Smart Conditioning with Venetoclax-Enhanced Sequential FLAMSA + RIC in Patients with High-Risk Myeloid Malignancies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient
2.2. Treatment
2.3. Monitoring and Definitions
2.4. Statistics
3. Results
3.1. Hematologic Reconstitution and Chimerism
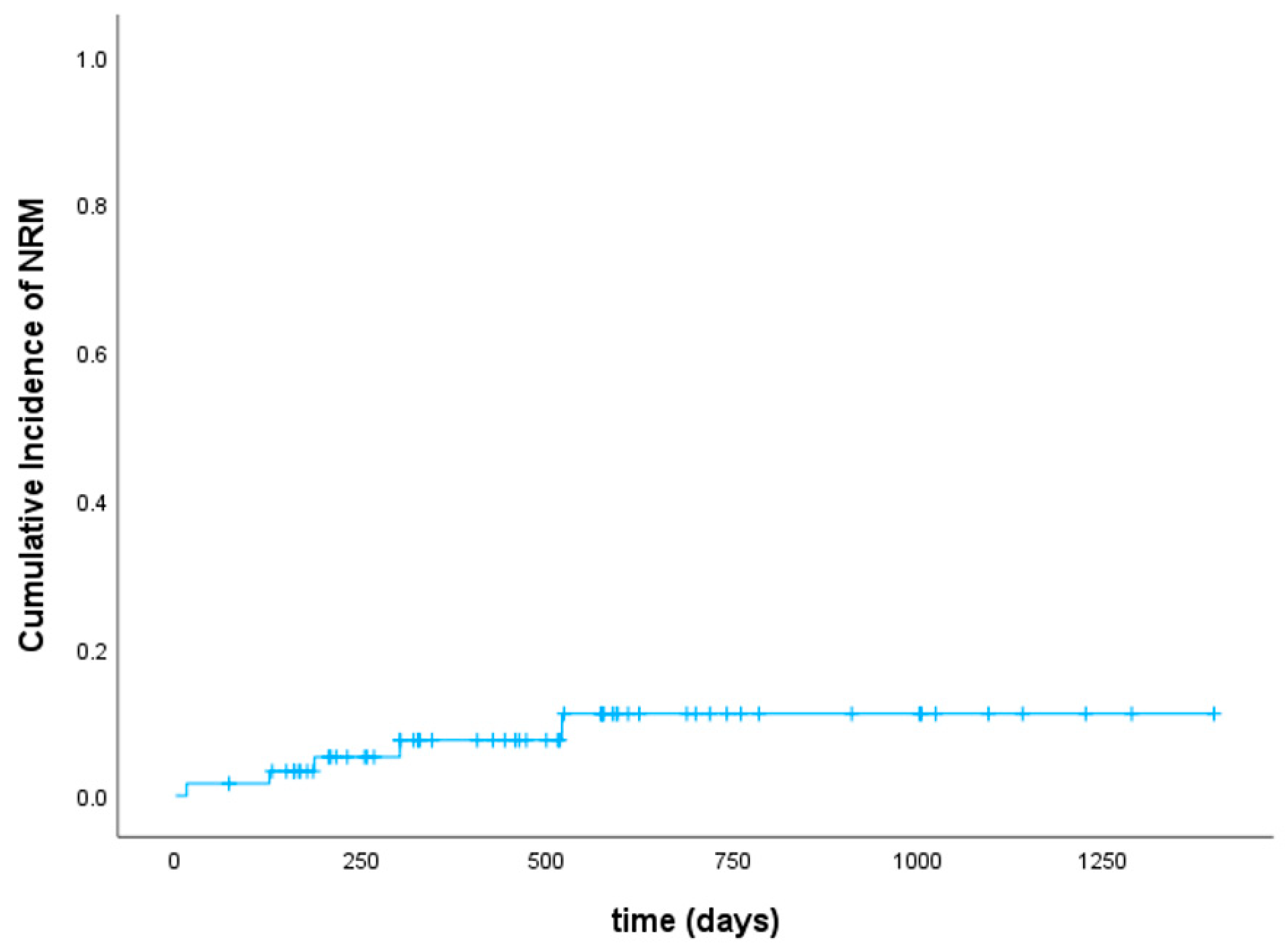
3.2. Toxicity and Infections
3.3. Acute and Chronic GVHD
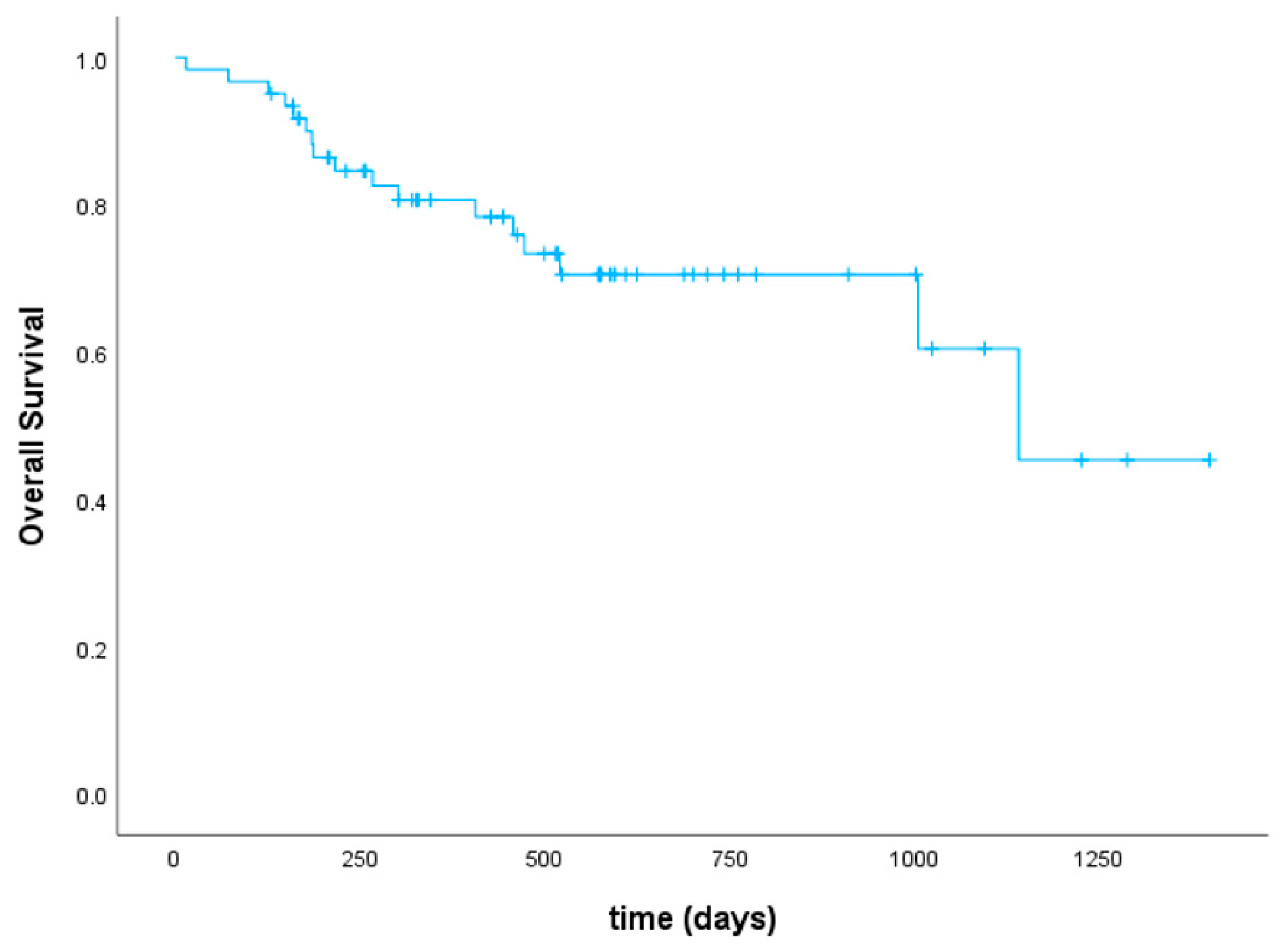
3.4. Disease Response, Relapse, and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; de la Cámara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: Monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021, 56, 1651–1664. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Corbacioglu, S.; de la Cámara, R.; Dolstra, H.; Glass, B.; Greco, R.; Mohty, M.; Neven, B.; et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: A report from the EBMT activity survey. Bone Marrow Transplant. 2022, 57, 742–752. [Google Scholar] [CrossRef]
- Kolb, H.J.; Schmid, C.; Barrett, A.J.; Schendel, D.J. Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004, 103, 767–776. [Google Scholar] [CrossRef]
- Schmid, C.; Schleuning, M.; Ledderose, G.; Tischer, J.; Kolb, H.J. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J. Clin. Oncol. 2005, 23, 5675–5687. [Google Scholar] [CrossRef]
- Horowitz, M.; Schreiber, H.; Elder, A.; Heidenreich, O.; Vormoor, J.; Toffalori, C.; Vago, L.; Kröger, N. Epidemiology and biology of relapse after stem cell transplantation. Bone Marrow Transplant. 2018, 53, 1379–1389. [Google Scholar] [CrossRef]
- Weller, J.F.; Lengerke, C.; Finke, J.; Schetelig, J.; Platzbecker, U.; Einsele, H.; Schroeder, T.; Faul, C.; Stelljes, M.; Dreger, P.; et al. Allogeneic hematopoietic stem cell transplantation in patients aged 60-79 years in Germany (1998–2018): A registry study. Haematologica 2023. [Google Scholar] [CrossRef]
- Meur, G.L.; Plesa, A.; Larcher, M.V.; Fossard, G.; Barraco, F.; Loron, S.; Balsat, M.; Ducastelle-Leprêtre, S.; Gilis, L.; Thomas, X.; et al. Impact on Outcome of Minimal Residual Disease after Hematopoietic Stem Cell Transplantation with Fludarabine, Amsacrine, and Cytosine Arabinoside-Busulfan Conditioning: A Retrospective Monocentric Study. Transplant. Cell. Ther. 2023, 29, 38.e1–38.e9. [Google Scholar] [CrossRef]
