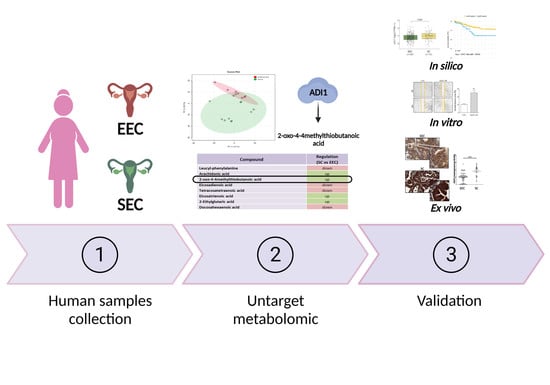
Metabolomic Analysis Points to Bioactive Lipid Species and Acireductone Dioxygenase 1 (ADI1) as Potential Therapeutic Targets in Poor Prognosis Endometrial Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sample Collection
2.2. Metabolite Extraction
2.3. Metabolome Analysis
2.4. Metabolomic Data Analysis and Statistics
2.5. Bioinformatic Analysis of Clinical Data
2.6. Inmunohistochemical Analysis
2.7. Cell Culture
2.8. Plasmid Transfection
2.9. Wound Healing Assay
2.10. Transwell Invasion Assay
3. Results
3.1. Clinical Parameters of Human Samples
3.2. Characterisation of Metabolomic Differences between SC and EEC
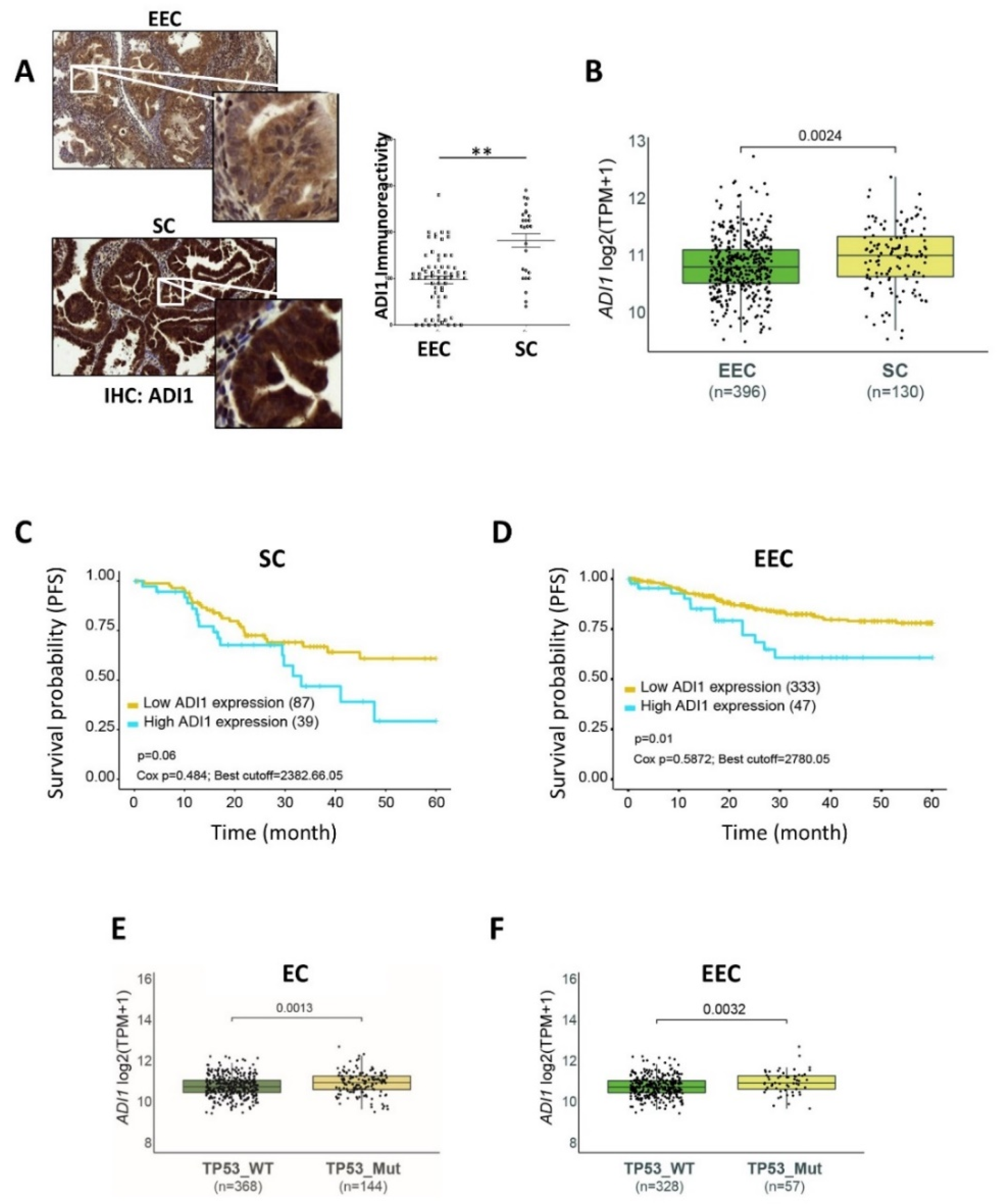
3.3. ADI1 Is Overexpressed in SC Patients
3.4. High ADI1 mRNA Levels Are Associated with Altered P53 Expression
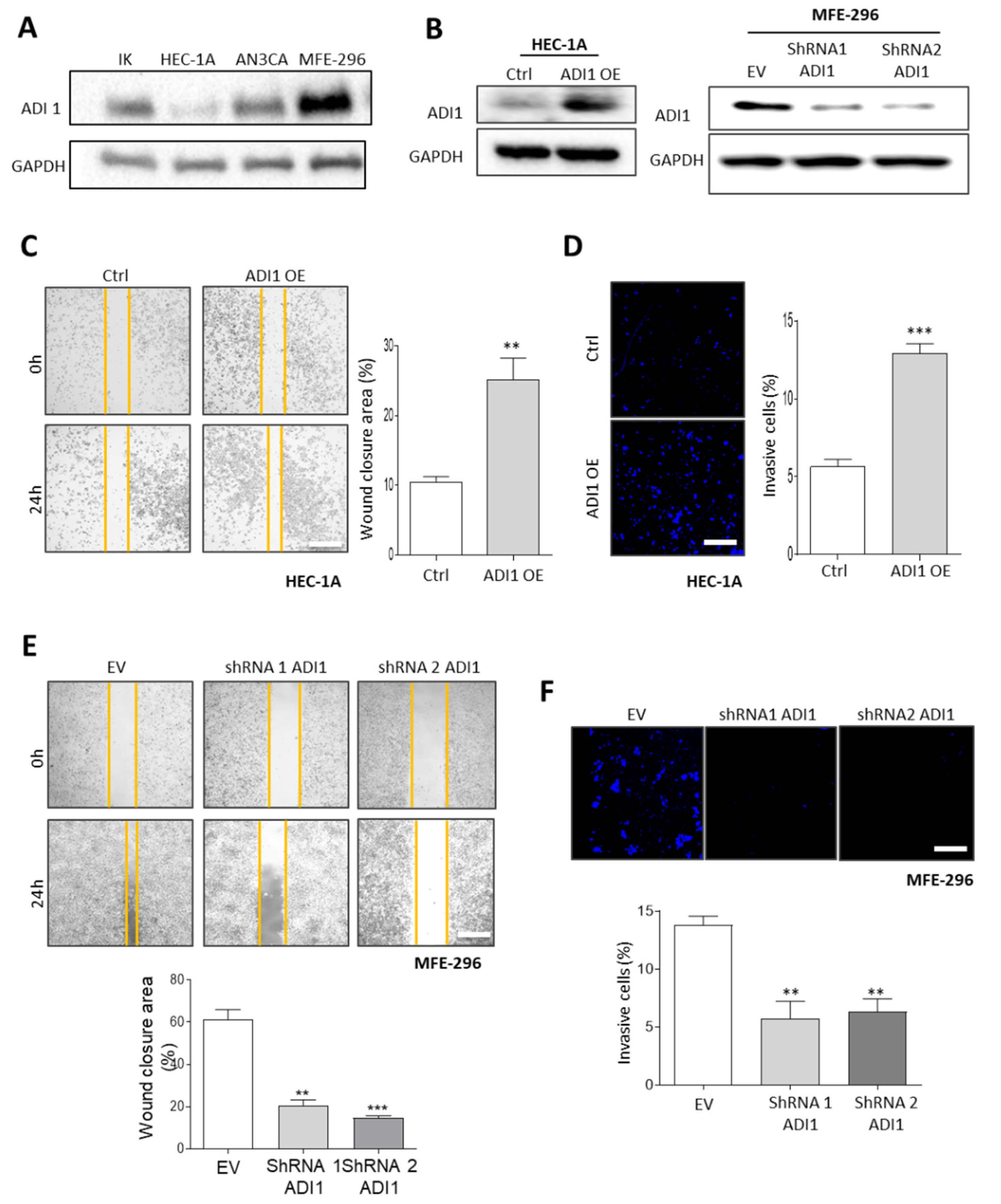
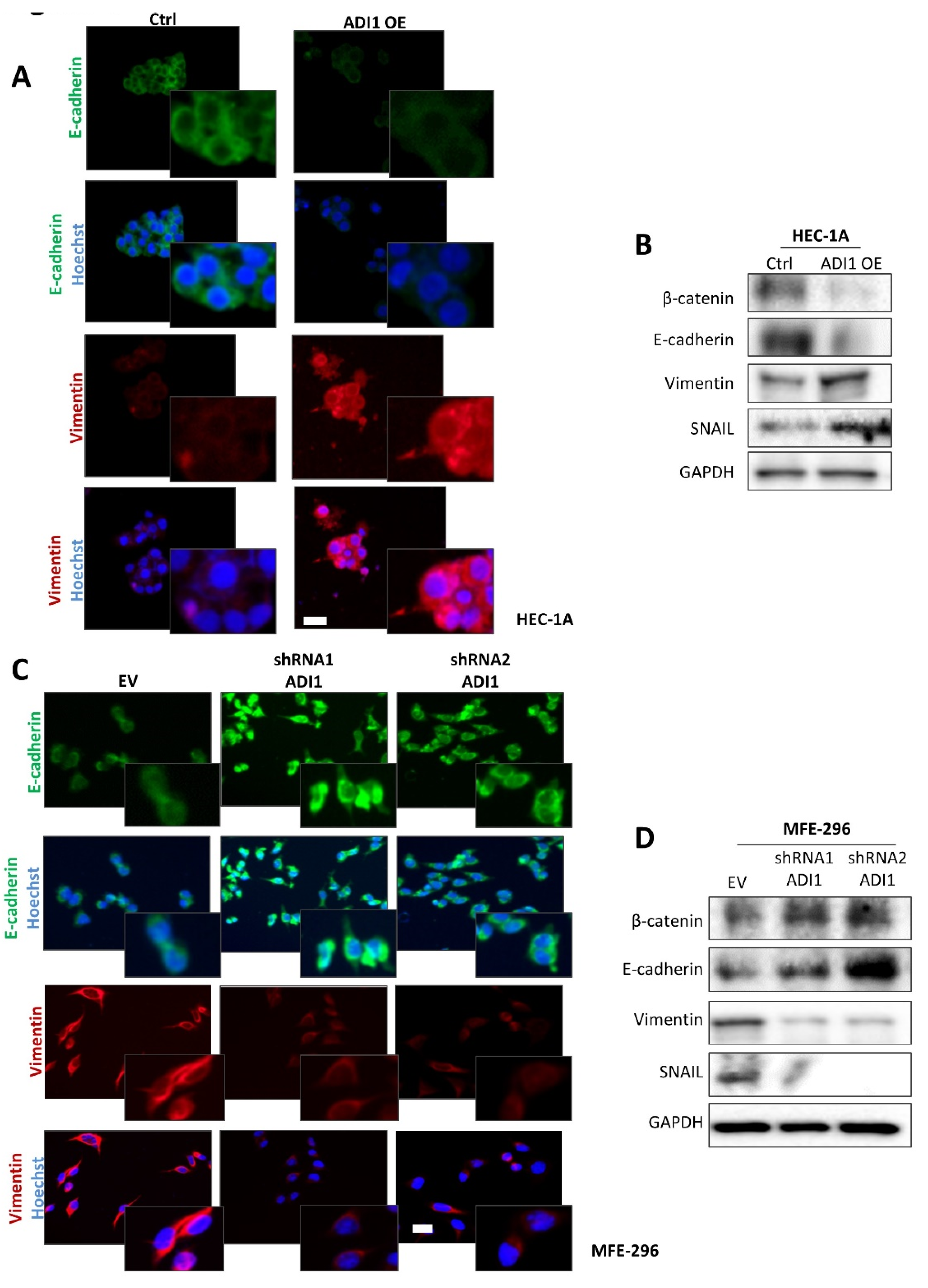
3.5. Alteration of ADI1 Expression Modulates Migration and Invasion Capabilities of EEC Cell Lines through Regulation of Epithelial–Mesenchymal Transition Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Eritja, N.; Chen, B.J.; Rodríguez-Barrueco, R.; Santacana, M.; Gatius, S.; Vidal, A.; Martí, M.D.; Ponce, J.; Bergada, L.; Yeramian, A.; et al. Autophagy orchestrates adaptive responses to targeted therapy in endometrial cancer. Autophagy 2017, 13, 608–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, G.F. Systemic chemotherapy for uterine carcinoma: Metastatic and adjuvant. J. Clin. Oncol. 2007, 25, 2983–2990. [Google Scholar] [CrossRef]
- Sherman, M.E. Theories of endometrial carcinogenesis: A multidisciplinary approach. Mod. Pathol. 2000, 13, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Azueta, A.; Gatius, S.; Matias-Guiu, X. Endometrioid carcinoma of the endometrium: Pathologic and molecular features. Semin. Diagn. Pathol. 2010, 27, 226–240. [Google Scholar] [CrossRef]
- Gatius, S.; Matias-Guiu, X. Practical issues in the diagnosis of serous carcinoma of the endometrium. Mod. Pathol. 2016, 29 (Suppl. S1), S45–S58. [Google Scholar] [CrossRef] [Green Version]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Benz, C.C.; Yau, C.; Laird, P.W.; Ding, L.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Piulats, J.M.; Guerra, E.; Gil-Martín, M.; Roman-Canal, B.; Gatius, S.; Sanz-Pamplona, R.; Velasco, A.; Vidal, A.; Matias-Guiu, X. Molecular approaches for classifying endometrial carcinoma. Gynecol. Oncol. 2017, 145, 200–207. [Google Scholar] [CrossRef]
- Singh, N.; Hirschowitz, L.; Zaino, R.; Alvarado-Cabrero, I.; Duggan, M.A.; Ali-Fehmi, R.; Euscher, E.; Hecht, J.L.; Horn, L.-C.; Ioffe, O.; et al. Pathologic Prognostic Factors in Endometrial Carcinoma (Other Than Tumor Type and Grade). Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S93–S113. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Esteban, F.J.; Stavreus-Evers, A.; Simón, C.; Giudice, L.; Lessey, B.A.; Horcajadas, J.A.; Macklon, N.S.; D'Hooghe, T.; Campoy, C.; et al. Guidelines for the design, analysis and interpretation of ‘omics’ data: Focus on human endometrium. Hum. Reprod. Update 2014, 20, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Day, R.S.; McDade, K.K.; Chandran, U.R.; Lisovich, A.; Conrads, T.P.; Hood, B.L.; Kolli, V.K.; Kirchner, D.; Litzi, T.; Maxwell, G.L. Identifier mapping performance for integrating transcriptomics and proteomics experimental results. BMC Bioinform. 