Stem Cell Renewal and Cell-Cell Communication; Turksen, K., Ed.; Springer: New York, NY, USA, 2014; pp. 171–181. [Google Scholar]
- Balbi, C.; Piccoli, M.; Barile, L.; Papait, A.; Armirotti, A.; Principi, E.; Reverberi, D.; Pascucci, L.; Becherini, P.; Varesio, L.; et al. First Characterization of Human Amniotic Fluid Stem Cell Extracellular Vesicles as a Powerful Paracrine Tool Endowed with Regenerative Potential: Amniotic Fluid Stem Cell Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1340–1355. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef]
- Shen, M.; Dong, C.; Ruan, X.; Yan, W.; Cao, M.; Pizzo, D.; Wu, X.; Yang, L.; Liu, L.; Ren, X.; et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019, 79, 3608–3621. [Google Scholar] [CrossRef]
- Figueroa, J.; Phillips, L.M.; Shahar, T.; Hossain, A.; Gumin, J.; Kim, H.; Bean, A.J.; Calin, G.A.; Fueyo, J.; Walters, A.T.; et al. Exosomes from Glioma-Associated Mesenchymal Stem Cells Increase the Tumorigenicity of Glioma Stem-like Cells via Transfer of miR-1587. Cancer Res. 2017, 77, 5808–5819. [Google Scholar] [CrossRef]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.-U.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, ra63. [Google Scholar] [CrossRef]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, P.; Li, J.; Peng, M.; Zhao, X.; Zhang, X.; Chen, K.; Zhang, Y.; Liu, H.; Gan, L.; et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene 2018, 37, 3822–3838. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, X.; Wang, M.; Qiao, Y.; Xu, Y.; Yang, J.; Dong, N.; He, J.; Sun, Q.; Lv, G.; et al. Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget 2016, 7, 22159–22173. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, Y.; Dong, L.; Ma, W.; Lv, L.; Zhang, J.; Wang, X. Glioblastoma Stem Cell-Derived Exosomes Enhance Stemness and Tumorigenicity of Glioma Cells by Transferring Notch1 Protein. Cell Mol. Neurobiol. 2020, 40, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Koh, E.J.; Kim, R.N.; Byun, J.W.; Phi, J.H.; Yang, J.; Wang, K.-C.; Park, A.K.; Hwang, D.W.; Lee, J.Y.; et al. Extracellular vesicle-associated miR-135b and -135a regulate stemness in Group 4 medulloblastoma cells by targeting angiomotin-like 2. Cancer Cell Int. 2020, 20, 558. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Guo, B.; Deng, J.; Wu, S.; Li, F.; Wang, Y.; Lu, J.; Zhou, Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol. Cancer 2019, 18, 22. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Provaznik, J.; Hackert, T.; Zöller, M. Pancreatic cancer-initiating cell exosome message transfer into noncancer-initiating cells: The importance of CD44v6 in reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 132. [Google Scholar] [CrossRef]
- Kuc, N.; Doermann, A.; Shirey, C.; Lee, D.D.; Lowe, C.-W.; Awasthi, N.; Schwarz, R.E.; Stahelin, R.V.; Schwarz, M.A. Pancreatic ductal adenocarcinoma cell secreted extracellular vesicles containing ceramide-1-phosphate promote pancreatic cancer stem cell motility. Biochem. Pharmacol. 2018, 156, 458–466. [Google Scholar] [CrossRef]
- Yan, T.; Mizutani, A.; Chen, L.; Takaki, M.; Hiramoto, Y.; Matsuda, S.; Shigehiro, T.; Kasai, T.; Kudoh, T.; Murakami, H.; et al. Characterization of Cancer Stem-Like Cells Derived from Mouse Induced Pluripotent Stem Cells Transformed by Tumor-Derived Extracellular Vesicles. J. Cancer 2014, 5, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-B.; Yan, C.; Mu, L.; Mi, Y.-L.; Zhao, H.; Hu, H.; Li, X.-L.; Tao, D.-D.; Wu, Y.-Q.; Gong, J.-P.; et al. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene 2019, 38, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chen, S.; He, P.; Herschman, H.; Li, H. PGE2/EP4 antagonism enhances tumor chemosensitivity by inducing extracellular vesicle-mediated clearance of cancer stem cells. Int. J. Cancer 2018, 143, 1440–1455. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Mu, L.; Huang, K.; Li, X.; Tao, D.; Wu, Y.; Qin, J. Fibroblast-Derived Exosomes Contribute to Chemoresistance through Priming Cancer Stem Cells in Colorectal Cancer. Heeschen, C., éditeur. PLoS ONE 2015, 10, e0125625. [Google Scholar]
