Endobronchial Ultrasound Staging During Navigation Bronchoscopy for Peripheral Pulmonary Nodules in the Real World: Which Patients Will Benefit?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
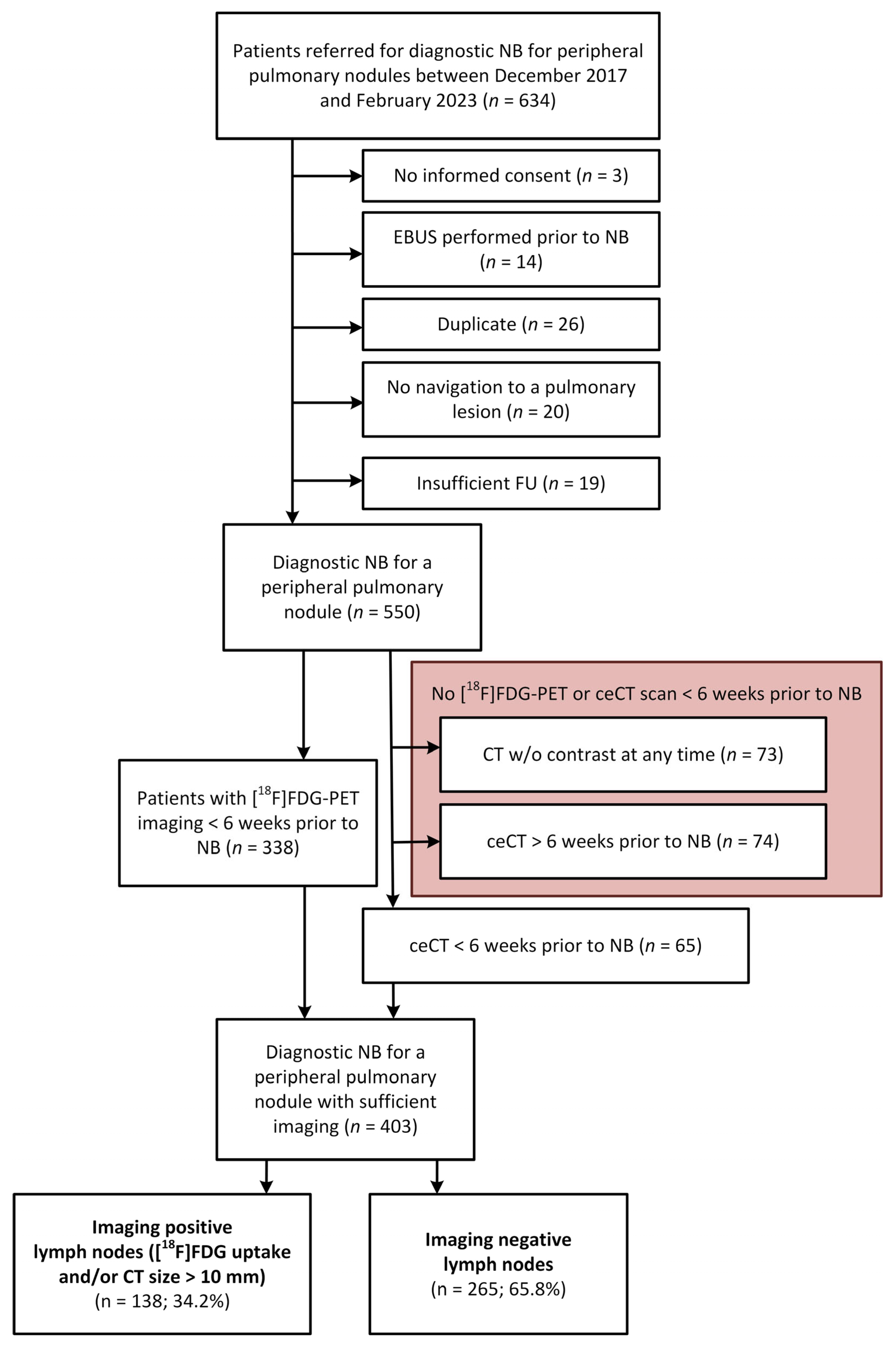
2.1. Data Collection and Case Selection Criteria
2.2. Patient Selection and Imaging Criteria for EBUS Performance Analysis
2.3. Diagnostic Procedures
2.4. Data Analysis
3. Results
3.1. EBUS, Imaging and Pathological Outcomes in All Patients
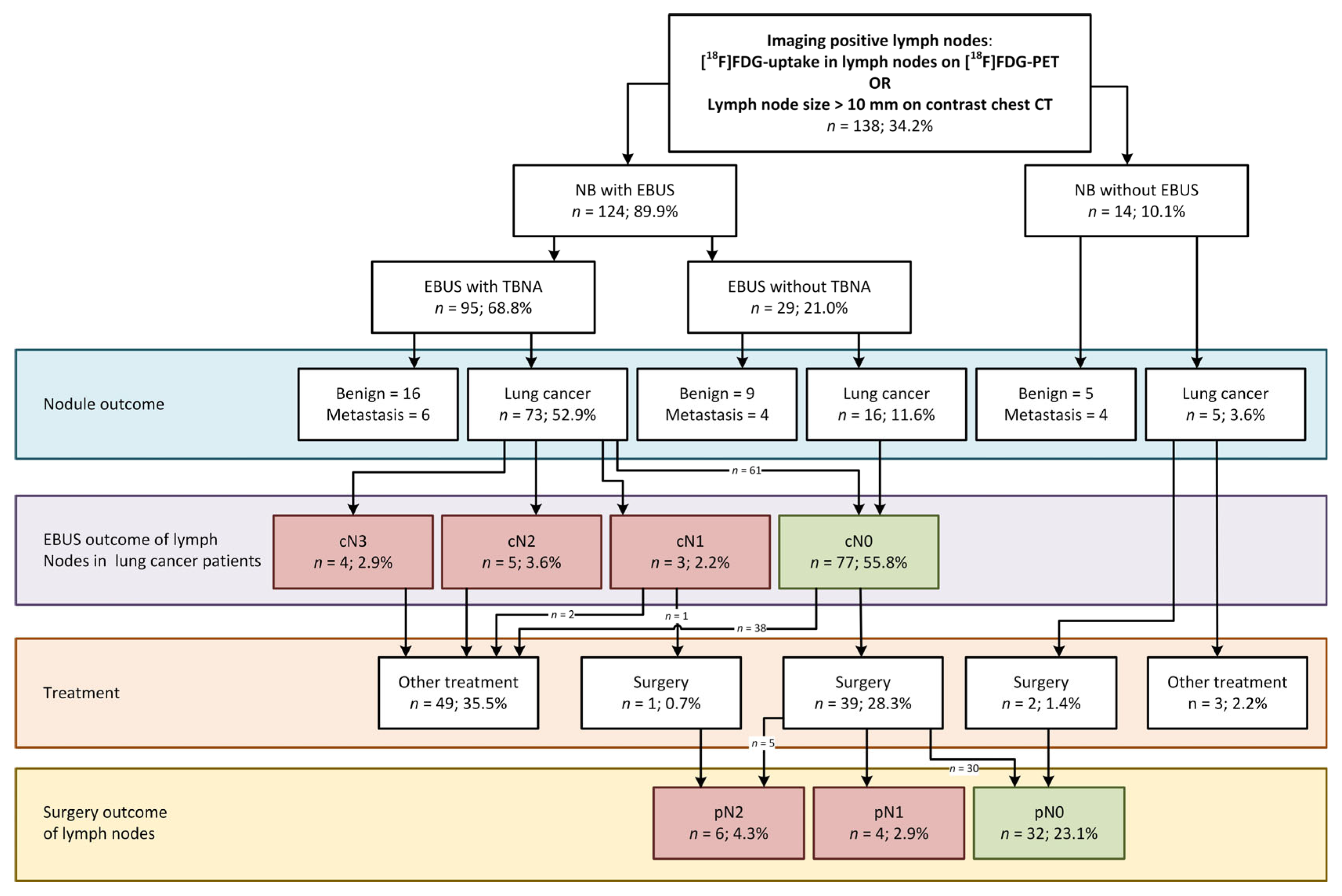
3.2. EBUS in the Cohort of Navigation Bronchoscopy Patients with Imaging-Positive Lymph Nodes
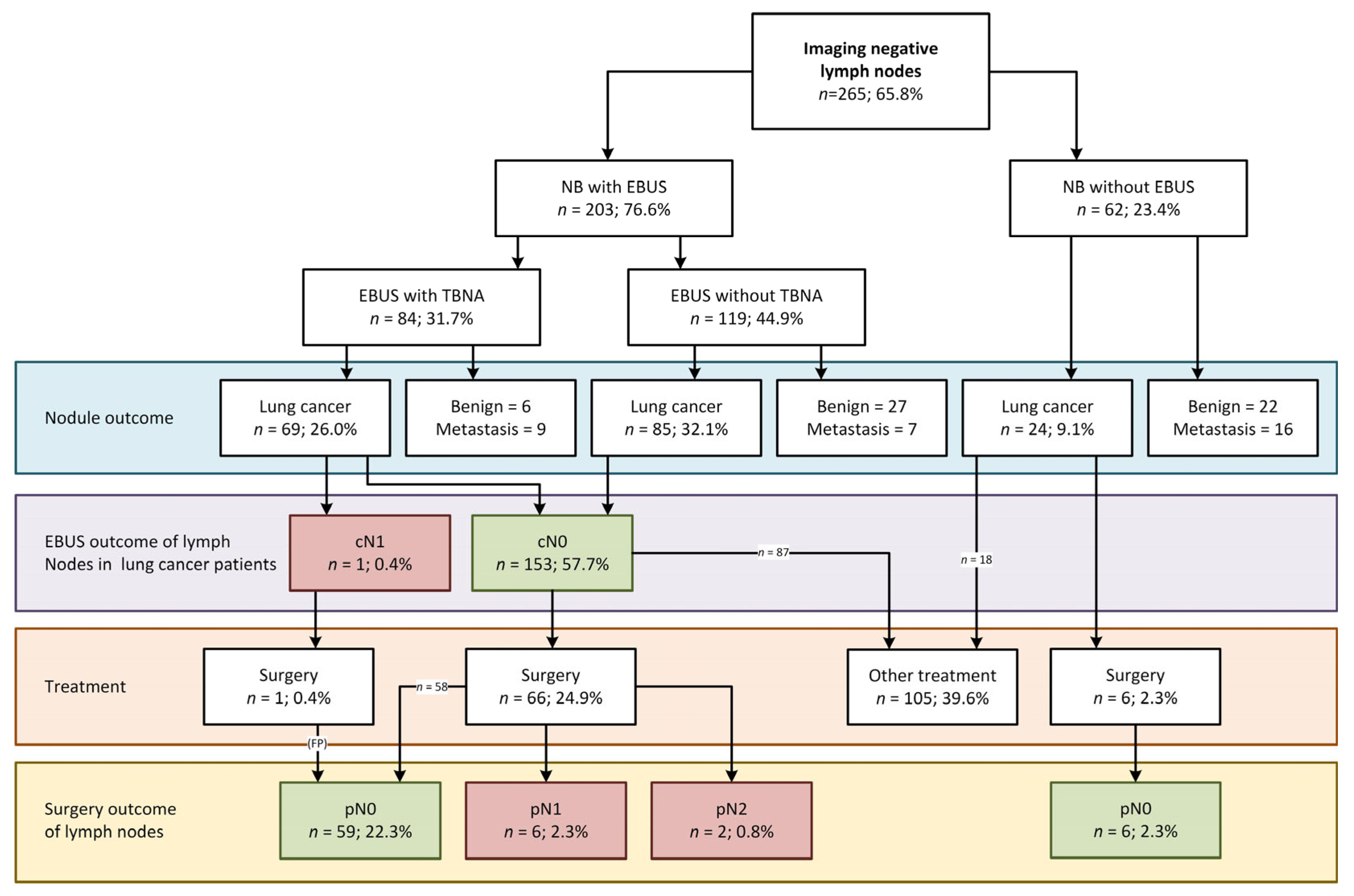
3.3. EBUS in the Cohort of Navigation Bronchoscopy Patients with Imaging-Negative Lymph Nodes
3.4. Surgically Verified EBUS Performance
4. Discussion
4.1. Accuracy of Imaging to Predict Lymph Node Involvement as Found by EBUS and/or Surgery
4.2. Accuracy of Imaging to Predict if EBUS Will Detect Lymph Node Involvement
4.3. Strengths and Limitations
4.4. Guidelines and Unexpected Nodal Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAH | Atypical Adenomatous Hyperplasia |
| AC | Adenocarcinoma |
| ACIS | Adeno Carcinoma in Situ |
| BMI | Body Mass Index |
| CBCT | Cone Beam Computed Tomography |
| ceCT | Contrast-enhanced Computed Tomography |
| CT | Computed Tomography |
| EBUS | Endobronchial Ultrasound |
| EBUS-TBNA | EBUS Transbronchial Needle Aspiration |
| [18F]FDG | 2′-[18F]fluoro-2′deoxyglucose |
| FU | Follow-up |
| MIA | Minimally Invasive Adenocarcinoma |
| IQR | Interquartile Range |
| NB | Navigation Bronchoscopy |
| NNT | Number Needed to Treat |
| NOS | Not Otherwise Specified |
| NPV | Negative Predictive Value |
| NSCLC | Non-Small Cell Lung Cancer |
| PET | Positron Emission Tomography |
| PPV | Positive Predictive Value |
| ROSE | Rapid On-Site Evaluation |
| SABR | Stereotactic ablative radiotherapy |
| SCC | Squamous Cell Carcinoma |
| SCLC | Small Cell Lung Cancer |
| SUVmax | Maximized Standardized Uptake Value |
Appendix A
Appendix A.1
- NNT calculation details
- EBUS performance analysis
- Imaging performance analysis for all EBUS and surgery patients
| Calculation of Number of EBUS Procedures Needed During NB to Prevent One Unexpected pN+ Disease During Surgery as Stated in Table 3, Numbers Derived from Figure 2 and Figure 3. | ||
|---|---|---|