2011, 12, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Gatius, S.; Yeramian, A.; Portero-Otin, M.; Eritja, N.; Santacana, M.; Colas, E.; Ruiz, M.; Pamplona, R.; Matias-Guiu, X. Metabotyping human endometrioid endometrial adenocarcinoma reveals an implication of endocannabinoid metabolism. Oncotarget 2016, 7, 52364–52374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altadill, T.; Dowdy, T.M.; Gill, K.; Reques, A.; Menon, S.S.; Moiola, C.P.; Lopez-Gil, C.; Coll, E.; Matias-Guiu, X.; Cabrera, S.; et al. Metabolomic and Lipidomic Profiling Identifies the Role of the RNA Editing Pathway in Endometrial Carcinogenesis. Sci. Rep. 2017, 7, 8803. [Google Scholar] [CrossRef] [PubMed]
- Trousil, S.; Lee, P.; Pinato, D.J.; Ellis, J.K.; Dina, R.; Aboagye, E.O.; Keun, H.C.; Sharma, R. Alterations of choline phospholipid metabolism in endometrial cancer are caused by choline kinase alpha overexpression and a hyperactivated deacylation pathway. Cancer Res. 2014, 74, 6867–6877. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e296. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laska, E.; Meisner, M.; Wanderling, J. A maximally selected test of symmetry about zero. Stat. Med. 2012, 31, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Eritja, N.; Mirantes, C.; Llobet, D.; Masip, G.; Matias-Guiu, X.; Dolcet, X. ERα-mediated repression of pro-inflammatory cytokine expression by glucocorticoids reveals a critical role for TNFα and IL1α in lumen formation and maintenance. J. Cell Sci. 2012, 125, 1929–1944. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.-D.; Lai, H.-Y.; Pai, L.-M.; Huang, Y.-H.; Lin, Y.-H.; Liang, K.-H.; Yeh, C.-T. The methionine salvage pathway-involving ADI1 inhibits hepatoma growth by epigenetically altering genes expression via elevating S-adenosylmethionine. Cell Death Dis. 2019, 10, 240. [Google Scholar] [CrossRef] [Green Version]
- Gagné, A.; Wang, E.; Bastien, N.; Orain, M.; Desmeules, P.; Pagé, S.; Trahan, S.; Couture, C.; Joubert, D.; Joubert, P. Characteristics on PD-L1 Testing in Non-Small Cell Lung Cancer: Validation of the IASLC PD-L1 Testing Recommendations. J. Thorac. Oncol. 2019, 14, 2062–2070. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Webster, J.D.; DuSold, D.; Miller, M.A. Immunohistochemical avaluation of the effects of paraffin section storage on biomarker stability. Vet. Pathol. 2014, 51, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Fang, Q.Q.; Wang, B. The age of paraffin block influences biomarker levels in archival breast cancer samples. Oncol. Lett. 2020, 20, 525–532. [Google Scholar] [CrossRef]
- Geisler, J.P.; Geisler, H.E.; Wiemann, M.C.; Zhou, Z.; Miller, G.A.; Crabtree, W. p53 expression as a prognostic indicator of 5-year survival in endometrial cancer. Gynecol. Oncol. 1999, 74, 468–471. [Google Scholar] [CrossRef]
- Alvarez, T.; Miller, E.; Duska, L.; Oliva, E. Molecular profile of grade 3 endometrioid endometrial carcinoma: Is it a type I or type II endometrial carcinoma? Am. J. Surg. Pathol. 2012, 36, 753–761. [Google Scholar] [CrossRef]
