Squamous Cell Carcinoma and Its Rare Variant, Carcinoma Cuniculatum: Insights and Case Studies
Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Clinical Presentation
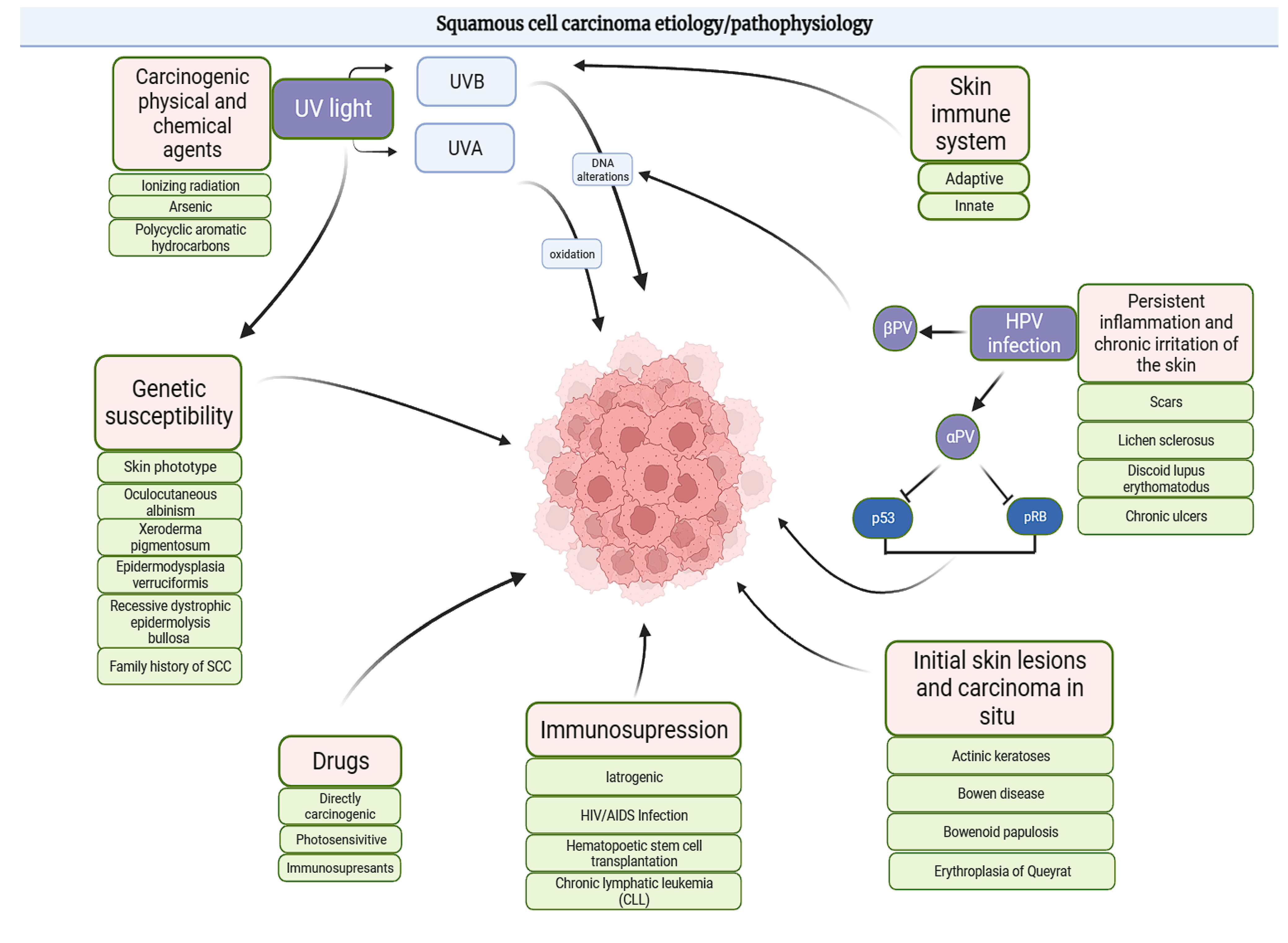
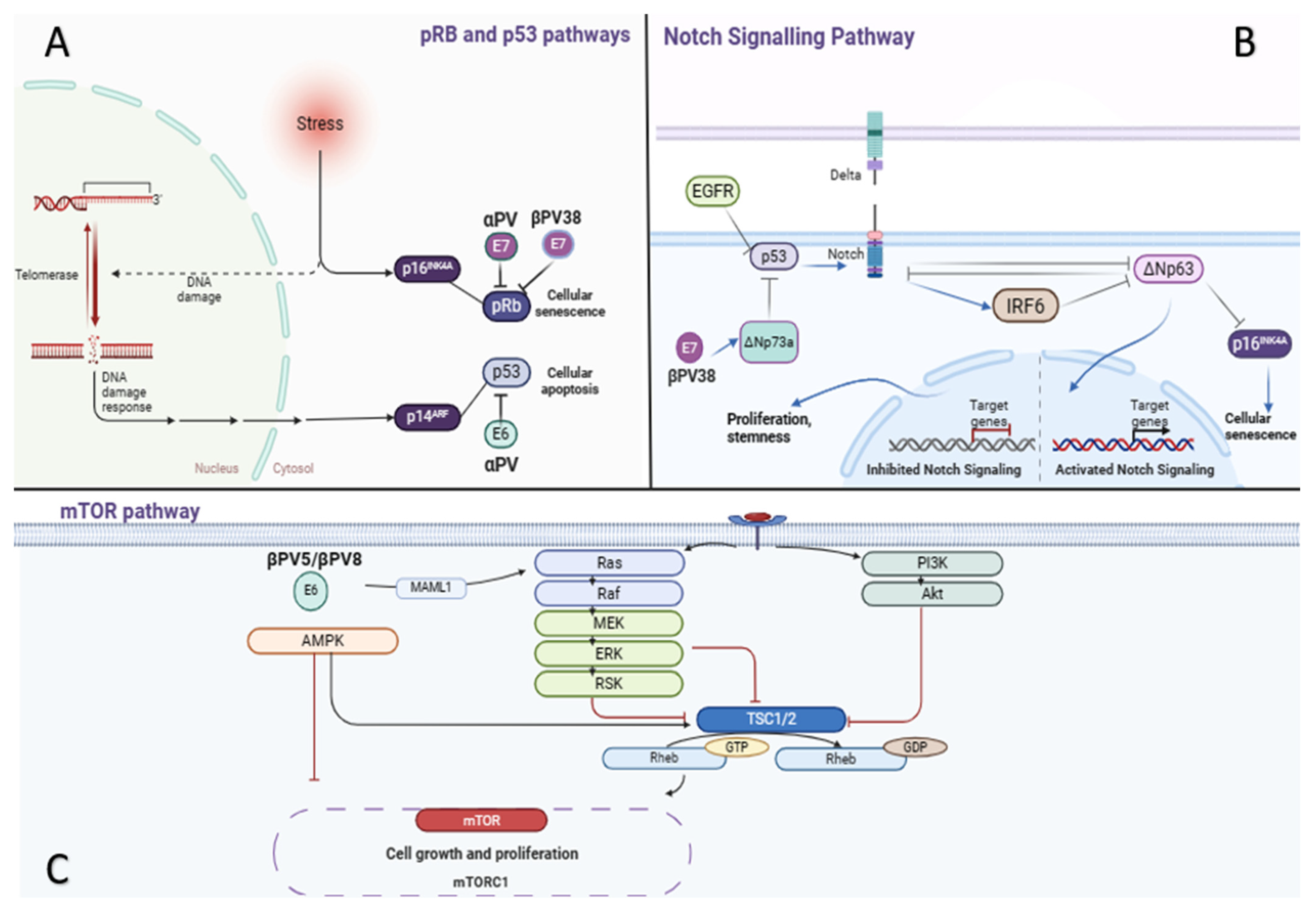
4. Pathogenesis
- Oral cavity (oral florid papillomatosis);
- Urogenital regions (giant condyloma acuminatum Buschke-Löwenstein);
- Surgical sites (amputation stumps);
- Sole of the foot (epithelioma cuniculatum, EC; also referred to as CC).
- Ball of the foot: 53% of cases;
- Toes: 21%;
- Heel: 16%.
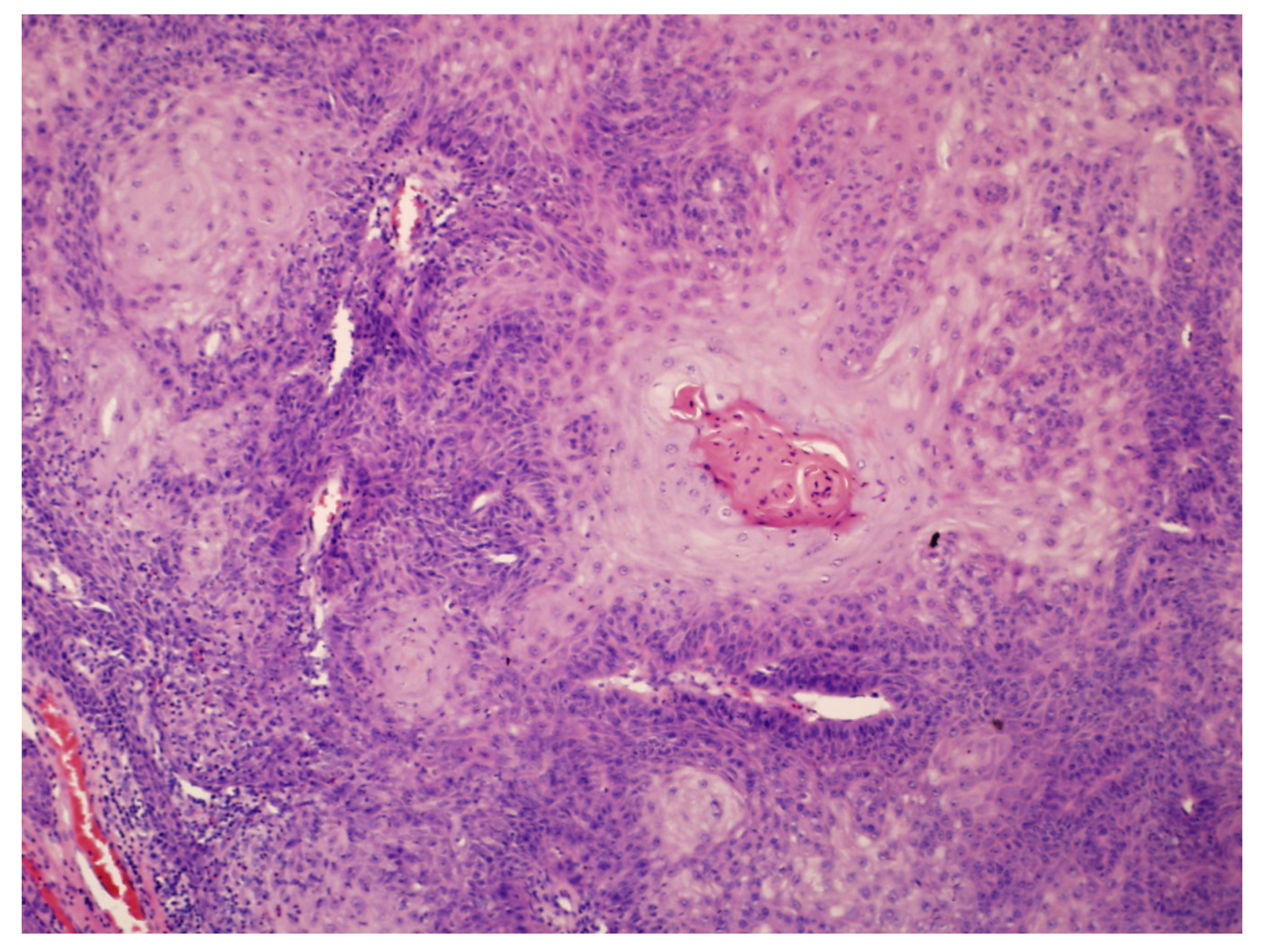
- Hyperkeratosis, papillomatosis, and acanthosis;
- Broad, blunt-ended rete ridges with a characteristic “bulldozing” architectural pattern;
- Minimal cellular atypia, though pleomorphic changes and eosinophilic cytoplasm may be observed, differentiating EC from common warts and conventional SCC.
SCC and CC Risk Factors
- Depletion of Langerhans cells in the epidermis;
- Impaired antigen presentation in skin-draining lymph nodes;
- Hindered tumor surveillance due to the expansion of regulatory T cells;
- A shift towards T-helper type 2 responses.
- Pharmacological treatments [44]:
- Prolonged use of photosensitizing drugs, such as antibiotics, fluoroquinolones, and triazole antifungals, increases SCC risk;
- Long-term UVA therapy for chronic skin conditions further exacerbates the risk, particularly in immunosuppressed individuals and those with sun-sensitive skin types.
- Immunosuppression [45]:
- Immunosuppressed patients, such as organ transplant recipients or individuals on long-term immunosuppressive therapy, show a marked increase in CC risk. This elevated risk likely results from impaired tumor surveillance and a higher incidence of chronic infections, including HPV.
- Chronic inflammation and carcinogens [46]:
- Occupational and environmental exposures to hydrocarbons, arsenic, or polycyclic aromatic hydrocarbons stimulate carcinogenic pathways, even without UV damage, underscoring the environmental contribution to CC pathogenesis.
- Genetic factors:
- ▪ Recessive dystrophic epidermolysis bullosa (RDEB) caused by COL7A1 mutations, leading to aggressive SCC;
- ▪ Other syndromes, such as Lynch syndrome, Muir–Torre syndrome, xeroderma pigmentosum, and Fanconi anemia, impair DNA repair and genomic stability, predisposing individuals to SCC.
5. The Role of HPV Infection
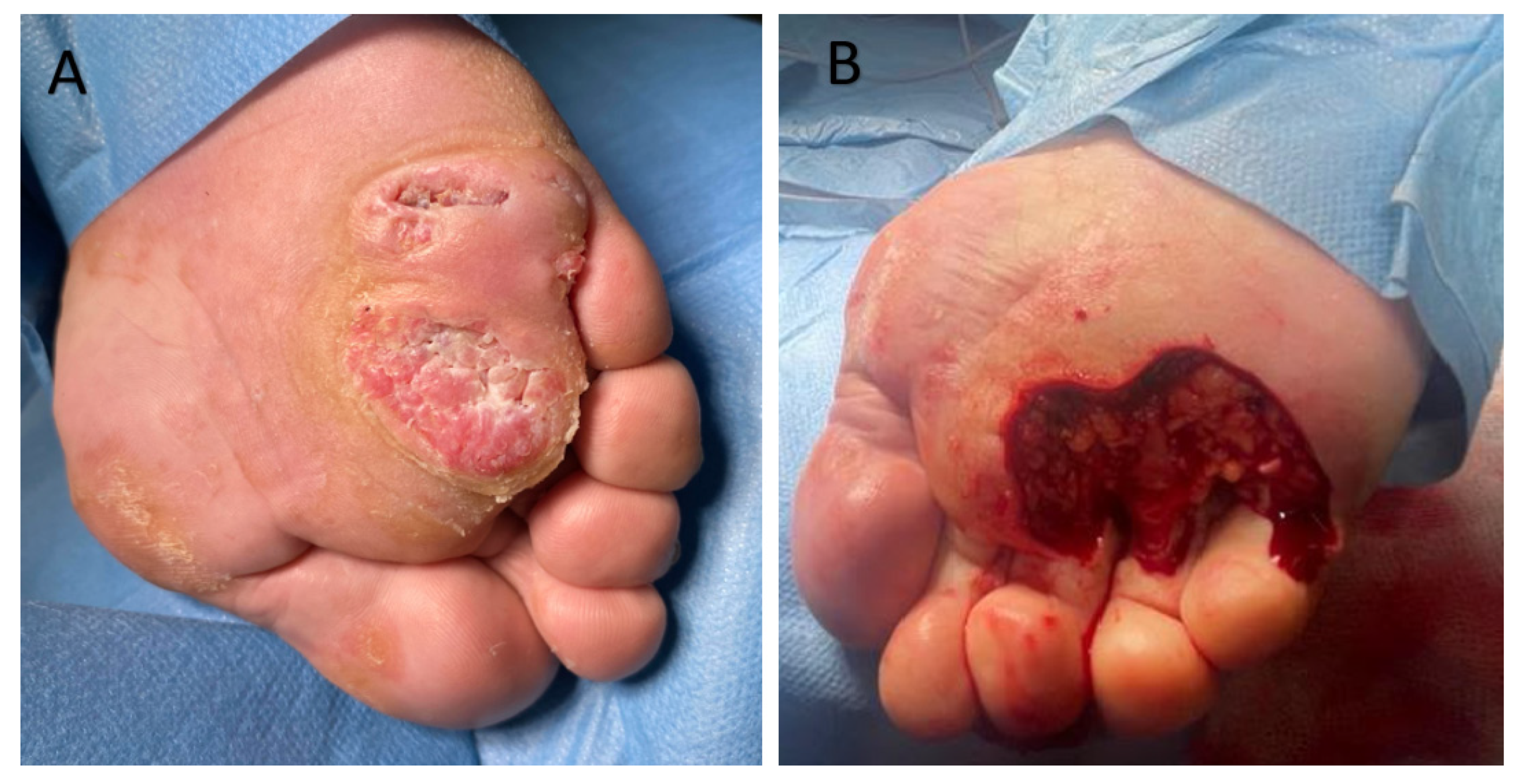
6. Clinical Cases
6.1. Clinical Case 1