| NNT (all patients) | ||
α one lymph node metastases from a colon cancer metastasis in the lung | ||
| NNT (iN+ subgroup) | ||
α one lymph node metastases from a colon cancer metastasis in the lung | ||
| NNT (iN+- sub-group) | ||
β The false negative lymph node is not taken into account | ||
| Calculation of number of EBUS procedures needed during NB to prevent one unexpected pN+ disease during surgery, in the subgroup of proven lung cancer patients only, as stated in online Table A2, numbers derived from Figure 2 and Figure 3. | ||
| NNT after final diagnosis (lung cancer patients) | ||
| NNT after final diagnosis (iN+ lung cancer patients) | ||
Appendix B
| Accuracy of EBUS in Surgically Treated cN0 Lung Cancer Patients (See Main Manuscript for cTNM and pTNM Details, Median Tumor Size 17 mm) | |||
|---|---|---|---|
| Lung cancer patient subgroup: NB+EBUS with surgical confirmation (n = 118) | Pathology N-status | ||
| pN+ | pN- | Total | |
| EBUS N+ | 12 | 1 a | 13 |
| EBUS N−, all (accessible b) EBUS N−, surgery < 6w after NB (accessible b) c | 17 (15) 12 (10) | 88 | 105 |
| Total | 29 | 89 | 118 |
| Sensitivity (all) | 12/(12 + 17) | 41.4% | |
| Sensitivity (EBUS accessible) | 12/(12 + 15) | 44.4% | |
| Sensitivity (surgery < 6w after NB) | 12/(12 + 12) | 50.0% | |
| Sensitivity (surgery < 6w after NB + EBUS accessible) | 12/(12 + 10) | 54.5% | |
| Specificity | 88/(1 + 88) | 98.9% | |
| PPV | 12/(12 + 1) | 92.3% | |
| NPV (all) | 88/(17 + 88) | 83.8% | |
| NPV (EBUS accessible) | 88/(15 + 88) | 85.4% | |
| NPV (surgery < 6w after NB) | 88/(12 + 88) | 88.0% | |
| NPV (surgery < 6w after NB + EBUS accessible) | 88/(10 + 88) | 89.8% | |
| Overall accuracy | (12 + 88)/118 | 84.7% | |
| NNT overall | 10 | ||
| NNT iN+ subgroup | 4 | ||
| NNT iN− subgroup | ∞ | ||
Appendix C
| Accuracy of Nodal Imaging in Patients for Lymph Nodal Involvement; EBUS and Surgical Staging Combined | |||
|---|---|---|---|
| Imaging status of all lung cancer patients with EBUS and/or surgery (n = 251) | N-status (EBUS + surgery) | ||
| N+ | N- | Total | |
| iN+ | 21 | 70 | 91 |
| iN− | 8 | 152 | 160 |
| Total | 29 | 222 | 251 |
| Sensitivity | 21/(21 + 8) | 72.4% | |
| Specificity | 199/(199 + 107) | 68.5% | |
| PPV | 21/(21 + 70) | 23.1% | |
| NPV | 152/(8 + 152) | 95.0% | |
| Overall accuracy | (21 + 152)/251 | 68.9% | |
References