- Muinelo-Romay, L.; Colas, E.; Barbazan, J.; Alonso-Alconada, L.; Alonso-Nocelo, M.; Bouso, M.; Curiel, T.; Cueva, J.; Anido, U.; Forteza, J.; et al. High risk endometrial carcinoma profiling identifies TGF-b1 as a key factor in the initiation of tumor invasion. Mol. Cancer Ther. 2011, 10, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, M.; Yang, J.X.; Cao, D.Y.; Shen, K.; Lang, J.H. Clinicopathological and survival analysis of uterine papillary serous carcinoma: A single institutional review of 106 cases. Cancer Manag. Res. 2018, 10, 4915–4928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvesen, H.B.; Haldorsen, I.S.; Trovik, J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012, 13, e353–e361. [Google Scholar] [CrossRef]
- Raffone, A.; Troisi, J.; Boccia, D.; Travaglino, A.; Capuano, G.; Insabato, L.; Mollo, A.; Guida, M.; Zullo, F. Metabolomics in endometrial cancer diagnosis: A systematic review. Acta Obstet. Et Gynecol. Scand. 2020, 99, 1135–1146. [Google Scholar] [CrossRef]
- Njoku, K.; Sutton, C.J.; Whetton, A.D.; Crosbie, E.J. Metabolomic Biomarkers for Detection, Prognosis and Identifying Recurrence in Endometrial Cancer. Metabolites 2020, 10, 314. [Google Scholar] [CrossRef]
- Moon, Y.; Choi, S.M.; Chang, S.; Park, B.; Lee, S.; Lee, M.-O.; Choi, H.-S.; Park, H. Chenodeoxycholic Acid Reduces Hypoxia Inducible Factor-1α Protein and Its Target Genes. PLoS ONE 2015, 10, e0130911. [Google Scholar]
- Carrasco, S.; Mérida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef]
- Almena, M.; Mérida, I. Shaping up the membrane: Diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 2011, 36, 593–603. [Google Scholar] [CrossRef]
- Sakane, F.; Hoshino, F.; Murakami, C. New Era of Diacylglycerol Kinase, Phosphatidic Acid and Phosphatidic Acid-Binding Protein. Int. J. Mol. Sci. 2020, 21, 6794. [Google Scholar] [CrossRef]
- Mérida, I.; Avila-Flores, A.; Merino, E. Diacylglycerol kinases: At the hub of cell signalling. Biochem. J. 2008, 409, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Wang, Y.; Luo, W. Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene 2020, 39, 6747–6756. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Sarno, L.; Landolfi, A.; Scala, G.; Martinelli, P.; Venturella, R.; Di Cello, A.; Zullo, F.; Guida, M. Metabolomic Signature of Endometrial Cancer. J. Proteome Res. 2018, 17, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Ihata, Y.; Miyagi, E.; Numazaki, R.; Muramatsu, T.; Imaizumi, A.; Yamamoto, H.; Yamakado, M.; Okamoto, N.; Hirahara, F. Amino acid profile index for early detection of endometrial cancer: Verification as a novel diagnostic marker. Int. J. Clin. Oncol. 2014, 19, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Audet-Delage, Y.; Villeneuve, L.; Grégoire, J.; Plante, M.; Guillemette, C. Identification of Metabolomic Biomarkers for Endometrial Cancer and Its Recurrence after Surgery in Postmenopausal Women. Front. Endocrinol. 2018, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Wray, J.W.; Abeles, R.H. The methionine salvage pathway in Klebsiella pneumoniae and rat liver. Identification and characterization of two novel dioxygenases. J. Biol. Chem. 1995, 270, 3147–3153. [Google Scholar] [CrossRef] [Green Version]