- Jondreville, L.; Roos-Weil, D.; Uzunov, M.; Boussen, I.; Grenier, A.; Norol, F.; Morel, V.; Nguyen, S.; Souchet, L. FLAMSA-Busulfan-Melphalan as a Sequential Conditioning Regimen in HLA-Matched or Haploidentical Hematopoietic Stem Cell Transplantation for High-Risk Myeloid Diseases. Transplant. Cell. Ther. 2021, 27, 915.e1–915.e8. [Google Scholar] [CrossRef]
- Holtick, U.; Herling, M.; Pflug, N.; Chakupurakal, G.; Leitzke, S.; Wolf, D.; Hallek, M.; Scheid, C.; Chemnitz, J.M. Similar outcome after allogeneic stem cell transplantation with a modified FLAMSA conditioning protocol substituting 4 Gy TBI with treosulfan in an elderly population with high-risk AML. Ann. Hematol. 2017, 96, 479–487. [Google Scholar] [CrossRef]
- Malard, F.; Labopin, M.; Stuhler, G.; Bittenbring, J.; Ganser, A.; Tischer, J.; Michallet, M.; Kröger, N.; Schmid, C.; Huynh, A.; et al. Sequential Intensified Conditioning Regimen Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Intermediate- or High-Risk Acute Myeloid Leukemia in Complete Remission: A Study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 278–284. [Google Scholar] [CrossRef]
- Zohren, F.; Czibere, A.; Bruns, I.; Fenk, R.; Schroeder, T.; Gräf, T.; Haas, R.; Kobbe, G. Fludarabine, amsacrine, high-dose cytarabine and 12 Gy total body irradiation followed by allogeneic hematopoietic stem cell transplantation is effective in patients with relapsed or high-risk acute lymphoblastic leukemia. Bone Marrow Transplant. 2009, 44, 785–792. [Google Scholar] [CrossRef]
- Chemnitz, J.M.; von Lilienfeld-Toal, M.; Holtick, U.; Theurich, S.; Shimabukuro-Vornhagen, A.; Krause, A.; Brossart, P.; Hallek, M.; Scheid, C. Intermediate intensity conditioning regimen containing FLAMSA, treosulfan, cyclophosphamide, and ATG for allogeneic stem cell transplantation in elderly patients with relapsed or high-risk acute myeloid leukemia. Ann. Hematol. 2012, 91, 47–55. [Google Scholar] [CrossRef]
- Saure, C.; Schroeder, T.; Zohren, F.; Groten, A.; Bruns, I.; Czibere, A.; Galonska, L.; Kondakci, M.; Weigelt, C.; Fenk, R.; et al. Upfront allogeneic blood stem cell transplantation for patients with high-risk myelodysplastic syndrome or secondary acute myeloid leukemia using a FLAMSA-based high-dose sequential conditioning regimen. Biol. Blood Marrow Transplant. 2012, 18, 466–472. [Google Scholar] [CrossRef]