- Hendrix, W.; Rutten, M.; Hendrix, N.; van Ginneken, B.; Schaefer-Prokop, C.; Scholten, E.T.; Prokop, M.; Jacobs, C. Trends in the incidence of pulmonary nodules in chest computed tomography: 10-year results from two Dutch hospitals. Eur. Radiol. 2023, 33, 8279–8288. [Google Scholar] [CrossRef] [PubMed]
- Callister, M.E.J.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: Accredited by NICE. Thorax 2015, 70, ii1–ii54. [Google Scholar] [CrossRef] [PubMed]
- Herder, G.J.; van Tinteren, H.; Golding, R.P.; Kostense, P.J.; Comans, E.F.; Smit, E.F.; Hoekstra, O.S. Clinical Prediction Model To Characterize Pulmonary Nodules: Validation and Added Value of 18 F-Fluorodeoxyglucose Positron Emission Tomography. Chest 2005, 128, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.; Dai, H.; Puri, A.; Liao, J.; Guo, S. Advancements in navigational bronchoscopy for peripheral pulmonary lesions: A review with special focus on virtual bronchoscopic navigation. Front. Med. 2022, 9, 989184. [Google Scholar] [CrossRef]
- Verhoeven, R.L.J.; Vos, S.; van der Heijden, E. Multi-modal tissue sampling in cone beam CT guided navigation bronchoscopy: Comparative accuracy of different sampling tools and rapid on-site evaluation of cytopathology. J. Thorac. Dis. 2021, 13, 4396–4406. [Google Scholar] [CrossRef]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef]
- Pritchett, M.A.; Schampaert, S.; de Groot, J.A.H.; Schirmer, C.C.; van der Bom, I. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J. Bronchol. Interv. Pulmonol. 2018, 25, 274–282. [Google Scholar] [CrossRef]
- Lentz, R.J.; Frederick-Dyer, K.; Planz, V.; Koyama, T.; Aboudara, M.; Swanner, B.; Roller, L.; Low, S.; Salmon, C.; Avasarala, S.K.; et al. Navigational Bronchoscopy Versus Computed Tomography-guided Transthoracic Needle Biopsy for the Diagnosis of Indeterminate Lung Nodules: Initial Results From the VERITAS Multicenter Randomized Trial. Am. J. Respir. Crit. Care Med. 2024, 209, A6665. [Google Scholar]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef]
- Bousema, J.E.; Dijkgraaf, M.G.W.; van der Heijden, E.; Verhagen, A.; Annema, J.T.; van den Broek, F.J.C.; group, M.E.s. Endosonography With or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial. J. Clin. Oncol. 2023, 41, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef]