- Oram, S.W.; Ai, J.; Pagani, G.M.; Hitchens, M.R.; Stern, J.A.; Eggener, S.; Pins, M.; Xiao, W.; Cai, X.; Haleem, R.; et al. Expression and function of the human androgen-responsive gene ADI1 in prostate cancer. Neoplasia 2007, 9, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.; Cao, H.; Chen, J.; Weller, S.G.; Krueger, E.W.; Zhang, L.; Razidlo, G.L.; McNiven, M.A. Pancreatic tumor cell metastasis is restricted by MT1-MMP binding protein MTCBP-1. J. Cell Biol. 2019, 218, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Yang, K.; Li, S.; Liu, C.; Han, J.; Zhang, Y.; Xu, G. Enolase-phosphatase 1 as a novel potential malignant glioma indicator promotes cell proliferation and migration. Oncol. Rep. 2018, 40, 2233–2241. [Google Scholar] [CrossRef]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.-K.; Amini, B.; Andersen, E.; Andersson, A.-C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.J.; Bae, D.-H.; Siafakas, A.R.; Rahmanto, Y.S.; Al-Akra, L.; Jansson, P.; Casero, R.; Richardson, D.R. Coupling of the polyamine and iron metabolism pathways in the regulation of proliferation: Mechanistic links to alterations in key polyamine biosynthetic and catabolic enzymes. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 2793–2813. [Google Scholar] [CrossRef]
- Mirza, A.; Wu, Q.; Wang, L.; McClanahan, T.; Bishop, W.R.; Gheyas, F.; Ding, W.; Hutchins, B.; Hockenberry, T.; Kirschmeier, P.; et al. Global transcriptional program of p53 target genes during the process of apoptosis and cell cycle progression. Oncogene 2003, 22, 3645–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oram, S.; Jiang, F.; Cai, X.; Haleem, R.; Dincer, Z.; Wang, Z. Identification and characterization of an androgen-responsive gene encoding an aci-reductone dioxygenase-like protein in the rat prostate. Endocrinology 2004, 145, 1933–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallares, J.; Llobet, D.; Santacana, M.; Eritja, N.; Velasco, A.; Cuevas, D.; Lopez, S.; Palomar-Asenjo, V.; Yeramian, A.; Dolcet, X.; et al. CK2beta is expressed in endometrial carcinoma and has a role in apoptosis resistance and cell proliferation. Am. J. Pathol. 2009, 174, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Pratt, J.; Iddir, M.; Bourgault, S.; Annabi, B. Evidence of MTCBP-1 interaction with the cytoplasmic domain of MT1-MMP: Implications in the autophagy cell index of high-grade glioblastoma. Mol. Carcinog. 2016, 55, 148–160. [Google Scholar] [CrossRef] [PubMed]

| Case | Age At Diagnosis | Histological Type | Grade | Stage | Molecular Classification | Recurrence | Follow Up | Duration Follow Up (Years) |
|---|---|---|---|---|---|---|---|---|
| 1 | 79 | EEC | II | pT3a | MSI | YES | DOD | 4 |
| 2 | 78 | EEC | I | pT1a | LCN | NO | NED | 4 |
| 3 | 56 | EEC | II | pT1a | MSI | NO | NED | 4 |
| 4 | 69 | EEC | II | pT1a | MSI | NO | NED | 4 |
| 5 | 85 | EEC | II | pT1a | LCN | NO | NED | 4 |
| 6 | 79 | EEC | II | pT1b | LCN | NO | NED | 4 |
| 7 | 76 | EEC | II | pT1a | LCN | NO | NED | 4 |
| 8 | 60 | EEC | I | pT1b | LCN | NO | NED | 4 |
| 9 | 65 | EEC | II | pT3c | LCN | YES | DOD | 4 |
| 10 | 84 | EEC | III | pT1b | LCN | NO | NED | 3 |
| 11 | 71 | EEC | III | pT1b | POLE | NO | DAD | 3 |
| 12 | 64 | EEC | I | pT1a | LCN | NO | NED | 3 |
| 13 | 85 | EEC | II | pT1b | MSI | NO | DAD | 2 |
| 14 | 86 | EEC | I | pT1b | MSI | NO | NED | 5 |
| 15 | 80 | EEC | II | pT1b | MSI | NO | DAD | 5 |
| 16 | 70 | EEC | I | pT1a | MSI | NO | NED | 6 |
| 17 | 45 | EEC | II | pT1a | POLE | NO | NED | 7 |
| 18 | 39 | EEC | II | pT3c | LCN | NO | NED | 7 |
| 19 | 86 | EEC | I | pT1a | LCN | NO | NED | 7 |
| 20 | 64 | EEC | II | pT1b | MSI | NO | NED | 7 |
| 21 | 81 | SC | III | pT3a | HCN | YES | DOD | 7 |
| 22 | 91 | SC | III | pT2 | HCN | NO | DAD | 7 |
| 23 | 76 | SC | III | pT1a | HCN | YES | DOD | 5 |
| 24 | 81 | SC | III | pT3b | HCN | YES | DOD | 4 |