- Liu, L.; Zhang, Z.; Zhou, L.; Hu, L.; Yin, C.; Qing, D.; Huang, S.; Cai, X.; Chen, Y. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp. Cell Res. 2020, 391, 111956. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Yan, I.K.; Lewis-Tuffin, L.; Patel, T. Targeting Liver Cancer Stem Cells Using Engineered Biological Nanoparticles for the Treatment of Hepatocellular Cancer. Hepatol. Commun. 2020, 4, 298–313. [Google Scholar] [CrossRef]
- Gernapudi, R.; Yao, Y.; Zhang, Y.; Wolfson, B.; Roy, S.; Duru, N.; Eades, G.; Yang, P.; Zhou, Q. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 2015, 150, 685–695. [Google Scholar] [CrossRef]
- Tanaka, C.; Kaji, H.; He, J.; Hazama, R.; Yokoyama, K.; Kinoshita, E.; Tsujioka, T.; Tohyama, K.; Yamamura, H.; Nishio, H.; et al. Rab27b regulates c-kit expression by controlling the secretion of stem cell factor. Biochem. Biophys. Res. Commun. 2012, 419, 368–373. [Google Scholar] [CrossRef]
- Bogard, N.; Lan, L.; Xu, J.; Cohen, R.S. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 2007, 134, 3413–3418. [Google Scholar] [CrossRef]
- Das, S.; Yu, S.; Sakamori, R.; Vedula, P.; Feng, Q.; Flores, J.; Hoffman, A.; Fu, J.; Stypulkowski, E.; Rodriguez, A.; et al. Rab8a vesicles regulate Wnt ligand delivery and Paneth cell maturation at the intestinal stem cell niche. Development 2015, 142, 2147–2162. [Google Scholar] [CrossRef]
- Sakamori, R.; Das, S.; Yu, S.; Feng, S.; Stypulkowski, E.; Guan, Y.; Douard, V.; Tang, W.; Ferraris, R.P.; Harada, A.; et al. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J. Clin Investig. 2012, 122, 1052–1065. [Google Scholar] [CrossRef]
- Chua, C.E.L.; Goh, E.L.K.; Tang, B.L. Rab31 is expressed in neural progenitor cells and plays a role in their differentiation. FEBS Lett. 2014, 588, 3186–3194. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Li, J.; Shao, J.; Fang, L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem. Biophys. Res. Commun. 2018, 501, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Li, L.; Ma, X.; Chen, Y.; Zhou, H.; Luo, Y.; Xiao, Y.; Liu, L. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 2018, 32, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Liu, T.; Huang, Y.; Qin, W.; Yang, H.; Chen, J. MicroRNA-134–3p is a novel potential inhibitor of human ovarian cancer stem cells by targeting RAB27A. Gene 2017, 605, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chano, T.; Kita, H.; Avnet, S.; Lemma, S.; Baldini, N. Prominent role of RAB39A-RXRB axis in cancer development and stemness. Oncotarget 2018, 9, 9852–9866. [Google Scholar] [CrossRef][Green Version]
- Gambarte Tudela, J.; Capmany, A.; Romao, M.; Quintero, C.; Miserey-Lenkei, S.; Raposo, G.; Goud, B.; Damiani, M.T. The late endocytic Rab39a GTPase regulates the interaction between multivesicular bodies and chlamydial inclusions. J. Cell Sci. 2015, 128, 3068–3081. [Google Scholar] [CrossRef]
- Seto, S.; Tsujimura, K.; Koide, Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic 2011, 12, 407–420. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Z.; Wang, Y.; Gao, S.; Li, Q.; Li, C.; Xu, W.; Chen, J.; Liu, T.; Song, Z.; et al. MiR-5100 increases the cisplatin resistance of the lung cancer stem cells by inhibiting the Rab6. Mol. Carcinog. 2018, 57, 419–428. [Google Scholar] [CrossRef]
- Hill, E.; Clarke, M.; Barr, F.A. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 2000, 19, 5711–5719. [Google Scholar] [CrossRef]
- Luo, M.-L.; Gong, C.; Chen, C.-H.; Lee, D.Y.; Hu, H.; Huang, P.; Yao, Y.; Guo, W.; Reinhardt, F.; Wulf, G.; et al. Prolyl isomerase Pin1 acts downstream of miR200c to promote cancer stem-like cell traits in breast cancer. Cancer Res. 2014, 74, 3603–3616. [Google Scholar] [CrossRef]