- Vilmann, P.; Clementsen, P.F.; Colella, S.; Siemsen, M.; De Leyn, P.; Dumonceau, J.M.; Herth, F.J.; Larghi, A.; Vazquez-Sequeiros, E.; Hassan, C.; et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015, 47, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Gould, M.K.; Margolis, M.L.; Tanoue, L.T.; McCrory, D.; Toloza, E.; Detterbeck, F.; American College of Chest, P. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007, 132, 178S–201S. [Google Scholar] [CrossRef]
- Beyaz, F.; Verhoeven, R.L.J.; Schuurbiers, O.C.J.; Verhagen, A.; van der Heijden, E. Occult lymph node metastases in clinical N0/N1 NSCLC; A single center in-depth analysis. Lung Cancer 2020, 150, 186–194. [Google Scholar] [CrossRef]
- Khandhar, S.J.; Bowling, M.R.; Flandes, J.; Gildea, T.R.; Hood, K.L.; Krimsky, W.S.; Minnich, D.J.; Murgu, S.D.; Pritchett, M.; Toloza, E.M.; et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: First results of the prospective, multicenter NAVIGATE study. BMC Pulm. Med. 2017, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Yu Lee-Mateus, A.; Reisenauer, J.; Garcia-Saucedo, J.C.; Abia-Trujillo, D.; Buckarma, E.H.; Edell, E.S.; Grage, R.A.; Bowman, A.W.; Labarca, G.; Johnson, M.M.; et al. Robotic-assisted bronchoscopy versus CT-guided transthoracic biopsy for diagnosis of pulmonary nodules. Respirology 2023, 28, 66–73. [Google Scholar] [CrossRef]
- Steinfort, D.P. Systematic mediastinal staging in non-small cell lung cancer: Filling in the guideline evidence gap. Respirology 2023, 29, 89. [Google Scholar] [CrossRef]
- Hoeijmakers, F.; Heineman, D.J.; Beck, N.; Klamer, J.; Tollenaar, R.; Wouters, M.; Schreurs, W.H. Mediastinoscopy for Staging of Non-Small Cell Lung Cancer: Surgical Performance in The Netherlands. Ann. Thorac. Surg. 2019, 107, 1024–1031. [Google Scholar] [CrossRef]
- Gonzalez, A.V.; Silvestri, G.A.; Korevaar, D.A.; Gesthalter, Y.B.; Almeida, N.D.; Chen, A.; Gilbert, C.R.; Illei, P.B.; Navani, N.; Pasquinelli, M.M.; et al. Assessment of Advanced Diagnostic Bronchoscopy Outcomes for Peripheral Lung Lesions: A Delphi Consensus Definition of Diagnostic Yield and Recommendations for Patient-centered Study Designs. An Official American Thoracic Society/American College of Chest Physicians Research Statement. Am. J. Respir. Crit. Care Med. 2024, 209, 634–646. [Google Scholar] [CrossRef]