| 25 | 58 | SC | III | pT3c | LCN | YES | DOD | 4 |
| 26 | 83 | SC | III | pT3a | HCN | YES | DOD | 4 |
| 27 | 74 | SC | III | pT3a | HCN | YES | DOD | 4 |
| 28 | NA | SC | III | NA | HCN | NA | NA | NA |
| 29 | NA | SC | III | NA | HCN | NA | NA | NA |
| 30 | NA | SC | III | NA | HCN | NA | NA | NA |
| 31 | 76 | SC | III | pT3b | HCN | NA | NA | NA |
| Case | Age at Diagnosis | Histological Type | Grade | Stage | Molecular Classification | Recurrence | Follow Up | Duration Follow Up (Years) |
|---|---|---|---|---|---|---|---|---|
| 1 | 80 | EEC | II | IB | MSI | NO | NED | 3 |
| 2 | 59 | EEC | I | IA | LCN | NO | NED | 4 |
| 3 | 55 | EEC | I | IA | MSI | NO | NED | 4 |
| 4 | 54 | EEC | III | IA | LCN | NO | NED | 3 |
| 5 | 62 | EEC | I | IA | LCN | NO | NED | 3 |
| 6 | 72 | EEC | I | IA | LCN | NO | NED | 3 |
| 7 | 63 | EEC | I | IA | LCN | NO | NED | 3 |
| 8 | 54 | EEC | I | IA | MSI | NO | NED | 3 |
| 9 | 64 | EEC | II | IA | LCN | NO | NED | 4 |
| 10 | 56 | EEC | I | IA | MSI | NO | NED | 3 |
| 11 | 60 | EEC | II | IA | MSI | NO | NED | 3 |
| 12 | 60 | EEC | III | IB | MSI | NO | NED | 3 |
| 13 | 69 | EEC | I | IA | MSI | NO | NED | 3 |
| 14 | 66 | EEC | II | IIIB | LCN | NO | NED | 3 |
| 15 | 59 | EEC | I | IA | MSI | SI | AWD | 3 |
| 16 | 80 | EEC | I | IB | LCN | NO | NED | 3 |
| 17 | 78 | EEC | I | IIIB | MSI | NO | NED | 3 |
| 18 | 68 | EEC | III | IB | MSI | NO | NED | 3 |
| 19 | 50 | EEC | I | IA | LCN | NO | NED | 3 |
| 20 | 80 | EEC | I | IA | MSI | NO | NED | 3 |
| 21 | 84 | EEC | I | IB | MSI | NO | NED | 3 |
| 22 | 61 | EEC | I | IA | LCN | NO | NED | 3 |
| 23 | 86 | EEC | II | IA | LCN | NO | NED | 3 |
| 24 | 86 | EEC | I | II | MSI | NO | NED | 3 |
| 25 | 57 | EEC | III | IB | MSI | NO | NED | 3 |
| 26 | 69 | EEC | II | IA | LCN | NO | NED | 3 |
| 27 | 69 | EEC | I | IA | POLE | NO | NED | 3 |
| 28 | 66 | EEC | I | IIIC | MSI | NO | NED | 2 |
| 29 | 63 | EEC | I | IA | MSI | NO | NED | 2 |
| 30 | 76 | EEC | II | IB | LCN | NO | NED | 2 |
| 31 | 62 | EEC | I | IA | MSI | NO | NED | 2 |
| 32 | 64 | EEC | III | II | P53 | NO | NED | 5 |
| 33 | 53 | EEC | III | II | NA | NO | NED | 7 |
| 34 | 70 | EEC | III | IB | POLE | NO | DAD | 7 |
| 35 | 83 | EEC | III | IB | LCN | NO | NED | 7 |
| 36 | 59 | EEC | III | II | MSI | NO | DAD | 4 |
| 37 | 79 | EEC | II | IA | NA | NO | NED | 5 |
| 38 | 88 | EEC | II | IA | MSI | NO | DOD | 5 |
| 39 | 80 | EEC | I | IB | NA | NO | DAD | 5 |
| 40 | 69 | EEC | II | II | NA | NO | NED | 7 |
| 41 | 68 | EEC | II | IB | NA | NO | NED | 7 |
| 42 | 64 | EEC | III | IB | MSI | NO | NED | 7 |
| 43 | 77 | EEC | I | IB | NA | NO | DAD | 6 |
| 44 | 67 | EEC | II | IB | NA | SI | AWD | 7 |
| 45 | 67 | EEC | I | IB | MSI | NO | NED | 7 |
| 46 | 49 | EEC | III | IV | MSI | NO | DAD | 7 |
| 47 | 82 | EEC | II | II | NA | NO | DAD | 7 |
| 48 | 82 | EEC | II | IB | NA | NO | NED | 7 |
| 49 | 77 | EEC | II | IB | NA | NO | DAD | 7 |
| 50 | 75 | EEC | II | IB | NA | NO | NED | 7 |
| 51 | 75 | EEC | II | IB | LCN | NO | NED | 7 |
| 52 | 62 | EEC | II | II | NA | NO | DAD | 5 |
| 53 | 85 | EEC | I | IB | NA | NO | NED | 8 |
| 54 | 54 | EEC | I | III | NA | NO | NED | 8 |
| 55 | 69 | EEC | I | IB | NA | NO | NED | 8 |
| 56 | 80 | EEC | II | IB | NA | NO | DAD | 5 |
| 57 | 64 | EEC | I | IA | NA | NO | NED | 8 |
| 58 | 47 | EEC | I | IA | NA | SI | DOD | 7 |
| 59 | 67 | EEC | I | IA | NA | NO | NED | 7 |