- Rustighi, A.; Zannini, A.; Tiberi, L.; Sommaggio, R.; Piazza, S.; Sorrentino, G.; Nuzzo, S.; Tuscano, A.; Eterno, V.; Benvenuti, F.; et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol. Med. 2014, 6, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.-L.; Gong, C.; Chen, C.-H.; Hu, H.; Huang, P.; Zheng, M.; Yao, Y.; Wei, S.; Wulf, G.; Lieberman, J.; et al. The Rab2A GTPase Promotes Breast Cancer Stem Cells and Tumorigenesis via Erk Signaling Activation. Cell Rep. 2015, 11, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhang, J.; Fan, X.; Yuan, F.; Jiang, Y.; Lv, R.; Ma, Y. Downregulation of Rab27A contributes to metformin-induced suppression of breast cancer stem cells. Oncol Lett. 2017, 14, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Jiang, Y.; Lu, H.; Lu, X.; Wang, S.; Wang, L.; Wei, M.; Lu, W.; Du, Z.; Ye, Z.; et al. Rab27A mediated by NF-κB promotes the stemness of colon cancer cells via up-regulation of cytokine secretion. Oncotarget 2016, 7, 63342–63351. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Cheng, H.-C.; Wang, Y.-S.; Lin, P.; Jen, J.; Kuo, I.-Y.; Chang, Y.-H.; Liao, P.-C.; Chen, R.-H.; Yuan, W.-C.; et al. Small GTPase Rab37 targets tissue inhibitor of metalloproteinase 1 for exocytosis and thus suppresses tumour metastasis. Nat. Commun. 2014, 5, 4804. [Google Scholar] [CrossRef]
- Tzeng, H.-T.; Tsai, C.-H.; Yen, Y.-T.; Cheng, H.-C.; Chen, Y.-C.; Pu, S.-W.; Wang, Y.-S.; Shan, Y.-S.; Tseng, Y.-L.; Su, W.-C.; et al. Dysregulation of Rab37-Mediated Cross-talk between Cancer Cells and Endothelial Cells via Thrombospondin-1 Promotes Tumor Neovasculature and Metastasis. Clin. Cancer Res. 2017, 23, 2335–2345. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kuo, I.-Y.; Lu, P.-J.F.; Tzeng, H.-T.; Lai, W.-W.; Su, W.-C.; Wang, Y.-C. Rab37 mediates exocytosis of secreted frizzled-related protein 1 to inhibit Wnt signaling and thus suppress lung cancer stemness. Cell Death Dis. 2018, 9, 868. [Google Scholar] [CrossRef]
- Xue, H.; Tian, G.-Y. MiR-429 regulates the metastasis and EMT of HCC cells through targeting RAB23. Arch. Biochem. Biophys. 2018, 637, 48–55. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; You, W.; Li, P.; Kuang, Y. Rab23 Promotes Hepatocellular Carcinoma Cell Migration Via Rac1/TGF-β Signaling. Pathol. Oncol. Res. 2020, 26, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Wei, W.-C.; Hung, C.-N.; Kuo, J.-F.; Hsu, C.-P.; Chang, K.-J.; Chao, W.-T. Rab11 collaborates E-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma. Eur. J. Clin. Investig. 2016, 46, 1002–1011. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Wei, W.-C.; Huang, S.-H.; Shih, C.-M.; Hsu, C.-P.; Chang, K.-J.; Chao, W.-T. Rab11 regulates E-cadherin expression and induces cell transformation in colorectal carcinoma. BMC Cancer 2014, 14, 587. [Google Scholar] [CrossRef]
- Zou, C.; Fan, J.; He, M.; Xu, Y.; Wang, K.; Cai, Y.; Li, M. Epigenetic silencing of Rab39a promotes epithelial to mesenchymal transition of cervical cancer through AKT signaling. Exp. Cell Res. 2019, 378, 139–148. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Ding, J.; Wang, J.; Zhang, J. Downregulation of Rab17 promotes cell proliferation and invasion in non-small cell lung cancer through STAT3/HIF-1α/VEGF signaling. Thorac Cancer 2020, 11, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, J.; Wang, X.; Liu, F.; Jiang, B.; Zhang, Y. Targeting Rabs as a novel therapeutic strategy for cancer therapy. Drug Discov Today 2017, 22, 1139–1147. [Google Scholar] [CrossRef]
- Gray, J.L.; von Delft, F.; Brennan, P.E. Targeting the Small GTPase Superfamily through Their Regulatory Proteins. Angew. Chem. 2020, 59, 6342–6366. [Google Scholar] [CrossRef]
- Spiegel, J.; Cromm, P.M.; Itzen, A.; Goody, R.S.; Grossmann, T.N.; Waldmann, H. Direct Targeting of Rab-GTPase-Effector Interactions. Angew. Chem. 2014, 53, 2498–2503. [Google Scholar] [CrossRef]
- Mitra, S.; Montgomery, J.E.; Kolar, M.J.; Li, G.; Jeong, K.J.; Peng, B.; Verdine, G.L.; Mills, G.B.; Moellering, R.E. Stapled peptide inhibitors of RAB25 target context-specific phenotypes in cancer. Nat. Commun. 2017, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Ramadass, M.; He, J.; Brown, S.J.; Zhang, J.; Abgaryan, L.; Biris, N.; Gavathiotis, E.; Rosen, H.; Catz, S.D. Identification of Neutrophil Exocytosis Inhibitors (Nexinhibs), Small Molecule Inhibitors of Neutrophil Exocytosis and Inflammation: DRUGGABILITY OF THE SMALL GTPase Rab27a. J. Biol. Chem. 2016, 291, 25965–25982. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).