6.2. Clinical Case 2
6.3. Molecular Pathway Speculation in Carcinoma Cuniculatum Case Studies
7. Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Incidence, Risk Factors, Diagnosis, and Staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kao, G.F.; Graham, J.H.; Helwig, E.B. Carcinoma Cuniculatum (Verrucous Carcinoma of the Skin): A Clinicopathologic Study of 46 Cases with Ultrastructural Observations. Cancer 1982, 49, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A. Verrucous Carcinoma of the Skin and Mucosa. J. Am. Acad. Dermatol. 1995, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Comune, R.; Ruggiero, A.; Portarapillo, A.; Villani, A.; Megna, M.; Tamburrini, S.; Masala, S.; Sica, G.; Sandomenico, F.; Bortolotto, C.; et al. Cutaneous Squamous Cell Carcinoma: From Diagnosis to Follow-Up. Cancers 2024, 16, 2960. [Google Scholar] [CrossRef]
- Wunderlich, K.; Suppa, M.; Gandini, S.; Lipski, J.; White, J.M.; Del Marmol, V. Risk Factors and Innovations in Risk Assessment for Melanoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma. Cancers 2024, 16, 1016. [Google Scholar] [CrossRef]
- Daniel, S.S.; Cox, S.V.; Kraus, C.N.; Elsensohn, A.N. Epithelioma Cuniculatum (Plantar Verrucous Carcinoma): A Systematic Review of Treatment Options. Cutis 2023, 111, E19–E24. [Google Scholar] [CrossRef]
- Ramesh, U.; Chiang, E.; Stafford, H.; Buell, J.; Materia, F.; Amit, M.; Yaniv, D. Cutaneous Squamous Cell Carcinoma of the Head and Neck: Pathological Features and What They Mean for Prognosis and Treatment. Cancers 2024, 16, 2866. [Google Scholar] [CrossRef]
- Bieniek, A.; Cisło, M.; Matusiak, Ł.; Woźniak, Z.; Maj, J.; Barancewicz-Łosek, M.; Szybejko-Machaj, G. Rak Brodawkujacy (Carcinoma Verrucosum)—Przeglad Objawów Klinicznych i Histologicznych. Postep. Dermatol. Alergol. 2006, 23, 57–66. [Google Scholar]
- Al Rudaisat, M.; Cheng, H. Dermoscopy Features of Cutaneous Warts. Int. J. Gen. Med. 2021, 14, 9903–9912. [Google Scholar] [CrossRef]
- Jordan Witchey, D.; Brianne Witchey, N.; Roth-Kauffman, M.M.; Kevin Kauffman, M. Plantar Warts: Epidemiology, Pathophysiology, and Clinical Management. J. Am. Osteopath. Assoc. 2018, 118, 92–105. [Google Scholar] [CrossRef]
- Zargaran, M.; Baghaei, F. A Clinical, Histopathological and Immunohistochemical Approach to the Bewildering Diagnosis of Keratoacanthoma. J. Dent. 2014, 15, 91–97. [Google Scholar]
- Tandel, J.J.; Polra, R.V.; Pillai, D.; Nair, P.A. Dermatoscopy of Solitary Keratoacanthoma. Indian J. Dermatol. 2022, 67, 628. [Google Scholar] [CrossRef] [PubMed]