| 60 | 46 | EEC | I | IA | LCN | NO | NED | 7 |
| 61 | 82 | EEC | I | IA | NA | NO | DAD | 6 |
| 62 | 55 | EEC | II | IA | NA | NO | NED | 7 |
| 63 | 62 | EEC | II | IA | NA | NO | NED | 7 |
| 64 | 63 | EEC | II | IA | NA | NO | NED | 7 |
| 65 | 68 | EEC | II | IA | MSI | NO | DAD | 6 |
| 66 | 70 | EEC | I | IA | LCN | NO | DAD | 6 |
| 67 | 70 | EEC | I | IA | NA | NO | NED | 7 |
| 68 | 68 | SC | III | IA | P53 | NO | NED | 3 |
| 69 | 75 | SC | III | IB | P53 | NO | NED | 3 |
| 70 | 84 | SC | III | IB | LCN | NO | DAD | 3 |
| 71 | 59 | SC | III | IA | P53 | NO | NED | 3 |
| 72 | 87 | SC | III | II | P53 | NO | NED | 3 |
| 73 | 76 | SC | III | IV | P53 | SI | AWD | 3 |
| 74 | 76 | SC | III | IIIA | P53 | NA | NA | NA |
| 75 | 70 | SC | III | IV | P53 | SI | DOD | 3 |
| 76 | 73 | SC | III | IIIB | P53 | SI | DOD | 3 |
| 77 | 67 | SC | III | IA | P53 | NO | NED | 2 |
| 78 | 58 | SC | III | IV | P53 | SI | DOD | 2 |
| 79 | 68 | SC | III | IIIC | P53 | NO | NED | 2 |
| 80 | 77 | SC | III | IA | P53 | NO | NED | 2 |
| 81 | 72 | SC | III | IA | P53 | NO | NED | 5 |
| 82 | 55 | SC | III | IV | P53 | SI | DOD | 5 |
| 83 | 77 | SC | III | IV | P53 | SI | DOD | 5 |
| 84 | 75 | SC | III | IIIA | P53 | SI | DOD | 4 |
| 85 | 84 | SC | III | IIIA | P53 | SI | DOD | 4 |
| 86 | 75 | SC | III | IIIB | P53 | SI | DOD | 4 |
| 87 | 91 | SC | III | II | P53 | NO | DAD | 4 |
| 88 | 70 | SC | III | IV | P53 | SI | DOD | 4 |
| 89 | 78 | SC | III | IIIC | P53 | SI | DOD | 4 |
| 90 | 78 | SC | III | IB | LCN | SI | DOD | 4 |
| 91 | 72 | SC | III | IIIV | P53 | SI | DOD | 4 |
| 92 | 71 | SC | III | IA | P53 | SI | DOD | 4 |
| 93 | 84 | SC | III | IV | P53 | SI | DOD | 4 |
| 94 | 87 | SC | III | II | P53 | SI | DOD | 5 |
| 95 | 77 | SC | III | IIIB | P53 | SI | DOD | 5 |
| 96 | 86 | SC | III | IB | P53 | SI | DOD | 5 |
| Class | Subclass | Compound | m/z | rt | p Value | FDR | Regulation (sc vs. eec) |
|---|---|---|---|---|---|---|---|
| Benzene and substituted derivatives | Benzoic acids and derivatives | Phthalic acid a | 149.0255 | 9.5 | 0.0096685 | 0.09476 | down |
| Diheptyl phthalate a | 363.2476 | 11.5 | 0.0041352 | 0.059558 | down | ||
| Phthalic acid mono-2-ethylhexyl ester b | 279.1545 | 9.5 | 0.003451 | 0.058653 | up | ||
| Carboxylic acids and derivatives | Amino acids, peptides and analogues | Glutathione b | 308.0837 | 0.4 | 0.040854 | 0.19345 | up |
| L-Isoleucine a | 249.1468 | 0.4 | 0.045153 | 0.20275 | up | ||
| Valine a | 118.0784 | 0.4 | 0.033932 | 0.17334 | up | ||
| N-Jasmonoylisoleucine b | 324.2122 | 9.5 | 0.045153 | 0.20275 | up | ||
| Leucyl-phenylalanine b | 279.1661 | 9.5 | 0.0065027 | 0.078944 | down | ||
| Fatty acyls | Fatty acids and conjugates | 2-oxo-4-methylthiobutanoic acid a | 149.0165 | 11.9 | 0.02082 | 0.13665 | up |
| Tetracosatetraenoic acid a | 343.2939 | 7.4 | 0.0009416 | 0.030514 | down | ||
| Eicosatrienoic acid a | 307.2528 | 11.9 | 0.0087278 | 0.090507 | up | ||
| FAHFA(16:0/13-O-16:0) a | 549.4234 | 12.9 | 0.010143 | 0.09476 | down | ||
| Eicosanoids | 8-iso-15-keto-PGE2 a | 373.2053 | 7.4 | 0.010132 | 0.09476 | down | |
| Lineolic acids and derivatives | alpha-Linolenic acid a | 279.2193 | 11.4 | 0.026493 | 0.15008 | down | |
| Fatty acid esters | Linoleyl carnitine a | 424.3364 | 9.8 | 0.020487 | 0.13665 | up | |
| Glycerolipids | Monoradylglycerols | MG(18:1) b | 357.293 | 11.3 | 0.013272 | 0.11253 | down |
| MG(18:3) b | 375.2435 | 10.4 | 0.0056723 | 0.070026 | down | ||