- Kops, S.E.P.; Heus, P.; Korevaar, D.A.; Damen, J.A.A.; Idema, D.L.; Verhoeven, R.L.J.; Annema, J.T.; Hooft, L.; van der Heijden, E. Diagnostic yield and safety of navigation bronchoscopy: A systematic review and meta-analysis. Lung Cancer 2023, 180, 107196. [Google Scholar] [CrossRef]
- Beyaz, F.; Verhoeven, R.L.J.; Hoogerwerf, N.; Mourisse, J.M.J.; van der Heijden, E. Cone Beam Computed Tomography-guided Navigation Bronchoscopy with Augmented Fluoroscopy for the Diagnosis of Peripheral Pulmonary Nodules: A step-by-step guide. Respiration 2025, 104, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Baldwin, D.; Beckles, M.; Duffy, J.; Entwisle, J.; Faivre-Finn, C.; Kerr, K.; Macfie, A.; McGuigan, J.; Padley, S.; et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010, 65 (Suppl. 3), iii1–iii27. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Evison, M.; Witt, A.; Tsaknis, G.; Kheir, F.; Manners, D.; Madan, K.; Sidhu, C.; Fantin, A.; Korevaar, D.A.; et al. Proposed quality indicators and recommended standard reporting items in performance of EBUS bronchoscopy: An official World Association for Bronchology and Interventional Pulmonology Expert Panel consensus statement. Respirology 2023, 28, 722–743. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public. Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Schmidt-Hansen, M.; Baldwin, D.R.; Hasler, E.; Zamora, J.; Abraira, V.; Roque, I.F.M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst. Rev. 2014, 2014, CD009519. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Zhang, H.; Shi, Y.; Wu, H.; Zhang, J.; Qian, Y.; Li, C.; Yang, J. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int. J. Cancer 2013, 132, E37–E47. [Google Scholar] [CrossRef]
- Serra Mitja, P.; Garcia-Cabo, B.; Garcia-Olive, I.; Radua, J.; Rami-Porta, R.; Esteban, L.; Barreiro, B.; Call, S.; Centeno, C.; Andreo, F.; et al. EBUS-TBNA for mediastinal staging of centrally located T1N0M0 non-small cell lung cancer clinically staged with PET/CT. Respirology 2024, 29, 158–165. [Google Scholar] [CrossRef]
- Cardoso, A.V.; Neves, I.; Magalhaes, A.; Sucena, M.; Barroca, H.; Fernandes, G. The value of rapid on-site evaluation during EBUS-TBNA. Rev Port Pneumol 2015, 21, 253–258. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, H.Y.; Lin, C.Y.; Chen, K.L.; Huang, T.W. High SUVmax Is an Independent Predictor of Higher Diagnostic Accuracy of ROSE in EBUS-TBNA for Patients with NSCLC. J. Pers. Med. 2022, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Shaolei, L.; Nan, L.; Yumei, L.; Shanyuan, Z.; Fangliang, L.; Yue, Y. Occult mediastinal lymph node metastasis in FDG-PET/CT node-negative lung adenocarcinoma patients: Risk factors and histopathological study. Thorac. Cancer 2019, 10, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Marziali, V.; Frasca, L.; Ambrogi, V.; Patirelis, A.; Longo, F.; Crucitti, P. Prognostic significance of uncertain resection for metastasis in the highest mediastinal lymph node after surgery for clinical N0 non-small cell lung cancer. Front. Surg. 2023, 10, 1115696. [Google Scholar] [CrossRef] [PubMed]
- DuComb, E.A.; Tonelli, B.A.; Tuo, Y.; Cole, B.F.; Mori, V.; Bates, J.H.T.; Washko, G.R.; San Jose Estepar, R.; Kinsey, C.M. Evidence for Expanding Invasive Mediastinal Staging for Peripheral T1 Lung Tumors. Chest 2020, 158, 2192–2199. [Google Scholar] [CrossRef]
| Patient Baseline Characteristics, All Included Patients (n = 403) | Frequency | ||
|---|---|---|---|
| Patient characteristics | Age, years, median (±IQR) | 67 (±11) | |
| Sex, n (%) | Male | 205 (50.9%) | |
| Female | 198 (49.1%) | ||
| BMI, median (±IQR) | 24.61 (±5.98) | ||
| Lesion < 2 cm from pleura, n (%) | Yes | 298 (72.2%) | |
| No | 105 (27.8%) | ||
| Radiologic nodule characteristics, n (%) | Solid | 317 (78.6%) | |
| Part-solid | 47 (11.7%) | ||
| GGO | 19 (4.7%) | ||
| Cystic | 20 (5.0%) | ||
| Nodule size, mm on CT, median (±IQR) | 17 (±12) | ||
| Total number of nodules navigated to, n | 504 | ||
| Number of nodules evaluated per patient, n (%) | One nodule | 308 (76.4%) | |
| Two nodules | 89 (22.1%) | ||
| Three nodules | 6 (1.5%) | ||
| Imaging data | [18F]FDG PET availability < 6 weeks prior to NB, n (%) | 338 (83.9%) | |
| Time between [18F]FDG-PET and NB, days, median (±IQR) | 22 days (±14) | ||
| [18F]FDG-uptake in lymph nodes, n (%) | Positive * | 122 (36.1%) | |
| Negative | 216 (63.9%) | ||
| In patients without PET or with PET > 6 weeks prior to NB: CT with contrast only < 6 weeks prior to NB, n (%) | 65 (16.1%) | ||
| Time between CT contrast and NB, days, median (±IQR) | 24 days (±15) | ||
| CT outcome of lymph nodes, n (%) | Positive | 19 (29.2%) | |
| Negative | 46 (70.8%) | ||
| EBUS | EBUS performed, n (%) | Yes | 327 (81.1%) |
| No | 76 (18.9%) | ||
| TBNA performed, n (%) | Yes | 179 (54.7%) | |