- Christopeit, M.; Badbaran, A.; Alawi, M.; Zabelina, T.; Zeck, G.; Wolschke, C.; Ayuk, F.; Kröger, N. Correlation of somatic mutations with outcome after FLAMSA-busulfan sequential conditioning and allogeneic stem cell transplantation in patients with myelodysplastic syndromes. Eur. J. Haematol. 2016, 97, 288–296. [Google Scholar] [CrossRef]
- Fraccaroli, A.; Prevalsek, D.; Fritsch, S.; Haebe, S.; Bücklein, V.; Schulz, C.; Hubmann, M.; Stemmler, H.J.; Ledderose, G.; Hausmann, A.; et al. Sequential HLA-haploidentical transplantation utilizing post-transplantation cyclophosphamide for GvHD prophylaxis in high-risk and relapsed/refractory AML/MDS. Am. J. Hematol. 2018, 93, 1524–1531. [Google Scholar] [CrossRef]
- Heinicke, T.; Labopin, M.; Polge, E.; Stelljes, M.; Ganser, A.; Tischer, J.; Brecht, A.; Kröger, N.; Beelen, D.W.; Scheid, C.; et al. Evaluation of six different types of sequential conditioning regimens for allogeneic stem cell transplantation in relapsed/refractory acute myelogenous leukemia—A study of the Acute Leukemia Working Party of the EBMT. Leuk. Lymphoma 2021, 62, 399–409. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef]
- Jiao, Y.; Davis, J.E.; Rautela, J.; Carrington, E.M.; Ludford-Menting, M.J.; Goh, W.; Delconte, R.B.; Souza-Fonseca-Guimaraes, F.; Koldej, R.; Gray, D.; et al. Recipient BCL2 inhibition and NK cell ablation form part of a reduced intensity conditioning regime that improves allo-bone marrow transplantation outcomes. Cell Death Differ. 2019, 26, 1516–1530. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Xiao, L.; Kadia, T.; Daver, N.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 2768–2778. [Google Scholar] [CrossRef]
- Garcia, J.S.; Kim, H.T.; Murdock, H.M.; Cutler, C.S.; Brock, J.; Gooptu, M.; Ho, V.T.; Koreth, J.; Nikiforow, S.; Romee, R.; et al. Adding venetoclax to fludarabine/busulfan RIC transplant for high-risk MDS and AML is feasible, safe, and active. Blood Adv. 2021, 5, 5536–5545. [Google Scholar] [CrossRef]
- Sorror, M.L.; Sandmaier, B.M.; Storer, B.E.; Maris, M.B.; Baron, F.; Maloney, D.G.; Scott, B.L.; Deeg, H.J.; Appelbaum, F.R.; Storb, R. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2007, 25, 4246–4254. [Google Scholar] [CrossRef]