| MG(20:5) b | 399.2496 | 11.7 | 0.006547 | 0.078944 | down | ||
| MG(22:0) b | 453.3387 | 5.9 | 0.011262 | 0.1022 | down | ||
| MG(22:6) b | 385.2849 | 10.4 | 0.0021001 | 0.042052 | down | ||
| MG(i-17:0) b | 367.2718 | 11.4 | 0.026493 | 0.15008 | down | ||
| Diradylglycerols | DG(34:4) b | 589.4824 | 13.2 | 0.00064951 | 0.021727 | down | |
| DG(36:6) b | 613.4706 | 13.1 | 0.013272 | 0.11253 | down | ||
| DG(44:11) b | 737.4961 | 10.9 | 0.033685 | 0.17334 | down | ||
| DG(37:6) b | 627.4929 | 13.2 | 0.025968 | 0.15008 | down | ||
| DG(36:5) b | 615.4996 | 13.2 | 0.017212 | 0.12309 | down | ||
| DG(40:4) b | 673.5836 | 15.4 | 0.024363 | 0.1494 | down | ||
| DG(40:8) b | 665.5056 | 13.2 | 0.015826 | 0.12123 | down | ||
| DG(i-28:0) b | 535.4314 | 12.9 | 0.0056723 | 0.070026 | down | ||
| DG(i-33:0) b | 583.5331 | 14.1 | 0.022169 | 0.13943 | down | ||
| Glycerophospholipids | Glycerophosphates | LysoPA(18:1) b | 419.2675 | 10.5 | 0.0000217 | 0.0037419 | down |
| LysoPA(P-16:0) b | 377.2554 | 10.9 | 0.044511 | 0.20275 | down | ||
| LysoPA(20:3) b | 499.2271 | 6.9 | 0.013508 | 0.11253 | down | ||
| LysoPA(22:1) b | 475.32 | 8.2 | 0.0041352 | 0.059558 | down | ||
| LysoPA(20:2) b | 463.2957 | 10.6 | 0.00027798 | 0.012533 | down | ||
| PA(P-34:2) b | 657.503 | 13 | 0.02976 | 0.16074 | down | ||
| PA(36:6) b | 693.4684 | 10.8 | 0.0056723 | 0.070026 | down | ||
| PA(28:1) b | 573.4021 | 12.6 | 0.025604 | 0.15008 | down | ||
| PA(29:1) b | 605.4157 | 10.7 | 0.0021604 | 0.042052 | down | ||
| PA(30:2) b | 617.4202 | 10.8 | 0.00015155 | 0.0098224 | down | ||
| PA(40:7) b | 747.4967 | 12.5 | 0.021704 | 0.13943 | up | ||
| PA(36:7) b | 691.4537 | 10.5 | 0.0037003 | 0.059558 | down | ||
| PA(38:5) b | 705.4727 | 10.9 | 0.0076209 | 0.084073 | down | ||
| PA(44:9) b | 781.5217 | 10.9 | 0.019748 | 0.13665 | down | ||
| PA(48:0) b | 855.7351 | 14.9 | 0.0000866 | 0.0081641 | down | ||
| PA(46:7) b | 831.5771 | 12.2 | 0.0076209 | 0.084073 | down | ||
| PA(48:1) b | 853.7199 | 14.6 | 0.0014506 | 0.035815 | down | ||
| PA(48:2) b | 891.6926 | 14.3 | 0.0098617 | 0.09476 | down | ||
| PA(i-27:0) b | 561.3887 | 10.7 | 0.00097426 | 0.030615 | down | ||
| Glycerophosphoglycerols | LysoPG(18:1) b | 493.3069 | 10.2 | 0.0000217 | 0.0037419 | down | |
| PG(40:5) b | 825.5453 | 10.9 | 0.0029877 | 0.052513 | down | ||
| PG(40:8) b | 801.4891 | 10.6 | 0.0000217 | 0.0037419 | down | ||
| PGP(40:4) b | 889.5384 | 10.7 | 0.0028316 | 0.052435 | down | ||
| PGP(i-30:0) b | 757.4626 | 10.6 | 0.0000433 | 0.0064146 | down | ||
| PGP(i-37:0) b | 873.5459 | 11.2 | 0.033685 | 0.17334 | down | ||
| PG(38:7) b | 793.5243 | 11 | 0.022018 | 0.13943 | down | ||
| PG(i-30:2) b | 713.4383 | 10.5 | 0.0000217 | 0.0037419 | down | ||
| PG(i-28:0) b | 649.4432 | 10.8 | 0.0005173 | 0.021458 | down | ||
| PG(42:9) b | 845.5149 | 10.7 | 0.0012528 | 0.03453 | down | ||
| Glycerophosphoinositols | LysoPI(18:2) b | 579.2943 | 9.5 | 0.003452 | 0.058653 | down | |
| Glycerophosphocholines | LysoPC(20:1) b | 532.3782 | 10.3 | 0.00064951 | 0.021727 | down | |
| LysoPC(22:2) a | 576.4025 | 10.4 | 0.0021001 | 0.042052 | down | ||
| LysoPC(P-18:1) a | 488.3517 | 10.2 | 0.022018 | 0.13943 | down | ||
| LysoPC(28:1) b | 644.487 | 10.8 | 0.045361 | 0.20275 | down | ||
| PC(34:5) b | 752.5083 | 10.6 | 0.0041352 | 0.059558 | down | ||
| PC(38:6) a | 806.5721 | 13.5 | 0.035678 | 0.17618 | down | ||