- Tsiogka, A.; Koumaki, D.; Kyriazopoulou, M.; Liopyris, K.; Stratigos, A.; Gregoriou, S. Eccrine Porocarcinoma: A Review of the Literature. Diagnostics 2023, 13, 1431. [Google Scholar] [CrossRef]
- Zalaudek, I. Amelanotic Melanoma. In Dermatoscopy of Non-Pigmented Skin Tumors: Pink-Think-Blink; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Koch, S.E.; Lange, J.R. Amelanotic Melanoma: The Great Masquerader. J. Am. Acad. Dermatol. 2000, 42, 731–734. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Steimle-Grauer, S.A.; Hein, R. Cutaneous Sarcomas. JDDG J. Der Dtsch. Dermatol. Ges. 2017, 15, 630–648. [Google Scholar] [CrossRef]
- Salerni, G.; Alonso, C.; Sanchez-Granel, G.; Gorosito, M. Dermoscopic Findings in an Early Malignant Fibrous Histiocytoma on the Face. Dermatol. Pr. Concept. 2017, 7, 44–46. [Google Scholar] [CrossRef]
- Di Brizzi, E.V.; Moscarella, E.; Piana, S.; Longo, C.; Franco, R.; Alfano, R.; Argenziano, G. Clinical and Dermoscopic Features of Pleomorphic Dermal Sarcoma. Australas. J. Dermatol. 2019, 60, E153–E154. [Google Scholar] [CrossRef]
- Joyce, K.; Leonard, N.; Theopold, C. Aggressive Digital Papillary Adenocarcinoma Mimicking a Giant Cell Tumour—A Case Report and Review of the Literature. Cureus 2020, 12, e9531. [Google Scholar] [CrossRef]
- Behera, B.; Kumari, R.; Gochhait, D.; Ayyanar, P. Dermoscopy of Pseudoepitheliomatous Hyperplasia Tattoo Reaction Pattern. Dermatol. Pr. Concept. 2022, 12, e2022121. [Google Scholar] [CrossRef]
- Zayour, M.; Lazova, R. Pseudoepitheliomatous Hyperplasia: A Review. Am. J. Dermatopathol. 2011, 33, 112–126. [Google Scholar] [CrossRef]
- Behera, B.; Jain, S.; Mohapatra, L.; Masatkar, V.; Panda, S. A Clinico-Histopathological Study of Lupus Vulgaris at a Tertiary Care Centre. Cureus 2023, 15, e42710. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Srivastava, P.; Khunger, N. Diascopy and Dermatoscopy of Lupus Vulgaris: The Tale of ‘Apple-Jelly’ Nodules. Indian J. Postgrad. Dermatol. 2022, 2, 54–56. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.M. Non-Melanoma Skin Cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Radiation; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2014; Volume 100D. [Google Scholar]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef]
- Wang, J.; Aldabagh, B.; Yu, J.; Arron, S.T. Role of Human Papillomavirus in Cutaneous Squamous Cell Carcinoma: A Meta-Analysis. J. Am. Acad. Dermatol. 2014, 70, 621–629. [Google Scholar] [CrossRef]