| No | 148 (45.3%) | ||
| Number of lymph nodes sampled, median (±IQR) | Imaging-positive group | 2 (±2) | |
| Imaging-negative group | 1 (±1) | ||
| Subgroup analysis in patients with a final diagnosis of lung cancer (n = 272) | Frequency | ||
| Pre-procedural characteristics | CT stage, n (%) | Tis | 5 (1.8%) |
| T1a | 39 (14.3%) | ||
| T1b | 100 (36.8%) | ||
| T1c | 46 (16.9%) | ||
| T2a | 18 (6.6%) | ||
| T2b | 12 (4.4%) | ||
| T3 | 15 (5.5%) | ||
| T4 | 4 (1.5%) | ||
| Multiple lesions (all T) | 33 (12.1%) | ||
| iN-stage (based on PET and or contrast CT imaging), n (%) | iN− | 178 (65.4%) | |
| iN+ | 94 (34.6%) | ||
| Pre-NB/-EBUS imaging-based cN-stage of all iN+ patients, n (%) | cN0 | 56 (59.6%) | |
| cN1 | 18 (19.1%) | ||
| cN2 | 15 (16.0%) | ||
| cN3 | 5 (5.3%) | ||
| Lesion location, n (%) | Right upper lobe | 98 (36.0%) | |
| Right middle lobe | 10 (3.7%) | ||
| Right lower lobe | 38 (14.0%) | ||
| Left upper lobe | 60 (22.1%) | ||
| Left lower lobe | 29 (10.7%) | ||
| Multiple lobes | 37 (13.6%) | ||
| Post-procedural characteristics | Post-NB+/-EBUS cN-stage, n (%) | cN0 | 259 (95.2%) |
| cN1 | 4 (1.5%) | ||
| cN2 | 5 (1.8%) | ||
| cN3 | 4 (1.5%) | ||
| Treatment, n (%) | Surgery | 115 (42.3%) | |
| SABR and/or cyberknife | 105 (38.6%) | ||
| Combination of treatments | 29 (10.7%) | ||
| Best supportive care | 15 (5.5%) | ||
| Immunotherapy | 4 (1.4%) | ||
| Chemotherapy | 3 (1.1%) | ||
| Unknown | 1 (0.4%) | ||
| Neo-adjuvant treatment, n (%) | Yes | 10 (8.7%) | |
| No | 105 (91.3%) | ||
| pN-stage after surgery, n (%) | pN0 | 97 (84.3%) | |
| pN1 | 10 (10.3%) | ||
| pN2 | 8 (8.2%) | ||
| Tumor histology, n (%) | AC (incl. AAH, ACIS, and MIA) | 138 (50.7%) | |
| SCC | 38 (14.0%) | ||
| Unknown (no tissue) | 21 (7.7%) | ||
| Carcinoid | 8 (2.9%) | ||
| NSCLC(-NOS) | 4 (1.5%) | ||
| SCLC | 8 (2.9%) | ||
| Mixed AC-SCC | 2 (0.7%) | ||
| Multiple (synchronous) histologically different lung cancers | 53 (19.5%) | ||
| Accuracy of Nodal Imaging in Navigation Bronchoscopy Patients | |||
|---|---|---|---|
| All NB + EBUS patients (n = 327) | EBUS outcome | ||
| cN+ | cN- | Total | |
| iN+ | 13 1 | 111 | 124 |
| iN− | 1 2 | 202 | 203 |
| Total | 14 | 313 | 327 |
| Sensitivity | 13/(13 + 1) | 92.9% | |
| Specificity | 202/(111 + 202) | 64.5% | |
| PPV | 13/(13 + 111) | 10.5% | |
| NPV | 202/(1 + 202) | 99.5% | |