| Glycerophosphoethanolamines | LysoPE(24:1) b | 546.392 | 10.7 | 0.010132 | 0.09476 | down | |
| LysoPE(O-16:3) a | 434.252 | 10.7 | 0.017016 | 0.12309 | down | ||
| PE(O-35:6) a | 708.481 | 10.5 | 0.0021001 | 0.042052 | down | ||
| PE(38:1) a | 774.6007 | 13.7 | 0.0035067 | 0.058653 | down | ||
| PE(36:1) a | 746.5744 | 13.5 | 0.0044838 | 0.062834 | down | ||
| PE(42:9) b | 796.534 | 10.6 | 0.01991 | 0.13665 | down | ||
| Glycerophosphoserines | PS(38:3) b | 814.5753 | 9.5 | 0.0028304 | 0.052435 | down | |
| PS(40:1) b | 884.5858 | 10.7 | 0.049943 | 0.22133 | down | ||
| Glycerophosphoinositol phosphates | PIP2(36:1) b | 1007.5233 | 10.6 | 0.02082 | 0.13665 | up | |
| CDP-glycerols | CDP-DG(38:4) b | 1068.5164 | 13.3 | 0.045361 | 0.20275 | up | |
| Organooxygen compounds | Carbonyl compounds | Kynurenine a | 209.0856 | 0.8 | 0.027929 | 0.15324 | up |
| Prenol lipids | Diterpenoids | Pristanic acid b | 299.2912 | 12.4 | 0.0041352 | 0.059558 | down |
| 3-hydroxypristanic acid a | 297.2762 | 12.1 | 0.00015155 | 0.0098224 | down | ||
| Purine nucleotides | Purine deoxyribonucleotides/purine ribonucleotides | 2’-Deoxyguanosine 5’-monophosphate/adenosine monophosphate a | 348.0631 | 0.6 | 0.0021898 | 0.042052 | up |
| Sphingolipids | Phosphosphingolipids | SM(d41:1) a | 801.6941 | 14.5 | 0.0041352 | 0.059558 | down |
| SM(d42:1) a | 797.6668 | 14.1 | 0.034987 | 0.17359 | down | ||
| SM(d43:2) a | 827.7039 | 14.6 | 0.0000217 | 0.0037419 | down | ||
| SM(d40:1) a | 787.6773 | 14.4 | 0.0056723 | 0.070026 | down | ||
| SM(d42:0) a | 839.7151 | 14.5 | 0.0002598 | 0.012246 | down | ||
| SM(d44:2) a | 841.7301 | 14.7 | 0.0002598 | 0.012246 | down | ||
| SM(d41:2) a | 799.6723 | 14.3 | 0.0002598 | 0.012246 | down | ||
| SM(d40:2) a | 785.6655 | 14.2 | 0.0076209 | 0.084073 | down | ||
| SM(d42:2) a | 813.6951 | 14.4 | 0.0056723 | 0.070026 | down | ||
| SM(d43:1) a | 829.7289 | 14.8 | 0.017212 | 0.12309 | down | ||
| Steroids and steroid derivatives | Bile acids, alcohols and derivatives | Ketodeoxycholic acid a | 391.2796 | 11.9 | 0.026276 | 0.15008 | up |
| Chenodeoxycholic acid 3-sulfate b | 473.2704 | 11.7 | 0.0076209 | 0.084073 | down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatius, S.; Jove, M.; Megino-Luque, C.; Albertí-Valls, M.; Yeramian, A.; Bonifaci, N.; Piñol, M.; Santacana, M.; Pradas, I.; Llobet-Navas, D.; et al. Metabolomic Analysis Points to Bioactive Lipid Species and Acireductone Dioxygenase 1 (ADI1) as Potential Therapeutic Targets in Poor Prognosis Endometrial Cancer. Cancers 2022, 14, 2842. https://doi.org/10.3390/cancers14122842
Gatius S, Jove M, Megino-Luque C, Albertí-Valls M, Yeramian A, Bonifaci N, Piñol M, Santacana M, Pradas I, Llobet-Navas D, et al. Metabolomic Analysis Points to Bioactive Lipid Species and Acireductone Dioxygenase 1 (ADI1) as Potential Therapeutic Targets in Poor Prognosis Endometrial Cancer. Cancers. 2022; 14(12):2842. https://doi.org/10.3390/cancers14122842
Chicago/Turabian StyleGatius, Sònia, Mariona Jove, Cristina Megino-Luque, Manel Albertí-Valls, Andree Yeramian, Nuria Bonifaci, Miquel Piñol, Maria Santacana, Irene Pradas, David Llobet-Navas, and et al. 2022. "Metabolomic Analysis Points to Bioactive Lipid Species and Acireductone Dioxygenase 1 (ADI1) as Potential Therapeutic Targets in Poor Prognosis Endometrial Cancer" Cancers 14, no. 12: 2842. https://doi.org/10.3390/cancers14122842