- Sperr, W.R.; Wimazal, F.; Kundi, M.; Baumgartner, C.; Nösslinger, T.; Makrai, A.; Stauder, R.; Krieger, O.; Pfeilstöcker, M.; Valent, P. Comorbidity as prognostic variable in MDS: Comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS Study Group. Ann. Oncol. 2010, 21, 114–119. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Paul Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Davis, J.E.; Du, K.; Ludford-Menting, M.J.; Prabahran, A.; Wong, E.; Huntington, N.D.; Koldej, R.M.; Ritchie, D.S. Venetoclax or Ruxolitinib in Pre-Transplant Conditioning Lowers the Engraftment Barrier by Different Mechanisms in Allogeneic Stem Cell Transplant Recipients. Front. Immunol. 2021, 12, 749094. [Google Scholar] [CrossRef]
- Bohl, S.; von Harsdorf, S.; Mulaw, M.; Hofmann, S.; Babiak, A.; Maier, C.P.; Schnell, J.; Hütter-Krönke, L.M.; Scholl, K.; Wais, V.; et al. Strong impact of extramedullary involvement in high-risk AML patients with active disease receiving the FLAMSA conditioning regimen for HSCT. Bone Marrow Transplant. 2016, 51, 994–996. [Google Scholar] [CrossRef]
- Schmid, C.; Schleuning, M.; Tischer, J.; Holler, E.; Haude, K.H.; Braess, J.; Haferlach, C.; Baurmann, H.; Oruzio, D.; Hahn, J.; et al. Early allo-SCT for AML with a complex aberrant karyotype--results from a prospective pilot study. Bone Marrow Transplant. 2012, 47, 46–53. [Google Scholar] [CrossRef]
- Krejci, M.; Doubek, M.; Dusek, J.; Brychtova, Y.; Racil, Z.; Navratil, M.; Tomiska, M.; Horky, O.; Pospisilova, S.; Mayer, J. Combination of fludarabine, amsacrine, and cytarabine followed by reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia. Ann. Hematol. 2013, 92, 1397–1403. [Google Scholar] [CrossRef]
- Schmid, C.; Schleuning, M.; Schwerdtfeger, R.; Hertenstein, B.; Mischak-Weissinger, E.; Bunjes, D.; Harsdorf, S.V.; Scheid, C.; Holtick, U.; Greinix, H.; et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006, 108, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schleuning, M.; Mayer, J.; Haude, K.H.; Tischer, J.; Buchholz, S.; Bunjes, D.; Bug, G.; Holler, E.; Meyer, R.G.; et al. Influence of molecular subgroups on outcome of acute myeloid leukemia with normal karyotype in 141 patients