- Asgari, M.M.; Kiviat, N.B.; Critchlow, C.W.; Stern, J.E.; Argenyi, Z.B.; Raugi, G.J.; Berg, D.; Odland, P.B.; Hawes, S.E.; De Villiers, E.M. Detection of Human Papillomavirus DNA in Cutaneous Squamous Cell Carcinoma among Immunocompetent Individuals. J. Investig. Dermatol. 2008, 128, 1409–1417. [Google Scholar] [CrossRef]
- Arron, S.T.; Ruby, J.G.; Dybbro, E.; Ganem, D.; Derisi, J.L. Transcriptome Sequencing Demonstrates That Human Papillomavirus Is Not Active in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2011, 131, 1745–1753. [Google Scholar] [CrossRef]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From Keratinocyte to Cancer: The Pathogenesis and Modeling of Cutaneous Squamous Cell Carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Gallo, A.; Fiorella, M.L.; Simonelli, M.; Della Rocca, C.; De Vincentiis, M. Carcinoma Cuniculatum: Verrucous Carcinoma of the Skin of the Face. Otolaryngol. Head. Neck Surg. 2005, 133, 640. [Google Scholar] [CrossRef]
- Aird, I.; Johnson, H.D.; Lennox, B.; Stansfeld, A.G. Epithelioma Cuniculatum a Variety of Squamous Carcinoma Peculiar to the Foot. Br. J. Surg. 1954, 42, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Penera, K.E.; Manji, K.A.; Craig, A.B.; Grootegoed, R.A.; Leaming, T.R.; Wirth, G.A. Atypical Presentation of Verrucous Carcinoma: A Case Study and Review of the Literature. Foot Ankle Spec. 2013, 6, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Pătrașcu, V.; Enache, O.; Ciurea, R. Verrucous Carcinoma—Observations on 4 Cases. Curr. Health Sci. J. 2016, 42, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, M.; Ferguson, J.E.; Hutchinson, C.E.; Muston, H.L. Carcinoma Cuniculatum of the Foot Assessed by Magnetic Resonance Scanning. Clin. Exp. Dermatol. 2001, 26, 419–422. [Google Scholar] [CrossRef]
- Nagai, M.; Pastwik, B.; Aguirre, A.; Burke, M.; Campbell, J. Carcinoma Cuniculatum: A Rare Malignancy with Unique Diagnostic Dilemmas. Cureus 2023, 15, e37453. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kashiwagi, K.; Nakamura, A.; Muto, M. Verrucous Carcinoma of the Foot Diagnosed Using P53 and Ki-67 Immunostaining in a Patient with Diabetic Neuropathy. Am. J. Dermatopathol. 2015, 37, 257–259. [Google Scholar] [CrossRef]
- García-Gavín, J.; González-Vilas, D.; Rodríguez-Pazos, L.; Sánchez-Aguilar, D.; Toribio, J. Verrucous Carcinoma of the Foot Affecting the Bone: Utility of the Computed Tomography Scanner. Dermatol. Online J. 2010, 16, 8. [Google Scholar] [CrossRef]