| Overall accuracy | (13 + 202)/327 | 65.7% | |
| NNT overall | 25 | ||
| NNT iN+ subgroup | 10 | ||
| NNT iN− subgroup | ∞ | ||
| Lymph Node Station (#) | 2L | 4L | 10L | 11L | ≥12L | 3 | 5 | 6 | 7 | 8 | 9 | 2R | 4R | 10R | 11Ri | 11Rs | ≥12R | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of times reported and measured, n | 7 | 239 | 12 | 218 | 0 | 0 | 0 | 0 | 253 | 0 | 0 | 14 | 235 | 53 | 145 | 201 | 0 | 1377 |
| Average short axis on EBUS (mm) | 3.60 | 4.40 | 5.05 | 5.35 | - | - | - | - | 6.20 | - | - | 4.90 | 4.95 | 4.60 | 5.30 | 5.40 | - | 5.10 |
| Number of times sampled, n | 0 | 41 | 2 | 64 | 83 | 5 | 59 | 6 | 29 | 71 | 360 | |||||||
| Benign, n | 0 | 20 | 2 | 41 | 66 | 3 | 41 | 2 | 22 | 45 | 242 | |||||||
| Insufficient specimen, n | 0 | 16 | 0 | 22 | 16 | 2 | 13 | 3 | 6 | 21 | 99 | |||||||
| Malignant, n | 0 | 5 | 0 | 1 | 1 | 0 | 5 | 1 | 1 | 5 | 19 | |||||||
| Malignant after surgery (pN+), n | 0 | 1 | 0 | 3 | 5 | 0 | 0 | 1 | 3 | 1 | 0 | 1 | 4 | 4 | 5 * | 6 | 34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ter Woerds, D.K.M.; Verhoeven, R.L.J.; Verhagen, A.F.T.M.; Aarntzen, E.H.J.G.; van der Heijden, E.H.F.M. Endobronchial Ultrasound Staging During Navigation Bronchoscopy for Peripheral Pulmonary Nodules in the Real World: Which Patients Will Benefit? Cancers 2025, 17, 1700. https://doi.org/10.3390/cancers17101700
ter Woerds DKM, Verhoeven RLJ, Verhagen AFTM, Aarntzen EHJG, van der Heijden EHFM. Endobronchial Ultrasound Staging During Navigation Bronchoscopy for Peripheral Pulmonary Nodules in the Real World: Which Patients Will Benefit? Cancers. 2025; 17(10):1700. https://doi.org/10.3390/cancers17101700
Chicago/Turabian Styleter Woerds, Desi K. M., Roel L. J. Verhoeven, Ad F. T. M. Verhagen, Erik H. J. G. Aarntzen, and Erik H. F. M. van der Heijden. 2025. "Endobronchial Ultrasound Staging During Navigation Bronchoscopy for Peripheral Pulmonary Nodules in the Real World: Which Patients Will Benefit?" Cancers 17, no. 10: 1700. https://doi.org/10.3390/cancers17101700
APA Styleter Woerds, D. K. M., Verhoeven, R. L. J., Verhagen, A. F. T. M., Aarntzen, E. H. J. G., & van der Heijden, E. H. F. M. (2025). Endobronchial Ultrasound Staging During Navigation Bronchoscopy for Peripheral Pulmonary Nodules in the Real World: Which Patients Will Benefit? Cancers, 17(10), 1700. https://doi.org/10.3390/cancers17101700