undergoing salvage allogeneic stem cell transplantation in primary induction failure or beyond first relapse. Haematologica 2013, 98, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Holtick, U.; Shimabukuro-Vornhagen, A.; Chakupurakal, G.; Theurich, S.; Leitzke, S.; Burst, A.; Hallek, M.; von Bergwelt-Baildon, M.; Scheid, C.; Chemnitz, J.M. FLAMSA reduced-intensity conditioning is equally effective in AML patients with primary induction failure as well as in first or second complete remission. Eur. J. Haematol. 2016, 96, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Schneidawind, D.; Federmann, B.; Faul, C.; Vogel, W.; Kanz, L.; Bethge, W.A. Allogeneic hematopoietic cell transplantation with reduced-intensity conditioning following FLAMSA for primary refractory or relapsed acute myeloid leukemia. Ann. Hematol. 2013, 92, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Pfrepper, C.; Klink, A.; Behre, G.; Schenk, T.; Franke, G.N.; Jentzsch, M.; Schwind, S.; Al-Ali, H.K.; Hochhaus, A.; Niederwieser, D.; et al. Risk factors for outcome in refractory acute myeloid leukemia patients treated with a combination of fludarabine, cytarabine, and amsacrine followed by a reduced-intensity conditioning and allogeneic stem cell transplantation. J. Cancer Res. Clin. Oncol. 2016, 142, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Platte, V.; Bergmann, A.; Hildebrandt, B.; Wieczorek, D.; Schuler, E.; Germing, U.; Kaivers, J.; Haas, R.; Kobbe, G.; Schroeder, T.; et al. Clinical and Cytogenetic Characterization of Early and Late Relapses in Patients Allografted for Myeloid Neoplasms with a Myelodysplastic Component. Cancers 2022, 14, 6244. [Google Scholar] [CrossRef]
- Venetoclax in Combination with Non-myeloablative Conditioning Allogeneic Haematopoietic Stem Cell Transplantation (VICTORY). Available online: https://clinicaltrials.gov/study/NCT05005299?cond=Venetoclax&term=conditioning&rank=1 (accessed on 15 September 2022).
- Venetoclax and Sequential Busulfan, Cladribine, and Fludarabine Phosphate Before Donor Stem Cell Transplant in Treating Patients with Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Available online: https://clinicaltrials.gov/study/NCT02250937?cond=Venetoclax&term=conditioning&rank=9 (accessed on 15 September 2022).