- Harwood, C.A.; Proby, C.M.; Inman, G.J.; Leigh, I.M. The Promise of Genomics and the Development of Targeted Therapies for Cutaneous Squamous Cell Carcinoma. Acta Derm. Venereol. 2016, 96, 3–16. [Google Scholar] [CrossRef]
- Hussein, M.R. Ultraviolet Radiation and Skin Cancer: Molecular Mechanisms. J. Cutan. Pathol. 2005, 32, 191–205. [Google Scholar] [CrossRef]
- Welsh, M.M.; Karagas, M.R.; Kuriger, J.K.; Houseman, A.; Spencer, S.K.; Perry, A.E.; Nelson, H.H. Genetic Determinants of UV-Susceptibility in Non-Melanoma Skin Cancer. PLoS ONE 2011, 6, e20019. [Google Scholar] [CrossRef]
- Green, A.C.; Olsen, C.M. Cutaneous Squamous Cell Carcinoma: An Epidemiological Review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Stukel, T.A.; Umland, V.; Tsoukas, M.M.; Mott, L.A.; Sorensen, H.T.; Jensen, A.O.; Nelson, H.H.; Spencer, S.K.; Perry, A.E.; et al. Reported Use of Photosensitizing Medications and Basal Cell and Squamous Cell Carcinoma of the Skin: Results of a Population-Based Case-Control Study. J. Investig. Dermatol. 2007, 127, 2901–2903. [Google Scholar] [CrossRef]
- Wu, J.H.; Cohen, D.N.; Rady, P.L.; Tyring, S.K. BRAF Inhibitor-Associated Cutaneous Squamous Cell Carcinoma: New Mechanistic Insight, Emerging Evidence for Viral Involvement and Perspectives on Clinical Management. Br. J. Dermatol. 2017, 177, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.M.; Hanke, C.W.; Zollinger, T.W.; Montebello, J.F.; Hornback, N.B.; Norins, A.L. Skin Cancer in Patients with Chronic Radiation Dermatitis. J. Am. Acad. Dermatol. 1989, 20, 608–616. [Google Scholar] [CrossRef]
- Nan, H.; Kraft, P.; Hunter, D.J.; Han, J. Genetic Variants in Pigmentation Genes, Pigmentary Phenotypes, and Risk of Skin Cancer in Caucasians. Int. J. Cancer 2009, 125, 909–917. [Google Scholar] [CrossRef]
- Kivisaari, A. Squamous Cell Carcinoma of the Skin: Emerging Need for Novel Biomarkers. World J. Clin. Oncol. 2013, 4, 85–90. [Google Scholar] [CrossRef]
- Karagas, M.R.; Nelson, H.H.; Sehr, P.; Waterboer, T.; Stukel, T.A.; Andrew, A.; Green, A.C.; Bavinck, J.N.B.; Perry, A.; Spencer, S.; et al. Human Papillomavirus Infection and Incidence of Squamous Cell and Basal Cell Carcinomas of the Skin. J. Natl. Cancer Inst. 2006, 98, 389–395. [Google Scholar] [CrossRef]
- Astori, G.; Lavergne, D.; Benton, C.; Höckmayr, B.; Egawa, K.; Garbe, C.; De Villiers, E.M. Human Papillomaviruses Are Commonly Found in Normal Skin of Immunocompetent Hosts. J. Investig. Dermatol. 1998, 110, 752–755. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; Den Breems, N.Y.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 Mutations Occur Early during Cutaneous Squamous Cell Carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.A.; White, E.A.; Sowa, M.E.; Harper, J.W.; Aster, J.C.; Howley, P.M. Cutaneous β-Human Papillomavirus E6 Proteins Bind Mastermind-like Coactivators and Repress Notch Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1473–E1480. [Google Scholar] [CrossRef]
- Nicolas, M.; Wolfer, A.; Raj, K.; Kummer, J.A.; Mill, P.; Van Noort, M.; Hui, C.C.; Clevers, H.; Dotto, G.P.; Radtke, F. Notch1 Functions as a Tumor Suppressor in Mouse Skin. Nat. Genet. 2003, 33, 416–421. [Google Scholar] [CrossRef]
- Dibble, C.C.; Elis, W.; Menon, S.; Qin, W.; Klekota, J.; Asara, J.M.; Finan, P.M.; Kwiatkowski, D.J.; Murphy, L.O.; Manning, B.D. TBC1D7 Is a Third Subunit of the TSC1-TSC2 Complex Upstream of MTORC1. Mol. Cell 2012, 47, 535–546. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, X.; Li, B.; Yang, H.J.; Miller, M.; Yang, A.; Dhar, A.; Pavletich, N.P. Mechanisms of MTORC1 Activation by RHEB and Inhibition by PRAS40. Nature 2017, 552, 368–373. [Google Scholar] [CrossRef]
- Campos, M.A.; Lopes, J.M.; Soares, P. The Genetics of Cutaneous Squamous Cell Carcinogenesis. Eur. J. Dermatol. 2018, 28, 597–605. [Google Scholar] [CrossRef]
- Thomas, E.J.; Graves, N.C.; Meritt, S.M. Carcinoma Cuniculatum: An Atypical Presentation in the Foot. J. Foot Ankle Surg. 2014, 53, 356–359. [Google Scholar] [CrossRef]
- Koch, H.; Kowatsch, E.; Hödl, S.; Smola, M.G.; Radl, R.; Hofmann, T.; Scharnagl, E. Verrucous Carcinoma of the Skin: Long-Term Follow-up Results Following Surgical Therapy. Dermatol. Surg. 2004, 30, 1124–1130. [Google Scholar] [CrossRef]
- Boettler, M.A.; Gray, A.N.; Brodsky, M.A.; Shahwan, K.T.; Carr, D.R. Mohs Micrographic Surgery for Verrucous Carcinoma: A Review of the Literature. Arch. Dermatol. Res. 2023, 315, 133–137. [Google Scholar] [CrossRef]
- Jungmann, J.; Vogt, T.; Müller, C.S.L. Giant Verrucous Carcinoma of the Lower Extremity in Women with Dementia. BMJ Case Rep. 2012, 2012, bcr2012006357. [Google Scholar] [CrossRef] [PubMed]