| Age in years, median (range) | 58 (20–74) |
| Sex, no. (%) | |
| Male | 32 (52.5) |
| Female | 29 (47.5) |
| Diagnosis, no. (%) AML (according to WHO 2022)
| 40 (65.6) 31 (50.8) 17 (27.9) 7 (11.5) 3 (4.9) 2 (3.3) 1 (1.6) 1 (1.6) 2 (3.3) 7 (11.5) 3 (4.9) 2 (3.3) 1 (1.6) 1 (1.6) |
MDS (according to WHO 2022)
| 18 (29.5) 6 (9.8) 6 (9.8) 5 (8.2) 1 (1.6) |
CMML
| 2 (3.3) 1 (1.6) 1 (1.6) |
| CML (in blast crisis) | 1 (1.6) |
| Risk stratification according to genetics, no. (%) | |
| High-risk | 45 (73.8) |
| Intermediate | 12 (19.7) |
| Favorable | 4 (6.6) |
| HCT-CI, no. (%) | |
| 0 | 21 (34.4) |
| 1–2 | 19 (31.1) |
| ≥3 | 19 (31.1) |
| Unknown | 2 (3.3) |
| Disease status at transplant, no. (%) | |
| Relapsed/refractory | 41 (67.2) |
| Untreated disease | 19 (31.1) |
| CR | 1 (1.6) |
| Number of transplant(s), no. (%) | |
| First | 58 (95.1) |
| Second | 3 (4.9) |
| Conditioning regimen, no. (%) | |
| FLAMSA + melphalan (100–200 mg/m2) | 29 (47.5) |
| FLAMSA + treosulfan (30 g/m2) | 22 (36.1) |
| FLAMSA + Cy 60 mg/kg + TBI 4 Gy | 9 (14.8) |
| FLAMSA + Cy 60 mg/kg + TBI 8 Gy | 1 (1.6) |
| FLAMSA + TBI 4 Gy | 1 (1.6) |
| Donor, no. (%) | |
| Matched unrelated donor (MUD) | 32 (52.5) |
| Matched related donor (MRD) | 12 (19.7) |
| Haploidentical | 10 (16.4) |
| Mismatched unrelated donor 9/10 (MMUD) | 7 (11.5) |
| GVHD prophylaxis, no. (%) | |
| Antithymocyte globulin 3 × 5 mg/kg BW | 5 (8.2) |
| Antithymocyte globulin 3 × 10 mg/kg BW | 30 (49.2) |
| Antithymocyte globulin 3 × 20 mg/kg BW | 7 (11.5) |
| Post-Transplant cyclophosphamide 50 mg/kg BW | 15 (24.6) |
| None | 4 (6.6) |
| No. (%) | |
| Infectious | |
| |
| 4 (6.6) 2 (3.3) 1 (1.6) 1 (1.6) 1 (1.6) 1 (1.6) 1 (1.6) |
| 4 (6.6) 3 (4.9) 1 (1.6) 1 (1.6) |
| 5 (8.2) |
| 2 (3.3) 2 (3.3) 1 (1.6) 1 (1.6) 1 (1.6) |
Cardiac
| 3 (4.9) 1 (1.6) 1 (1.6) |
Hemorrhagic
| 1 (1.6) 1 (1.6) |
Other
| 2 (3.3) 1 (1.6) 1 (1.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, F.; Jäger, P.; Tischer, J.; Fraccaroli, A.; Bug, G.; Hausmann, A.; Baermann, B.-N.; Tressin, P.; Hoelscher, A.; Kasprzak, A.; et al. Smart Conditioning with Venetoclax-Enhanced Sequential FLAMSA + RIC in Patients with High-Risk Myeloid Malignancies. Cancers 2024, 16, 532. https://doi.org/10.3390/cancers16030532
Schulz F, Jäger P, Tischer J, Fraccaroli A, Bug G, Hausmann A, Baermann B-N, Tressin P, Hoelscher A, Kasprzak A, et al. Smart Conditioning with Venetoclax-Enhanced Sequential FLAMSA + RIC in Patients with High-Risk Myeloid Malignancies. Cancers. 2024; 16(3):532. https://doi.org/10.3390/cancers16030532
Chicago/Turabian StyleSchulz, Felicitas, Paul Jäger, Johanna Tischer, Alessia Fraccaroli, Gesine Bug, Andreas Hausmann, Ben-Niklas Baermann, Patrick Tressin, Alexander Hoelscher, Annika Kasprzak, and et al. 2024. "Smart Conditioning with Venetoclax-Enhanced Sequential FLAMSA + RIC in Patients with High-Risk Myeloid Malignancies" Cancers 16, no. 3: 532. https://doi.org/10.3390/cancers16030532