- Imko-Walczuk, B.; Cegielska, A.; Placek, W.; Kaszewski, S.; Fiedor, P. Human Papillomavirus-Related Verrucous Carcinoma in a Renal Transplant Patient after Long-Term Immunosuppression: A Case Report. Transplant. Proc. 2014, 46, 2916–2919. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Sileni, V.C.; Dutriaux, C.; de Groot, J.W.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Wruhs, M.; Peinhaupt, T.; Stella, A.; Breier, F.; Steiner, A. Carcinoma Cuniculatum of the Right Thenar Region with Bone Involvement and Lymph Node Metastases. Case Rep. Dermatol. 2017, 9, 225–230. [Google Scholar] [CrossRef]
- Mitsuishi, T.; Ohara, K.; Kawashima, M.; Kobayashi, S.; Kawana, S. Prevalence of Human Papillomavirus DNA Sequences in Verrucous Carcinoma of the Lip: Genomic and Therapeutic Approaches. Cancer Lett. 2005, 222, 139–143. [Google Scholar] [CrossRef]
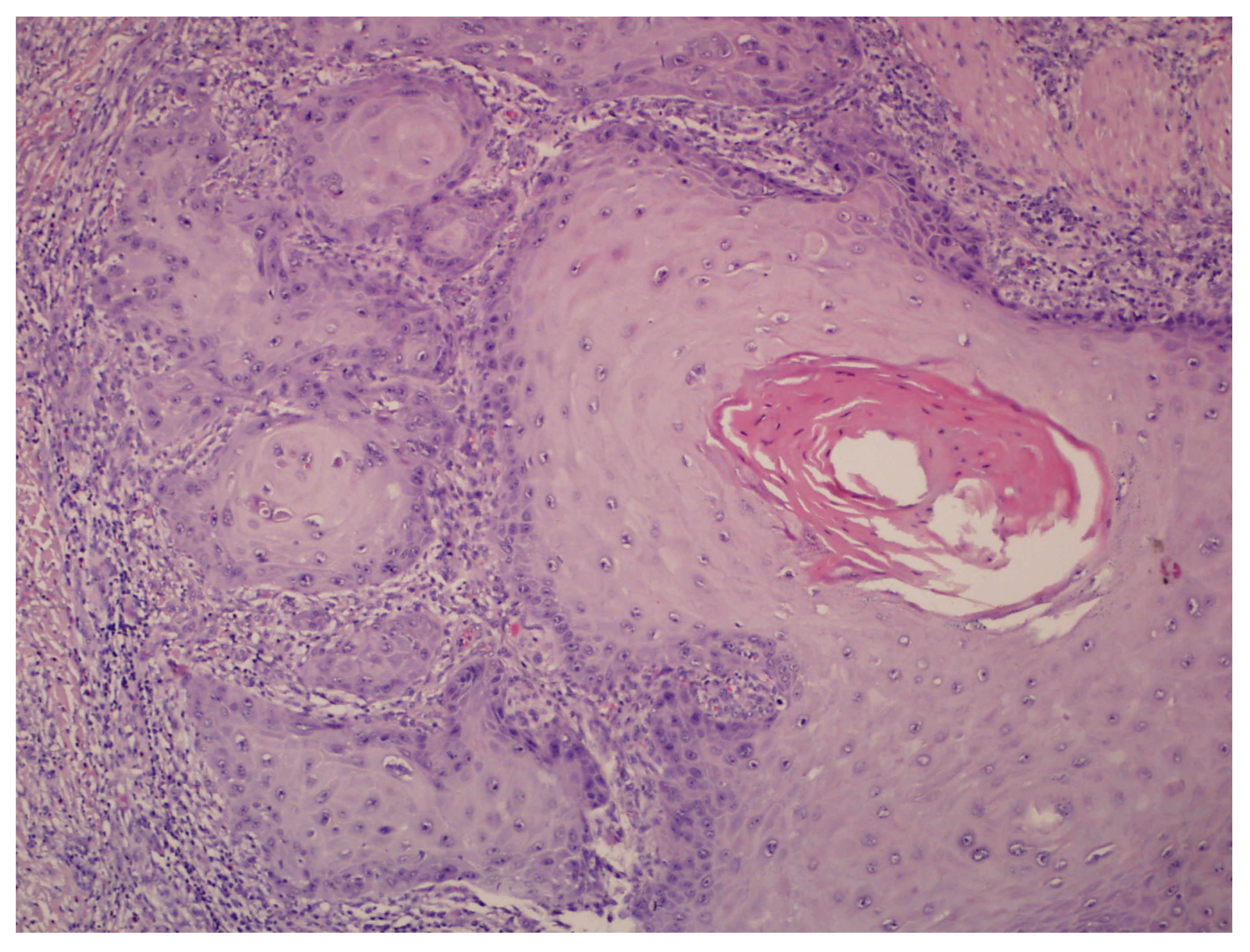

| Differential Diagnosis | Clinical Features | Histological Findings | Dermoscopic Findings | Sources |
|---|---|---|---|---|
| Refractory Verruca Plantaris | Plaque with hyperkeratotic surface painful under pressure Numerous black dots on the surface Lesions may coalesce into a “paving stone” pattern | Hyperkeratosis, acanthosis, koilocytosis, papillomatosis | Mosaic pattern with papillae surrounded by haloes Interrupted skin lines and brownish background | [9,10] |
| Keratoacanthoma | Rapidly growing dome-shaped nodules with central keratin plug | Well-differentiated squamous cells Keratin-filled plug | Keratin-filled invagination White keratin surface crown vessels | [11,12] |
| Eccrine Poroma or Porocarcinoma | Solitary, firm, slow-growing, dome-shaped papule or nodule skin-colored to erythematous lesion May ulcarate | Basaloid cells with ductal differentiation Hyperkeratosis | Dilated vessels, structureless pink-whitish areas Ulceration Blue-gray nests | [13] |
| Amelanotic Melanoma | Asymmetrical macular lesions, with uniform pink or red coloration with peripheral brown pigment | Atypical melanocytes, absence of melanin pigment epithelioid cells | Irregular polymorphous vessels Blue-whitish structures Milky-red areas Scar-like depigmentation | [14,15] |
| Cutaneous Sarcoma: AFX | Rapidly enlarging, dome-shaped nodules Sun-exposed areas Eroded surface | Spindle cells arranged in a storiform pattern Infiltrating the subcutaneous tissues Poorly defined architecture | Red and white featureless regions Irregular linear vessels White areas | [16,17,18] |
| Cutaneous Sarcoma: CUPS | Subcutaneous nodule with a gradual enlargement Substantial size | Pleomorphic histiocyte-like and spindled cells Atypical mitotic activity | Red and white structureless areas Thick and irregular vessels | [16,17,18] |
| Aggressive Digital Papillary Adenocarcinoma | Painful nodular lesions on fingers prone to recurrence | Papillary structures Cuboidal or columnar cells Invasion into deep dermis tubuloalveolar and ductal structures Papillary projections extending into the cystic lumina | None or nonspecific findings, requires histopathology | [19] |
| Pseudoepitheliomatous Hyperplasia | Skin-colored or tan pink Well-demarcated plaque or nodule scaling and crusting | Hyperkeratosis, acanthosis pseudoepitheliomatous hyperplasia Exuberant growth of squamous epithelium with invagination into the dermis nodular masses, composed of sheets of cells and nests with large keratin pearls and keratin cysts no dysplasia | Scales, keratin, and white circles surrounding a dilated and plugged follicular infundibulum white structureless area Hairpin, linear, irregular, glomerular, or polymorphic vessels White homogenous area, along with keratotic follicular plugging surrounded by white circles | [20,21] |
| Lupus vulgaris | Soft, solitary plaque Brownish to reddish hue, advancing edge with scarring | Formation of tuberculoid granulomas With or without caseation surrounded by epithelioid Histiocytes and multinucleated giant cells | Linear focussed telangiectasias on a yellow-brown background white scales | [22,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knecht-Gurwin, K.; Stefaniak, A.A.; Chlebicka, I.; Matusiak, L.; Woźniak, Z.; Szepietowski, J.C. Squamous Cell Carcinoma and Its Rare Variant, Carcinoma Cuniculatum: Insights and Case Studies. Cancers 2025, 17, 1217. https://doi.org/10.3390/cancers17071217
Knecht-Gurwin K, Stefaniak AA, Chlebicka I, Matusiak L, Woźniak Z, Szepietowski JC. Squamous Cell Carcinoma and Its Rare Variant, Carcinoma Cuniculatum: Insights and Case Studies. Cancers. 2025; 17(7):1217. https://doi.org/10.3390/cancers17071217
Chicago/Turabian StyleKnecht-Gurwin, Klaudia, Aleksandra A. Stefaniak, Iwona Chlebicka, Lukasz Matusiak, Zdzisław Woźniak, and Jacek C. Szepietowski. 2025. "Squamous Cell Carcinoma and Its Rare Variant, Carcinoma Cuniculatum: Insights and Case Studies" Cancers 17, no. 7: 1217. https://doi.org/10.3390/cancers17071217
APA StyleKnecht-Gurwin, K., Stefaniak, A. A., Chlebicka, I., Matusiak, L., Woźniak, Z., & Szepietowski, J. C. (2025). Squamous Cell Carcinoma and Its Rare Variant, Carcinoma Cuniculatum: Insights and Case Studies. Cancers, 17(7), 1217. https://doi.org/10.3390/cancers17071217








